Abstract
Amino acid exchanges in the virus capsid protein VP1 allow the coxsackievirus B3 variant PD (CVB3 PD) to replicate in decay accelerating factor (DAF)-negative and coxsackievirus-adenovirus receptor (CAR)-negative cells. This suggests that molecules other than DAF and CAR are involved in attachment of this CVB3 variant to cell surfaces. The observation that productive infection associated with cytopathic effect occurred in Chinese hamster ovary (CHO-K1) cells, whereas heparinase-treated CHO-K1 cells, glucosaminoglycan-negative pgsA-745, heparan sulfate (HS)-negative pgsD-677, and pgsE-606 cells with significantly reduced N-sulfate expression resist CVB3 PD infection, indicates a critical role of highly sulfated HS. 2-O-sulfate-lacking pgsF-17 cells represented the cell line with minimum HS modifications susceptible for CVB3 PD. Inhibition of virus replication in CHO-K1 cells by polycationic compounds, pentosan polysulfate, lung heparin, and several intestinal but not kidney HS supported the hypothesis that CVB3 PD uses specific modified HS for entry. In addition, recombinant human hepatocyte growth factor blocked CVB3 PD infection. However, CAR also mediates CVB3 PD infection, because this CVB3 variant replicates in HS-lacking but CAR-bearing Raji cells, infection could be prevented by pretreatment of cells with CAR antibody, and HS-negative pgsD-677 cells transfected with CAR became susceptible for CVB3 PD. These results demonstrate that the amino acid substitutions in the viral capsid protein VP1 enable CVB3 PD to use specific modified HS as an entry receptor in addition to CAR.
Coxsackievirus B3 (CVB3) belongs to the family of nonenveloped picornaviruses. Like other picornaviruses, CVB3 particles show icosahedral structure. The capsid is formed by the virus proteins VP1, VP2, VP3, and VP4 enclosing a single strand of positive-sense RNA (22). A canyon-like structure in the viral capsid is proposed to enable virus attachment by interaction with cell surface molecules (27). Until now, two different cellular molecules for attachment of CVB3 were detected. Human decay accelerating factor (DAF/CD55) was identified as an attachment but not entry receptor for CVB1, CVB3, and CVB5 (5, 33). Murine DAF does not bind CVB3 (37). Binding and entry of all six coxsackievirus B serotypes and of swine vesicular disease virus is mediated by both human and murine coxsackievirus-adenovirus receptor (CAR) at the cellular level (3, 4, 19, 39). CAR expression patterns are species, tissue, and age dependent and may vary during inflammation (15, 23, 24, 38). In both polarized cells and mucosal epithelium, the CAR protein is expressed on the basolateral surface (7, 34, 43). CAR-negative, nonpolarized cells are considered to be nonpermissive for CVB3 infection in vitro. In contrast, multiple passages of a persistent coxsackievirus B3 Nancy variant in human embryonic fibroblasts resulted in an increase of virulence and capability to infect CAR-negative cells by the derived CVB3 variant PD (41). CVB3 PD binds strongly to DAF and weakly to CAR, is able to infect both DAF- and CAR-deficient cells, and possesses several unique amino acid substitutions at the surface of VP1 which were shown to be responsible for the changes in cell tropism (31). This implied that the substituted amino acids in VP1 are involved in virus binding to the cell surface and led to an extension in receptor usage of CVB3 PD. The glucosaminoglycan heparan sulfate (HS) was considered to be a likely candidate. HS are widely expressed molecules, conserved between humans and rodents that bind a number of viruses, including also echoviruses, Theilers murine encephalomyelitis virus, and foot-and-mouth disease virus, belonging like CVB3 to the picornaviridae (11, 16, 25). During this study we tried to clarify the role of CAR for CVB3 PD replication and to identify the yet-unknown receptor.
MATERIALS AND METHODS
Cell lines and viruses.
Chinese hamster ovary cells (CHO-K1; DSMZ, no. ACC-110) were used for cytotoxic and cytopathic effect (CPE)-inhibitory tests. The replication pattern of CVB3 variants was studied in CHO-K1, pgsA-745 (9), pgsD-677 (17), pgsE-606 (2), and pgsF-17 cells (1) and in the human lymphoblastoid cell line Raji.
CVB3 PD was derived after serial passages of the persistent CVB3 strain Nancy P (CVB3 P) in human embryonic fibroblasts H (41). CVB3 P (40), CVB3 M2 (cDNA-generated) (18), and CVB3 Bgl/Spe (cDNA-generated from a pCVB3 M2 construct containing the genome region coding for VP1 of CVB3 PD which was shown to be responsible for the enhanced cell tropism) (31) were included as controls in these studies. Virus stocks were prepared in monolayers of HeLa Ohio (CVB3 P and M2) or CHO-K1 cells (CVB3 PD and Bgl/Spe). Titers of virus were determined on HeLa cell (ATCC, no. CCL-2) monolayers using the endpoint method of Reed and Muench (26). Aliquots were stored at −80°C until use.
Compounds.
Pentosan polysulfate (PPS) (Bene Arzneimittel GmbH, Munich, Germany), heparin from bovine lung, mesoglycane, sulodexide, HS from bovine kidney, HS from porcine intestinal mucosa, protamine sulfate, apolactoferrin (all from Sigma), and the dispirotriperazine derivative 27 (DSTP 27) (29) were dissolved in sterile water (10 mg/ml) and stored at 4°C until dilution in test media. The recombinant human hepatocyte growth factor (rhHGF) (R&D Systems) was dissolved in Dulbecco's modified Eagle medium (20 μg/ml) and stored at −20°C.
Determination of cell line-specific replication.
To determine the capability of CVB3 variants to replicate in different CHO cell mutants as well as in Raji cells, three virus passages were performed in each cell line. During the first passage, the cells were infected with virus at a multiplicity of infection (MOI) of 1. After 3 days of incubation, 50 μl of virus-containing cell supernatant was harvested and transferred to fresh monolayers to perform the next passage. An additional 50 μl of supernatant per well was harvested for virus titration. At day 6 postinfection (p.i.), crystal violet staining was used to quantify the CPE by estimation of the optical density at 570/630 nm as described previously (31). The vitality of Raji cells was estimated with the Cell Counting Kit-8 from Alexis Biochemicals according to the manufacturer's instructions.
Blockade of Raji infection with CAR antibodies.
Raji cells (5 × 105 cells/ml) were seeded into 96-well tissue culture plates. Cells were incubated for 60 min at room temperature with monoclonal anti-CAR antibody (RmcB diluted 1:500; Biomol), RmcB from ascites (1:100 dilution of ascites fluids kindly provided by J. Bergelson), monoclonal antibody to HS (DPC Biermann), or RPMI without antibodies, followed by inoculation with CVB3 PD (MOI of 1). The presence of antibodies in the culture medium was maintained throughout the experiment. Cell vitality was estimated spectrophotometrically with the Cell Counting Kit-8 (Alexis Biochemicals) at 24 h p.i.
Immunofluorescence.
Two-day-old cells grown in chamber slides (Nunc Inc.) were infected with the indicated CVB3 variants at a MOI of 1. Indirect immunofluorescence was performed using a monoclonal mouse anti-CVB3 antibody, 948 (DPC Biermann) and a Cy3-labeled goat antimouse antibody (Dianova) as described previously (31).
Transfection experiments.
CHO-K1, pgsD-677, and pgsE-606 cells were transfected with pCVB3-M2 plasmid (18) using Lipofectamine Plus reagent (Gibco BRL Life Technologies) according to the manufacturer's instructions. Transfections were performed in 24-well microtiter plates with plasmid concentrations of 0.4, 0.6, and 0.8 μg/ml, each in duplicate. At 1, 2, 3, and 6 days after transfection, 50 μl of cell supernatant per well was harvested and checked for infectious virus on HeLa cell monolayers.
Full-length human CAR (nucleotides 1 to 1164, accession no. Y07593) was amplified from human heart RNA using a single-step reverse transcription (RT)-PCR kit (Invitrogen, Carlsbad, Calif.) and sense (5′ TTAATCTAGAGAATTCCCAGGAGCGAGAG 3′) and antisense (5′ TTAAGGTACCGGAGGCTCTATACTATAGACC 3′) primers and cloned into the XbaI/KpnI sites of pcDNA3.1 (Invitrogen). HS-negative pgsD-677 cells were transfected with the resulting plasmid, pcDNA3.1HCAR, using Lipofectamine Plus reagent. Transfected cells were selected with 800 μg of Geneticin (GIBCO)/ml. Human CAR expression was proven by RT-PCR according to the protocol of Mena et al. (20) and indirect immunofluorescence staining with RmcB and Cy3-conjugated goat antimouse antibody (Dianova).
Cytotoxicity assay.
The 50% cytotoxic concentration of test compounds was determined on 2-day-old confluent CHO-K1 cell monolayers grown in 96-well microtiter plates as described previously (30).
CPE-inhibitory assay.
The CPE-inhibitory assay was described in detail by Schmidtke et al. (30). It was carried out using the following modifications: CHO-K1 cells were grown for 2 days in 96-well Falcon microtiter plates to confluence. Fifty microliters of drug solution and 50 μl of virus (MOI of 3.5 for CVB3 PD and of 4.5 for CVB3 Bgl/Spe) were added to the cell monolayers. The inhibition of viral CPE by the test compounds was scored spectrophotometrically 2 days postinfection when the six virus control wells showed maximal CPE.
Heparinase treatment.
Heparinase I and III (Sigma) were solved and diluted in phosphate-buffered saline containing 1 mM CaCl2, 0.5 mM MgCl2, 0.1% glucose, 1% fetal calf serum, and 0.5% bovine serum albumin. After thorough washing with phosphate-buffered saline, 2-day-old confluent CHO-K1 cells were incubated with 10 U of heparinase I or III per ml of buffer or with only the solvent solution for 90 min at 37°C. Thereafter, CVB PD was added at a MOI of 3.5 to enzyme- or mock-treated cells and incubated for 4 h at 37°C. Virus-containing supernatant was removed, the cells were washed three times with medium to eliminate unbound virus, and fresh medium with 2% fetal calf serum was added. About 36 h after infection, the virus-induced CPE was determined spectrophotometrically as described above.
RESULTS
Both CAR and HS can mediate infection.
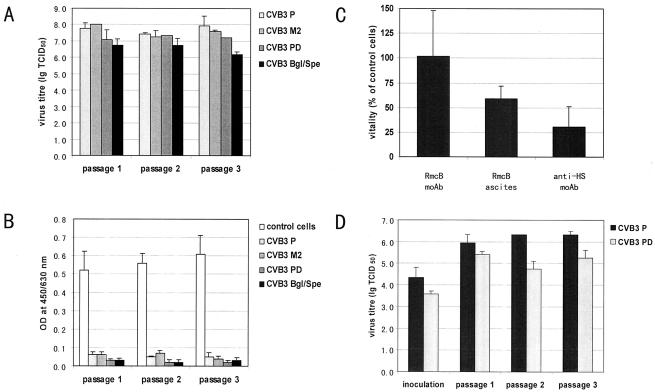
The results shown in Fig. 1A and B indicate that CVB3 PD, like all other tested CVB3 variants, replicated with a high titer of virus and nearly complete CPE in CAR-positive Raji cells in the absence of HS during three virus passages. CVB3 PD infection in Raji cells was blocked after pretreatment of cells with CAR-specific antibody (Fig. 1C). Furthermore, CVB3 P and CVB PD replicated efficiently in pgsD-677 cells transfected with CAR (Fig. 1D). These results demonstrate that CAR can mediate CVB3 PD infection in the absence of HS.
FIG. 1.
CAR mediates infection of CVB3 PD. Replication of CVB3 P, CVB3 M2, CVB3 PD, and CVB3 Bgl/Spe was studied by determination of virus titers (A) and quantification of the virus-induced CPE (B) during three passages in CAR-positive but HS-negative Raji cells. The data show the means ± standard deviation from three experiments. Furthermore, CVB3 PD replication in Raji cells was blocked with CAR antibody (C), and CAR-negative HS negative pgsD-677 cells transfected with CAR became susceptible to CVB3 P and CVB3 PD (D). OD, optical density; lg TCID50, log 50% tissue culture infective dose.
In contrast to Raji cells, CAR-negative but HS-positive CHO-K1 cells were susceptible only to CVB3 PD and CVB3 Bgl/Spe (Fig. 2) but not to CVB3 P and M2 under the same experimental conditions. Neither CVB3 PD nor CVB3 Bgl/Spe replicated in glycosaminoglycan (GAG)-negative pgsA-745, HS-negative pgsD-677, or pgsE-606 cells with undersulfated HS. These results indicate that CVB3 PD and CVB3 Bgl/Spe need highly sulfated GAG for productive infection of Chinese hamster ovary cells. The fact that both virus variants also failed to infect pgsD-677 cells producing chondroitin sulfate but not HS points out that these GAGs are HS. The resistance of pgsE-606 cells displays the crucial role of highly N-sulfated HS chains. However, 2-O-sulfates seem not to be essential for infection because 2-O-sulfotransferase-deficient pgsF-17 cells were susceptible to CVB3 PD infection in almost the same manner as CHO-K1 cells. The replication of CVB3 PD in CHO-K1 and pgsF-17 but not in pgsA-745, pgsD-677, and pgsE-606 was confirmed by indirect immunofluorescence staining at 6, 24, and 48 h p.i. For example, the immunofluorescence photographs of CVB3 PD-infected cells (48 h p.i.) are shown in Fig. 2B. Surprisingly, the CVB3 Bgl/Spe-induced CPE as well as titer of virus continuously decreased during passages and were not detectable at passage three.
FIG. 2.
Influence of HS pattern on replication of CVB3 variants in CHO cells. CHO cell mutants were infected with the indicated viruses at a MOI of 1. Three virus passages were performed. At the end of the third passage, cells were stained with crystal violet formalin to visualize the virus-induced CPE. Titers of virus were estimated by endpoint titration (A). In addition, virus replication in CHO mutant cells was verified by indirect immunfluorescence of virus-infected cells after infection with CVB3 PD (MOI of 1) at 48 h p.i. (B).
Resistant cells transfected with pCVB3-M2 or CAR produce infectious CVB3.
To exclude that a lack of crucial intracellular factors give rise to the failure of CVB3 replication in HS-negative pgsD-677 and in pgsE-606 cells with under-sulfated HS, the pCVB3-M2 plasmid encoding the complete cDNA of CVB3 M2 was transfected into these and CHO-K1 cells (for control). At 1, 2, 3, and 6 days after transfection, 50 μl of cell supernatant per well was harvested and checked for infectious virus on HeLa cell monolayers. Infectious virus was detected at different times after transfection (results not shown). This demonstrates indirectly that the blockade of CHO-K1, pgsE-606, and pgsD-677 cell infection results from a block of entry. In addition, CVB3 P and CVB3 PD replicated in HS-negative pgsD-677 cells expressing CAR after transfection (Fig. 1D). These two transfection experiments provide evidence that attachment and/or entry but not intracellular replication is blocked in HS-deficient cells.
Heparinase treatment of cells reduces infection.
To further verify the role of HS for virus adsorption, we treated CHO-K1 cells before virus inoculation with heparinase I or III or with medium only for a control. Heparinase III and heparinase I treatment of cells reduced the CVB3 PD-induced CPE by 57.7 and 48.2%, respectively, in comparison to results with untreated virus-infected cells (results not shown). These results underline the impact of HS on CVB3 PD infection.
Polycations, rhHGF, heparin, and soluble HS prevent CVB3 PD infection.
To provide additional evidence that cell surface-associated HS can be used by CVB3 PD for entry, various polycations and rhHGF suggested to bind to HS as well as polyanions widely used as soluble HS receptor analogues were tested for compatibility and their ability to prevent CHO-K1 cell infection by CVB3 PD (Table 1; Fig. 3).
TABLE 1.
Cytotoxicity and antiviral activity of polycations, polyanions, and hepatocyte growth factor against CVB3 PD in CHO-K1 cellsa
| Compound | CVB3 PD, IC50 (μg/ml) | Cytotoxicity, CC50 (μg/ml) |
|---|---|---|
| Polyanions | ||
| Bovine renal HS | No inhibition | >800 |
| Mesoglycane | 65.57 | >800 |
| Sulodexide | 32.56 | >800 |
| Porcine intestinal HS | 34.83 | >800 |
| Bovine pulmonary heparin | 29.94 | >800 |
| Pentosan polysulfate | 18.92 | >800 |
| Polycations | ||
| Protamine sulfate | 50% not reached | 378.04 |
| DSTP 27 | 50% not reached | >3,200 |
| Apolactoferrin | No inhibition | >1,000 |
| HS-binding growth factor | ||
| rhHGF | 4.39 | >10 |
IC50, 50% inhibitory concentration. CC50, 50% cytotoxic concentration.
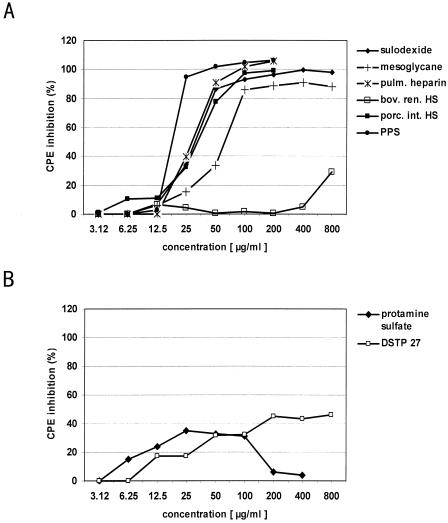
FIG. 3.
Inhibition of virus-induced CPE (34) by various polycations and polyanions. (A) Effects of the polycations protamine sulfate and DSTP 27 on the CVB3 PD-induced CPE in CHO-K1 cells. Increasing concentrations of polycations were added to confluent CHO-K1 cells immediately before virus application. Mean percentages of CPE reduction calculated from at least three experiments are shown. (B) Dose-dependent inhibition of CVB3 PD-induced CPE by the polycations PPS, heparin, and HS from different organs. CHO-K1 cells were incubated with increasing concentrations of polyanions and infected with CVB3 PD. Three independent tests were taken to calculate mean values.
The polycationic compounds protamine sulfate, DSTP 27, and apolactoferrin affected CVB PD replication only weakly or not at all at noncytotoxic concentrations. Protamine sulfate, clinically used as a heparin antidote, inhibited the CVB3 PD-induced CPE by only 40%. DSTP 27, blocking herpesvirus adsorption by binding to HS (28), showed moderate antiviral activity with only 50% CPE inhibition using 800 μg/ml. Apolactoferrin did not exhibit antiviral effects even at 1,000 μg/ml. In addition, inhibition of virus replication by rhHGF, which binds specifically to N- and 6-O-sulfated glucosamines within HS chains at the cell surface, was studied using 0.15 to 10 μg/ml. Interestingly, rhHGF very effectively prevented CVB3 PD replication in CHO-K1 cells, with a 50% inhibitory concentration of 4.39 μg/ml (Table 1).
Figure 3B indicates concentration- and sulfation-dependent inhibitory effects of the tested polyanionic compounds. PPS, possessing a high degree of sulfation, exhibited the best antiviral effect. It is derived from beech wood lignine and is composed of polysulfated β(1-4)glycosidic-connected β-d-xylopyranose moieties. The sulfate content is about 10 times higher and the arrangement of the polar groups is more regular than in heparin. The HS analogues heparin from bovine lung and heparin-like compounds derived from intestine, such as mesoglycane, sulodexide, and porcine intestinal HS, also strongly inhibited CVB3 PD infection. In contrast, kidney HS possessing fewer O-sulfate groups, N-sulfate groups, and iduronic acid moieties showed no inhibitory potential. The antiviral activity of tested polycationic and polyanionic compounds was completely confirmed in control experiments with CVB3 Bgl/Spe (results not shown).
DISCUSSION
Previously, amino acid substitutions in VP1 were identified as the molecular basis of the extended cell tropism of CVB3 PD infecting both DAF- and CAR-negative cell lines (31). The aim of this study was the identification of the yet-unknown cell surface molecule mediating CVB3 PD attachment and entry.
The results demonstrate that in addition to CAR, cell surface HS mediate entry of CVB3 PD into cells. This conclusion is based on evidence that both CVB3 PD and CVB3 Bgl/Spe replicate efficiently in Raji cells lacking HS but expressing CAR as well as in CHO-K1 cells lacking CAR but expressing HS. Viral replication associated with CPE was not visible after infection of glucosaminoglycan (pgsA745)-negative or HS (pgsD677)-negative cells. Treatment with heparinase I or III reduces coxsackievirus-induced destruction of CHO-K1 cell monolayers. Furthermore, the soluble receptor analogues heparin as well as porcine intestinal HS inhibited the virus-induced CPE in CHO-K1 cells very effectively in a dose-dependent manner. GAGs, such as HS, are polyanionic carbohydrate chains consisting of as many as 150 repeating disaccharides and are linked to the cell surface by core proteins, such as the syndecanes. HS are expressed nearly ubiquitously, but they vary in sulfate content and charge among different tissues and cells and also at different developmental stages (42). Moreover, cells can alter the HS structure they make in response to extracellular signals, e.g., growth factors. HS bind several growth factors, chemokines, cytokines, and enzymes. They are involved in blood clotting, wound healing, and embryonic development. Numerous protozoa and bacteria attach to host tissues via HS (6). HS are also used by members of herpesviruses (36), retroviruses (21), papillomaviruses (10, 32), respiratory syncytial virus (12), adenoviruses 2 and 5 (8), and picornaviruses (11, 16, 25) for attachment. Moreover, HS might be sufficient for entry in the absence of known protein-based receptors (16, 25, 35). Interestingly, the structural requirements of HS for attachment may differ from that for entry of one and the same virus (14, 35) and seem to vary for different virus types of the same family (13). These observations imply an important role of sugar specificities and sulfation for binding of viruses to HS and in this way for cell tropism of viruses.
In contrast to the case with herpes simplex virus 1 (35), a critical role of HS modified by 3-O-sulfotransferase-3 could be excluded, because both CVB3 PD and Bgl/Spe replicate in CHO-K1 cells lacking this enzyme. During this study, with CHO mutant cells defective in N-sulfation (pgsE-606) or 2-O- sulfation (pgsF-17) and in antiviral assays with polycations, several tissue-specific HS and HS-binding growth factors were used to analyze the critical structural features of HS chains that interact with CVB3 PD. The suggestion that differences in the sulfation pattern of HS may influence virus attachment was verified by examination of virus replication in pgsE-606 cells lacking the isoenzyme N-deacetylase/N-sulfotransferase-1 but expressing N-deacetylase/N-sulfotransferase-2. PgsE-606 cells produce undersulfated HS (2), which renders this cell line completely resistant to CVB3 PD. The observed susceptibility of 2-O-sulfotransferase-deficient pgsF-17 cells for CVB3 PD indicate that 2-O-sulfates do not play an essential role in infection. Obviously, the presence of N-sulfoglucosamines and 6-O-sulfates (1) is sufficient for pgsF-17 cell infection. Taken together, the results from these studies with pgsE-606 and pgsF-17 cells indicate that CVB3 PD needs highly sulfated HS containing N- and 6-O-sulfates for infection. The results from antiviral investigations with polycations, several tissue-specific HS, and rhHGF support the essential role of N-sulfoglucosamine moieties with 6-O-sulfates and sulfated iduronic acid for CVB3 PD entry. Whereas polycations that are known to inhibit HSV-1 adsorption to HS and kidney HS possessing fewer O-sulfate groups, N-sulfate groups, and iduronic acid moieties moderate or do not at all affect CVB3 PD replication, intestine HS, mesoglycane, and sulodexide block the replication of CVB3 PD. However, neither the additional involvement of other, less-characterized HS nor the transfer of HS-attached CVB3 PD to a secondary receptor subsequently mediating infection can be completely excluded.
In summary, the results demonstrate that the determined amino acid substitutions in VP1 of CVB3 PD allow the virus to use both specific cell surface HS and CAR as entry receptors, which enhances its virulence in vitro. It is interestingly now to elucidate whether binding of CVB3 to HS is only a cell culture phenomenon, as in the case of foot-and-mouth disease virus (25), or is also true for clinical isolates, as shown for echoviruses recently (11).
Acknowledgments
The mutant derivative CHO cells were kindly provided by J. Esko, University of California, San Diego, and we are grateful to K. Wright for her great patience in working with these cells. We also thank V. Güntzschel for excellent technical assistance.
This study was supported by grants from the DFG (SCHM 1594/1) and from the Jenoptik, Jena, Germany.
REFERENCES
- 1.Bai, X., and J. D. Esko. 1996. An animal cell mutant defective in heparan sulfate hexuronic acid 2-O-sulfation. J. Biol. Chem. 271:17711-17717. [DOI] [PubMed] [Google Scholar]
- 2.Bame, K. J., and J. D. Esko. 1989. Undersulfated heparan sulfate in a Chinese hamster ovary cell mutant defective in heparan sulfate N-sulfotransferase. J. Biol. Chem. 264:8059-8065. [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., A. Krithivas, L. Celi, G. Droguett, M. S. Horwitz, T. Wickham, R. L. Crowell, and R. W. Finberg. 1998. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelson, J. M., J. G. Mohanty, R. L. Crowell, N. F. St John, D. M. Lublin, and R. W. Finberg. 1995. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J. Virol. 69:1903-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, C. J., J. T. Shieh, R. J. Pickles, T. Okegawa, J. T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268:382-390. [DOI] [PubMed] [Google Scholar]
- 9.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giroglou, T., L. Florin, F. Schafer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodfellow, I. G., A. B. Sioofy, R. M. Powell, and D. J. Evans. 2001. Echoviruses bind heparan sulfate at the cell surface. J. Virol. 75:4918-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallak, L. K., P. L. Collins, W. Knudson, and M. E. Peeples. 2000. Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology 271:264-275. [DOI] [PubMed] [Google Scholar]
- 13.Herold, B. C., S. I. Gerber, B. J. Belval, A. M. Siston, and N. Shulman. 1996. Differences in the susceptibility of herpes simplex virus types 1 and 2 to modified heparin compounds suggest serotype differences in viral entry. J. Virol. 70:3461-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold, B. C., S. I. Gerber, T. Polonsky, B. J. Belval, P. N. Shaklee, and K. Holme. 1995. Identification of structural features of heparin required for inhibition of herpes simplex virus type 1 binding. Virology 206:1108-1116. [DOI] [PubMed] [Google Scholar]
- 15.Ito, M., M. Kodama, M. Masuko, M. Yamaura, K. Fuse, Y. Uesugi, S. Hirono, Y. Okura, K. Kato, Y. Hotta, T. Honda, R. Kuwano, and Y. Aizawa. 2000. Expression of coxsackievirus and adenovirus receptor in hearts of rats with experimental autoimmune myocarditis. Circ. Res. 86:275-280. [DOI] [PubMed] [Google Scholar]
- 16.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lidholt, K., J. L. Weinke, C. S. Kiser, F. N. Lugemwa, K. J. Bame, S. Cheifetz, J. Massague, U. Lindahl, and J. D. Esko. 1992. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. USA 89:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindberg, A. M., R. L. Crowell, R. Zell, R. Kandolf, and U. Pettersson. 1992. Mapping of the RD phenotype of the Nancy strain of coxsackievirus B3. Virus Res. 24:187-196. [DOI] [PubMed] [Google Scholar]
- 19.Martino, T. A., M. Petric, H. Weingartl, J. M. Bergelson, M. A. Opavsky, C. D. Richardson, J. F. Modlin, R. W. Finberg, K. C. Kain, N. Willis, C. J. Gauntt, and P. P. Liu. 2000. The coxsackie-adenovirus receptor (CAR) is used by reference strains and clinical isolates representing all six serotypes of coxsackievirus group B and by swine vesicular disease virus. Virology 271:99-108. [DOI] [PubMed] [Google Scholar]
- 20.Mena, I., C. Fischer, J. R. Gebhard, C. M. Perry, S. Harkins, and J. L. Whitton. 2000. Coxsackievirus infection of the pancreas: evaluation of receptor expression, pathogenesis, and immunopathology. Virology 271:276-288. [DOI] [PubMed] [Google Scholar]
- 21.Mondor, I., M. Moulard, S. Ugolini, P. J. Klasse, J. Hoxie, A. Amara, T. Delaunay, R. Wyatt, J. Sodroski, and Q. J. Sattentau. 1998. Interactions among HIV gp120, CD4, and CXCR4: dependence on CD4 expression level, gp120 viral origin, conservation of the gp120 COOH- and NH2-termini and V1/V2 and V3 loops, and sensitivity to neutralizing antibodies. Virology 248:394-405. [DOI] [PubMed] [Google Scholar]
- 22.Muckelbauer, J. K., M. Kremer, I. Minor, G. Diana, F. J. Dutko, J. Groarke, D. C. Pevear, and M. G. Rossmann. 1995. The structure of coxsackievirus B3 at 3.5 A resolution. Structure 3:653-667. [DOI] [PubMed] [Google Scholar]
- 23.Nalbantoglu, J., G. Pari, G. Karpati, and P. C. Holland. 1999. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum. Gene Ther. 10:1009-1019. [DOI] [PubMed] [Google Scholar]
- 24.Noutsias, M., H. Fechner, H. de Jonge, X. Wang, D. Dekkers, A. B. Houtsmuller, M. Pauschinger, J. Bergelson, R. Warraich, M. Yacoub, R. Hetzer, J. Lamers, H. P. Schultheiss, and W. Poller. 2001. Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v)beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections. Circulation 104:275-280. [DOI] [PubMed] [Google Scholar]
- 25.Reddi, H. V., and H. L. Lipton. 2002. Heparan sulfate mediates infection of high-neurovirulence Theiler's viruses. J. Virol. 76:8400-8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed, J., and H. Muench. 1938. A simple method of estimation of fifty per cent end points. Am. J. Hyg. 493-497.
- 27.Rossmann, M. G. 1989. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J. Biol. Chem. 264:14587-14590. [PubMed] [Google Scholar]
- 28.Schmidtke, M., A. Karger, A. Meerbach, R. Egerer, A. Stelzner, and V. Makarov. 2003. Binding of a N,N′-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology 311:134-143. [DOI] [PubMed] [Google Scholar]
- 29.Schmidtke, M., O. Riabova, H. M. Dahse, A. Stelzner, and V. Makarov. 2002. Synthesis, cytotoxicity and antiviral activity of N,N′-bis-5-nitropyrimidyl derivatives of dispirotripiperazine. Antivir. Res. 55:117-127. [DOI] [PubMed] [Google Scholar]
- 30.Schmidtke, M., U. Schnittler, B. Jahn, H. Dahse, and A. Stelzner. 2001. A rapid assay for evaluation of antiviral activity against coxsackie virus B3, influenza virus A, and herpes simplex virus type 1. J. Virol. Methods 95:133-143. [DOI] [PubMed] [Google Scholar]
- 31.Schmidtke, M., H. C. Selinka, A. Heim, B. Jahn, M. Tonew, R. Kandolf, A. Stelzner, and R. Zell. 2000. Attachment of coxsackievirus B3 variants to various cell lines: mapping of phenotypic differences to capsid protein VP1. Virology 275:77-88. [DOI] [PubMed] [Google Scholar]
- 32.Selinka, H., T. Giroglou, and M. Sapp. 2002. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology 299:279-287. [DOI] [PubMed] [Google Scholar]
- 33.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shieh, J. T., and J. M. Bergelson. 2002. Interaction with decay-accelerating factor facilitates coxsackievirus B infection of polarized epithelial cells. J. Virol. 76:9474-9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 36.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiller, O. B., I. G. Goodfellow, D. J. Evans, J. W. Almond, and B. P. Morgan. 2000. Echoviruses and coxsackie B viruses that use human decay-accelerating factor (DAF) as a receptor do not bind the rodent analogue of DAF. J. Infect. Dis. 181:340-343. [DOI] [PubMed] [Google Scholar]
- 38.Tomko, R. P., C. B. Johansson, M. Totrov, R. Abagyan, J. Frisen, and L. Philipson. 2000. Expression of the adenovirus receptor and its interaction with the fiber knob. Exp. Cell Res. 255:47-55. [DOI] [PubMed] [Google Scholar]
- 39.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonew, M., M. Hartmann, M. Schmidtke, and A. Stelzner. 1995. Replication and persistence of coxsackieviruses B3 in human fibroblasts. Zentbl. Bakteriol. 282:92-101. [DOI] [PubMed] [Google Scholar]
- 41.Tonew, M., B. Wagner, M. Wagner, M. Schmidtke, and A. Stelzner. 1996. Permissiveness of human embryonal fibroblasts for coxsackieviruses B3. Investigations on virus genetic markers in vitro and localization of virus receptor distribution by immunogold replica technique. Zentbl. Bakteriol. 284:443-456. [DOI] [PubMed] [Google Scholar]
- 42.Turnbull, J., A. Powell, and S. Guimond. 2001. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 11:75-82. [DOI] [PubMed] [Google Scholar]
- 43.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]