Abstract
To enhance the efficiency of antigen uptake at mucosal surfaces, CTB was conjugated to simian immunodeficiency virus (SIV) virus-like particles (VLPs). We characterized the immune responses to the Env and Gag proteins after intranasal administration. Intranasal immunization with a mixture of VLPs and CTB as an adjuvant elicited higher levels of SIV gp160-specific immunoglobulin G (IgG) in sera and IgA in mucosae, including saliva, vaginal-wash samples, lung, and intestine, as well as a higher level of neutralization activities than immunization with VLPs alone. Conjugation of CTB to VLPs also enhanced the SIV VLP-specific antibodies in sera and in mucosae to similar levels. Interestingly, CTB-conjugated VLPs showed higher levels of cytokine (gamma interferon)-producing splenocytes and cytotoxic-T-lymphocyte activities of immune cells than VLPs plus CTB, as well as an increased level of both IgG1 and IgG2a serum antibodies, which indicates enhancement of both Th1- and Th2-type cellular immune responses. These results demonstrate that CTB can be an effective mucosal adjuvant in the context of VLPs to induce enhanced humoral, as well as cellular, immune responses.
Numerous pathogens, including respiratory, gastrointestinal, and sexually transmitted agents, infect their hosts by interaction with mucosal surfaces. Human immunodeficiency virus (HIV) can be transmitted by infected seminal fluid, vaginal secretions, or rectal tissue through sexual intercourse, affecting populations worldwide. Several studies have indicated a crucial role for local mucosal immune responses in inhibiting HIV or simian immunodeficiency virus (SIV) infection (12, 33). It is also reported that secretory immunoglobulin A (IgA) inhibits epithelial transcytosis of HIV type 1 (HIV-1) (1, 19). The presence of SIV-specific antibodies and cytotoxic T lymphocytes (CTL) in the mucosa was able to be protective or to delay disease progression upon mucosal SIV challenge (15, 48). Therefore, it is of prime importance to develop immunization strategies that will induce protective mucosal immunity to prevent transmission of HIV across the genital mucosa.
A major problem in mucosal immunization is the low efficiency of antigen uptake. In addition, mucosally administered antigens are frequently not immunogenic and may induce immune tolerance. Previous studies demonstrated that it is advantageous to increase the immunogenicity of vaccine antigens by coadministration of an appropriate adjuvant. Cholera toxin (CT) is a well-known mucosal adjuvant, stimulating antigen-specific secretory IgA and systemic IgG antibody responses to unrelated antigens when coadministered via the oral, nasal, or genital route (14, 32, 47). The CT holotoxin consists of two subunits: a toxigenic A subunit (CTA), which activates the adenylate cyclase system, and a pentameric B subunit (CTB), which is responsible for CT binding to GM1 gangliosides on the cell membrane. Unfortunately, in humans, oral ingestion of microgram quantities of CT can induce toxic effects (23). Because of the association of toxicity with CTA, several investigators have analyzed the potential of CTB to act as a mucosal adjuvant. Purified natural CTB, as well as recombinant CTB, has a mucosal adjuvant effect when used together with bovine serum albumin as a model antigen (9, 44). Other reports indicate that the immunogenicities of protein antigens can be enhanced when they are conjugated to CTB rather than simply mixed with it (3, 11, 35). However, the precise mechanism of CTB adjuvanticity for many antigens of interest, including virus-like particles (VLPs), needs to be further studied.
When the gag and env genes of HIV or SIV are coexpressed in cells using a baculovirus (10, 50, 51) or vaccinia virus (18, 45) expression system or in cell lines (28), these proteins are able to assemble to form VLPs containing viral core and Env proteins. VLPs derived from several viruses (hepatitis B virus, papillomavirus, Norwalk virus, and HIV) have been shown to induce neutralizing antibodies and CTL responses (5, 26, 40, 46). Most of these studies using VLP antigens were performed through systemic immunizations, which do not induce immune responses at mucosal surfaces. Moldoveanu et al. reported the induction of humoral immune responses using SIV VLPs by mucosal routes after priming with vaccinia virus expressing the SIV Env protein (37). A recent study demonstrated that intranasal immunization with SIV VLPs plus CT induced both humoral and cellular immune responses (52).
In the present study, taking advantage of the ability of nontoxic CTB to bind with high affinity to GM1 gangliosides on epithelial surfaces, we examined the effects on immune responses of targeting VLPs to mucosal surfaces. Using SIV VLPs produced from a baculovirus expression system, we conjugated CTB to SIV VLPs and determined whether the CTB conjugation enhanced cellular and/or humoral immune responses.
MATERIALS AND METHODS
SIV Env and Gag proteins.
To evaluate the immune responses to SIV VLPs, the SIV Env protein was purified from a vaccinia virus expression system. A recombinant vaccinia virus (rVV) expressing the SIV Env protein (rVV-SIV Env) was previously described (45). SIVmac239 Env protein was purified from Hep2 cells infected with rVV-SIV Env using a lectin affinity column as described previously(13, 25) and used for SIV Env-specific antibody enzyme-linked immunosorbent assay (ELISA) analysis. Gag p55 protein was obtained from the National Institutes of Health (NIH) AIDS Reagent Program (catalog no. 1845).
Production of SIV VLPs.
Spodoptera frugiperda insect (Sf9) cells (Invitrogen) were maintained in serum-free SF900 II medium (GIBCO-BRL) at 28°C. The construction and characterization of SIVmac239 VLPs using the recombinant-baculovirus expression system were previously described in detail (50). For VLP production, Sf9 cells were coinfected with two independent recombinant baculoviruses expressing SIV Gag and SIV Env at multiplicities of infection of 2 and 5, respectively. On day 3 postinfection, the culture medium was collected and centrifuged at 1,500 × g for 20 min. The supernatant was filtered through a 0.45-μm-pore-size filter, and the VLPs were pelleted at 120,000 × g for 2 h at 4°C. The pelleted VLPs were resuspended in a small volume of phosphate-buffered saline (PBS) buffer. The total protein concentration of the VLPs was determined by a Bio-Rad (Hercules, Calif.) protein assay. The incorporation of SIV Env protein into VLPs was determined by Western blot analysis using monkey anti-SIV sera as described previously (50).
Conjugation of CTB to VLPs.
For CTB conjugation to VLPs, both VLPs and CTB (Sigma) were biotinylated using sulfo-NHS-biotin (Pierce, Rockford, Ill.) by following the manufacturer's manual. The biotinylated VLPs (1 mg) were mixed with 0.25 mg of biotinylated CTB for 30 min at 4°C. Streptavidin (0.25 mg) was added to the mixture of biotinylated VLPs and CTB, and the mixture was incubated for 1 h at 4°C. The efficiency of conjugation of CTB to VLPs was assessed by ELISA plates coated with GM1 ganglioside (Calbiochem) and by determining the bound SIV-VLPs with monkey anti-SIV serum. After all steps of CTB conjugation to VLPs, the CTB-conjugated VLPs were precipitated by ultracentrifugation and the supernatants were analyzed to determine unbound CTB or dissociated SIV Env proteins. SIV Env proteins were found in the precipitates and not in the supernatants. Most biotinylated CTB was found to be bound to biotinylated VLPs via streptavidin at a 1 (CTB)-to-4 (VLPs) ratio, conjugated VLPs, after extensive dialysis, were used for immunization without further purification.
Immunizations and sample collection.
Female BALB/c mice (Charles River) aged 6 to 8 weeks were used. Each group consisted of six mice. The mice were immunized intranasally in both nares at 0, 2, 4, and 6 weeks with 40 μg of VLPs, VLPs (40 μg) plus CTB (10 μg) or CT (10 μg), or VLPs (40 μg) conjugated with 10 μg of CTB. All immunizing reagents were suspended in 45 μl of PBS buffer, and individual mice received 15 μl (7.5 μl in each naris) three times with a 3-h rest interval between injections. The mice were slightly anesthetized with isoflurane and held inverted with nose down until the droplets of vaccine that were applied to both external nares were completely inhaled.
Samples were collected before immunization and 2 weeks after every immunization. All samples were stored at −20°C prior to antibody titration. Blood samples were collected by retro-orbital plexus puncture. Saliva was collected after intraperitoneal injection of 2 μg of carbamylcholine chloride to stimulate flow, and vaginal lavage fluids were collected by washing the vagina with 200 μl of PBS buffer. Lungs and large intestines with feces were collected at 8 weeks (2 weeks after the final immunization), and a perfusion-extraction method was employed for IgA determination (24). Lung tissue was washed in a large volume of PBS to wash out any contaminating blood. Lung and intestine tissues were weighed, cut into small pieces (2 to 3 mm diameter), suspended in extraction buffer (2% saponin, 0.1% NaN3), and rocked overnight (100 mg of lung in 400 μl of extraction buffer and 100 mg of intestine in 200 μl of extraction buffer). The supernatants were collected for antibody assay.
ELISA.
For the analysis of CTB conjugation to VLPs, ELISA plates (Costar) were coated with ganglioside GM1 (Calbiochem) at a concentration of 5 μg/ml of PBS overnight at 4°C. In all washing steps, PBS with 0.05% Tween 20 was used. After being washed, the coated plate was blocked with 2% bovine serum albumin (BSA) in PBS for 2 h at 37°C. CTB-conjugated VLPs were diluted with PBS and added to both uncoated and GM1-coated wells. The numbers of bound VLPs were determined using monkey anti-SIV sera following horseradish peroxidase (HRP)-conjugated secondary antibodies. The efficiency of conjugation was represented as the percentage of VLPs in GM1-coated wells compared to that in uncoated wells (total VLPs). HRP substrate, ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] containing H2O2 (Sigma), was used to develop color, and the optical density at 405 nm was read by ELISA reader (MTX Lab Systems). For determination of antibodies specific for the SIV Env or Gag protein, ELISA plates were coated with purified SIV Env at 3 μg/ml of PBS or Gag p55 protein at 2.5 μg/ml of PBS and blocked with 2% BSA. Samples diluted in PBS with 1% BSA were added to SIV Env- or Gag pp55-coated ELISA plates and incubated overnight at 4°C or for 4 h at room temperature. After being washed, HRP-conjugated goat anti-mouse IgG, IgG1, and IgG2a antibodies (Southern Biotechnology) were used for serum samples and goat anti-mouse IgA antibody was used for mucosal samples (vaginal wash, saliva, lung, and intestine), and color was developed as described above. Antibody concentrations were determined by comparing the readings for experimental samples with the standard curves for purified mouse IgG, IgG1, IgG2a, and IgA antibodies and are represented as the arithmetic mean ± 1 standard error. The statistical significance was calculated by Student's two-tailed t test for two groups.
Cell preparations.
The spleen, cervical and mesenteric lymph nodes, and Peyer's patches were obtained from individual mice and minced between the frosted ends of glass slides. To remove erythrocytes, the spleen cells were treated with 0.1 M ammonium chloride buffer at pH 7.4 for 2 min at room temperature, and the remaining cells were then washed in RPMI media with 10% fetal bovine serum (FBS). The cells, suspended in medium, were filtered through fine metal screens or nylon membranes (Fisher) to remove cellular debris. After being stained with trypan blue, live cells were counted by light microscopy and used for enzyme-linked immunospot (ELISPOT), cell proliferation, and CTL analyses.
ELISPOT and proliferation assays.
All antibodies against mouse cytokines used in ELISPOT assays were purchased from PharMingen (San Diego, Calif.). Anti-mouse gamma interferon (IFN-γ) (R4-6A2) and interleukin-4 (IL-4) (BVD4-1D11) antibodies (4 μg/ml in PBS) were used to coat Multiscreen 96-well filtration plates (Millipore, Bedford, Mass.) at 4°C overnight. After the cytokine-coated plates were blocked with 10% FBS in RPMI 1640, freshly isolated splenocytes (1 × 106 to 2 × 106) were added to each well. SIV Gag peptide (amino acids [aa] 186 to 205; INQMLNCVGDHQAAMQIIRD) or a mixture of SIV Env peptides (aa 211 to 230, CNTSVIQESCDKHYWDAIRF, and aa 231 to 250, RYCAPPGYALLRCNDTNYSG) (29, 31, 52) was added at a concentration of 5 μg/ml, and the plates were incubated for 36 h at 37°C. After the plates were washed with PBS with 0.05% Tween 20 six times, biotinylated anti-mouse IFN-γ (XMG1.2) and IL-4 (BVD6-24G2) antibodies (1 μg/ml) were added to the plates. After the plates were washed, spots in the plates were developed with stable DAB (Research Genetics) for 3 to 5 min and counted in an ImmunoSpot ELISPOT reader (Cellular Technology, Ltd.). Splenocytes from naive mice were used as a negative control. For proliferation assays, 50 μl containing 5 × 105 cells was mixed with 50 μl of CD4 T-cell epitope peptide (RQIINTWHKVGKNVYL; 20 μg/ml) (53) and incubated for 3 days, and live cells were determined by using a CellTiter96 nonradioactive cell proliferation assay (Promega, Madison, Wis.). This method has been widely used to measure the proliferation of various cell types, including lymphocytes (17, 39, 43). The degree of cell proliferation is presented as the ratio of the optical density at 570 nm for cells from immunized mice to that for control cells from nonimmunized mice.
CTL analysis.
P815 target cells (major histocompatibility complex class I; H-2d) were prepared by infecting them with rVV expressing SIVmac239 Env protein at a multiplicity of infection of 1. Infected P815 cells (104) were harvested 5 to 7 h postinfection and incubated with freshly isolated lymphocytes from immunized or naïve mice at different effector-to-target-cell ratios. The amount of cell lysis was measured using the CytoTox96 nonradioactive cytotoxicity assay (Promega). This assay measures lactate dehydrogenase, a stable cytosolic enzyme that is released upon cell lysis in the same way that 51Cr is released in radioactive assays. The results from these two assays were shown to be almost identical (8, 27), and several studies have used the nonradioactive cytotoxic assay for CTL analysis (2, 6, 36). The percentage of specific target cell lysis was represented based on total cell lysis with 0.1% Triton X-100.
Neutralization assay.
For neutralization assays, we used sMAGI cells (7) and SIVmac1A11 grown in HUT78 cells (American Type Culture Collection). In brief, sMAGI cells (3.5 × 103 per well) were added to a 96-well plate 1 day before infection. Serum samples were heat inactivated (56°C; 30 min) and serially twofold diluted in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, and a final volume of 25 μl was mixed with 25 μl of diluted virus stock containing 100 infectious particles. The virus-serum mixtures were incubated at 37°C for 1 h and then added to sMAGI cells with DEAE-Dextran at a final concentration of 15 μg/ml. After a 2-h incubation, 150 μl of DMEM was added. After 1 day of incubation, the medium was replaced with 10% FBS DMEM containing 10 μM zidovudine (Sigma). On day 3 postinfection, the medium was removed and the cells were fixed and stained as described previously (7). The blue cells in the wells without antisera indicated the total number of infectious virus particles. Neutralization titers are presented as the dilutions giving a 50% reduction in the number of blue cells. Vaginal-wash samples from three mice were pooled, and the resulting two pools from each group (six mice per group) were analyzed for neutralization activities.
RESULTS
Serum IgG antibody responses after intranasal immunization with SIV VLPs.
As an approach to increase immune responses to VLPs utilizing the ability of CTB to bind to GM1 gangliosides on the surfaces of mucosal epithelial cells, we conjugated CTB to VLPs via streptavidin-biotin interaction. A similar method has been utilized to conjugate tumor necrosis factor alpha protein to papillomavirus VLPs (7). The efficiency of conjugation was ∼50%, as determined using GM1-coated (conjugated VLPs) and uncoated (total VLPs) wells in ELISA plates (data not shown). For comparison, groups immunized with VLPs alone, or VLPs plus CTB or CT, were included.
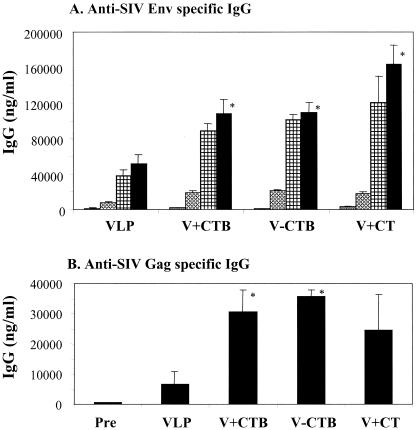
To examine the effects of CTB conjugation on immune responses to VLPs, serum samples were collected 2 weeks after intranasal immunizations, and SIV Env-specific total IgG antibody was determined as shown in Fig. 1. The levels of anti-SIV Env were greatly increased in all groups after the second boost (6 weeks), which is consistent with studies of hepatitis virus surface B antigen together with recombinant CTB adjuvant (21). Mice immunized with SIV VLPs in the absence of any adjuvant showed a relatively high level of serum IgG specific to the SIV Env protein. CTB conjugation to SIV VLPs induced a twofold-enhanced level of SIV Env-specific IgG antibody (P < 0.02), and a similar increase was induced by VLPs mixed with unconjugated CTB (P < 0.02). Mice immunized with VLPs plus CT showed the highest level of SIV Env-specific antibodies, but the difference between the groups of mice which received CTB or conjugated CTB as the adjuvant and the group of mice which received CT was not significant (P = 0.237 for CTB and P = 0.286 for conjugated CTB). Gag-specific serum IgG antibody responses were also found to be significantly higher in the VLP-plus-CTB and VLP-CTB (conjugated) groups compared to the VLP group (Fig. 1B), although the Gag-specific antibody concentrations were lower than those of Env-specific antibody. These results indicate that both conjugated CTB and free CTB have an adjuvant effect on inducing antibody responses to VLPs.
FIG. 1.
Humoral immune responses after SIV-VLP intranasal immunizations. (A) Anti-SIV Env-specific IgG. Serum samples were collected before immunization (0 weeks [shaded bars]) and at 2 weeks after the second (4 weeks [cross-hatched bars]), third (6 weeks [checked bars]), and fourth (8 weeks [solid bars]) immunizations and analyzed using ELISA plates coated with purified SIV Env gp160 protein. (B) Anti-SIV Gag protein-specific IgG. Serum samples were collected after the fourth (8 weeks) immunizations and analyzed using ELISA plates coated with purified SIV p55 Gag protein. Standard error bars are indicated for six mice per group. Pre, preimmune samples; VLP, SIV VLPs only; V+CTB, SIV VLPs plus CTB (mixture); V-CTB, SIV VLP-CTB (conjugated); V+CT, SIV VLPs plus CT (mixture). *, P < 0.02 (calculated by Student's two-tailed t test compared to VLP alone).
Effects of CTB on induction of IgG1 and IgG2a antibodies.
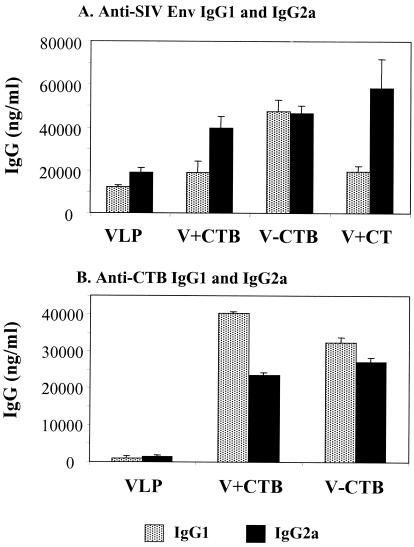
To determine the subclass distribution of serum IgG antibody, we determined SIV Env-specific IgG1 and IgG2a levels in sera after the third immunization (Fig. 2). We observed levels of IgG2a antibody specific to SIV Env higher than those of IgG1 antibody (Fig. 2A). The addition of CTB or CT together with VLPs increased the levels of both IgG1 and IgG2a antibodies but more prominently enhanced the levels of IgG2a. Conjugation of CTB to VLPs resulted in similar increases in both IgG1 and IgG2a antibodies.
FIG. 2.
Isotypes of IgG1 and IgG2a in sera after the third immunization (6 weeks). (A) SIV Env-specific IgG1 and IgG2a antibodies were determined. (B) CTB-specific IgG1 and IgG2a were determined using ELISA plates coated with purified CTB protein. The relative ratios of IgG1 and IgG2a antibodies in the serum were maintained at similar levels following the fourth immunization (data not shown). Standard error bars are indicated for six mice per group. VLP, SIV VLPs only; V+CTB, SIV VLP plus CTB; V-CTB, SIV VLP-CTB (conjugated); V+CT, SIV VLPs plus CT.
To further examine the induction of IgG1 and IgG2a, we compared the serum IgG1 and IgG2a antibodies specific to CTB (Fig. 2B). Sera from mice immunized with VLPs without CTB showed very low levels of IgG1 and IgG2a, like the negative preimmune serum controls. CTB conjugated to VLPs elicited the production of IgG1 and IgG2a antibodies specific to CTB in a ratio similar to those specific to the SIV Env protein on VLPs. However, in mice immunized with free CTB as an adjuvant mixed with VLPs, the level of IgG1 specific to CTB was much higher than that of IgG2a, which is opposite to the antibody profile seen with the SIV Env protein (Fig. 2A). The levels of total IgG specific to CTB were similar in the groups immunized with VLPs conjugated with CTB or mixed with free CTB (data not shown). These results suggest that intranasal immunization with a particulate antigen like VLPs induces levels of IgG2a serum antibodies higher than those of IgG1. Immunization with CTB-conjugated VLPs induced both types of antibodies, indicating that Th1- and Th2-like helper cell responses are elicited. The difference in antibody isotypes specific to CTB and VLPs also implies that the induction pathway used by VLPs may be different from that for soluble protein antigen.
Mucosal IgA responses to intranasally administered VLPs.
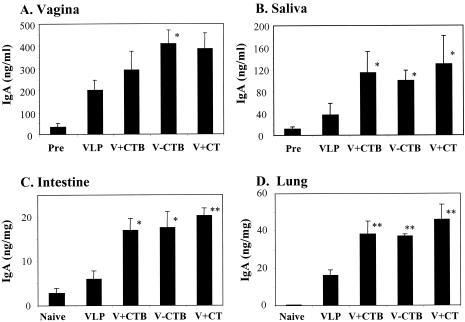
To determine mucosal immune responses after intranasal immunization with VLPs, SIV Env-specific IgA antibody induction was analyzed in mucosal secretions of vaginal washes and saliva collected after the third immunization (at week 6), as shown in Fig. 3. Intranasal immunization with VLPs without any adjuvant elicited SIV-specific IgA antibody responses in saliva, vaginal-wash samples, and lung lavage fluid and a low level of IgA in the large intestine. CTB conjugation to VLPs showed enhanced levels of SIV-specific IgA in vaginal-wash samples and saliva (both P < 0.05) and in extracts of lung (P < 0.02) and large intestines (P < 0.05). Importantly, free CTB included in VLPs elicited levels of SIV-specific mucosal IgA production similar to those elicited by VLPs with CT as an adjuvant. Analysis of mucosal IgA immune responses indicated that groups of mice intranasally immunized with CTB-conjugated VLPs, VLPs plus CTB, or VLPs plus CT were similarly effective in enhancing the levels of mucosal IgA antibody responses against VLPs.
FIG. 3.
Mucosal IgA responses to SIV Env after intranasal immunization with VLPs. For vaginal-wash samples and saliva, preimmune samples (Pre) were used as controls. For intestine and lung extracts, samples from naive (unimmunized) mice (Naive) were used as controls. (A) IgA in vaginal-wash samples after the third immunization (6 weeks). (B) IgA in saliva after the third immunization (6 weeks). The SIV Env-specific IgA antibodies in vaginal-wash samples and saliva after the fourth immunization were similar to those observed after the third immunization (data not shown). (C) IgA in large-intestine extracts after the fourth immunization (8 weeks). (D) IgA in lung extracts after the fourth immunization (8 weeks). Standard error bars are indicated for six mice per group. VLP, SIV VLPs only; V+CTB, SIV VLP plus CTB; V-CTB, SIV VLP-CTB (conjugated); V+CT, SIV VLPs plus CT. *, P < 0.05; **, P < 0.02 (calculated by Student's two-tailed t test compared to VLP alone).
Neutralization activities in sera and secretions.
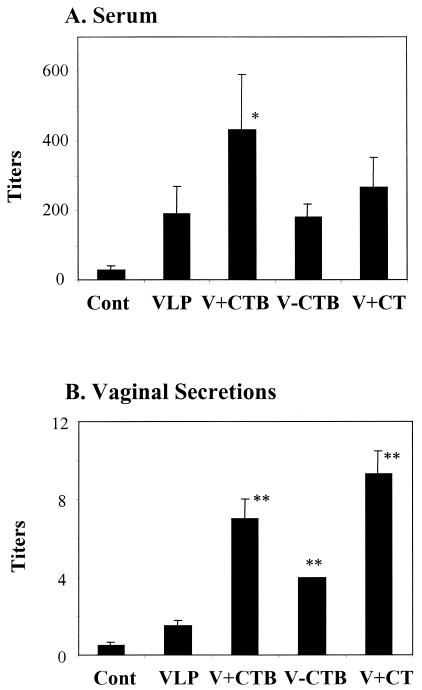
To determine the neutralizing activities of immune sera, 50% neutralization titers to SIVmac 1A11, a strain closely related to SIVmac239 but more sensitive to neutralization, were determined (Fig. 4). Sera from mice intranasally immunized with VLPs without CTB induced neutralizing antibody titers reaching 200. The addition of free CTB to VLPs increased the neutralizing activities by twofold (P < 0.05). CTB conjugation to VLPs or CT mixed with VLPs did not increase serum neutralizing activities significantly compared to those in sera of mice immunized with VLPs alone.
FIG. 4.
SIV neutralizing titers in sera and in secretions of mice immunized with VLPs. The results represent averages (plus standard errors) from three independent experiments. The data are 50% neutralization titers. (A) Serum neutralizing titers. Individual serum samples (six samples per group) collected after the fourth immunization (at 8 weeks) were serially diluted and incubated with SIVmac1A11 prior to being added to sMAGI cells. (B) Neutralizing titers in vaginal secretions. Vaginal-wash samples (collected in weeks 6 and 8) were pooled (two pools per group), and serially diluted samples were incubated with SIVmac1A11 prior to being added to sMAGI cells. The titer of V-CTB did not show variation in three independent experiments. Cont, control (preimmune samples); VLP, SIV VLPs only; V+CTB, SIV VLP plus CTB; V-CTB, SIV VLP-CTB (conjugated); V+CT, SIV VLPs plus CT. *, P < 0.05; **, P < 0.02 (calculated by Student's two-tailed t test compared to VLP alone).
The induction of neutralizing antibody activities in mucosal secretions is not well studied, even though it is of prime importance in mucosal immunization. The pools of vaginal-wash samples in each group were analyzed to determine the neutralizing activities, as shown in Fig. 4B. Weak neutralizing activities were observed in vaginal-wash sample pools from mice immunized with VLPs alone. The mice immunized with CTB-conjugated VLPs showed a twofold increase in neutralizing activity compared to mice immunized with VLPs in the absence of adjuvant (P < 0.02). The group of mice immunized with VLPs plus CTB or CT showed even higher neutralizing activities, with an increase of three- to fivefold compared to the group immunized with VLPs alone (P < 0.02). The results indicate that CTB in a conjugated or free form can enhance the induction of neutralizing antibodies to SIV in mucosal secretions.
Cellular immune responses after intranasal immunization with VLPs.
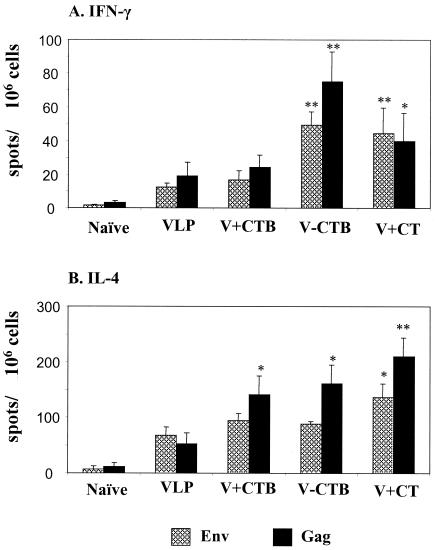
The mechanism of induction of cellular immune responses after mucosal immunization with VLPs is not well understood. Determining the cytokine production profile by ELISPOT assay is one of the approaches to assess the cellular immune responses. Two weeks after the final immunization, the splenocytes were freshly isolated and used to determine SIV Env- or Gag-specific IFN-γ-producing cells as an indicator for Th1-type cellular immune responses (Fig. 5A). In all groups of mice immunized with VLPs intranasally, IFN-γ-producing spots were easily detected and the numbers of Gag-specific IFN-γ-producing spots were found to be higher than those of Env-specific IFN-γ-producing spots. Conjugation of CTB to VLPs or coimmunization of VLPs with CT greatly increased the number of IFN-γ spots (threefold) compared to the group of mice immunized with VLPs only (P < 0.02). However, the addition of free CTB to VLPs did not increase the number of IFN-γ spots significantly, in contrast to its effect on humoral immune responses.
FIG. 5.
Cytokine-producing lymphocytes determined by an ELISPOT assay. Splenocytes were isolated 2 weeks after the final (fourth) immunization and stimulated for 2 days with SIV Env or SIV Gag peptide. (A) The spots for IFN-γ-producing cells from the spleen were counted and expressed based on 106 cells. (B) The spots for IL-4-producing cells from the spleen were expressed based on 106 cells. Standard error bars are indicated for six mice per group. Naïve, unimmunized control; VLP, SIV VLPs only; V+CTB, SIV VLP plus CTB; V-CTB, SIV VLP-CTB (conjugated); V+CT, SIV VLPs plus CT. *, P < 0.05; **, P < 0.02 (calculated by Student's two-tailed t test compared to VLP alone).
IL-4 plays an important role in inducing Th2-type helper CD4 T-cell immune responses. To determine the IL-4 production in VLP-immunized mice, a method similar to that used for IFN-γ was employed, using an IL-4-detecting ELISPOT assay. The numbers of IL-4 spot-producing spleen cells were relatively high in all mice immunized with VLPs compared to those of IFN-γ-producing cells. The number of Gag-specific but not Env-specific IL-4-producing cells was increased by the addition of free CTB or by conjugating CTB to VLPs (Fig. 5B).
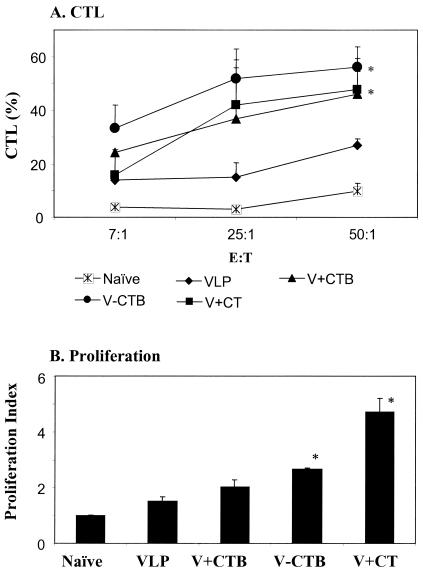
To directly determine the activity of CTL, cytolysis of target cells by lymphocytes from immunized or naïve mice was analyzed using a nonradioactive CTL assay (2, 6, 36). P815 cells infected with rVV expressing SIV Env were used as target cells. Since the unfractionated lymphocytes used in this study were heterogeneous cell types containing both T and B cells, higher ratios of effector to target cells are required for CTL activity. Spleen cells from mice immunized with VLPs alone could achieve ∼20% lysis of target cells at the effector-to-target-cell ratio of 50, whereas cells from naïve mice showed <10% CTL activity (Fig. 6A). Conjugation of CTB to VLPs increased CTL activity up to 55% in splenocytes (P < 0.05), as well as in lymph node cells (data not shown). The addition of CTB or CT to VLPs for intranasal immunization produced CTL activity higher than that in the group immunized with VLPs alone but consistently lower than that in the group immunized with VLPs conjugated to CTB.
FIG. 6.
(A) CTL activities of spleen cells. P815 cells (major histocompatibility complex class I; H-2d) infected with rVV expressing SIVmac239 Env protein were used as target cells. Target cell lysis was determined using a nonradioactive CTL assay. CTL activities are represented as percentages of target cell lysis. The results are shown with standard error bars for six mice per group. The ratio on the x axis is the effector-to-target-cell ratio. (B) Assessment of T-cell proliferation. Splenocytes isolated from naïve and immune mice were incubated for 3 days in media containing T helper cell epitope peptide. Proliferation of cells was determined using a nonradioactive proliferation assay. The proliferation index is presented as the factor of increase in absorbance (570 nm) compared with lymphocytes from nonimmunized-mouse controls. Standard error bars are indicated for six mice per group. Naïve, unimmunized control; VLP, SIV VLPs only; V+CTB, SIV VLP plus CTB; V-CTB, SIV VLP-CTB (conjugated); V+CT, SIV VLPs plus CT. *, P < 0.05 (calculated by Student's two-tailed t test compared to VLP alone).
Assay of proliferation of splenocytes isolated from naïve and immune mice was performed by incubating them for 3 days in medium containing helper T-cell epitope peptide (53) and then determining the viable cells by using a nonradioactive proliferation assay (17, 39, 43). As shown in Fig. 6B, splenocytes from mice immunized with VLPs conjugated to CTB showed a moderate but significant increase in proliferation compared to those from mice immunized with VLPs alone (P < 0.03). The addition of CT to VLPs increased the proliferation of lymphocytes by fourfold compared to the group immunized with VLPs alone (P < 0.03).
These results indicate that intranasal immunization with VLPs, even at low levels, can induce cellular immune responses. Conjugation of CTB to VLPs greatly increased the number of SIV Env-specific IFN-γ-producing spleen and lymph node cells, CTL activities, and proliferation of splenocytes. In these assays, conjugated CTB was found to be superior to uncoupled CTB in enhancing cellular immune responses.
DISCUSSION
We have determined the effects of the addition of free CTB or conjugation with CTB on immune responses to a particulate antigen, SIV VLPs, after intranasal immunization. The results indicate that either inclusion of free CTB or conjugation of CTB to VLPs enhances the humoral immune responses to SIV Env. Conjugated CTB was found to be superior to free CTB in enhancing the production of the serum IgG1 isotype and secretory vaginal IgA antibodies. Importantly, conjugated CTB was also found to be more effective than free CTB in enhancing cellular immune responses.
In contrast to toxic CT, CTB is an attractive mucosal adjuvant, because it can be safely administered to humans (22, 23). CTB binds to cell membrane GM1 gangliosides, thus enhancing its uptake by mucosa-associated lymphoid tissue. It is likely that conjugating CTB to an antigen would enhance the immune responses to the antigen, as shown in previous studies (4, 11, 35, 42). However, most of these studies focused on comparing the induction of binding antibodies after immunization with conjugated or free antigen. By conjugating CTB to the VLPs, it was expected that the same cells taking up CTB would capture VLPs, thus enhancing mucosal uptake of VLPs. However, we found that both approaches similarly enhanced the humoral immune responses to SIV Env in blood, intestine, and lung. Consistent with our results, using streptococcal surface antigen or ovalbumin as a model antigen, it was reported that free or coupled CTB had similar adjuvant effects on inducing humoral immune responses (9, 41, 49).
The detection of neutralizing antibodies in mucosal secretions has significant implications for AIDS vaccine-induced mucosal protection. In contrast to serum neutralizing activities, we observed enhanced neutralizing activities in vaginal-wash pools from mice immunized with CTB-conjugated VLPs. The vaginal-wash pools from mice coimmunized with VLPs and free CTB or CT showed the highest levels of neutralizing activities to SIV. Conjugation of CTB to VLPs did not enhance the serum neutralization titers, although higher levels of binding antibodies against SIV Env protein were induced. The structural integrity of envelope antigens on VLPs should not be affected after CTB conjugation, since conjugation was carried out under very mild conditions. However, we cannot rule out the possibility that some epitopes of SIV Env on VLPs might be blocked on the CTB-conjugated particles. This might have resulted in apparently lower conjugation efficiency due to the lower binding reactivities of monkey antisera (used to evaluate conjugation efficiency) to the conjugated VLPs compared to the unconjugated VLPs. The potential to induce neutralizing antibody may be greater if CTB is incorporated into VLPs during the budding process at the cell surface rather than by in vitro conjugation.
In contrast to humoral immune responses, our results indicate that the VLP-CTB conjugate was superior to free CTB mixed with VLPs in inducing cellular immune responses. Most previous studies of CTB conjugation to protein antigens focused on analyzing humoral immune responses. We observed that IFN-γ-producing lymphocytes, CTL activities in the spleen, and lymphocyte proliferation responses were higher in mice immunized with the VLP-CTB conjugate than in mice immunized with VLPs plus free CTB. It has also been reported that conjugating CTB to influenza virus hemagglutinin or ovalbumin increased the CD40 and CD86 expression of antigen-presenting cells, T-cell proliferation, and IFN-γ secretion (16). Thus, enhancing cell-mediated immune responses would provide an advantage in developing CTB-conjugated VLP-based vaccines.
Our results provide two lines of evidence that, as a particulate antigen, VLPs may interact with mucosal immune cells differently from soluble protein antigens in inducing immune responses. First, most protein antigens, when administered intranasally in the absence of adjuvant, induce low or undetectable levels of immune responses (20, 34, 38, 44). In contrast, VLPs alone administered intranasally induced both systemic and mucosal immune responses at relatively high levels. Second, VLPs elicited higher IgG2a isotype antibody responses specific to SIV Env than IgG1 isotype antibody responses. This is quite interesting, considering that soluble protein antigens with CT or CTB adjuvant are reported to preferentially induce IgG1 rather than IgG2a (30, 44). In another recent study consistent with our results, HIV Env in lipid vesicles conjugated with CTB induced both IgG1 and IgG2a antibodies (30). Our results indicate that intranasal immunization with CTB-conjugated VLPs can induce both Th1- and Th2-type responses, as determined by antigen-specific IgG1 and IgG2a production; production of cytokines, such as IFN-γ- and IL-4-producing lymphocytes; and CTL activities in spleen cells.
In summary, VLPs produced from a baculovirus expression system are immunogenic and can induce systemic, as well as mucosal, immune responses by intranasal immunization. CTB, in the context of VLPs, is effective as a mucosal adjuvant, and CTB conjugation to VLPs enhances both humoral and cellular immune responses. Considering the practical advantages and the capacity to stimulate mucosal as well as systemic immunity, intranasal immunization protocols have considerable promise for the design of vaccines inducing protective responses against HIV. The present results support the feasibility of further efforts to develop such immunization strategies.
Acknowledgments
We thank Karen Chocho and Ann Vincent for technical assistance.
This work has been supported by NIH/NIAID grants AI28147 and AI43045. SIV Gag p55 protein, SIV Env, and Gag peptides were obtained through the NIH AIDS Research and Reference Reagent Program.
REFERENCES
- 1.Alfsen, A., P. Iniguez, E. Bouguyon, and M. Bomsel. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166:6257-6265. [DOI] [PubMed] [Google Scholar]
- 2.Behl, C., J. B. Davis, R. Lesley, and D. Schubert. 1994. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77:817-827. [DOI] [PubMed] [Google Scholar]
- 3.Bergquist, C., T. Lagergard, M. Lindblad, and J. Holmgren. 1995. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect. Immun. 63:2021-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi, A. T., R. J. Zwart, and P. J. van der Heijden. 1990. Induction of an enteric Ig-response against ovalbumin and stimulation of the response by cholera toxin and its B-subunit in mice. Reg. Immunol. 3:131-138. [PubMed] [Google Scholar]
- 5.Boisgerault, F., G. Moron, and T. Lehner. 2002. Virus-like particles: a new family of delivery systems. Expert Rev. Vaccines 1:101-109. [DOI] [PubMed] [Google Scholar]
- 6.Brander, C., T. Wyss-Coray, D. Mauri, F. Bettens, and W. J. Pichler. 1993. Carrier-mediated uptake and presentation of a major histocompatibility complex class I-restricted peptide. Eur. J. Immunol. 23:3217-3223. [DOI] [PubMed] [Google Scholar]
- 7.Chackerian, B., D. R. Lowy, and J. T. Schiller. 2001. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J. Clin. Investig. 108:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decker, T., and M. L. Lohmann-Matthes. 1988. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 115:61-69. [DOI] [PubMed] [Google Scholar]
- 9.de Geus, B., M. Dol-Bosman, J. W. Scholten, W. Stok, and A. Bianchi. 1997. A comparison of natural and recombinant cholera toxin B subunit as stimulatory factors in intranasal immunization. Vaccine 15:1110-1113. [DOI] [PubMed] [Google Scholar]
- 10.Deml, L., G. Kratochwil, N. Osterrieder, R. Knuchel, H. Wolf, and R. Wagner. 1997. Increased incorporation of chimeric human immunodeficiency virus type 1 gp120 proteins into Pr55gag virus-like particles by an Epstein-Barr virus gp220/350-derived transmembrane domain. Virology 235:10-25. [DOI] [PubMed] [Google Scholar]
- 11.Dertzbaugh, M. T., and C. O. Elson. 1993. Comparative effectiveness of the cholera toxin B subunit and alkaline phosphatase as carriers for oral vaccines. Infect. Immun. 61:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devito, C., J. Hinkula, R. Kaul, L. Lopalco, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2000. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 14:1917-1920. [DOI] [PubMed] [Google Scholar]
- 13.Edinger, A. L., M. Ahuja, T. Sung, K. C. Baxter, B. Haggarty, R. W. Doms, and J. A. Hoxie. 2000. Characterization and epitope mapping of neutralizing monoclonal antibodies produced by immunization with oligomeric simian immunodeficiency virus envelope protein. J. Virol. 74:7922-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elson, C. O., and W. Ealding. 1984. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 132:2736-2741. [PubMed] [Google Scholar]
- 15.Fuller, D. H., P. A. Rajakumar, L. A. Wilson, A. M. Trichel, J. T. Fuller, T. Shipley, M. S. Wu, K. Weis, C. R. Rinaldo, J. R. Haynes, and M. Murphey-Corb. 2002. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J. Virol. 76:3309-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George-Chandy, A., K. Eriksson, M. Lebens, I. Nordstrom, E. Schon, and J. Holmgren. 2001. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect. Immun. 69:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez, R. S., J. E. da Costa, T. M. Lorentz, A. Garrocho Ade, and J. A. Nogueira-Machado. 1994. Chemoluminescence generation and MTT dye reduction by polymorphonuclear leukocytes from periodontal disease patients. J. Periodontal Res. 29:109-112. [DOI] [PubMed] [Google Scholar]
- 18.Haffar, O., J. Garrigues, B. Travis, P. Moran, J. Zarling, and S. L. Hu. 1990. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J. Virol. 64:2653-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hocini, H., L. Belec, S. Iscaki, B. Garin, J. Pillot, P. Becquart, and M. Bomsel. 1997. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res. Hum. Retrovir. 13:1179-1185. [DOI] [PubMed] [Google Scholar]
- 20.Horner, A. A., A. Ronaghy, P. M. Cheng, M. D. Nguyen, H. J. Cho, D. Broide, and E. Raz. 1998. Immunostimulatory DNA is a potent mucosal adjuvant. Cell Immunol. 190:77-82. [DOI] [PubMed] [Google Scholar]
- 21.Isaka, M., Y. Yasuda, M. Mizokami, S. Kozuka, T. Taniguchi, K. Matano, J. Maeyama, K. Mizuno, K. Morokuma, K. Ohkuma, N. Goto, and K. Tochikubo. 2001. Mucosal immunization against hepatitis B virus by intranasal co-administration of recombinant hepatitis B surface antigen and recombinant cholera toxin B subunit as an adjuvant. Vaccine 19:1460-1466. [DOI] [PubMed] [Google Scholar]
- 22.Jertborn, M., I. Nordstrom, A. Kilander, C. Czerkinsky, and J. Holmgren. 2001. Local and systemic immune responses to rectal administration of recombinant cholera toxin B subunit in humans. Infect. Immun. 69:4125-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1992. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine 10:130-132. [DOI] [PubMed] [Google Scholar]
- 24.Johansson, E. L., C. Rask, M. Fredriksson, K. Eriksson, C. Czerkinsky, and J. Holmgren. 1998. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun. 66:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, D. H., B. W. McBride, M. A. Roff, V. Maloney, and G. H. Farrar. 1994. Purification and characterization of simian immunodeficiency virus (SIVmac) envelope glycoprotein gp130 from virus-infected cells. Vaccine 12:250-258. [DOI] [PubMed] [Google Scholar]
- 26.Kang, C. Y., L. Luo, M. A. Wainberg, and Y. Li. 1999. Development of HIV/AIDS vaccine using chimeric gag-env virus-like particles. Biol. Chem. 380:353-364. [DOI] [PubMed] [Google Scholar]
- 27.Korzeniewski, C., and D. M. Callewaert. 1983. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 64:313-320. [DOI] [PubMed] [Google Scholar]
- 28.Krausslich, H. G., C. Ochsenbauer, A. M. Traenckner, K. Mergener, M. Facke, H. R. Gelderblom, and V. Bosch. 1993. Analysis of protein expression and virus-like particle formation in mammalian cell lines stably expressing HIV-1 gag and env gene products with or without active HIV proteinase. Virology 192:605-617. [DOI] [PubMed] [Google Scholar]
- 29.Lagranderie, M., N. Winter, A. M. Balazuc, B. Gicquel, and M. Gheorghiu. 1998. A cocktail of Mycobacterium bovis BCG recombinants expressing the SIV Nef, Env, and Gag antigens induces antibody and cytotoxic responses in mice vaccinated by different mucosal routes. AIDS Res. Hum. Retrovir. 14:1625-1633. [DOI] [PubMed] [Google Scholar]
- 30.Lian, T., T. Bui, and R. J. Ho. 1999. Formulation of HIV-envelope protein with lipid vesicles expressing ganglioside GM1 associated to cholera toxin B enhances mucosal immune responses. Vaccine 18:604-611. [DOI] [PubMed] [Google Scholar]
- 31.Lim, E. M., M. Lagranderie, R. Le Grand, J. Rauzier, M. Gheorghiu, B. Gicquel, and N. Winter. 1997. Recombinant Mycobacterium bovis BCG producing the N-terminal half of SIVmac251 Env antigen induces neutralizing antibodies and cytotoxic T lymphocyte responses in mice and guinea pigs. AIDS Res. Hum. Retrovir. 13:1573-1581. [DOI] [PubMed] [Google Scholar]
- 32.Lycke, N. 1997. The mechanism of cholera toxin adjuvanticity. Res. Immunol. 148:504-520. [DOI] [PubMed] [Google Scholar]
- 33.Mazzoli, S., D. Trabattoni, C. S. Lo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 34.McCluskie, M. J., and H. L. Davis. 2001. Oral, intrarectal and intranasal immunizations using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine 19:413-422. [DOI] [PubMed] [Google Scholar]
- 35.McKenzie, S. J., and J. F. Halsey. 1984. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J. Immunol. 133:1818-1824. [PubMed] [Google Scholar]
- 36.Meyer, D., and J. V. Torres. 1999. Induction of cytotoxic and helper T cell responses by modified simian immunodeficiency virus hypervariable epitope constructs. Viral Immunol. 12:117-129. [DOI] [PubMed] [Google Scholar]
- 37.Moldoveanu, Z., A. N. Vzorov, W. Q. Huang, J. Mestecky, and R. W. Compans. 1999. Induction of immune responses to SIV antigens by mucosally administered vaccines. AIDS Res. Hum. Retrovir. 15:1469-1476. [DOI] [PubMed] [Google Scholar]
- 38.Morris, C. B., E. Cheng, A. Thanawastien, L. Cardenas-Freytag, and J. D. Clements. 2000. Effectiveness of intranasal immunization with HIV-gp160 and an HIV-1 env CTL epitope peptide (E7) in combination with the mucosal adjuvant LT(R192G). Vaccine 18:1944-1951. [DOI] [PubMed] [Google Scholar]
- 39.Niks, M., M. Otto, B. Busova, and J. Stefanovic. 1990. Quantification of proliferative and suppressive responses of human T lymphocytes following ConA stimulation. J. Immunol. Methods 126:263-271. [DOI] [PubMed] [Google Scholar]
- 40.Paliard, X., Y. Liu, R. Wagner, H. Wolf, J. Baenziger, and C. M. Walker. 2000. Priming of strong, broad, and long-lived HIV type 1 p55gag-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res. Hum. Retrovir. 16:273-282. [DOI] [PubMed] [Google Scholar]
- 41.Russell, M. W., Z. Moldoveanu, P. L. White, G. J. Sibert, J. Mestecky, and S. M. Michalek. 1996. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect. Immun. 64:1272-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen, X., T. Lagergard, Y. Yang, M. Lindblad, M. Fredriksson, and J. Holmgren. 2000. Systemic and mucosal immune responses in mice after mucosal immunization with group B streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate vaccine. Infect. Immun. 68:5749-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi, Y. F., B. M. Sahai, and D. R. Green. 1989. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature 339:625-626. [DOI] [PubMed] [Google Scholar]
- 44.Tochikubo, K., M. Isaka, Y. Yasuda, S. Kozuka, K. Matano, Y. Miura, and T. Taniguchi. 1998. Recombinant cholera toxin B subunit acts as an adjuvant for the mucosal and systemic responses of mice to mucosally co-administered bovine serum albumin. Vaccine 16:150-155. [DOI] [PubMed] [Google Scholar]
- 45.Vzorov, A. N., and R. W. Compans. 1996. Assembly and release of SIV env proteins with full-length or truncated cytoplasmic domains. Virology 221:22-33. [DOI] [PubMed] [Google Scholar]
- 46.Wagner, R., V. J. Teeuwsen, L. Deml, F. Notka, A. G. Haaksma, S. S. Jhagjhoorsingh, H. Niphuis, H. Wolf, and J. L. Heeney. 1998. Cytotoxic T cells and neutralizing antibodies induced in rhesus monkeys by virus-like particle HIV vaccines in the absence of protection from SHIV infection. Virology 245:65-74. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, A. D., C. J. Clarke, and C. R. Stokes. 1990. Whole cholera toxin and B subunit act synergistically as an adjuvant for the mucosal immune response of mice to keyhole limpet haemocyanin. Scand. J. Immunol. 31:443-451. [DOI] [PubMed] [Google Scholar]
- 48.Wilson, L. A., M. Murphey-Corb, L. N. Martin, R. M. Harrison, M. S. Ratterree, and R. P. Bohm. 2000. Identification of SIV env-specific CTL in the jejunal mucosa in vaginally exposed, seronegative rhesus macaques (Macaca mulatta). J. Med. Primatol. 29:173-181. [DOI] [PubMed] [Google Scholar]
- 49.Wu, H. Y., and M. W. Russell. 1993. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect. Immun. 61:314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamshchikov, G. V., G. D. Ritter, M. Vey, and R. W. Compans. 1995. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology 214:50-58. [DOI] [PubMed] [Google Scholar]
- 51.Yao, Q., F. M. Kuhlmann, R. Eller, R. W. Compans, and C. Chen. 2000. Production and characterization of simian-human immunodeficiency virus-like particles. AIDS Res. Hum. Retrovir. 16:227-236. [DOI] [PubMed] [Google Scholar]
- 52.Yao, Q., V. Vuong, M. Li, and R. W. Compans. 2002. Intranasal immunization with SIV virus-like particles (VLPs) elicits systemic and mucosal immunity. Vaccine 20:2537-2545. [DOI] [PubMed] [Google Scholar]
- 53.Yasutomi, Y., T. J. Palker, M. B. Gardner, B. F. Haynes, and N. L. Letvin. 1993. Synthetic peptide in mineral oil adjuvant elicits simian immunodeficiency virus-specific CD8+ cytotoxic T lymphocytes in rhesus monkeys. J. Immunol. 151:5096-5105. [PubMed] [Google Scholar]