Abstract
ICP27 is an essential herpes simplex virus type 1 (HSV-1) immediate-early protein that stimulates viral mRNA expression from many viral delayed-early and late genes during infection. One HSV-1 late gene which is highly dependent on ICP27 during infection is that encoding the glycoprotein C (gC). Here we report that the gC gene is specifically transactivated by ICP27 in transfected Vero cells. Using various gC plasmid constructs, we show that ICP27's stimulatory effects are independent of the gC gene's endogenous promoter and polyadenylation site. This suggests that ICP27-responsive elements lie in the transcribed body of the gC gene. We also show that transactivation of the gC gene by ICP27 is independent of other viral proteins, as ICP27 alone can transactivate the gC gene when its transcription is mediated by the human cytomegalovirus immediate-early gene promoter. However, when gC gene expression is driven by its endogenous promoter, the stimulatory effect of ICP27 requires additional transactivators. To explore the level at which ICP27 transactivates the gC gene, we established stably transfected Vero cell lines that have integrated copies of the gC gene under control of the cytomegalovirus immediate-early gene promoter. These gC genes are not constitutively expressed but can be efficiently induced by HSV-1 infection. Using nuclear run-on transcription assays, we show that transcriptional induction of the stably transfected genes is ICP27 independent. In contrast, accumulation of gC mRNA is very highly dependent on ICP27. Together, these results demonstrate that ICP27 posttranscriptionally activates mRNA expression from a biologically relevant viral target gene.
Herpesviruses encompass a large group of medically important, nuclear-replicating double-stranded DNA viruses. Herpes simplex virus type 1 (HSV-1) is the most extensively studied of theherpesviruses and serves as a prototype for characterizing their fundamental replication mechanisms. In particular, much is understood about how HSV-1 regulates the expression of its genes during productive infection (reviewed in references 52 and 72). During such infections, HSV-1 efficiently commandeers the host RNA polymerase II machinery and other host components to express its own genes at high levels, while simultaneously suppressing host gene expression. Moreover, the virus is able to coordinate the expression of its approximately 80 genes in a temporally regulated cascade, consisting of the sequential expression of three viral gene sets: the immediate-early (IE), delayed-early (DE), and late (L) genes. The five IE genes are expressed immediately upon infection. Their transcription does not require any newly synthesized viral proteins but is enhanced by a virion protein,VP16, working in concert with cellular transcription factors. The IE genes encode four gene regulatory factors: ICP4, ICP0, ICP22, and ICP27. These proteins induce the expression of the DE genes, the majority of which encode proteins that are involved in viral DNA replication. Expression of L genes, which predominantly encode structural proteins, comprises the final wave of viral gene expression. Viral L gene expression requires the previous expression of IE genes and is further stimulated by the process of viral DNA synthesis. L genes have been subdivided into two categories based on their DNA replication dependence: true-L genes are strictly dependent on viral DNA replication for their expression, whereas leaky-L genes are only partially dependent.
The IE protein ICP27 is essential for viral replication (35, 53) and has been implicated in a variety of regulatory functions. First, it activates, to various extents, the expression of several DE and L genes (35, 37, 49, 53, 68). Second, ICP27 down-regulates the expression of certain IE and DE genes as infection proceeds (35, 39, 49, 53). Third, ICP27 contributes to the shutoff of host gene expression (21, 53). Finally, in certain human cells, ICP27 prevents apoptotic cell death which is otherwise induced by early events in viral infection (1).
ICP27 is a multifunctional protein and can modulate gene expression through a variety of mechanisms. Many of ICP27's effects appear to be mediated posttranscriptionally. In various experimental settings, it has been demonstrated to (i) enhance expression of genes which contain weak poly(A) signals by stimulating 3′-end processing (37, 38, 56); (ii) stabilize beta interferon mRNA expressed from a transfected gene (2, 43); (iii) inhibit pre-mRNA splicing (22, 32); and (iv) enhance mRNA transport out of the nucleus (5, 6, 31, 55, 63). As might be expected for a posttranscriptional regulatory protein, ICP27 binds directly to RNA (2, 25, 41, 55). In addition to its posttranscriptional functions, there is also evidence that ICP27 can regulate transcription (26, 65). Two recent reports demonstrate that ICP27 associates with RNA polymerase II complexes in infected cells (27, 73).
The present study was designed to characterize the molecular mechanism(s) by which ICP27 activates responsive viral DE and L genes during productive infection. At least three different mechanisms have been proposed. First, it has been hypothesized that ICP27 activates some L genes by stimulating the usage of their poly(A) sites, which appear to be inherently weak (37). Consistent with this, ICP27 can increase the efficiency with which a weak poly(A) site is utilized during infection (38) and can modestly stimulate expression of chloramphenicol acetyltransferase (CAT) reporter genes bearing weak or noncanonical poly(A) sequences (4, 56). Second, evidence has been presented that ICP27 increases the transcription rates of at least two viral L genes (26). Third, several studies suggest that ICP27 enhances viral gene expression by mediating the transport of DE and L mRNAs out of the nucleus (5, 6, 31, 55, 63). Since mRNA export is normally linked to pre-mRNA splicing (reviewed in reference 47), the intronless mRNAs of most HSV-1 DE and L genes may require a special mechanism for their transport.
To investigate the mechanism of gene induction by ICP27, we have focused on a model viral target gene: the true-L gene encoding glycoprotein C (gC). This gene is highly dependent upon ICP27 during viral infection, at both the protein and mRNA levels (37, 49, 53, 61). In this study, we show that the gC gene is also strongly transactivated by ICP27 in transfected cells. We further show that this effect can occur independently of other viral proteins, and does not require the gC gene's endogenous promoter or poly(A) site. In addition, using a nuclear run-on transcription assay, we demonstrate that transactivation of the transfected gC gene occurs via a posttranscriptional mechanism.
MATERIALS AND METHODS
Cells, viruses, and infections.
Vero cells (African green monkey kidney cells) were obtained from the American Type Culture Collection. V27 cells are derivatives of Vero cells that have been stably transfected with the ICP27 gene (49). The generation of Vero cell lines stably transfected with the HSV-1 gC gene (VgC cells) is described below. Vero cells were grown in Dulbecco modified Eagle medium supplemented to contain 5% heat-inactivated fetal bovine serum (FBS), penicillin (50 U/ml), and streptomycin (50 mg/ml). The medium for V27 and VgC cells was the same as for Vero cells except that it also contained G418 (300 μg/ml). All tissue culture reagents were purchased from Life Technologies/Invitrogen (Carlsbad, Calif.).
Strain KOS1.1 (24) was the HSV-1 strain used in these studies. The ICP27 deletion mutant d27-1 has been previously described (49). The derivation of the HSV-1 gC-negative mutants d44 and d44/27 is described below. All infections were carried out at a multiplicity of infection of 10 PFU per cell in phosphate-buffered saline containing 0.1% glucose and 1% heat-inactivated FBS. Virus was allowed to adsorb to the cells for 1 h at 37°C, at which time the viral inoculum was replaced with 199 medium containing 2% heat-inactivated FBS, penicillin (50 U/ml), and streptomycin (50 mg/ml). Infected cells were then incubated at 37°C.
To construct the gC-negative viruses d44 and d44/27, a marker transfer protocol was used (49, 50). Briefly, infectious viral DNA was isolated from wild-type (WT) strain KOS1.1 or the ICP27 deletion mutant d27-1. The viral DNAs were then separately cotransfected into V27 cells along with DNA from plasmid pgClacZ (described below) that had been digested with PstI and HindIII to release the modified viral insert. The transfected cultures were harvested after 6 days, and small viral stocks were made. Beta-galactosidase-encoding recombinants in the stocks were identified by plaque assay of the stocks on V27 cells in the presence of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (300 μg/ml). The resulting isolates were plaque purified three times in V27 cells. The KOS1.1-derived recombinant was designated d44, whereas the d27-1-derived recombinant was designated d44/27. The structure of the engineered viral genomes was verified by Southern blotting. In addition, immunoblotting experiments with antibodies specific for gC, ICP27, and β-galactosidase confirmed that both mutants expressed the predicted proteins in Vero and V27 cells.
Plasmids.
Plasmid pgC contains a 3.0-kb PstI-HindIII restriction fragment from HSV-1 strain KOS1.1 DNA cloned into the PstI and HindIII sites of pUC19. For expression of ICP27 in transfected cells, the plasmid pC27 (40) or pBH27 (48) was used. pC27 contains the an ICP27 gene under control of an enhancerless cytomegalovirus (CMV) IE gene promoter, whereas pBH27 contains the intact ICP27 gene with its endogenous promoter. For coexpression of ICP4 and ICP0, the plasmid pSG1 (14) was used. Plasmids pK1-2 (8) and pSHZ (44) were used for individual expression of ICP4 and ICP0, respectively. Plasmid pSG18 (14) was used for experiments involving the intact ICP8 gene. Plasmid pUHD15-1 (15) was used to express the tetracycline transactivator (tet-TA). The plasmid pCMVβ-c expresses an intronless β-galactosidase gene from the CMV IE promoter. It was constructed from pCMVβ (Clontech) by deleting a 192-bp XhoI-SmaI fragment which corresponds to a simian virus 40 (SV40) intron sequence.
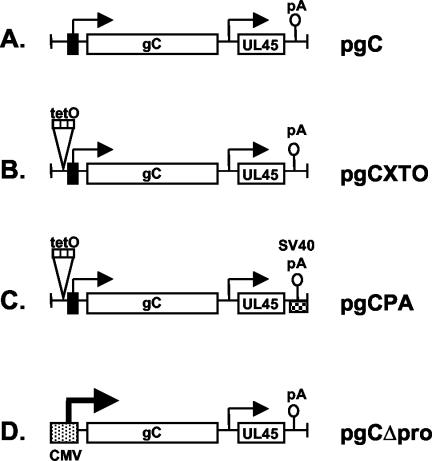
Several derivatives of pgC were constructed. Plasmid pgCXTO contains three copies of the tetracycline repressor operator sequence (TetO) inserted upstream of the gC gene TATA box. To construct pgCXTO, multiple steps were carried out. First, an XcmI site 15 bp upstream of the gC gene's TATA box was converted into an XhoI site. This was accomplished by digesting pgC with XcmI, converting the overhanging 3′ ends to blunt ends using T4 DNA polymerase, ligating on XhoI linkers, digesting with XhoI, and religating. The resulting plasmid was designated pgCX. Next, a 307-bp fragment containing a trimeric TetO sequence was obtained from plasmid pUHC13-3 (15) by digestion with SmaI and XhoI. An XhoI linker was added to the blunt-ended SmaI end. After XhoI digestion, the resulting TetO fragment was inserted into the XhoI site of pgCX. The resulting plasmid was named pgCXTO. Plasmid pgCPA is a derivative of pgC in which the SV40 late poly(A) site replaces the endogenous poly(A) site. Several steps were carried out to construct pgCPA. First, oligonucleotide-directed mutagenesis was used to engineer an AgeI site approximately 40 bp upstream of the gC gene poly(A) site (at nucleotide 98623 according to the numbering system of McGeoch et al. [36]). This AgeI site is found at the analogous position of HSV-1 strain 17 (36) but is not present in strain KOS1.1 (K. Perkins and S. Rice, unpublished data). The resulting plasmid was designated pgCAge. pgCAge was digested with AgeI and HindIII, and the large AgeI-HindIII fragment was gel purified. The ends of this fragment were made blunt using the Klenow fragment, BglII linkers were ligated on, and the DNA was digested with BglII. This DNA was ligated to an ∼250-bp DNA fragment corresponding to the SV40 late poly(A) signal, obtained from plasmid pRL-CMV (Promega Corp.). The latter DNA was prepared by digestion of pRL-CMV with XbaI, treatment with the Klenow fragment to create blunt ends, ligation of BamHI linkers, digestion with BamHI, and gel purification. The resulting plasmid was designated pgCPA. Plasmid pgCΔpro is a derivative of pgC in which the endogenous gC gene promoter is replaced by the human CMV IE gene enhancer-promoter. This plasmid was constructed by the following steps. Oligonucleotide-directed mutagenesis was used to engineer an XhoI restriction site 20 bp downstream of the gC transcription start site (23), creating plasmid pgCΔss. Next, a plasmid in which the gC promoter was deleted was engineered by digesting pgCΔss with PstI and XhoI, converting the sticky DNA ends to blunt ends using T4 DNA polymerase, ligating on BglII linkers, digesting with BglII, and religating. This plasmid was termed pgCdpro. The CMV enhancer-promoter, derived from plasmid pRL-CMV, was then inserted into the BglII site of pgCdpro. To accomplish this, a 753-bp HindIII-BglII fragment from pRL-CMV was converted into a BamHI fragment using BamHI linkers and ligated to pgCdpro that had been digested with BglII. Transformants were screened to find an isolate which had the CMV promoter inserted in the correct orientation to drive gC expression. This plasmid was designated pgCΔpro. The plasmid pgClacZ is a derivative of pgC which was engineered for the purpose of constructing gC-negative viruses. It was constructed in a series of steps. First, pgC was modified by converting its EcoRV restriction site, which is in the 3′ end of the gC gene, into a BglII site. To do this, pgC was linearized with EcoRV, BglII linkers were ligated on, and the DNA was digested with BglII and religated. The resulting plasmid was designated pgC-Bgl. Second, a mutation was introduced into the 5′ end of the gC open reading frame in pgC-Bgl by oligonucleotide-directed mutagenesis. The alteration changed the first three codons of gC from 5′CCGGGGCGG to 5′CCAGATCTG, creating a BglII site. This plasmid was designated pgC-BX2. Next, the gC open reading frame was replaced with the Escherichia coli lacZ gene. To do this, pgC-BX2 was digested with BglII, and the resulting large fragment was ligated to the 3.1-kb BamHI fragment from plasmid pMC1871 (59), which contains the lacZ gene. A plasmid in which the lacZ insert was in the same orientation as the deleted gC gene was isolated and designated pgClacZ.
Transfections.
Cells were transiently transfected using the calcium phosphate precipitation procedure as previously described (16, 48). For immunoblot analysis of transfected cells, 25-cm2 culture flasks of Vero or VgC cells were transfected with 4 μg of each plasmid, and salmon sperm DNA was added to bring the total amount of DNA in each transfection to 16 μg. Total protein extracts were prepared at 2 days posttransfection. For Northern analysis of transfected cells, 10-cm2 cell culture dishes of Vero cells were transfected with 12 μg of each plasmid, and salmon sperm DNA was added to bring the total amount of DNA to 48 μg. Total RNA was prepared at 2 days posttransfection using the Trizol reagent (Invitrogen) and the protocol supplied by the manufacturer. The RNA was treated with RNase-free DNase (Roche Diagnostics Corp.) to remove contaminating DNA. The RNA was then extracted with phenol-chloroform-isoamyl alcohol and precipitated with ethanol.
Vero cells were stably transfected with pgCΔpro as follows. Slightly subconfluent monolayers of Vero cells in 25-cm2 flasks were cotransfected with 2.5 μg of pgcΔpro and 0.5 μg of pSV2neo (64) as described above. Two days posttransfection, the cells were trypsinized and divided into three 10-cm2 cell culture dishes in Dulbecco modified Eagle medium containing G418 (400 μg/ml). Four weeks after transfection, the G418 concentration was raised to 600 μg/ml. Sixteen G418-resistant clones from two dishes were isolated and grown into mass culture. The ∼200 colonies from the remaining dish were trypsinized and pooled in a culture that was designated VgCpool. The 16 cell lines were tested for the ability to produce gC protein when infected with the gC-negative virus d44. Four of the 16 cell lines (VgC2, VgC3, VgC14, and VgC16) tested positively in this assay and were saved for further study.
Immunoblotting.
Protein samples for immunoblotting were prepared from transfected or infected cells by scraping the cells in ice-cold phosphate-buffered saline containing protease inhibitors (50 μg of Nα-p-tosyl-l-lysine chloromethyl ketone per ml and 25 μg of phenylmethylsulfonyl fluoride per ml). The cells were pelleted by low-speed centrifugation and then lysed in sodium dodecyl sulfate (SDS)-polyacrylamide gel sample buffer. Equal cell equivalents of protein samples were separated by SDS-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose filters (Bio-Rad). The filters were washed once with TBST (10 mM Tris [pH 8.0], 150 mM NaCl, 0.05% Tween 20) and blocked overnight at 4°C with 10% nonfat dry milk. The filters were washed three times with TBST for 15 min each wash and treated at room temperature with primary antibodies. To monitor gC expression, a 1:300 dilution of gC-specific monoclonal antibody H1104 was used. The ICP8-specific monoclonal antibody H1115 was used at a dilution of 1:300. The above antibodies were purchased from the Rumbaugh-Goodwin Institute for Cancer Research (Plantation, Fla.). Beta-galactosidase production was monitored by immunoblotting with a 1:10,000 dilution of a specific monoclonal antibody (Promega). After 1 h of antibody exposure each blot was washed three times with TBST. The blots were then treated with a 1:7,500 dilution of peroxidase-labeled anti-mouse immunoglobulin G secondary antibody (Amersham Pharmacia) and then washed three more times with TBST. Immunoreactive proteins were detected by enhanced chemiluminescence using a commercially available kit (Amersham Pharmacia).
Northern blots.
Five micrograms of each total RNA sample was subjected to electrophoresis through a denaturing formaldehyde-agarose gel. The RNA was transferred to GeneScreen Plus (DuPont) filters and UV-cross-linked in a Stratalinker (Stratagene). The filters were prehybridized with 10 ml of prewarmed (60°C) ULTRAhyb hybridization solution (Ambion) for at least half an hour. Hybridization of 32P-labeled DNA probes was performed overnight at 42°C in ULTRAhyb solution. The filters were then washed according to the ULTRAhyb manufacturer's instructions and exposed to X-ray film. An 813-bp EcoRV-EcoNI fragment from pgC, corresponding to a section of the gC coding region, was used as the hybridization probe for some blots (see Fig. 3, 4, 5, and 7). For another blot (see Fig. 2), the probe corresponded to the entire 3.0-kb HSV-1 insert of pgC. Radioactive labeling of the probes with 32P was done using a random-primer labeling kit (Invitrogen).
FIG. 3.
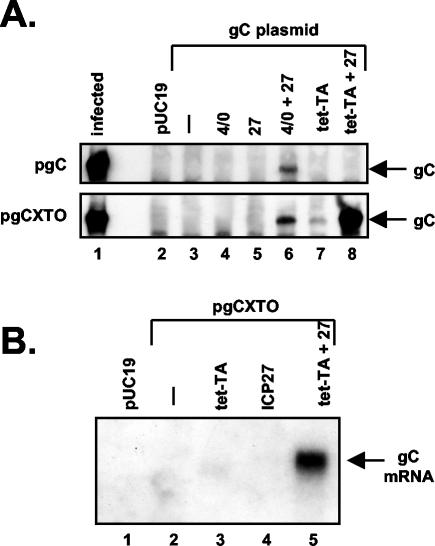
ICP27 and the tet-TA can cooperate to induce gC gene expression. (A) Induction of gC protein expression. Vero cells were transfected with pUC19 only (lane 2) or with a gC-encoding plasmid (lanes 3 to 8), either pgC or pgCXTO (top and bottom panels, respectively). Transfections also included pSG1, which encodes ICP4 and ICP0 (lane 4); pBH27, which encodes ICP27 (lane 5); pSG1 plus pBH27 (lane 6); pUHD15-1, which encodes tet-TA (lane 7); or pUHD15-1 plus pBH27 (lane 8). Protein analysis was carried out as described for Fig. 2. Lane 1 contains protein extract from HSV-1-infected cells. (B) Induction of gC mRNA expression. Vero cells were transfected with the plasmids indicated, as in panel A. RNA was prepared at 2 days posttransfection, and equal amounts were subjected to Northern analysis using a gC gene-specific probe.
FIG. 4.
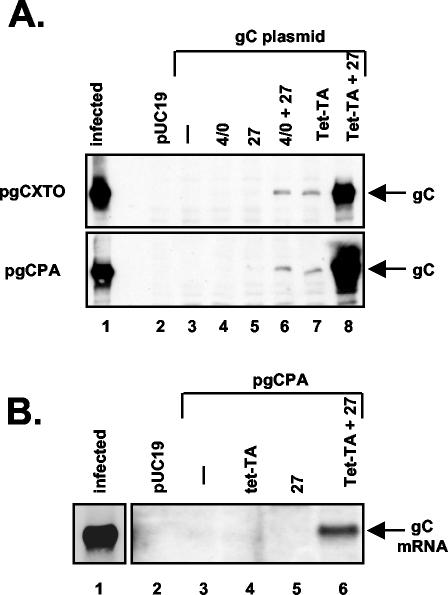
The endogenous poly(A) site is not required for transactivation of the gC gene by ICP27. (A) Induction of gC protein expression. Vero cells were transfected with pUC19 only (lane 2) or with pgCXTO or pgCpA (lanes 3 to 8, top and bottom panels, respectively). Transfections also included pSG1, which encodes ICP4 and ICP0 (lane 4); pBH27, which encodes ICP27 (lane 5); pSG1 plus pBH27 (lane 6); pUHD15-1, which encodes tet-TA (lane 7); or pUHD15-1 plus pBH27 (lane 8). Expression of gC was assessed by immunoblotting as described for Fig. 2. Lane 1 contains protein extract from HSV-1-infected cells. (B) Induction of gC mRNA expression. Vero cells were transfected with pUC19 only (lane 2) or with pgCPA (lanes 3 to 6). Transactivator plasmids were added as indicated, as in panel A. RNA was prepared at 2 days posttransfection, and equal amounts were subjected to Northern analysis using a gC gene-specific probe. Lane 1 contains RNA from HSV-1-infected Vero cells. A shorter autoradiographic exposure of this lane is shown.
FIG. 5.
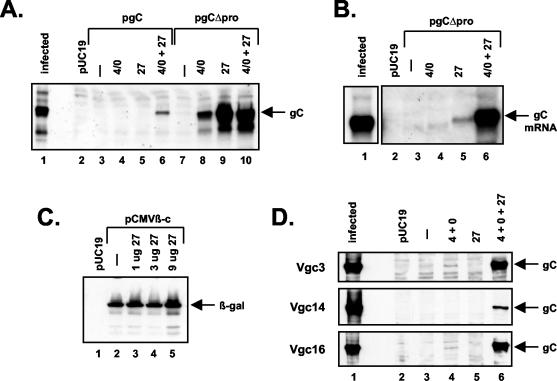
ICP27 transactivates the gC gene when its transcription is driven by the CMV IE promoter. (A) Induction of protein expression from the transiently transfected gCΔpro gene. Vero cells were transfected with pUC19 only (lane 2) or with pgC (lanes 3 to 6) or pgCΔpro (lanes 7 to 10). Transfections also included pSG1, which encodes ICP4 and ICP0 (lanes 4 and 8); pC27, which encodes ICP27 (lanes 5 and 9); or pSG1 plus pC27 (lanes 6 and 10). Protein analysis was carried out as done for Fig. 2. (B) Induction of mRNA from the transiently transfected gCΔpro gene. Vero cells were transfected with the plasmids indicated, as in panel A. RNA was prepared at 2 days posttransfection, and equal amounts were subjected to Northern analysis using a gC gene-specific probe. Lane 1 contains RNA from HSV-1-infected Vero cells. A shorter autoradiographic exposure of this lane is shown. (C) Expression of an intronless CMV-β-galactosidase gene is unaffected by ICP27. Vero cells were transfected with pUC19 only (lane 1) or with pCMVβ-c (lanes 2 to 5). Increasing amounts of pC27 were added to the indicated transfections (lanes 3 to 5). Protein analysis was carried out as in Fig. 2, except that detection was done using a monoclonal antibody specific for β-galactosidase. (D) Induction of the stably transfected gCΔpro gene. VgC3, VgC14 and VgC16 cells were transfected with pUC19 only (lane 2) or with 4 μg of each of the following plasmids: pK1-2 and pSHX, which encode ICP4 and ICP0, respectively (lane 4); pC27, which encodes ICP27 (lane 5); and pC27, pK1-2, and pSHZ (lane 6). No plasmid was added to the transfection shown in lane 3. Protein analysis was carried out as done for Fig. 2. Lane 1 contains protein extract from HSV-1-infected Vero cells.
FIG. 7.
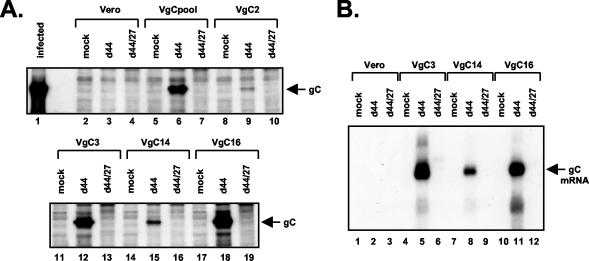
HSV-1 infection induces the stably transfected gC gene in an ICP27-dependent manner. (A) gC protein expression in infected VgC cells. Vero, VgC cell lines, or a pool of gCΔpro transfectants (VgCpool) were mock infected or infected with d44 or d44/27, as indicated. PAA (400 μg/ml) was added to all cultures at 1 hpi to inhibit viral DNA replication. Total cell proteins, harvested at 6 hpi, were subjected to immunoblot analysis using a gC-specific monoclonal antibody. As a control, a protein extract from WT HSV-1-infected Vero cells was also analyzed (lane 1). (B) gC mRNA accumulation in stably transfected cell lines. Vero and VgC cell lines were mock infected or infected with d44- or d44/27, as indicated. Total RNA was isolated at 6 hpi, and equal amounts were subjected to Northern analysis using a gC gene-specific probe.
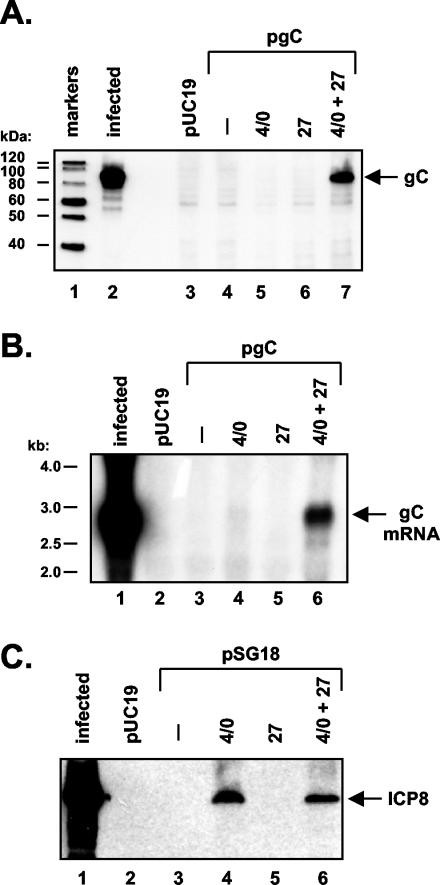
FIG. 2.
ICP27 transactivates the gC gene, in combination with ICP4 and ICP0. (A). Induction of gC protein expression. Vero cells were transfected with pUC19 only (lane 3) or with pgC (lanes 4 to 7). Transfections also included pSG1, which encodes ICP4 and ICP0 (lane 5); pC27, which encodes ICP27 (lane 6); or pSG1 plus pC27 (lane 7). After 2 days, cell lysates were prepared and equal amounts were subjected to immunoblotting using a monoclonal antibody specific for gC. Lane 1 shows protein size standards; lane 2 contains protein extract from HSV-1-infected Vero cells, harvested at 6 hpi. (B) Induction of gC mRNA expression. Vero cells were transfected with the plasmids indicated, as in panel A. RNA was prepared at 2 days posttransfection, and equal amounts were subjected to Northern analysis using a gC gene-specific probe. Lane 1 contains RNA from HSV-1-infected Vero cells. (C) ICP8 gene expression is not affected by ICP27. Vero cells were transfected as in panel A, except that plasmid pSG18, which encodes ICP8, replaced pgC. Protein analysis was carried out as in panel A, except the blot was probed with an ICP8-specific monoclonal antibody.
Nuclear run-on transcription assays.
Nuclear run-on transcription assays were performed as previously described (65, 66). Briefly, Vero cells or VgC16 cells were either mock infected or infected with d44 or d44/27. Infections were performed in the presence of phosphonoacetic acid (PAA) (400 μg/ml) to inhibit viral DNA replication. Nuclei were harvested at 4 h postinfection (hpi) and frozen at −80°C. Equal numbers of thawed nuclei were allowed to undergo run-on transcription in the presence of [32P]UTP. Radiolabeled transcripts were purified and hybridized to single-stranded bacteriophage M13 probes corresponding to the viral gC and ICP8 genes (13) or the cellular gamma actin gene (65). A probe consisting of the M13 vector mp19 served as an additional negative control and showed no significant hybridization (data not shown).
RESULTS
ICP27-dependent expression of the HSV-1 gC gene in transfected cells.
ICP27 stimulates the expression of some but not all viral DE and L genes during productive infection. To begin to investigate the nature of this gene specificity, we asked whether a viral gene known to be responsive to ICP27 during infection is also responsive in the context of transfected cells. We chose to analyze the HSV-1 true-L gene encoding gC, which is highly dependent on ICP27 during viral infection (37, 49, 53, 61). A 3.0-kb restriction fragment containing the intact gC gene from HSV-1 strain KOS1.1 was cloned in the plasmid vector pUC19, giving rise to plasmid pgC. The viral insert in pgC (Fig. 1A) contains all known functional elements of the gC gene, including its promoter (23) and poly(A) site (36, 37). pgC also contains the downstream UL45 gene, a true-L gene which has an independent promoter but shares a poly(A) site with the gC gene (36, 69).
FIG. 1.
Cloned gC genes used in this study. (A) Plasmid pgC. The 3.0-kb viral DNA insert of pgC, which contains the intact gC gene, is shown. Arrows represent transcriptional start sites, open blocks represent coding regions, the black bar represents the gC promoter, and the lollipop represents the gC/UL45 poly(A) site. (B) Plasmid pgCXTO. The position at which the trimeric tetracycline repressor operator sequence (tetO) is inserted is shown. (C) Plasmid pgCPA. The checked bar with lollipop represents the sequence containing the SV40 late poly(A) signal. (D) Plasmid pgCΔpro. The stippled bar represents the CMV IE promoter-enhancer, and the bold arrow represents the transcriptional start site from this promoter.
In initial experiments, pgC was transfected into Vero cells alone, or together with a plasmid encoding ICP27 and/or a plasmid encoding both ICP4 and ICP0. ICP4 and ICP0 are known to activate many HSV-1 genes in transfection assays and have been shown to stimulate transcription (13, 18, 29). Total protein extracts were prepared after 2 days, and gC protein levels were measured by immunoblotting using a gC-specific monoclonal antibody. The results of a typical experiment are shown in Fig. 2A. In the absence of any cotransfected IE genes, no expression of gC could be detected (lane 4). Furthermore, expression was not induced by cotransfection of either the ICP4/0 or ICP27 plasmids (lanes 5 and 6, respectively). However, when the ICP4/0 and ICP27-encoding plasmids were combined, gC expression was readily apparent (lane 7). The gC protein comigrated with gC expressed from infected Vero cells (lane 2) and appeared to correspond predominantly to the ∼86-kDa pgC form of the protein (3). In additional experiments, we determined that an ICP4- but not ICP0-encoding plasmid could partially substitute for the ICP4/0 plasmid to induce gC expression in the presence of ICP27 (data not shown).
To see if the increase in gC protein expression was due to an increase in encoding mRNA, we repeated the transfection experiment but assayed mRNA levels by Northern blotting (Fig. 2B). The results were very similar to those of the protein analysis, i.e., both ICP27- and ICP4/0-encoding plasmids were required for detectable expression of gC at the mRNA level (lane 6). The mRNA was approximately 2.7 kb, as expected (12), and comigrated with gC mRNA isolated from HSV-1-infected cells (lane 1). Together, these experiments demonstrate that the transfected gC gene is responsive to ICP27 at the level of both protein and mRNA expression. However, the stimulatory effect also requires expression of other HSV-1 transactivators.
It has been proposed that ICP27 is a viral export factor that promotes the transport of HSV-1 intronless RNAs (55, 62). To see if ICP27 stimulates expression of all intact, intronless HSV-1 genes in transfected cells, we studied the effect of ICP27 on the DE ICP8 gene. During infection, this gene does not require ICP27 for its expression (35, 51, 68). ICP8 expression from plasmid pSG18 (14) was undetectable in the absence of HSV-1 transactivators (Fig. 2C, lane 3) but was stimulated by coexpression of ICP4 and ICP0 (lane 4), consistent with previous studies (46). Cotransfection of the ICP27 plasmid did not activate ICP8 expression (lane 5), nor did expression of ICP27 enhance the ability of ICP4/0 to stimulate ICP8 expression (compare lane 6 to lane 4). Thus, the response of the intact gC gene to ICP27 appears to be specific, in that another intronless HSV-1 gene is nonresponsive.
Stimulation of gC gene expression by ICP27 does not require ICP4 or ICP0.
There are at least two possible explanations for why ICP27-dependent transactivation of the transfected gC gene also requires ICP4/0. First, ICP4 and/or ICP0 may be directly involved in the mechanism by which ICP27 stimulates gene expression. This is consistent with previous studies which have demonstrated physical and functional interactions between ICP27 and ICP4 or ICP0 (45, 54, 74). Alternatively, ICP27 and ICP4/0 might stimulate distinct steps in gene expression, making them both required for gC expression. For example, ICP4 and ICP0 are known to stimulate transcription, whereas ICP27 may have a posttranscriptional effect. To distinguish between these two general possibilities, we asked whether ICP4/0 could be replaced in the transfection assay by an unrelated transcriptional transactivator. To accomplish this, the gC gene was modified by the insertion of three copies of the tetracycline repressor operator sequence 15 bp upstream of the gC gene's TATA box. This new plasmid was designated pgCXTO (Fig. 1B). This addition was intended to make the gC gene responsive to the tet-TA, an artificial regulatory molecule composed of the tetracycline repressor DNA-binding domain fused to the HSV-1 VP16 transcriptional activation domain (15).
Next, we carried out a transfection experiment in which we compared the responses of pgC and pgCXTO to ICP27 and other transactivators. Both plasmids responded very similarly to the HSV-1 transactivators, requiring both the ICP27 and ICP4/ICP0 plasmids for detectable expression of gC (Fig. 3A, lane 6, top and bottom panels). However, the two plasmids responded quite differently to the tet-TA. Whereas tet-TA had no effect on gC expression from pgC, either alone or in combination with ICP27 (Fig. 3A, top panel, lanes 7 and 8, respectively), it had a strong effect on expression from pgCXTO (bottom panel). In the absence of ICP27, tet-TA led to low but detectable gC expression (Fig. 3A, lane 7). Moreover, when the tet-TA- and ICP27-encoding plasmids were combined, gC expression was dramatically stimulated (Fig. 3A, lane 8). In fact, the combination of ICP27 and the tet-TA was significantly more efficient at inducing gC expression than the combination of ICP27 and ICP4/0. Northern analysis of total RNA extracted from a similar transfection experiment gave results that paralleled the protein analysis; i.e., tet-TA and ICP27 acted synergistically to induce gC mRNA expression from pgCXTO (Fig. 3B, lane 5). tet-TA alone led to low but detectable gC mRNA expression (barely visible in lane 3, but apparent in longer exposures). Together, the results of these experiments demonstrate that tet-TA can substitute for ICP4/0 to help ICP27 stimulate gC expression from a transfected plasmid, provided that the tet-TA binding site is present in the gC promoter. We conclude that ICP27's stimulatory effect on the transfected gC gene does not require a specific interaction with ICP4 or ICP0.
The endogenous poly(A) site is not required for ICP27's stimulatory effect on the transfected gC gene.
Previous work has demonstrated that ICP27 can stimulate the expression of transfected reporter genes which possess weak poly(A) signals (4, 56). Moreover, the gC gene has a relatively weak poly(A) site, as judged by its inefficient processing in vitro (37). Together, these findings have led to the proposal that the weak poly(A) signal of the gC gene and other L genes may be critical for their transactivation by ICP27 (37). To test this, we engineered pgCPA, a derivative of pgCXTO in which the endogenous poly(A) site has been replaced by the strong poly(A) site from the SV40 late transcription unit (Fig. 1C). This poly(A) signal is considered to be especially efficient in mammalian cells (20) and has been shown to be nonresponsive to ICP27 in the context of a reporter gene transfection assay (56). When pgCPA was tested (Fig. 4A, bottom panel), its response to transactivators was very similar to that of pgCXTO (top panel). Specifically, gC expression from pgCPA was unresponsive to ICP4/0 or ICP27 (lanes 4 and 5), but was modestly induced by the tet-TA (Fig. 4A, lane 7). Moreover, ICP27 was able to cooperate with either ICP4/0 (Fig. 4A, lane 6) or the tet-TA (Fig. 4A, lane 8) to induce gC expression. Northern blot analysis of RNA isolated from a similar transfection experiment indicated that the primary effect of ICP27 on pgCPA was to stimulate mRNA expression (Fig. 4B), similar to what was seen for pgCXTO (Fig. 3B). Therefore, regulation of the transfected gC gene by ICP27 is not significantly affected by replacing the endogenous weak poly(A) site with the highly efficient one from the SV40 late transcription unit. We conclude that the endogenous poly(A) signal is not required for the response of the transfected gene to ICP27.
A gC gene driven by the CMV IE promoter is highly responsive to ICP27.
ICP0 and ICP4 are known to activate gene expression at the level of transcription (13, 18, 29), as is the tet-TA (15). It is perhaps not surprising that transcriptional activation of the gC gene may be required, as the gC promoter and other HSV-1 true-L promoters are weak in uninfected cells (58, 72). With this in mind, we hypothesized that replacement of the gC promoter with a strong constitutive promoter might alter the requirements for viral transactivators in the transfection assay. To test this, we constructed a derivative of pgC, designated pgCΔpro, in which the strong promoter-enhancer of the CMV IE gene was attached to the body of the gC gene (Fig. 1D). The CMV sequences were joined to the gC gene at nucleotide +22 relative to the gC transcription start site (23), leaving the modified gC gene with most of its normal 5′ untranslated region. When pgCΔpro was transfected into Vero cells, no gC was detected (Fig. 5A, lane 7), indicating that the gene was not constitutively expressed. Interestingly, ICP27 alone was now able to induce high-level expression of the gC gene (Fig. 5A, lane 9). We found that the ICP4/0-encoding plasmid also was able to induce gC expression from pgCΔpro (Fig. 5A, lane 8), albeit not as efficiently as ICP27. Further experiments with separate ICP4- and ICP0-encoding plasmids indicated that the stimulatory effect of the ICP4/0 plasmid was predominantly due to ICP0 (data not shown).
To examine whether ICP27 stimulated mRNA accumulation from the CMV promoter-driven gC gene, we next carried out Northern analysis on Vero cells transfected with pgCΔpro (Fig. 4B). In the absence of transactivators, no gC mRNA could be detected (Fig. 4B, lane 3). ICP27 clearly stimulated mRNA expression from pgCΔpro (Fig. 4B, lane 5), as did ICP4/0 (Fig. 4B, lane 4), although to a lesser extent than ICP27. Interestingly, the combination of ICP27 and ICP4/0 (Fig. 4B, lane 6) dramatically stimulated mRNA expression over that induced by ICP27 alone Fig. 4B, (lane 5). This was somewhat surprising as there was little difference when protein levels were compared (Fig. 5A). This observation suggests that one or more of the HSV-1 transactivators may affect the stability or translation of gC expressed from pgCΔpro. This possibility is currently being investigated.
The above experiments indicate that ICP27 can stimulate mRNA and protein expression from a transfected gC gene driven by the CMV promoter. We considered the possibility that this effect might be due to a general stimulation of CMV IE promoter activity by ICP27. To test this idea, we asked whether ICP27 could stimulate expression of another intronless CMV IE promoter-driven gene. We used the plasmid pCMVβ-c, a construct in which the CMV IE promoter drives expression of beta-galactosidase. This plasmid was transfected into Vero cells, plus or minus increasing amounts of ICP27 plasmid, and beta-galactosidase expression was assayed after 2 days by immunoblotting (Fig. 5C). Unlike the gC gene in pgCΔpro, which was not expressed at all in the absence of ICP27, the β-galactosidase gene of pCMVβ-c was constitutively expressed (lane 2). Moreover, its expression appeared unresponsive to a cotransfected ICP27 gene at several doses (lanes 3 to 5). Thus, the stimulatory effect of ICP27 appears to be specific for the pgCΔpro gene, rather than a general induction of genes driven by the CMV IE promoter.
Response of the stably transfected gC gene to ICP27.
The response of transfected HSV-1 genes to HSV-1 trans-regulators can differ depending on whether the gene is on a transiently transfected plasmid or is stably integrated into the cellular genome (34, 60). To see if an integrated gCΔpro gene is also responsive to ICP27, we introduced it into Vero cells by stable transfection. To do this, Vero cells were cotransfected with pgCΔpro and pSV2neo (64), and G418-resistant colonies were isolated. Of 16 G418-resistant cell lines, 4 had at least one integrated gCΔpro gene, as demonstrated by their ability to express gC following infection with a gC-negative HSV-1 mutant (see below). These lines were designated VgC2, VgC3, VgC14, and VgC16.
We first asked whether the integrated pgCΔpro genes are responsive to ICP27. To do this, we transiently transfected the VgC3, VgC14, and VgC16 cell lines with HSV IE plasmids and assessed gC expression at 2 days posttransfection (Fig. 5D). On its own, ICP27 was unable to induce gC expression from the integrated genes (Fig. 5D, lane 5, all three panels). However, the integrated genes were clearly responsive to ICP27, as expression of ICP27 greatly enhanced gC expression when it was coexpressed with ICP4 and ICP0 (Fig. 5D, compare lanes 6 and 4). For the VgC3 and VgC16 cell lines, a small level of gC expression was induced by introduction of ICP4 and ICP0 plasmids, even in the absence of ICP27. This was not observed for VgC14 cells (Fig. 5D, lane 4, middle panel), but the significance of this is unclear, since the overall efficiency of gC induction by the combination of ICP27, ICP4, and ICP0 was reduced for this cell line compared to the others (Fig. 5D, lane 6). We conclude from these data that ICP27 is able to stimulate expression of the stably integrated CMV promoter-driven gC genes in VgC3, VgC14, and VgC16 cells, in combination with ICP4 and ICP0. Preliminary analysis indicates that ICP0, not ICP4, plays the major role in stimulating expression of the integrated genes in combination with ICP27 (data not shown).
HSV-1 infection induces expression of the stably transfected CMV-gC gene in an ICP27-dependent manner.
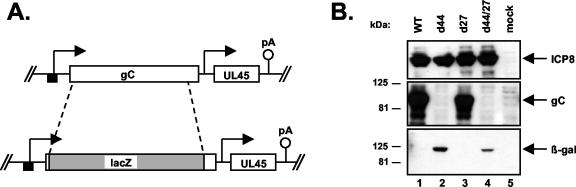
The above results indicate that both the transiently and stably transfected gC genes are responsive to ICP27. We asked next whether the stably transfected gC genes could be activated by ICP27 expressed from an infecting virus. However, this question is complicated by the fact that an infecting virus would carry in its own gC gene, making it difficult to follow expression of the transfected gene. To avoid this complication, we engineered derivatives of WT HSV-1 and the ICP27 null mutant d27-1 (49) which have their nonessential gC genes replaced by the E. coli lacZ gene (Fig. 6A). These mutants were designated d44 and d44/27, respectively. To confirm that gC-negative mutants express β-galactosidase in place of gC, we examined the gene expression of these viruses under permissive conditions. To do this, we infected V27 cells, which express ICP27 upon infection and thus complement the growth of ICP27 mutants, with d44, d44/27, or their parental viruses. Proteins were collected at 6 hpi and subjected to immunoblot analysis (Fig. 6B). All the virus-infected cells expressed similar amounts of ICP8 (top panel), indicating that the cells were successfully infected. However, and as expected, d44- and d44/27-infected cells did not express gC but did express β-galactosidase (middle and bottom panels, respectively).
FIG. 6.
Isolation of HSV-1 gC-negative mutants. (A) Schematic representation of the gC gene modification in the virus mutants d44 and d44/27. The gC gene open reading frame was replaced with that of the E. coli lacZ gene, as shown. The mutant d44 was derived from WT HSV-1, whereas d44/27 was derived from the ICP27 deletion mutant d27-1. (B) The d44 and d44/27 mutants express β-galactosidase but not gC. V27 cells were mock infected or infected with the viruses indicated. Proteins were harvested at 6 hpi and subjected to immunoblot analysis using monoclonal antibodies specific for ICP8, gC, and β-galactosidase (top, middle, and bottom panels, respectively).
To see if HSV-1 infection activates expression of the stably transfected CMV-gC gene, the four VgC cell lines were mock infected or infected with either d44 or d44/27. We also analyzed a pool of stable pgcΔpro transfectants, designated VgCpool, representing a mixture of ∼200 colonies. As an additional control, Vero cells were infected. All infections were carried out in the presence of PAA (400 μg/ml), a specific inhibitor of HSV-1 DNA replication. This treatment prevents infections from progressing into the L phase and thus eliminates any differences between d44 and d44/27 infections which would be due to differences in the rate of viral DNA replication. The expression of gC at 6 hpi was analyzed by immunoblotting (Fig. 7A). As expected, no gC was expressed in infected Vero cells (lanes 3 and 4). However, all four stably transfected lines, as well as the transfectant pool, exhibited similar patterns of gC gene expression. No expression was seen in the absence of infection (Fig. 7A, lanes 5, 8, 11, 14, and 17), nor in d44/27 infections, lacking ICP27 (Fig. 7A, lanes 7, 10, 13, 16, and 19). However, gC expression was efficiently induced in the d44 infections, wherein ICP27 was expressed (Fig. 7A, lanes 6, 9, 12, 15, and 18). These results indicate that HSV-1 infection efficiently activates the stably transfected gCΔpro gene and that ICP27 is required for this effect.
To determine whether activation of the stably transfected gC genes is at the level of mRNA accumulation, a similar infection experiment was carried out in the VgC3, VgC14, and VgC16 lines or in Vero cells. Total RNA was prepared at 6 hpi, and gC mRNA was analyzed by Northern blotting (Fig. 7B). No gC mRNA could be detected in mock- or d44/27-infected VgC cells. However, abundant gC mRNA accumulated in d44-infected VgC cells (Fig. 7B, lanes 5, 8, and 11). Thus, ICP27-dependent activation of the stably transfected gC gene during viral infection appears to occur primarily at the level of steady-state mRNA accumulation.
HSV-1-induced transcription of the stably transfected gC gene is independent of ICP27.
We next asked whether ICP27 stimulates mRNA production from the transfected gC genes by stimulating transcription. One well-accepted method for measuring transcription rates in mammalian cells is the nuclear run-on assay, wherein nuclei are isolated and subsequently incubated in vitro with radioactive RNA precursors (17, 19). RNA polymerases that are actively engaged in transcription at the time of nuclear isolation extend their RNA chains under these conditions, leading to the synthesis of short radioactive RNA molecules. Hybridization of these labeled RNAs to single-stranded DNA probes can be used to quantitate transcription rates on individual genes.
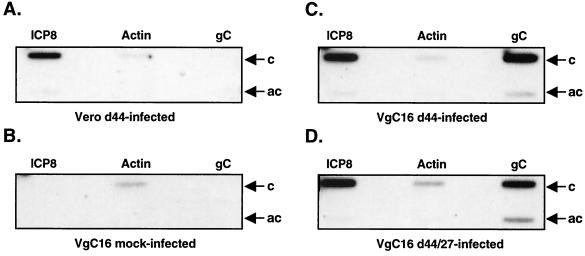
Nuclear runoff transcription assays performed on transiently transfected cells are problematic, due to background unregulated transcription over both strands of the transfected plasmid (C. Spencer, personal communication). However, we reasoned that we could apply the nuclear run-on assay to study the transcription of the stably transfected gC gene, since it is integrated into cellular chromatin. We decided to carry out the analysis in VgC16 cells, since these showed high gC expression following d44 infection (Fig. 7). VgC16 cells were mock infected or infected with d44 or d44/27. As a negative control, Vero cells were also infected with d44. As in the RNA analysis, PAA was included in all infections to prevent viral DNA replication. At 4 hpi, nuclei were isolated and subjected to nuclear run-on analysis. Radiolabeled run-on transcripts were hybridized to single-stranded bacteriophage M13 probes corresponding to three different genes: the DE ICP8 gene, the cellular gamma actin gene, and the gC gene (Fig. 8). Complementary probes measure transcription of the relevant mRNAs, whereas anticomplementary probes measure transcription from the opposite strand. In d44-infected-Vero cells (Fig. 8A), ICP8 transcription was readily apparent, and there appeared to be low-level transcription of the cellular gamma actin gene. Transcription of the gC gene was not detected, as expected, since the gC coding region is deleted from the d44 genome. In mock-infected VgC16 cells (Fig. 8B), only gamma actin transcription was detected. Thus, the stably transfected gCΔpro gene is not detectably transcribed in uninfected cells. In d44-infected VgC16-infected cells (Fig. 8C), the gC gene is transcriptionally active, as is the viral ICP8 gene. gC transcription is expected in this case, since d44-infected VgC16 cells accumulate abundant gC mRNA (Fig. 7B). Significantly, gC gene transcription is also strong in d44/27-infected VgC16 cells (Fig. 8D), even though no stable gC mRNA accumulates in these cells (Fig. 7B). Quantitation of these results by phosphorimaging indicated that the gC transcription was only slightly increased (1.6-fold) in d44-infected cells compared to d44/27-infected cells. This experiment was repeated twice more, with very similar results. In the three replicate experiments, transcription of the integrated gC gene in VgC16 cells occurred efficiently in both d44- and d44/27 infections. However, there was a consistent modest ICP27-dependent increase in gC transcription (average of 2.6-fold). This relatively small difference cannot account for the qualitative induction of gC mRNA that is observed in d44-infected cells. We conclude from these data that ICP27 posttranscriptionally stimulates expression of the gC mRNA from the stably transfected gC gene in VgC16 cells.
FIG. 8.
ICP27 is not required for HSV-1-induced transcription of the stably transfected gC gene. Vero cells were d44 infected (A), or VgC16 cells were mock infected (B), d44 infected (C), or d44/27 infected (D). Nuclei were isolated at 4 hpi, and nuclear run-on transcription assays were performed. The radiolabeled RNAs were hybridized to single-stranded DNA probes complementary (c) or anticomplementary (ac) to mRNA from the HSV-1 ICP8 gene, the cellular gamma actin gene, or the gC gene. An autoradiograph of the hybridized filters is shown.
DISCUSSION
The transfected HSV-1 gC gene is highly responsive to ICP27.
During viral infection, expression of the HSV-1 true-L gene encoding gC is regulated at multiple levels and by several factors. First, like nearly all DE and L genes, gC expression depends on the IE transcriptional activator protein ICP4 (30, 70). Second, like other true-L genes, gC expression is strongly activated by viral DNA synthesis (23), an effect which appears to result from a cis-acting change in the viral template which is conferred by the process of DNA replication (33). Third, gC expression is highly dependent upon ICP27 (35, 37, 49, 53). As ICP27 stimulates viral DNA synthesis approximately 5- to 10-fold (35, 49), at least some of ICP27's stimulatory effects on gC expression are an indirect result of its ability to enhance viral DNA replication. However, ICP27 also directly stimulates gC expression via a mechanism which is independent of viral DNA replication. This has been demonstrated by the isolation of several viral ICP27 mutants which are proficient at viral DNA replication but which are unable to express significant levels of gC mRNA (49, 50). The studies described here on the transfected gC gene also demonstrate that ICP27 directly transactivates the gC gene, independent of effects on DNA replication. Moreover, our experiments indicate that no viral factors other than ICP27 are required for this effect, since ICP27 alone dramatically transactivates the transiently transfected gC gene when its transcription is driven by the CMV IE promoter.
Transfection assays have been used previously to study the effects of ICP27 on gene expression (2, 4, 11, 48, 56, 57, 67). Our study differs from these past studies in two important ways. First, the target genes utilized previously have generally corresponded to chimeric genes consisting of HSV-1 regulatory sequences [promoters, introns, poly(A) sites] attached to nonbiologically relevant reporter genes such as that encoding CAT. In our experiments, an intact and biologically relevant HSV-1 gene was used. Second, in most cases, the positive effects of ICP27 on transfected target genes have been absent or relatively weak in the absence of other transactivators such as ICP4 or ICP0 (4, 11, 48, 57). However, we found that the CMV promoter-driven gC gene is highly induced by ICP27 alone. The dramatic response of this gene to ICP27 is reminiscent of studies on the transfected beta interferon gene, which is also highly responsive to ICP27 in the absence of other transactivators (2).
ICP27 posttranscriptionally activates expression of the transfected gC gene.
Several studies have provided evidence that ICP27 activates the gC gene and other L genes during infection via a posttranscriptional mechanism (37, 55, 61). However, one recent study concluded that ICP27 activates the gC gene by increasing its transcription rate (26). To date, it has not been possible to use the nuclear run-on transcription assay (19), a standard method for measuring transcription rates in mammalian cells, to directly measure the transcription rates of L genes during infection. This is because, at late times of infection, both strands and nearly all regions of the HSV-1 genome appear transcriptionally active when assayed by the nuclear run-on assay (13, 26, 61, 65, 71). This effect is associated with viral DNA replication, since transcription remains strand and gene specific if infections are carried out in the presence of an HSV-1 DNA synthesis inhibitor such as PAA. However, since L genes are highly dependent on viral DNA replication, the use of PAA itself blocks expression of L genes. It is not known whether the promiscuous run-on transcription seen on replicating viral genomes reflects bona fide transcription, or is an experimental artifact associated with viral DNA synthesis. However, this phenomenon makes it difficult if not impossible to use the nuclear run-on assay to study L gene transcription in HSV-1-infected cells.
Our stable transfection system allowed us to avoid the above complication since we were able to study the transcription of an ICP27-responsive L gene that is integrated into cellular chromatin. In addition, since the stably transfected gC gene is transactivated by ICP27 even in the presence of PAA, we were able to include this drug in our experiments. The results of our nuclear run-on experiments showed that the stably transfected gC gene is transcriptionally activated by HSV-1 infection. However, this effect is largely independent of ICP27, since transcription of the gC gene is similarly efficient in both the presence and absence of ICP27. In contrast, accumulation of gC mRNA was found to be highly dependent upon ICP27. We conclude from these data that activation of the transfected gC gene by ICP27 is primarily posttranscriptional.
Our findings contrast with those of Jean et al. (26), who used a [3H]uridine pulse-labeling technique to measure transcription rates of the gC and UL47 genes in WT- and ICP27-mutant virus-infected cells (26). These investigators concluded that ICP27 stimulates transcription of these two L genes, and that this effect is sufficient to account for the differences in corresponding steady-state mRNAs. In our nuclear run-on assays, we found that ICP27 stimulated gC transcription by a factor of ∼2.6-fold, clearly not enough to account for the qualitative induction of mRNA accumulation that is dependent on ICP27 (Fig. 7B). There are at least two possible explanations for why our results differ from those of Jean et al. First, the apparent discrepancy could reflect the two different transcription assays that were used. For example, in the studies of Jean et al. (26), it is conceivable that the apparent lower rate of in vivo [3H]uridine incorporation into gC mRNA in ICP27-mutant-infected cells was due to very rapid turnover of gC mRNA after its synthesis. Indeed, our results suggest that ICP27 may prevent gC mRNA from being rapidly degraded following its transcription (see below). The second explanation, which we do not favor but cannot exclude, is that transfected gC gene is transactivated by ICP27 via a posttranscriptional mechanism, whereas the endogenous viral gene is transactivated during infection via a transcriptional mechanism.
Possible mechanisms by which ICP27 could posttranscriptionally stimulate gC gene expression.
What posttranscriptional mechanism could account for the effect of ICP27 on gC mRNA accumulation? One model which has been proposed to explain how ICP27 transactivates the gC gene and other L genes is that it stimulates the usage of inherently weak L gene polyadenylation sites, thus leading to increased mRNA accumulation (37). We performed an experiment to test this hypothesis by replacing the gC gene's endogenous poly(A) site, shown to be weak in an in vitro processing assay (37), with that from the SV40 L gene region. This sequence has been shown to be an especially efficient poly(A) signal in mammalian cells (20). Furthermore, a CAT reporter gene bearing the SV40 late poly(A) site is unresponsive to ICP27-dependent stimulation in a transfection assay (56). We found that the gC gene bearing the SV40 poly(A) site is still strongly activated by ICP27. Thus, the endogenous, weak poly(A) site of the gC gene is not required for the response of the gene to ICP27, at least in transfected cells. However, our results do not exclude the possibility that the poly(A) site of the gC gene plays a role in ICP27-responsiveness, in combination with other sequences.
Another possible posttranscriptional mechanism to account for ICP27-dependent transactivation is that ICP27 stimulates gC expression by functioning as a nuclear export factor for the transport of gC mRNA to the cytoplasm, as suggested by several recent studies (5, 31, 55, 63). Our results are consistent with such a model, especially if ICP27-dependent transport of gC mRNA out of the nucleus also prevents the message from being otherwise rapidly degraded.
There are some interesting parallels between our work and earlier studies on the effects of HSV-1 infection and ICP27 on the transfected beta-interferon gene (2, 43). Similar to our study, these studies found that a stably transfected CMV-IE promoter-driven beta-interferon gene was expressed at undetectable levels in uninfected cells, a result that is unexpected given the powerful nature of the CMV IE promoter (42, 43). HSV-1 infection was shown to induce beta-interferon mRNA expression by more than 100-fold, and nuclear run-on analysis indicated that the effect is predominantly posttranscriptional (43). A subsequent study by Brown et al. demonstrated that ICP27 is the HSV-1 factor responsible for induction of the transfected beta interferon gene (2). The effect of ICP27 was mapped to the 3′ untranslated region of the beta interferon gene, which contains an AU-rich element (ARE) that is known to mediate cytoplasmic mRNA instability. These studies suggest that ICP27 can posttranscriptionally stabilize the beta-interferon mRNA, possibly by interfering with its normal ARE-dependent degradation. Given the similarities between our studies and those on the beta-interferon gene, it is tempting to speculate that ICP27 has a direct effect in stabilizing gC mRNA. Although an inspection of the sequence of the gC gene does not reveal any sequences which resemble ARE instability elements, it is possible that one or more other types of mRNA instability element exist in the gC transcript. Further experiments will be required to test these ideas.
The roles of ICP4 and ICP0 in stimulation of the transfected gC gene.
The finding that ICP27 posttranscriptionally activates the transfected gC gene suggests an explanation for why the various versions of the gC gene that we constructed showed different requirements for other trans-acting factors. The WT gC gene promoter and other L promoters have minimal promoter structures and are generally weak or inactive in uninfected cells (28, 58). Thus, the transfected gC gene likely requires both transcriptional and posttranscriptional activation. Transcriptional activation can be mediated by ICP4 and ICP0, (13, 18, 29), whereas ICP27 mediates posttranscriptional activation. ICP4 and ICP0, however, are not needed when a distinct transcriptional activator such as tet-TA can function, or when transcription is driven by the strong cis-acting signals of the CMV IE promoter-enhancer.
One interesting finding was that ICP27 is able to efficiently transactivate the transiently transfected CMV IE promoter-driven gC gene but is unable on its own to stimulate the stably transfected gene. In this case, ICP4 and ICP0 were found to also be required (Fig. 5C). We suggest that the packaging of the CMV-gC gene into cellular chromatin represses transcription of the gene and that this effect is overcome by ICP4 and ICP0. Consistent with this, ICP0 has been proposed to counteract a cellular gene repression mechanism via its ability to induce degradation of cellular target proteins (10). A related finding from our nuclear run-on studies was that there is little if any transcription of the stably transfected CMV-gC gene in VgC16 cells, but HSV-1 infection strongly activates transcription. This effect is largely independent of ICP27, since it occurs in both d44 and d44/27 infections. It has previously been noted that HSV-1 infection can induce the expression of stably transfected viral and cellular genes, or of certain endogenous cellular genes, and that ICP4 and ICP0 appear to have important roles in this phenomenon (7, 9, 34). Based on this and on the results of our transfection studies, we suggest that ICP4 and/or ICP0 are involved in activating the transcription of the integrated CMV-gC gene.
Mapping ICP27-responsive sequences in the gC gene.
It is clear from this and previous studies that there is great deal of gene-specificity in the stimulatory effects of ICP27, with the HSV-1 gC gene being an example of a highly responsive gene. Responsiveness is not merely due to lack of introns (55), as we have found that the intronless ICP8 gene and an intronless CMV-β-galactosidase gene are unresponsive to ICP27. Thus, there must be other sequences in responsive genes which mediate their regulation by ICP27. Such sequences might be positively acting elements which are recognized directly or indirectly by ICP27 to stimulate expression. Alternatively, they may be negatively acting elements that normally inhibit expression, e.g., sequences which activate mRNA degradation. Indeed, it is surprising that the CMV IE promoter-driven gC gene is not expressed in the absence of ICP27, since this promoter is one of the strongest RNA polymerase II promoters known. This strongly suggests that the gC gene contains a negatively acting element that is overcome by ICP27.
Given the dramatic responsiveness of the transfected CMV-gC gene to ICP27, it should be possible to use this gene to map the ICP27-responsive element or elements. Such experiments are currently in progress. The present work demonstrates that neither the promoter of the gC gene nor its poly(A) signal harbors a sequence which is the sole mediator of ICP27 response. Instead, our results suggest that key responsive sequences map to the transcribed body of the gene. Given our finding that regulation is posttranscriptional, we suspect that the relevant cis-acting sequences will function at the level of mRNA, and it is conceivable that they correspond to binding sites for ICP27.
Acknowledgments
We are grateful to Joy Lengyel, Chandra Guy, and Katy Hansen for expert technical assistance and to members of the Rice and Schiff laboratories for stimulating discussions. We also thank Anna Strain and Leslie Schiff for insightful reviews of the manuscript and Scott Bunnell for construction and early analysis of the pCMVβ-c plasmid. Finally, we acknowledge Charlotte Spencer (University of Alberta) for nuclear run-on transcription assay protocols as well as invaluable help and advice with this technique.
This research was supported by a grant from the NIH (RO1-AI42737) to S.A.R. K.D.P. was supported by NIH training grant 2T32 AI07421.
REFERENCES
- 1.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, C. R., M. S. Nakamura, J. D. Mosca, G. S. Hayward, S. E. Straus, and L. P. Perera. 1995. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J. Virol. 69:7187-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campadelli-Fiume, G., and F. Serafini-Cessi. 1985. Processing of the oligosaccharides chains of herpes simplex virus type 1 glycoproteins, p. 357-382. In B. Roizman (ed.), The herpesviruses, vol. 3. Plenum Press, New York, N.Y.
- 4.Chapman, C. J., J. D. Harris, M. A. Hardwicke, R. M. Sandri-Goldin, M. K. Collins, and D. S. Latchman. 1992. Promoter-independent activation of heterologous virus gene expression by the herpes simplex virus immediate-early protein ICP27. Virology 186:573-578. [DOI] [PubMed] [Google Scholar]
- 5.Chen, I. H., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 76:12877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung, P., K. S. Ellison, R. Verity, and J. R. Smiley. 2000. Herpes simplex virus ICP27 induces cytoplasmic accumulation of unspliced polyadenylated alpha-globin pre-mRNA in infected HeLa cells. J. Virol. 74:2913-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung, P., B. Panning, and J. R. Smiley. 1997. Herpes simplex virus immediate-early proteins ICP0 and ICP4 activate the endogenous human alpha-globin gene in nonerythroid cells. J. Virol. 71:1784-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLuca, N. A., and P. A. Schaffer. 1987. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 15:4491-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett, R. D. 1985. Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J. 4:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 11.Everett, R. D. 1986. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J. Gen. Virol. 67:2507-2513. [DOI] [PubMed] [Google Scholar]
- 12.Frink, R. J., R. Eisenberg, G. Cohen, and E. K. Wagner. 1983. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J. Virol. 45:634-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godowski, P. J., and D. M. Knipe. 1986. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc. Natl. Acad. Sci. USA 83:256-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldin, A. L., R. M. Sandri-Goldin, M. Levine, and J. C. Glorioso. 1981. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J. Virol. 38:50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham, F. L., and A. J. van der Eb. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg, M. E., and E. B. Ziff. 1984. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 311:433-438. [DOI] [PubMed] [Google Scholar]
- 18.Grondin, B., and N. DeLuca. 2000. Herpes simplex virus type 1 ICP4 promotes transcription preinitiation complex formation by enhancing the binding of TFIID to DNA. J. Virol. 74:11504-11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groudine, M., M. Peretz, and H. Weintraub. 1981. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol. Cell. Biol. 1:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hans, H., and J. C. Alwine. 2000. Functionally significant secondary structure of the simian virus 40 late polyadenylation signal. Mol. Cell. Biol. 20:2926-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardwicke, M. A., and R. M. Sandri-Goldin. 1994. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J. Virol. 68:4797-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homa, F. L., T. M. Otal, J. C. Glorioso, and M. Levine. 1986. Transcriptional control signals of a herpes simplex virus type 1 late (γ2) gene lie within bases −34 to +124 relative to the 5′ terminus of the mRNA. Mol. Cell. Biol. 6:3652-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes, R. G., Jr., and W. H. Munyon. 1975. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J. Virol. 16:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingram, A., A. Phelan, J. Dunlop, and J. B. Clements. 1996. Immediate early protein IE63 of herpes simplex virus type 1 binds RNA directly. J. Gen. Virol. 77:1847-1851. [DOI] [PubMed] [Google Scholar]
- 26.Jean, S., K. M. LeVan, B. Song, M. Levine, and D. M. Knipe. 2001. Herpes simplex virus 1 icp27 is required for transcription of two viral late (gamma2) genes in infected cells. Virology 283:273-284. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins, H. L., and C. A. Spencer. 2001. RNA polymerase II holoenzyme modifications accompany transcription reprogramming in herpes simplex virus type 1-infected cells. J. Virol. 75:9872-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, P. A., and R. D. Everett. 1986. The control of herpes simplex virus type-1 late gene transcription: a ‘TATA-box’/cap site region is sufficient for fully efficient regulated activity. Nucleic Acids Res. 14:8247-8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan, R., and P. A. Schaffer. 1997. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J. Virol. 71:6850-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, D. B., S. Zabierowski, and N. A. DeLuca. 2002. The initiator element in a herpes simplex virus type 1 late-gene promoter enhances activation by ICP4, resulting in abundant late-gene expression. J. Virol. 76:1548-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindberg, A., and J. P. Kreivi. 2002. Splicing inhibition at the level of spliceosome assembly in the presence of herpes simplex virus protein ICP27. Virology 294:189-198. [DOI] [PubMed] [Google Scholar]
- 33.Mavromara-Nazos, P., and B. Roizman. 1987. Activation of herpes simplex virus 1 gamma 2 genes by viral DNA replication. Virology 161:593-598. [DOI] [PubMed] [Google Scholar]
- 34.Mavromara-Nazos, P., S. Silver, J. Hubenthal-Voss, J. L. McKnight, and B. Roizman. 1986. Regulation of herpes simplex virus 1 genes: alpha gene sequence requirements for transient induction of indicator genes regulated by beta or late (gamma 2) promoters. Virology 149:152-164. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy, A. M., L. McMahan, and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J. Virol. 63:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 37.McGregor, F., A. Phelan, J. Dunlop, and J. B. Clements. 1996. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J. Virol. 70:1931-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLauchlan, J., A. Phelan, C. Loney, R. M. Sandri-Goldin, and J. B. Clements. 1992. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J. Virol. 66:6939-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahan, L., and P. A. Schaffer. 1990. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J. Virol. 64:3471-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242:128-137. [DOI] [PubMed] [Google Scholar]
- 41.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 70:7445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosca, J. D., K. T. Jeang, P. M. Pitha, and G. S. Hayward. 1987. Novel induction by herpes simplex virus of hybrid interferon gene transcripts driven by the strong cytomegalovirus IE94 promoter. J. Virol. 61:819-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosca, J. D., P. M. Pitha, and G. S. Hayward. 1992. Herpes simplex virus infection selectively stimulates accumulation of beta interferon reporter gene mRNA by a posttranscriptional mechanism. J. Virol. 66:3811-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nabel, G. J., S. A. Rice, D. M. Knipe, and D. Baltimore. 1988. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science 239:1299-1302. [DOI] [PubMed] [Google Scholar]
- 45.Panagiotidis, C. A., E. K. Lium, and S. J. Silverstein. 1997. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J. Virol. 71:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinlan, M. P., and D. M. Knipe. 1985. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol. Cell. Biol. 5:957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed, R., and E. Hurt. 2002. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell 108:523-531. [DOI] [PubMed] [Google Scholar]
- 48.Rice, S. A., and D. M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 62:3814-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice, S. A., V. Lam, and D. M. Knipe. 1993. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J. Virol. 67:1778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roizman, B., and D. M. Knipe. 2001. Herpes simplex virus and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 53.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samaniego, L. A., A. L. Webb, and N. A. DeLuca. 1995. Functional interactions between herpes simplex virus immediate-early proteins during infection: gene expression as a consequence of ICP27 and different domains of ICP4. J. Virol. 69:5705-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandri-Goldin, R. M. 1998. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandri-Goldin, R. M., and G. E. Mendoza. 1992. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 6:848-863. [DOI] [PubMed] [Google Scholar]
- 57.Sekulovich, R. E., K. Leary, and R. M. Sandri-Goldin. 1988. The herpes simplex virus type 1 α protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J. Virol. 62:4510-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shapira, M., F. L. Homa, J. C. Glorioso, and M. Levine. 1987. Regulation of the herpes simplex virus type 1 late (gamma 2) glycoprotein C gene: sequences between base pairs −34 to +29 control transient expression and responsiveness to transactivation by the products of the immediate early (alpha) 4 and 0 genes. Nucleic Acids Res. 15:3097-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shapira, S. K., J. Chou, F. V. Richaud, and M. J. Casadaban. 1983. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene 25:71-82. [DOI] [PubMed] [Google Scholar]
- 60.Silver, S., and B. Roizman. 1985. Gamma 2-thymidine kinase chimeras are identically transcribed but regulated a gamma 2 genes in herpes simplex virus genomes and as beta genes in cell genomes. Mol. Cell. Biol. 5:518-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74-86. [DOI] [PubMed] [Google Scholar]
- 62.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soliman, T. M., and S. J. Silverstein. 2000. Herpesvirus mRNAs are sorted for export via Crm1-dependent and -independent pathways. J. Virol. 74:2814-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Southern, P. J., and P. Berg. 1982. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J. Mol. Appl. Genet. 1:327-341. [PubMed] [Google Scholar]
- 65.Spencer, C. A., M. E. Dahmus, and S. A. Rice. 1997. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J. Virol. 71:2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spencer, C. A., R. C. LeStrange, U. Novak, W. S. Hayward, and M. Groudine. 1990. The block to transcription elongation is promoter dependent in normal and Burkitt's lymphoma c-myc alleles. Genes Dev. 4:75-88. [DOI] [PubMed] [Google Scholar]
- 67.Su, L., and D. M. Knipe. 1989. Herpes simplex virus alpha protein ICP27 can inhibit or augment viral gene transactivation. Virology 170:496-504. [DOI] [PubMed] [Google Scholar]
- 68.Uprichard, S. L., and D. M. Knipe. 1996. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J. Virol. 70:1969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Visalli, R. J., and C. R. Brandt. 1993. The HSV-1 UL45 18 kDa gene product is a true late protein and a component of the virion. Virus Res. 29:167-178. [DOI] [PubMed] [Google Scholar]
- 70.Watson, R. J., and J. B. Clements. 1980. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature 285:329-330. [DOI] [PubMed] [Google Scholar]
- 71.Weinheimer, S. P., and S. L. McKnight. 1987. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J. Mol. Biol. 195:819-833. [DOI] [PubMed] [Google Scholar]
- 72.Weir, J. P. 2001. Regulation of herpes simplex virus gene expression. Gene 271:117-130. [DOI] [PubMed] [Google Scholar]
- 73.Zhou, C., and D. M. Knipe. 2002. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J. Virol. 76:5893-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu, Z., N. A. DeLuca, and P. A. Schaffer. 1996. Overexpression of the herpes simplex virus type 1 immediate-early regulatory protein, ICP27, is responsible for the aberrant localization of ICP0 and mutant forms of ICP4 in ICP4 mutant virus-infected cells. J. Virol. 70:5346-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]