Abstract
Avian reovirus and Nelson Bay reovirus are two unusual nonenveloped viruses that induce extensive cell-cell fusion via expression of a small nonstructural protein, termed p10. We investigated the importance of the transmembrane domain, a conserved membrane-proximal dicysteine motif, and an endodomain basic region in the membrane fusion activity of p10. We now show that the p10 dicysteine motif is palmitoylated and that loss of palmitoylation correlates with a loss of fusion activity. Mutational and functional analyses also revealed that a triglycine motif within the transmembrane domain and the membrane-proximal basic region were essential for p10-mediated membrane fusion. Mutations in any of these three motifs did not influence events upstream of syncytium formation, such as p10 membrane association, protein topology, or surface expression, suggesting that these motifs are more intimately associated with the membrane fusion reaction. These results suggest that the rudimentary p10 fusion protein has evolved a mechanism of inducing membrane merger that is highly dependent on the specific interaction of several different motifs with donor membranes. In addition, cross-linking, coimmunoprecipitation, and complementation assays provided no evidence for p10 homo- or heteromultimer formation, suggesting that p10 may be the first example of a membrane fusion protein that does not form stable, higher-order multimers.
Protein-mediated membrane fusion is an essential step in numerous cellular processes and in entry of enveloped viruses into cells (20, 22, 53). The viral fusion proteins involved in enveloped virus entry and virus-induced syncytium formation have been extensively characterized. Membrane fusion is dependent on extensive conformational changes in these complex, multimeric viral proteins that serve to regulate and drive the fusion reaction (46, 52). Much effort has been focused on defining the roles of specific fusion protein motifs in the membrane fusion reaction. Numerous studies have revealed the essential role that viral fusion peptides play in the membrane fusion reaction, serving to destabilize lateral lipid interactions and alter membrane curvature, leading to fusion pore formation (15, 19, 31, 39, 48). Studies on the influence of the transmembrane (TM) domains or endodomains of viral fusion proteins on membrane fusion, however, have yielded variable or contradictory results, and specific roles for these motifs remain unclear (1, 9, 34, 35, 38).
Avian reovirus (ARV) and Nelson Bay reovirus (NBV) are two of only a limited number of nonenveloped viruses that induce cell-cell fusion and multinucleated-syncytium formation (12). Both viruses encode homologous 10-kDa proteins (p10) that, when expressed by themselves in transfected cells, induce extensive cell-cell fusion (45). These fusion-associated small transmembrane (FAST) proteins are significantly smaller than the enveloped virus fusion proteins, containing only 95 to 98 amino acids. The reovirus FAST proteins are also the only examples of nonstructural viral proteins that induce syncytium formation and, as such, are not involved in virus entry. The primary, if not sole, purpose of the p10 FAST proteins is to induce fusion of virus-infected cells with neighboring uninfected cells (13, 45). Since p10 is not required for entry of the nonenveloped reoviruses, its evolution may have been freed from constraints imposed on enveloped virus fusion proteins, which require triggered conformational changes to regulate their fusion activity during virus entry (20, 22, 46). Consequently, the rudimentary p10 fusion proteins may offer a more minimalist system for analysis of those domains that are essential and directly involved in promoting membrane merger.
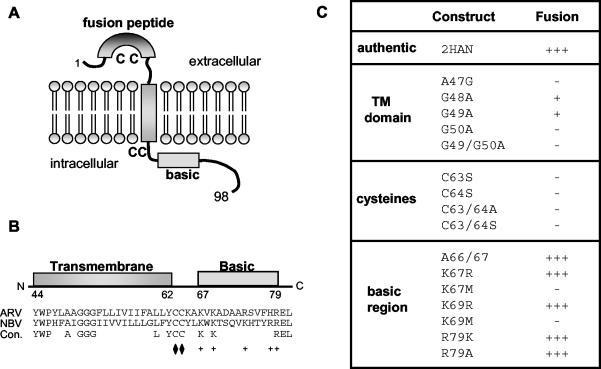
The p10 protein assumes a surface-localized type I topology (45), with a central TM domain that separates small (about 40 residues), approximately equal-size ecto- and endodomains (Fig. 1A). The topological arrangement of p10 contrasts markedly with that of the enveloped virus fusion proteins, which contain large, multidomain ectodomains representing the majority of the protein (20, 52, 53). Similar to enveloped virus fusion proteins, p10 contains a hydrophobic patch in its ectodomain. Mutations in this hydrophobic patch eliminate p10-induced cell fusion, suggesting that it may function as a fusion peptide via interactions with target membranes during the fusion reaction (45). The unusual topological arrangement of p10 suggests that the p10 endodomain and TM domain, which represent two-thirds of the protein, may play as significant a role in the fusion process as the ectodomain. The p10 endodomain contains two motifs of interest, a membrane-proximal dicysteine motif and an adjacent basic region, while the TM domain contains an unusual triglycine motif located near the exoplasmic face of the lipid bilayer (Fig. 1A and B). Preliminary mutagenic analysis indicated that alterations in the TM domain, cysteine residues, and basic region affect the membrane fusion activity of p10, but the basis for such effects was not determined (45).
FIG. 1.
p10 motifs and mutant constructs. (A) p10 structural motifs and membrane topology. The locations of the ectodomain fusion peptide, endodomain basic region, and conserved cysteine residues (C) are indicated. Numbers refer to amino acid residues. (B) Locations of the TM domain and basic region of p10. Numbers refer to amino acid residues. The aligned sequences of the ARV and NBV p10 proteins (residues 44 to 81) are shown, along with the locations of the conserved (Con.) residues. The locations of the membrane-proximal conserved dicysteine motif (diamonds) and conserved basic residues (+) are indicated. (C) Summary of p10 expression plasmid constructs. Mutant constructs containing substitutions in the TM domain, dicysteine motif, or basic region are indicated. The nomenclature for the mutant constructs refers to the identity of authentic residue, its location in the p10 sequence, and the identity of the substituted residue. Mutant fusogenic activity was qualitatively assessed on a + scale of − to +++ by immunostaining transfected cells and scoring the extent of syncytium formation relative to that of the parental p10-2HAN construct (see Fig. 2).
We now show that the p10 dicysteine motif is palmitoylated, a modification that is essential for p10-induced membrane fusion. Mutational and functional analyses indicated that the endodomain basic region affects cell fusion independent of any influence on p10 topology, providing the first evidence for the specific role of basic residues in the fusion activity of a viral membrane fusion protein. The TM domain of p10 also exerts a sequence-specific effect on membrane fusion. These results imply that the p10 fusion reaction is highly dependent on the interaction of several motifs with donor membranes. In addition, several different approaches failed to provide evidence for p10 multimer formation, suggesting that p10 may be the first example of a viral membrane fusion protein that does function as a higher-order multimer. The unusual involvement of a membrane fusion protein in the replication cycle of a nonenveloped virus may have contributed to the evolution of a membrane fusion reaction that is highly dependent on donor membrane interactions mediated by the rudimentary p10 membrane fusion protein.
MATERIALS AND METHODS
Cells and transfection.
The continuous quail cell line QM5 (14) was maintained in growth medium consisting of medium 199 supplemented with 10% fetal bovine serum and penicillin-streptomycin (50 U/ml and 50 μg/ml, respectively). Six- or 12-well cluster plates containing cells at 70% confluence were transfected by using 1 to 2 μg of plasmid DNA and 3 to 6 μl of Lipofectamine (Life Technologies) as previously described (45).
Plasmid constructs.
A functional p10 construct with an N-terminal hemagglutinin (HA) epitope tag was previously cloned into the eukaryotic expression vector pcDNA3 (45). An additional HA epitope tag was added to the N terminus of this construct. This construct retains membrane fusion activity, albeit with reduced fusion kinetics, but increases the sensitivity of the immunoassays (i.e., immunoprecipitation, surface immunofluorescence, and protein topology analysis). The p10-2HAN construct was used as the template for creation of 16 substituted p10 constructs. A similar series of HA epitope tags was added to the C terminus of a previously reported functional p10 construct carrying a single C-terminal epitope tag (45). All site-specific substitutions were made by using a previously described rapid PCR-based technique (45). The sequences of all constructs were confirmed.
Immunocytochemical and cell surface staining.
Expression of p10 constructs in transfected cells and p10-induced syncytium formation were detected immunocytochemically at 36 to 96 h posttransfection by using anti-HA monoclonal antibody and alkaline phosphatase-conjugated secondary antibody as previously described (45). Stained cells were photographed at a magnification of ×100 under bright-field microscopy. Cell-cell fusion was monitored for 4 days, the end point for maximal syncytium formation, before concluding that a particular construct was incapable of inducing fusion. The extent of cell-cell fusion was qualitatively assessed on a scale of − to +++ based on the number and size of syncytia detected by microscopy in immunostained transfected-cell monolayers.
Surface immunofluorescence was performed with live cells at 26 h posttransfection, as previously described (45). Briefly, cells were incubated with concentrated anti-HA antibody supernatants (5 mg of protein per ml) diluted 1:40 in Hanks' balanced salt solution (HBSS) containing 10% fetal bovine serum for 45 min at 4°C. Following extensive cold washes with HBSS, cells were fixed with ice-cold methanol for 2 min and incubated for 45 min with fluorescein isothiocyanate-labeled goat anti-mouse immunoglobulin G (IgG) (Life Technologies) diluted 1:25 in phosphate-buffered saline (PBS)-bovine serum albumin. Following extensive washes, cells were mounted, visualized with a Zeiss inverted microscope, and photographed at a magnification of ×630. The specificity of the surface staining protocol was confirmed by using a p10 construct with HA tags in the C-terminal endodomain, as previously reported (45).
Radiolabeling and immunoprecipitation.
QM5 cells were transfected and labeled with [35S]methionine (50 μCi/ml) for 30 min at 30 h posttransfection. For cotransfection studies, cells were transfected with 1 μg of each p10-expression plasmid. Radiolabeled cells were lysed on ice with 1 ml of radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 1 μg each of aprotinin, leupeptin, and pepstatin per ml). The cell lysates were incubated for 1 h with shaking at room temperature with 4 μl of concentrated anti-HA antibodies previously preincubated for 1 h with 15 μl of protein G-agarose (Life Technologies) or with a polyclonal anti-p10 rabbit antiserum followed by IgGsorb (The Enzyme Center) as previously described (45). Immune complexes were washed twice with each of the following buffers (each containing protease inhibitors): RIPA buffer, high-salt buffer (50 mM Tris-HCl [pH 7.5], 500 mM NaCl, 0.1% NP-40, 0.05% sodium deoxycholate), and low-salt buffer (50 mM Tris-HCl [pH 7.5], 0.1% NP-40, 0.05% sodium deoxycholate). Immune complexes were released by boiling in sodium dodecyl sulfate (SDS) protein sample buffer (28) before SDS-polyacrylamide gel electrophoresis (SDS-PAGE) with 15% acrylamide gels.
Membrane fractionation.
Transfected QM5 cells were labeled with [35S]methionine (50 μCi/ml) for 30 min when extensive syncytium was observed. The membrane fraction was obtained following cell disruption by syringing cells 10 times through a 30-gauge needle as previously described (45). The membrane pellet was treated with 100 mM Na2CO3 (pH 11.3) for 30 min on ice to extract peripheral membrane-associated proteins, followed by centrifugation at 100,000 × g for 25 min to recover the membrane fraction and associated integral membrane proteins. The final membrane pellet was dissolved in RIPA buffer with protease inhibitors, and membrane-associated p10 was detected by immunoprecipitation as described above. Controls for the membrane purification protocol included the absence of the soluble viral σC protein from the membrane pellet fractions and the absence of p10 in the soluble fraction.
Palmitoylation assay.
At 36 h posttransfection, 6 × 106 QM5 cells, transfected with either p10-2HAN or various cysteine mutants, were prelabeled with serum-free Dulbecco's modified Eagle's medium for 30 min. [3H]palmitic acid (500 μCi) was dried under nitrogen, resuspended in 20 μl of dimethyl sulfoxide (DMSO), diluted to 2 ml in serum-free DMEM, and added to cells for 1 h. The cells were lysed and immunoprecipitated as described above. Immune complexes were disrupted by addition of protein sample buffer (28) without reducing agents and were not heated prior to fractionation by SDS-PAGE with 15% acrylamide gels. Gels were processed for fluorography by using 2,5-diphenyloxazole (PPO)-DMSO as previously described (11). In parallel, a duplicate flask of transfected cells was preincubated with leucine-free medium, labeled with 100 μCi of [3H]leucine per ml, and similarly processed.
Chemical cross-linking.
Two million cells were transfected and labeled with [35S]methionine (75 μCi/ml) for 30 min at 30 h posttransfection. The cells were suspended by incubation in PBS for 20 min at room temperature and collected by centrifugation at 700 × g for 7 min. Rather than determining conditions for cross-linking of a positive control protein, where such conditions are unlikely to reflect those that would be appropriate for the heterologous p10 protein, we chose instead to use seven different cross-linking reagents with different reactive-group specificities, spacer arm lengths, and membrane permeation properties. Sulfo-N-hydroxysuccinimide-SS-biotin (NHS), polyethylene oxide-malemide activated biotin (PEO), dithiobis(succinimidylpropionate) (DSP), ethylene glycolobis(succinimidylsuccinate) (EGS), disuccinimidyl tartrate (DST), disuccinimidyl suberate (DSS), and dithio-bismaleimidoethane (DTME) (all from Pierce Biochemical) were dissolved in water (NHS, PEO, and DSS) or DMSO (DSP, EGS, DST, and DTME) at a concentration of 200 mM immediately prior to use, diluted to 2 mM in cross-linking buffer (HBSS with 10 mM HEPES [pH 7.0]), and added to suspended cell pellets. The cross-linking reaction mixture was incubated with shaking at room temperature for 60 min. Reactions were stopped with 40 mM glycine-PBS. The cells were lysed by addition of an equal volume of 2× RIPA buffer followed by immunoprecipitation with HA-specific monoclonal antibodies. Immune complexes were disrupted by addition of protein sample buffer (28) without reducing agents and were not heated prior to fractionation by SDS-PAGE with 15% acrylamide gels.
RESULTS
Substitution analysis of the p10 TM and cytoplasmic domains.
As shown in Fig. 1, a triglycine motif in the TM domain, a membrane-proximal dicysteine motif, and an adjacent endodomain basic region are all conserved in the p10 proteins of ARV and NBV. Previous mutagenic analysis indicated that replacement of Gly49 in the TM domain, of Cys63 in the dicysteine motif, and of Lys69 in the basic region all inhibited the syncytium-inducing activity of p10 (45). Whether these substitutions directly affected the membrane fusion reaction or exerted their influence indirectly by affecting upstream events (e.g., p10 membrane topology or localization) was not determined.
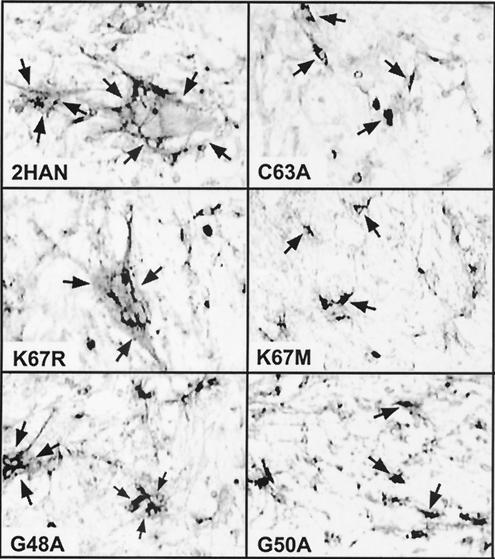
To gain a clearer understanding of the role of specific TM domain and endodomain motifs in the syncytium-inducing ability of p10, we undertook a more comprehensive mutagenic analysis of these regions by using a functional p10-2HAN construct that permitted determination of the membrane topologies and surface localizations of the substituted proteins. The double-tagged p10 construct reduced the rate of p10-induced syncytium formation (from +++ to ++ in the qualitative fusion assay described in the legend to Fig. 2) (M. Shmulevitz et al., submitted for publication) but improved the sensitivity of the immunoassays for p10 surface expression and membrane topology (Fig. 2; see Fig. 4 and 5). A series of 16 p10 constructs that carried site-specific substitutions in the TM domain or endodomain of p10-2HAN were created (Fig. 1). These constructs were transfected into QM5 quail fibroblasts, and the extent of syncytium formation was assessed by immunostaining transfected-cell monolayers (Fig. 2). The extent of cell-cell fusion was qualitatively assessed on a scale of − to +++ (summarized in Fig. 1C) based on the number and size of syncytia detected by microscopy. Cell-cell fusion was monitored for 4 days, the end point for maximal syncytium formation, before concluding that a particular construct was fusion negative. The effects of specific substitutions on p10-induced membrane fusion are described below.
FIG. 2.
Syncytium-inducing activity of p10 constructs. Cells were transfected with various p10 constructs and fixed at 36 h posttransfection. The fixed cells were immunostained with anti-HA monoclonal antibodies and goat anti-mouse IgG conjugated with alkaline phosphatase. Arrows in the left panels indicate the boundaries of antigen-positive syncytial foci. The brighter areas surrounded by darkly stained areas indicate antigen-positive cytoplasm surrounding multiple antigen-negative nuclei in a single syncytium. Arrows in the right panels indicate single, darkly staining antigen-positive cells in monolayers transfected with syncytium-negative p10 constructs. Magnification, ×100. A subset of p10 constructs are shown, which include substitutions that did not significantly alter the fusogenic activity of p10 (K67R), those that drastically reduced both the number and size of syncytia (G48A), and those that eliminated p10-induced syncytium formation (C63A, K67 M, and G50A). The qualitative fusion activities of all of the p10 constructs, assessed in a similar manner, are summarized in Fig. 1C.
FIG. 4.
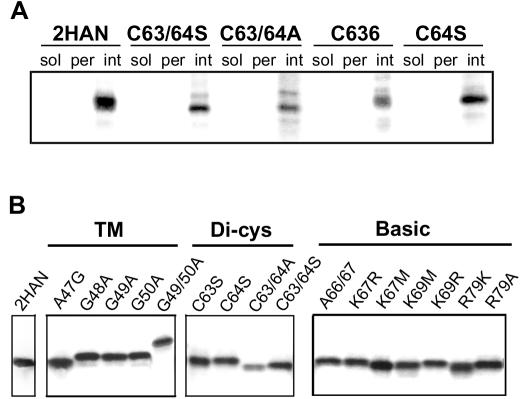
Integral membrane association of p10 constructs. (A) Transfected, radiolabeled cells were disrupted and fractionated into the soluble (sol) and membrane pellet fractions by ultracentrifugation. The membrane pellet was resuspended in high-pH extraction buffer and repelleted to separate the extracted, peripheral membrane-associated proteins (per) from the integral membrane proteins (int). The presence of HA-tagged p10 (2HAN) and the indicated substituted p10 constructs in each of the subcellular fractions was detected by immunoprecipitation, SDS-PAGE, and fluorography. (B) The presence of all of the substituted p10 constructs in the integral membrane fraction was confirmed, as described for panel A.
FIG. 5.
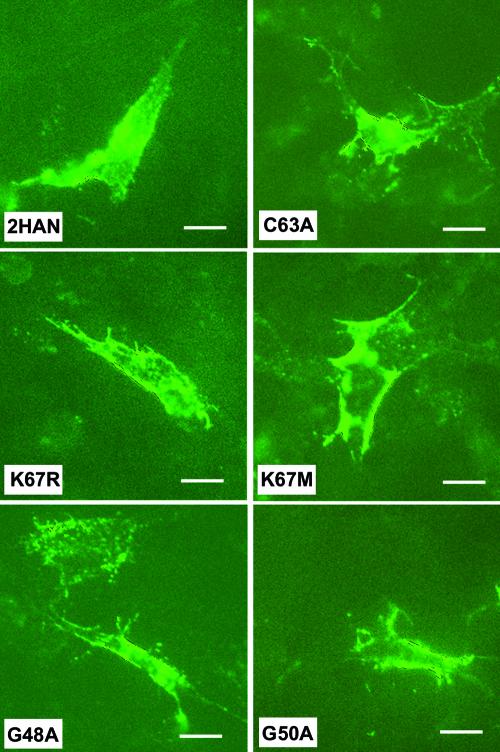
Surface localization of p10 constructs. Live cells transfected with various p10 constructs were treated with anti-HA monoclonal antibodies to detect the presence of the surface-localized N-terminal HA epitope tags on the p10 constructs. Surface-bound anti-HA antibodies were detected by immunofluorescence with fluorescein isothiocyanate-conjugated goat anti-mouse IgG. A subset of the p10 constructs are shown. All of the p10 constructs, assessed in the same manner, yielded similar results. Images were captured under identical parameters. Magnification, ×630; bars, 20 μm. The specificity of the surface-staining protocol was confirmed by using a p10 construct with HA tags in the C-terminal endodomain, as previously described (45), and is evident from the low level of background staining of the surrounding nontransfected cells in the confluent monolayer.
Palmitoylation of the p10 dicysteine motif is essential for fusion activity.
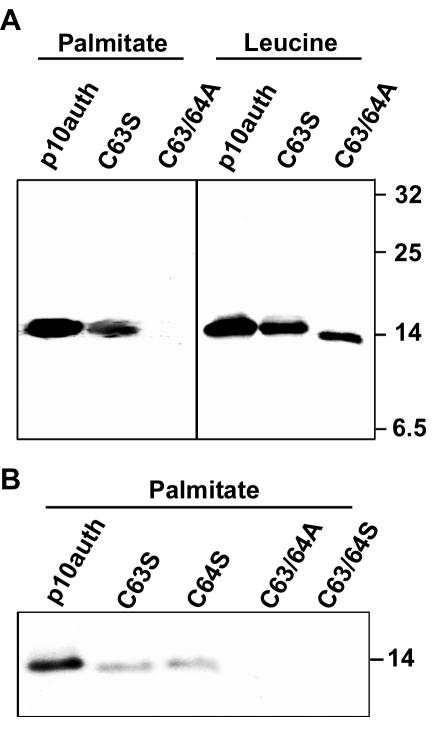
Both ARV and NBV contain two conserved cysteines adjacent to the transmembrane domain (Fig. 1). HA-tagged p10 containing either substitutions of cysteine to serine (C63S and C64S) or dual substitution of both cysteines to either alanines or serines (C63/64A and C63/64S) produced p10 proteins that were unable to induce cell-cell fusion (Fig. 1 and 2). The C63S substitution in the context of authentic, untagged p10 retains a low level of fusion activity, while the C63/64A construct in the same background is fusion negative (45), further indicating the importance of the dicysteine motif. Since palmitoylation of transmembrane-proximal cysteine residues is common to many enveloped virus fusion proteins (6, 37, 55), we used [3H]palmitic acid to determine whether the p10 dicysteine motif is palmitoylated. As shown in Fig. 3, p10 was labeled by [3H]palmitic acid, suggesting that the protein is palmitoylated. The p10-2HAN constructs carrying the C63S or C64S substitutions showed reduced [3H]palmitic acid incorporation, while the C63/64A and C63/64S constructs, where both cysteines were replaced with either serines or alanines, exhibited a complete loss of labeling with [3H]palmitic acid (Fig. 3), indicating that both cysteines are targets for palmitoylation. The loss of labeling by [3H]palmitic acid in the C63/64A and C63/64S constructs also confirmed that the labeling of p10-2HAN was specific and not due to metabolic redistribution of the radiolabel. As shown for the C63S and C63/64A constructs (Fig. 3A), analysis of transfected cells labeled with [3H]leucine demonstrated that the p10 constructs were expressed at similar levels and revealed a slight mobility shift in the double cysteine substitution, presumably due to the loss of both palmitates (Fig. 3A). These results clearly indicated that the p10 dicysteine motif is palmitoylated and that loss of palmitoylation correlates with a loss of fusion activity.
FIG. 3.
The endodomain cysteines of p10 are palmitoylated. Cells were transfected with the authentic p10-2HAN construct or with p10 constructs containing the indicated substitutions in the endodomain dicysteine motif. Transfected cells were radiolabeled with [3H]palmitic acid or [3H]leucine (A) or with [3H]palmitic acid (B), and the radiolabeled p10 proteins were detected by immunoprecipitation, SDS-PAGE, and fluorography. The relative migration of molecular mass markers (in kilodaltons) is indicated on the right.
The membrane-proximal basic region of p10 is involved in membrane fusion.
Five of the six basic residues present within ARV p10 are located in a membrane-proximal basic region in the endodomain (Fig. 1). Previous results indicated that a nonconservative substitution of Lys69 eliminated the syncytium-inducing ability of p10 (45). To further explore the influence of the conserved basic region on p10 function, a series of seven substitutions in this region were analyzed. Conservative substitutions of the lysine residues (K67R or K69R) or insertion of a single alanine spacer between the TM domain and the basic region (A66/67) had no effect on the fusogenic activity of p10 (Fig. 1 and 2). However, nonconservative substitutions (K67M or K69M) blocked p10-induced cell fusion. Conversely, a nonconservative substitution of Arg79 (R79A) had no effect on p10 function, indicating that conservation of this basic residue in the ARV and NBV p10 proteins is not essential. Hence, a minimum of two, and possibly as many as five (Lys65, Arg74, and His78 were not investigated), basic residues adjacent to the p10 TM domain are required for p10-induced membrane fusion.
The triglycine motif within the TM domain of p10 is essential for membrane fusion.
The ARV and NBV p10 fusion proteins contain a conserved triglycine motif within the TM domain (Fig. 1). A G49A substitution in p10 eliminated syncytium formation over a 36-h time course, suggesting the importance of p10 TM domain glycines in fusion activity, similar to results of previous studies with enveloped virus fusion proteins (9, 34). Additional alanine substitutions (G48A and G50A) further demonstrated the importance of the p10 TM triglycine motif. Over the extended 4-day time course of syncytium analysis, both the G48A and G49A constructs displayed a drastic reduction in syncytium formation (Fig. 1), yielding far fewer syncytial foci than authentic p10-2HAN. A field showing two such small syncytia is presented in Fig. 2. Replacement of the glycine at position 50 by alanine (G50A) abolished its fusogenic activity, as did the double mutation of glycines 49 and 50 (G49/50A). Interestingly, increasing the glycine stretch to four consecutive glycines (A47G construct) also abolished p10-induced fusion (Fig. 1). These results confirmed and extended previous data indicating that the TM triglycine motif exerts a sequence-specific effect on the fusogenic activity of p10.
Substitutions in the p10 TM domain or endodomain do not influence p10 membrane insertion, surface expression, or topology.
To determine whether the various substitutions in p10 directly or indirectly influence the fusion activity of the protein, we examined several known properties of p10 that indirectly contribute to cell-cell fusion. The p10 protein is most likely targeted to the endoplasmic reticulum-Golgi transport pathway via its TM domain, which serves as an internal signal-anchor sequence (32, 45). All of the p10 mutants remained as integral membrane proteins following cell fractionation of transfected cells under conditions that displace peripheral proteins (Fig. 4). Slight differences in the gel mobilities of various substituted p10 constructs presumably reflected variable binding of amino acids to SDS. We therefore excluded the possibility that the substitutions might indirectly influence p10-induced membrane fusion by altering the insertion of the protein into the endoplasmic reticulum membrane.
To determine whether the p10 substitutions abolished syncytium formation by altering p10 membrane topology or trafficking to the plasma membrane, we used anti-HA monoclonal antibodies to detect the N-terminal HA epitope tags present on all p10 constructs. Attempts to quantify surface fluorescence by flow cytometry were unsuccessful, presumably due to a low level of surface expression of p10. Therefore, immunofluorescence of nonpermeabilized transfected cells was used to examine p10 surface expression and topology, as previously reported (45). Images were captured under identical parameters such that fluorescence intensity served as a qualitative indicator of p10 surface expression. The specificity of the surface staining protocol was previously demonstrated by using p10 constructs with HA tags in the C-terminal endodomain (45) and is evident from the low level of background staining of nontransfected cells surrounding the antigen-positive foci in the monolayer (Fig. 5). As shown in Fig. 5 for a subset of the p10 constructs, none of the substitutions in the p10 endodomain or TM domain significantly altered the ability of p10 to assume the correct type I surface topology. This observation was particularly noteworthy for the altered basic region constructs, since basic residues are known to be major determinants of membrane protein topology (32, 51, 54).
Based on these results, it is unlikely that the loss of membrane fusion activity displayed by any of the substituted p10 constructs reflected alterations in p10 membrane insertion, trafficking, or topology. These results suggested that the p10 TM domain, palmitoylated dicysteine motif, and basic region may all specifically influence the p10 membrane fusion reaction.
Evidence that p10 may function as a monomer.
All of the enveloped virus membrane fusion proteins function as multimers, usually assuming a functional trimeric structure (20, 53). Since the multimeric status of p10 has not been determined, we could not exclude the possibility that the loss of syncytium-inducing activity of any of the substituted p10 constructs reflected an inhibition of p10 multimer formation. We therefore undertook a series of experiments to determine whether p10 functions as a multimer. Surprisingly, several different approaches all failed to provide evidence that p10 functions as a higher-order multimer.
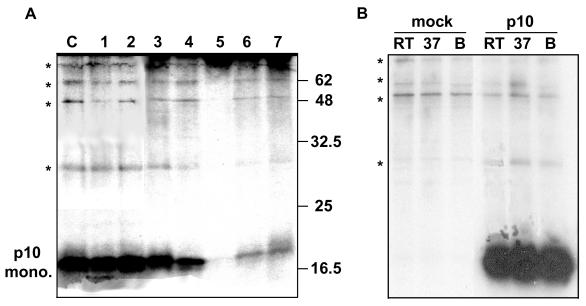
Determinations of the multimeric status of membrane proteins by using biophysical approaches, such as gel filtration or sedimentation analysis, are confounded by the presence of protein-detergent micelles. Rather than relying on a single cross-linker with a heterologous positive control protein (since conditions that cross-link one protein are unlikely to be the same for a different protein), we used a series of seven different cross-linkers to detect p10 homo- or heteromultimers. These cross-linkers included reagents that react with either amino (NHS, DSP, EGS, DST, and DSS) or sulfhydryl (DTME and PEO) groups, that are either membrane permeative (DSP, EGS, DST, and DTME) or impermeative (NHS, DSS, and PEO), and that contain a range of spacer arm lengths between 6.4 and 29.3 Å. Gel analysis of the cross-linked cell lysates was performed under nonreducing conditions and without prior boiling of the protein samples to prevent cleavage of susceptible chemical groups. Despite these precautions and the diversity of cross-linkers, the only form of p10 detected in transfected cells was the monomer (Fig. 6A). A series of slower-migrating, minor protein bands were present in all samples analyzed (Fig. 6A), including the control p10-transfected samples that were not treated with a cross-linker. Additional studies with mock-transfected cells treated at three different temperatures before gel analysis indicated that the slower-migrating species represented nonspecific precipitation of host cell proteins and not p10 homo- or heteromultimers (Fig. 6B).
FIG. 6.
Cross-linking analysis of p10. (A) Radiolabeled cell suspensions were prepared from transfected cells expressing the p10-2HAN construct. The suspended cells were treated with one of seven different chemical cross-linking reagents (lane 1, PEO; lane 2, DTME; lane 3, DSS; lane 4, DST; lane 5, EGS; lane 6, DSP; lane 7, NHS). Control cell suspensions (lane C) were mock-treated with cross-linkers. The cells were disrupted, and the presence of p10 monomers or multimers was detected by SDS-PAGE under nonreducing conditions following immunoprecipitation with anti-HA antibody. The relative migration of molecular mass markers (in kilodaltons) is indicated on the right. Asterisks indicate the locations of background cell proteins present in all samples, including the control. (B) Mock- or p10-transfected cells were treated as described for the cross-linking control in panel A. Samples were treated at room temperature (RT), 37°C, or boiling (B) prior to gel analysis. *, locations of the faint high-molecular-weight protein species, indicative of background precipitation of host cell proteins rather than multimeric species of p10.
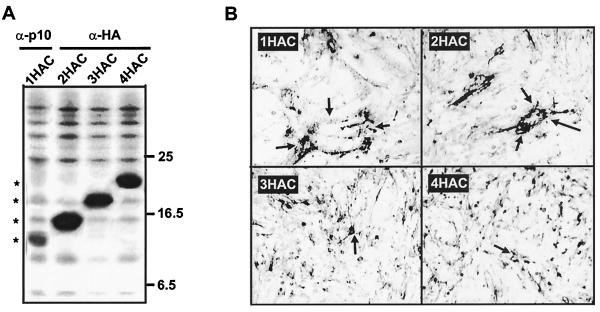
The inability to detect p10 homo- or hetromultimers by cross-linking was surprising given that all enveloped virus fusion proteins function as multimers. However, cross-linking studies are highly dependent on experimental conditions and the accessibility and spacing of reactive groups, making negative results difficult to interpret. Therefore, two additional lines of investigation were undertaken to probe the multimeric status of p10. We had available various HA-tagged p10 constructs containing one to four HA epitope tags at either the N or C terminus of the protein. As shown for the C-terminally tagged p10 constructs, sequential addition of epitope tags led to decreased gel mobility of p10 and a progressive loss in syncytium formation (Fig. 7). A double HA tag allowed efficient detection of p10 by immunoprecipitation while retaining the syncytium-inducing ability of the protein (Fig. 7). If p10 forms homomultimers, then cotransfection of authentic p10 and a functional HA-tagged p10 construct would be expected to lead to the formation of mixed multimers consisting of both species of p10. Since the HA monoclonal antibody recognizes only the tagged version of p10, coprecipitation of the authentic and HA-tagged p10 by the HA monoclonal antibody would be indicative of multimer formation between the two species of p10. A similar approach was previously used to demonstrate the trimeric nature of the mammalian reovirus σ1 protein (47).
FIG. 7.
Sequential addition of HA epitope tags to the C terminus of p10. The authentic p10 expression construct was modified by sequential addition of HA epitope tags to the C terminus to generate the p10-1HAC, p10-2HAC, p10-3HAC, and p10-4HAC constructs. (A) Radiolabeled cell lysates were prepared from cells transfected with each of the HA-tagged constructs, immunoprecipitated with anti-HA (α-HA) monoclonal antibody, and detected by SDS-PAGE and fluorography. The p10-1HAC construct was not precipitated by the anti-HA monoclonal antibody and was therefore precipitated with a low-avidity polyclonal anti-p10 antiserum. Asterisks indicate the electrophoretic mobilities of the various tagged p10 constructs. The relative migration of molecular mass markers (in kilodaltons) is indicated on the right. (B) Cell monolayers transfected with the same constructs were immunostained with anti-p10 or anti-HA and alkaline phosphatase-conjugated secondary antibody. Magnification, ×100. Arrows in the top two panels indicate the boundaries of syncytial foci induced by the functional 1HAC and 2HAC constructs, while arrows in the lower panels indicate single antigen-positive cells present in monolayers transfected with the nonfunctional 3HAC and 4HAC constructs.
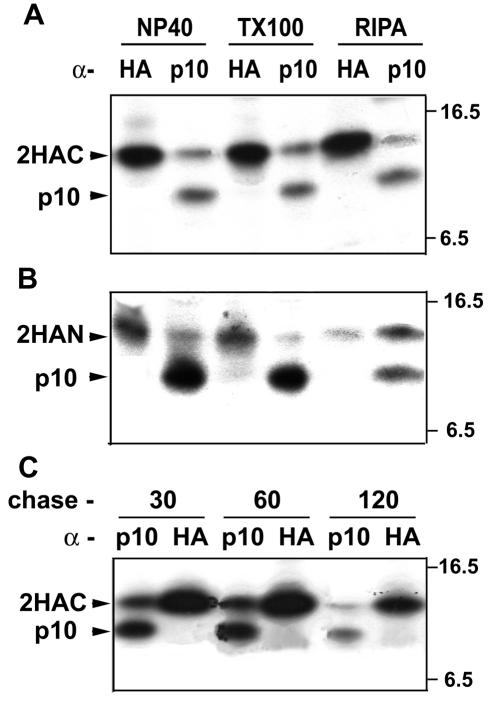
Cells were cotransfected with plasmids expressing authentic p10 and the functional p10-2HAC construct. Immunoprecipitation of the cotransfected cells with a polyclonal anti-p10 antiserum clearly revealed the presence of both species of p10, which were distinguished based on the slower gel mobility of the extended p10-2HAC protein (Fig. 8A). Conversely, the HA monoclonal antibody precipitated only the HA-tagged version of p10 under a variety of detergent conditions, including such gentle extractions as those with 0.1% NP-40 or Triton X-100 (Fig. 8A) or with Tween 20 or saponin (data not shown). The same results were obtained with the N-terminally tagged p10 construct (p10-2HAN) used for mutational and topology studies (Fig. 8B). Extended chases of 2 h or more following the pulse-labeling, to allow time for p10 transport to the cell surface and slow multimer formation, also failed to detect p10 multimerization (Fig. 8C). Identical results were obtained with a threefold increase in the ratio of plasmid expressing authentic p10 to plasmid expressing HA-tagged p10 (data not shown). These results supported the cross-linking studies suggesting that p10 may function as a monomer.
FIG. 8.
Authentic p10 and HA-tagged p10 do not form mixed multimers. Authentic p10 and the p10-2HAC construct (A and C) or p10-2HAN construct (B) were coexpressed in transfected cells. (A and B) Radiolabeled cell lysates were prepared by cell disruption with either 0.1% NP-40, 0.1% Triton X-100 (TX100), or RIPA buffer. (C) Transfected cells were pulse-labeled for 15 min, followed by chases of 30, 60, or 120 min before preparation of cell lysates with 0.1% NP-40. In all panels, the cell lysates were immunoprecipitated with anti-p10 polyclonal antiserum or anti-HA monoclonal antibodies, and the immunoprecipitates were detected by SDS-PAGE and fluorography. The locations of the HA-tagged p10 and authentic p10 are indicated on the left, while the relative migration of molecular mass markers (in kilodaltons) is indicated on the right.
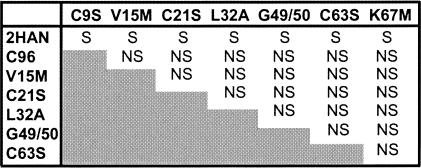
In addition to the above-described direct approaches to detect p10 multimers, pairwise cotransfections with different syncytium-negative p10 constructs, which included substitutions in the TM domain, ectodomain, or endodomain, revealed that none of the mutants complemented each other (Fig. 9). Furthermore, cotransfection of the syncytium-negative p10 constructs with functional p10-2HAN revealed that none of the mutants exerted a dominant-negative effect on p10-induced cell fusion, as might be expected if p10 formed homomultimers (Fig. 9).
FIG. 9.
Syncytium-negative p10 constructs do not complement each other or exert a dominant-negative effect on syncytium formation. Cells were cotransfected in a pairwise fashion either with equal proportions of plasmids expressing different syncytium-negative p10 constructs or with functional p10-2HAN in combination with each of the syncytium-negative p10 constructs. Syncytium-negative p10 constructs included the substitutions in the TM domain (G49/50A), dicysteine motif (C63S), or basic region (K67M) described in this report, as well as several syncytium-negative constructs carrying substitutions in the p10 ectodomain. Transfected monolayers were observed for transfection efficiency and syncytium formation over time by immunostaining as described in the legend to Fig. 2 and were scored as syncytial (S) or nonsyncytial (NS). None of the syncytium-negative p10 constructs exerted a dominant-negative effect on syncytium formation induced by p10-2HAN, and the syncytium-negative p10 constructs were unable to complement each other.
Based on the cumulative evidence, we infer that p10 may not function as a stable higher-order multimer. Therefore, the loss of fusion activity displayed by p10 carrying substitutions in the TM domain or endodomain is unlikely to be the result of alterations in the multimeric status of p10, further supporting a direct role for these motifs in the fusion reaction.
DISCUSSION
Donor membrane interaction motifs specifically influence p10-induced syncytium formation.
Substitutions in the TM triglycine motif, the palmitoylated cysteines, and the basic region of the reovirus p10 fusion protein severely restricted or eliminated p10-induced cell-cell fusion. Alterations in these motifs affected p10-induced syncytium formation independent of any effects on protein membrane insertion, topology, or surface localization. Evidence obtained by using several different approaches also implied that p10 may not form higher-order multimers; therefore, the syncytium-negative substitutions are unlikely to exert their effect by altering p10 multimer formation. These results are consistent with a direct role for the p10 TM domain, basic region, and palmitoylated dicysteine motif in the fusion reaction. Consequently, membrane fusion induced by p10 appears to be highly dependent on several sequence-specific interactions with the donor membrane.
The essential role of palmitoylation in p10-induced syncytium formation.
Numerous membrane proteins are palmitoylated, including several enveloped virus fusion proteins, though the influence of palmitoylation on their membrane fusion activity is variable and the specific role of the fatty acids remains unclear (6, 17, 23, 24, 43). As we have shown, a membrane-proximal dicysteine motif in p10 is palmitoylated. The conservative nature of the Cys-Ser substitutions is consistent with a role for the fatty acid, rather than an essential role for the conserved cysteine residues per se, in fusion activity of p10. The reduced fusogenic activity of a single substituted cysteine in the context of authentic, untagged p10 (45) and the loss of fusion activity of the same substitution in the p10-2HAN context (this study) implies that both palmitates exert an influence on p10 fusion activity. Insertion of the monoacylated palmitate residues of p10 into the donor membrane would be expected to lead to localized alterations in lipid packing (42, 50) that may be required to facilitate p10-induced membrane fusion.
Implications of the potential monomeric status of p10.
A diversity of approaches all failed to detect p10 homo- or heteromultimerization. Although atomic-level structural determination of p10 is required to conclusively state that p10 exists as a monomer, our present results suggest that p10 may be the first viral membrane fusion protein that does not function in a stable, higher-order structure. The potential monomeric nature of p10 suggests a second possible role for palmitoylation in the fusion process. It is highly unlikely that a single monomer of p10 would be capable of mediating cell-cell fusion. It is more probable that some minimal number of p10 monomers must congregate in a localized region on the plasma membrane in order to initiate and drive the fusion reaction. In this regard, the palmitate residues could provide essential interactions with membrane cholesterol, conceivably mediating concentration of p10 in membrane microdomains (4, 21).
Interestingly, a second, unrelated reovirus FAST protein (the baboon reovirus p15 protein) lacks membrane-proximal cysteine residues, suggesting that it is not palmitoylated, but it is N-terminally myristylated, and this modification is essential for syncytium formation (11). Consequently, acylation represents an essential feature of the reovirus FAST proteins. Analysis of the association of p10 and p15 FAST proteins with lipid microdomains and of the influence of the acyl groups on any such association may provide additional insights into the role of acylation in FAST-induced syncytium formation.
The p10 TM domain exerts a sequence-specific effect on syncytium formation.
The inability of glycophosphatidylinositol-anchored enveloped virus fusion proteins and soluble N-ethylmalemide-sensitive attachment protein receptor proteins to mediate membrane fusion suggests that there may be a universal need for a membrane-spanning anchor sequence to facilitate complete membrane merger (25, 33, 38). Whether TM domains exert a sequence-dependent or -independent role on membrane fusion varies for different viral fusion proteins (1, 9, 34, 38). As previously shown, TM domains can contribute to multimer formation, frequently influenced by glycine residues that promote tight packing of adjacent TM domains (30, 36, 44). Our evidence that p10 may function as a monomer argues against such a role for the p10 TM glycine residues. If membrane fusion requires substantial stress perpendicular to the membrane surface, then a membrane-spanning TM domain may be required to anchor the protein firmly within the donor membrane (1). A nonhelical conformation of the triglycine motif could serve to lengthen the TM domain to permit optimal spanning of the membrane, as previously suggested for the TM anchors of various enveloped virus and intracellular vesicle fusion proteins (1, 26, 33, 34, 38). If the length of the TM domain is the deciding factor, then the length requirement is fairly precise, since addition of an extra glycine residue (G49/50A) to the triglycine motif leads to loss of fusion activity. A stringent length requirement for the TM domain of influenza virus HA has been previously reported, with a minimum of 17 residues required to retain fusion activity (1).
In addition to length, TM domains may exert a sequence-dependent effect by altering lipid packing or membrane curvature within the donor lipid bilayer as part of the fusion process (8, 9, 29, 34, 49). In the case of p10, the small volume of the glycine residues and free mobility around the glycine-glycine bonds could facilitate additional mobility of the fatty acyl groups of adjacent lipids. Furthermore, helical depictions of the p10 TM domain indicate a sided structure, with the alignment of bulky, hydrophobic aromatic residues along one side of the transmembrane domain and predominantly aliphatic amino acids on the other face of the helix (data not shown). This sided structure, in conjunction with the triglycine motif, may exert localized influences on membrane structure in a manner similar to that proposed for other peptides that alter lipid packing (3, 16).
Basic residues in the endodomain of p10 are required for syncytium formation.
A minimal number of basic residues, located in the endodomain and adjacent to the cytoplasmic face of the membrane, are essential for the fusion activity of p10. Both Lys67 and Lys69 are essential for p10 membrane fusion activity independent of any role they may play in determining protein topology (32, 51, 54). As far as we are aware, this is the first example of basic residues playing an essential role in the activity of a membrane fusion protein, independent of effects on determining protein topology. Since cationic peptides can partially penetrate through the lipid head groups of lipid bilayers (16, 40, 44), interactions between the p10 cationic residues and the anionic phospholipid head groups of the inner leaflet may serve to increase the head group/acyl chain volume ratio and promote the correct curvature necessary for fusion (10, 40, 41).
An assessment of the cytoplasmic regions of various enveloped virus fusion proteins (data not shown) showed a variable quantity of basic residues, ranging from two to seven, with no similarity in their arrangement or spacing. Although the clustering of basic residues adjacent to a TM domain may not occur in all enveloped virus fusion proteins, it is a hallmark feature of the viroporins, a family of small viral proteins involved in increased membrane permeability and virion release (5, 7, 18, 27). We previously noted the similarity between p10 and viroporins, with a basic region adjacent to a TM domain (45). A recent study provided direct evidence that p10 may share the membrane permeabilization property of the viroporins, and deletion analysis implicated the p10 TM domain or endodomain in this process (2). It is conceivable that the palmitoylated dicysteine motif, TM triglycine motif, or endodomain basic motif of p10 may influence the proposed viroporin-like activity via interactions with the donor membrane.
Potential relationship between the role of p10 in the virus replication cycle and the evolution of p10 structure and function.
For enveloped viruses, motifs that alter lipid interactions in the donor membrane may need to be minimized to ensure that the integrity of the viral envelope is not compromised. As a result, enveloped viruses have evolved to be highly dependent on the activity of very hydrophobic and efficient fusion peptides. In contrast, NBV and ARV are nonenveloped viruses that are not dependent on p10 for virus entry. In fact, p10 and the other reovirus FAST proteins are not components of the virion but are nonstructural proteins expressed in virus-infected cells (11, 45). Therefore, the donor membrane for the FAST proteins represents the plasma membrane of a virus-infected cell, not a virus envelope. This fact may have reduced limitations on the number and nature of motifs acquired by p10 that function on the donor membrane, allowing p10 to evolve by assembling a diversity of motifs that provide the minimum sum of forces necessary for fusion of biological membranes. These motifs include a potential fusion peptide that is only moderately hydrophobic (45) and whose reduced fusogenic activity may be compensated for by several motifs that act in concert on the donor membrane to overcome the barriers that prevent spontaneous membrane fusion. Between the polybasic, dipalmitate, triglycine, and fusion peptide motifs, p10 could exert fusion-promoting lipid alterations on both leaflets of the donor membrane in addition to the outer leaflet of the target membrane.
In addition, the multimeric status of enveloped virus fusion proteins is an essential feature involved in regulating their fusion activity via complex conformational changes that regulate the exposure of a sequestered fusion peptide motif (46, 52, 53). The enveloped virus fusion proteins also frequently serve as the viral receptor-binding protein. The nonstructural nature of the FAST proteins suggests that p10 fusion activity may not be subject to the same regulatory requirements as enveloped virus fusion proteins. Furthermore, p10 is a promiscuous fusogen that fuses numerous cell types, suggesting that p10 may not possess specific receptor-binding activity but rather may depend on cellular adhesion proteins to mediate cell-cell contact and close membrane apposition. These properties of p10-induced membrane fusion may allow p10 to function as a monomer. The atypical relationship of the reovirus FAST proteins to the virus replication cycle suggests that additional structural and functional analysis of these unusual membrane fusion proteins will continue to offer new insights into altered mechanisms of protein-mediated membrane fusion.
Acknowledgments
We thank Jingyun Shou for expert technical assistance and Jennifer Corcoran and Sandra Dawe for insightful discussions and comments on the manuscript.
This research was funded by a grant from the Canadian Institutes of Health research (CIHR). R.D. is the recipient of a CIHR-RPP Investigators Award. M.S. was funded by scholarships from the Natural Sciences and Engineering Research Council (NSERC) of Canada and from the Killam Foundation. J.S. was funded by a scholarship from Cancer Care Nova Scotia.
REFERENCES
- 1.Armstrong, R. T., A. S. Kushnir, and J. M. White. 2000. The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J. Cell Biol. 151:425-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodelon, G., L. Labrada, J. Martinez-Costas, and J. Benavente. 2002. Modification of late membrane permeability in avian reovirus-infected cells. J. Biol. Chem. 277:17789-17796. [DOI] [PubMed] [Google Scholar]
- 3.Brasseur, R., T. Pillot, L. Lins, J. Vandekerchkhove, and M. Rosseneu. 1997. Peptides in membranes: tipping the balance of membrane stability. Trends Biochem. Sci. 22:167-171. [DOI] [PubMed] [Google Scholar]
- 4.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 5.Browne, E. P., R. Bellamy, and J. A. Taylor. 2000. Membrane-destabilizing activity of rotavirus NSP4 is mediated by a membrane-proximal amphipathic domain. J. Gen. Virol. 81:1955-1959. [DOI] [PubMed] [Google Scholar]
- 6.Caballero, M., J. Carabana, J. Ortego, R. Fernandez-Munoz, and M. L. Celma. 1998. Measles virus fusion protein is palmitoylated on transmembrane-intracytoplasmic cysteine residues which participate in cell fusion. J. Virol. 72:8198-8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrasco, L. 1995. Modification of membrane permeability by animal viruses. Adv. Virus Res. 45:61-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, K. W., Y. Sheng, and J. L. Gombold. 2000. Coronavirus-induced membrane fusion requires the cysteine-rich domain in the spike protein. Virology 269:212-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleverley, D. Z., and J. Lenard. 1998. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA 95:3425-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dathe, M., M. Schumann, T. Wiepprecht, A. Winkler, M. Beyermann, E. Drause, K. Matsuzaki, O. Murase, and M. Bienert. 1996. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry 35:12612-12622. [DOI] [PubMed] [Google Scholar]
- 11.Dawe, S. J., and R. Duncan. 2002. The S4 genome segment of baboon reovirus is bicistronic and encodes a novel fusion-associated small transmembrane protein. J. Virol. 76:2131-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan, R. 1999. Extensive sequence divergence and phylogenetic relationships between the fusogenic and nonfusogenic orthoreoviruses: a species proposal. Virology 260:316-328. [DOI] [PubMed] [Google Scholar]
- 13.Duncan, R., Z. Chen, S. Walsh, and S. Wu. 1996. Avian reovirus-induced syncytium-formation is independent of infectious progeny virus production and enhances the rate, but is not essential, for virus-induced cytopathology and virus egress. Virology 224:453-464. [DOI] [PubMed] [Google Scholar]
- 14.Duncan, R., and K. Sullivan. 1998. Characterization of two avian reoviruses that exhibit strain-specific quantitative differences in their syncytium-inducing and pathogenic capabilities. Virology 250:263-272. [DOI] [PubMed] [Google Scholar]
- 15.Durell, S. R., I. Martin, J. M. Ruysschaert, Y. Shai, and R. Blumenthal. 1997. What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion. Mol. Membr. Biol. 14:97-112. [DOI] [PubMed] [Google Scholar]
- 16.Epand, R. M., Y. Shair, J. P. Segrest, and G. M. Anantharamaiah. 1995. Mechanisms for the modulation of membrane bilayers properties by amphipathic helical peptides. Biopolymers 37:319-338. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, C., B. Schroth-Diez, A. Herrmann, W. Garten, and H. Klenk. 1998. Acylation of the influenza hemagglutinin modulates fusion activity. Virology 248:284-294. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez, M. E., and L. Carrasco. 1998. The human immunodeficiency virus type 1 Vpu protein enhances membrane permeability. Biochemistry 37:13710-13719. [DOI] [PubMed] [Google Scholar]
- 19.Han, X., J. H. Bushweller, D. S. Cafiso, and L. K. Tamm. 2001. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat. Struct. Biol. 8:715-720. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 21.Hooper, N. M. 2000. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol. Membr. Biol. 16:145-156. [DOI] [PubMed] [Google Scholar]
- 22.Hughson, F. M. 1997. Enveloped viruses: a common mode of membrane fusion? Curr. Biol. 7:R565-R569. [DOI] [PubMed] [Google Scholar]
- 23.Ito, H., S. Watanabe, A. Takada, and Y. Kawaoka. 2001. Ebola virus glycoprotein: proteolytic processing, acylation, cell tropism, and detection of neutralizing antibodies. J. Virol. 75:1576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin, H., K. Subbarao, S. Bagai, G. P. Leser, B. R. Murphy, and R. A. Lamb. 1996. Palmitylation of the influenza virus hemagglutinin (H3) is not essential for virus assembly or infectivity. J. Virol. 70:1406-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemble, G. W., Y. I. Henis, and J. M. White. 1993. GPI- and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J. Cell Biol. 122:1253-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozerski, C., E. Ponimaskin, B. Schroth-Diez, M. F. G. Schmidt, and A. Herrmann. 2000. Modification of the cytoplasmic domain of influenza virus hemagglutinin affects enlargement of the fusion pore. J. Virol. 74:7529-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuppeveld, F. J. M., J. G. J. Hoenderop, R. L. L. Smeets, P. H. G. M. Willems, H. B. P. M. Dijkman, J. M. D. Glalma, and W. J. G. Melchers. 1997. Coxsackievirus protein 2B modifies endoplasmic reticulum membranes and plasma membrane permeability and facilitates virus release. EMBO J. 16:3519-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Langosch, D., B. Brosig, and R. Pipkorn. 2001. Peptide mimics of the vesicular stomatitis virus G-protein transmembrane segment drive membrane fusion in vitro. J. Biol. Cell. 276:32016-32021. [DOI] [PubMed] [Google Scholar]
- 30.Lemmon, M. A., and D. M. Engelman. 1994. Specificity and promiscuity in membrane helix interactions. FEBS Lett. 346:17-20. [DOI] [PubMed] [Google Scholar]
- 31.Martin, I., J.-M. Ruysschaert, and R. M. Epand. 1999. Role of the N-terminal peptides of viral envelope proteins in membrane fusion. Adv. Drug Del. Rev. 38:233-255. [DOI] [PubMed] [Google Scholar]
- 32.Matlack, K. E. S., W. Mothes, and T. A. Rapoport. 1998. Protein translocation: tunnel vision. Cell 92:381-390. [DOI] [PubMed] [Google Scholar]
- 33.McNew, J. A., T. Weber, F. Parlati, R. J. Johnston, T. J. Melia, T. H. Sollner, and J. E. Rothman. 2000. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J. Cell Biol. 150:105-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melikyan, G. B., S. Lin, M. G. Roth, and F. S. Cohen. 1999. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol. Biol. Cell 10:1821-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melikyan, G. B., R. M. Markosyan, S. A. Brener, Y. Rozenberg, and F. S. Cohen. 2000. Role of the cytoplasmic tail of ecotropic Moloney murine leukemia virus Env protein in fusion formation. J. Virol. 74:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mingarro, I., P. Whitley, M. A. Lemmon, and G. von Heijne. 1996. Ala-insertion scanning mutagenesis of the glycophorin A transmembrane helix: a rapid way to map helix-helix interactions in integral membrane proteins. Protein Sci. 5:1339-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naim, H. Y., B. Amarneh, N. T. Ktistakis, and M. G. Roth. 1992. Effects of altering palmitoylation sites on biosynthesis and function of the influenza virus hemagglutinin. J. Virol. 66:7585-7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odell, D., E. Wanas, J. S. Yan, and H. P. Ghosh. 1997. Influence of membrane anchoring and cytoplasmic domains on the fusogenic activity of vesicular stomatitis virus glycoprotein G. J. Virol. 71:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pecheur, E. I., J. Sainte-Marie, A. Bienvenue, and D. Hoekstra. 1999. Peptides and membrane fusion: towards an understanding of the molecular mechanisms of protein-induced fusion. J. Membr. Biol. 167:1-17. [DOI] [PubMed] [Google Scholar]
- 40.Pecheur, E. I., J. Sainte-Marie, A. Bienvenue, and D. Hoekstra. 1999. Lipid headgroup spacing and peptide penetration, but not peptide oligomerization, modulate peptide-induced fusion. Biochemistry 38:364-373. [DOI] [PubMed] [Google Scholar]
- 41.Plozov, I. V., A. I. Polozova, J. G. Molotkovsky, and R. M. Epand. 1997. Amphipathic peptide affects the lateral domain organization of lipid bilayers. Biochim. Biophys. Acta 1328:125-139. [DOI] [PubMed] [Google Scholar]
- 42.Razinkov, V. I., and F. S. Cohen. 2000. Sterols and sphingolipids strongly affect the growth of fusion pore induced by the hemagglutinin of influenza virus. Biochemistry 39:13462-13468. [DOI] [PubMed] [Google Scholar]
- 43.Schroth-Diez, B., E. Ponimaskin, H. Reverey, M. F. G. Schmidt, and A. Herrmann. 1998. Fusion activity of transmembrane and cytoplasmic domain chimeras of the influenza virus glycoprotein hemagglutinin. J. Virol. 72:133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shai, Y. 1995. Molecular recognition between membrane-spanning polypeptides. Trends Biochem. Sci. 11:460-464. [DOI] [PubMed] [Google Scholar]
- 45.Shmulevitz, M., and R. Duncan. 2000. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the nonenveloped fusogenic reoviruses. EMBO J. 19:902-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza haemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 47.Strong, J. E., G. Leone, R. Duncan, R. K. Sharma, and P. W. K. Lee. 1991. Biochemical and biophysical characterization of the reovirus cell attachment protein F1: evidence that it is a homotrimer. Virology 184:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamm, L. K., X. Han, Y. Li, and A. L. Lai. 2002. Structure and function of membrane fusion peptides. Biopolymers 66:249-260. [DOI] [PubMed] [Google Scholar]
- 49.Tatulian, S. A., and L. K. Tamm. 1999. Secondary structure, orientation, oligomerization and lipid interactions of the transmembrane domain of influenza hemagglutinin. Biochemistry 39:496-507. [DOI] [PubMed] [Google Scholar]
- 50.Uittenbogaard, A., and E. J. Smart. 2000. Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation and rapid transport of cholesterol to caveolae. J. Biol. Chem. 275:25595-25599. [DOI] [PubMed] [Google Scholar]
- 51.Von Heijne, G. 1992. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487-494. [DOI] [PubMed] [Google Scholar]
- 52.Weissenhorn, W., A. Dessen, L. J. Calder, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 53.White, J. M. 1990. Viral and cellular membrane fusion proteins. Annu. Rev. Physiol. 52:675-679. [DOI] [PubMed] [Google Scholar]
- 54.Whitely, P., I. Nilsson, and G. von Heijne. 1993. Three-dimensional model for the membrane domain of Escherichia coli leader peptidase based on disulfide mapping. Biochemistry 32:8534-8539. [DOI] [PubMed] [Google Scholar]
- 55.Yang, C., C. P. Spies, and R. W. Compans. 1995. The human and simian immunodeficiency virus envelope glycoprotein transmembrane subunits are palmitoylated. Proc. Natl. Acad. Sci. USA 92:9871-9875. [DOI] [PMC free article] [PubMed] [Google Scholar]