Abstract
Antigenic alterations to the dentin organic matrix may be detected by an immunohistochemical approach. We hypothesized that alterations in the antigenicity of type I collagen and proteoglycans occur in sclerotic dentin under caries lesions. Transverse sections were prepared from carious teeth in the sclerotic zone and normal hard dentin. A double-immunolabeling technique was performed on these sections, with anti-type I collagen and anti-chondroitin 4/6 sulfate monoclonal primary antibodies. We used gold-conjugated secondary antibodies to visualize the distribution of intact collagen fibrils and proteoglycans by high-resolution SEM. For sclerotic dentin, labeling densities were 19.57 ± 3.01/µm² for collagen and 9.84 ± 2.62/µm² for proteoglycans. For normal hard dentin, values were 35.20 ± 2.73/µm² and 17.03 ± 1.98/µm², respectively. Distribution of intact collagen fibrils and proteoglycans in sclerotic dentin was significantly lower than in normal hard dentin. Reductions in antigenicity from the organic matrix of sclerotic dentin under caries lesions raise concern about the potential of intrafibrillar remineralization.
Keywords: caries, collagen fibrils, proteoglycans, immunohistochemistry, FEISEM
INTRODUCTION
The dentin organic matrix contains different extracellular proteins, such as type I collagen, proteoglycans, dentin phosphoproteins, and sialoproteins. Type I collagen fibrils provide a three-dimensional scaffold for the deposition of the apatite mineral phase (Marshall et al., 1997). Collagen fibrils are linked together by proteoglycans that act like bridges between contiguous fibrils (Scott, 1988). Proteoglycans are perpendicularly oriented with respect to collagen fibril direction, linking to the latter every 68 nm (Bourdon et al., 1985). They are high-molecular-weight (ca. 11-220 kDa) carbohydrate-containing polyanions consisting of a polypeptide core with lateral glycosaminoglycan chains. Glycosaminoglycans regulate the biophysical properties of dentin by filling spaces, binding and organizing water molecules, and repelling negatively charged molecules (Bourdon et al., 1985). Due to their high affinity for water, proteoglycans play a pivotal role in determining the shrinkage properties of the demineralized dentin matrix. They help to maintain the hydration status of dentin (Breschi et al., 2003b) following demineralization of the dentin organic matrix.
Carious dentin consists of a soft, outer layer of caries-infected dentin, in which caries is actively in progress, and a relatively harder inner layer of sclerotic dentin that is bacteria-free (Arends et al., 1997). So that the grossly denatured caries-infected dentin can be mechanically removed, a dye solution consisting of 0.5% basic fuchsin or 1% acid red is used clinically to differentiate between the denatured outer layer and the remineralizable inner layer of carious dentin. Previous in vitro (Okuda et al., 2003) and in vivo studies (Arends et al., 1989) have reported the effect of carious attack on the apatite phase of the dentin matrix. However, alterations to the dentin organic matrix during the carious process remain controversial (Tjäderhane et al., 1998; Van Strijp et al., 2003; Nakornchai et al., 2004; Pashley et al., 2004). In particular, the fate of proteoglycans in dentinal caries remains obscure. In light of the minimally invasive strategy for the retention of sclerotic dentin for remineralization and dentin permeability reduction (Kawasaki et al., 1999), preservation of the structural integrity of collagen fibrils and proteoglycans in carious dentin is paramount if the integrity of adhesive-bonded caries-affected dentin is to be ensured over time.
Thus, the objective of this study was to examine the changes in type I collagen and proteoglycan distribution in sclerotic dentin under caries lesions by an immunohistochemical approach (Breschi et al., 2003b), in conjunction with field-emission in-lens scanning electron microscopy (FEISEM). The null hypothesis tested was that there are no differences in the distribution of antigenically intact collagen fibrils and proteoglycans between normal hard dentin and sclerotic dentin under caries lesions.
MATERIALS & METHODS
We selected for study 20 human molars with active enamel and dentin caries of 1.5 to 2.5 mm in diameter (measured on preoperative radiographs) and scheduled for extraction for periodontal or orthodontic reasons. Informed consent was received from each patient, under a protocol approved by the Ethics Committee of the University of Bologna, Italy.
Immediately after extraction, teeth were examined under an optical stereomicroscope for identification of areas with carious dentin. The soft caries-infected dentin was removed with a low-speed handpiece (Castellini, Bologna, Italy) equipped with a 0.7-mm carbide bur, followed by hand excavation to expose the underlying sclerotic dentin under the caries lesion. A custom-prepared solution of 0.5% basic fuchsin was used to discriminate between the caries-affected and sound dentin. The teeth were transversely sectioned with a low-speed diamond saw (Remet, Casalecchio di Reno, Italy) under water irrigation. Two 1-mm-thick dentin slices were retrieved from each tooth. The first slice was obtained at the level of the surface sclerotic dentin, and the second at the level of the underlying normal hard dentin. The sclerotic (N = 20) and sound (N = 20) dentin slices were polished with silicon carbide papers of decreasing abrasiveness (120–4000 grit) under irrigation with de-ionized water. The specimens were then ultrasonicated for 1 min in de-ionized water (pH 7.4) and exposed to 10% citric acid for 15 sec (Breschi et al., 2003b).
Immunohistochemistry
A double-immunolabeling procedure (Breschi et al., 2003b) was performed with 2 monoclonal primary antibodies: an IgG anti-type I collagen and an IgM anti-chondroitin 4/6 sulfate (mouse monoclonal; Sigma Chemical Co., St. Louis, MO, USA), for simultaneous detection of the distribution of antigenically intact collagen fibrils and proteoglycans. The normal hard and sclerotic dentin slices were immersed in 0.05 M Tris HCl buffered solution (TBS; pH 7.6) with 0.15 M NaCl and 0.1% bovine serum albumin, and then pre-incubated for 30 min in TBS 0.05 M normal goat serum (British BioCell International, Cardiff, United Kingdom) at pH 7.6. Overnight incubation was subsequently performed with the primary antibodies at 4°C. After incubation, the specimens were rinsed with 0.05 M TBS at pH 7.6 and 0.02 M TBS at pH 8.2 (0.02 M Tris HCl, buffered at pH 8.2 with 0.15 M NaCl and 0.1% bovine serum albumin). Gold labeling was performed with 2 secondary antibodies conjugated with gold particles of different sizes: an IgG goat anti-mouse-IgG conjugated with 30-nm gold particles (British BioCell International) for type I collagen identification, and an IgG goat anti-mouse-IgM conjugated with 15 nm colloidal gold for chondroitin 4/6 sulfate identification (British BioCell International). Reaction of the secondary antibodies was performed in 0.02 M TBS at pH 8.2 for 90 min at room temperature. The specimens were then rinsed in 0.02 M TBS at pH 8.2 and, finally, in de-ionized water prior to further laboratory processing.
Ultrastructural Processing
The specimens were fixed in 2.5% glutaraldehyde in 0.1 M Sorensen's phosphate buffer (PB) at pH 7.2 for 4 hrs, rinsed in 0.15 M PB, dehydrated in ascending ethanol series, and dried with hexamethyldisilazane (Sigma Chemical Co.). They were coated with carbon and examined under a FEISEM (JSM 890, JEOL, Tokyo, Japan) at 7 KeV and 1 × 10−12 Amp. Final images were obtained as a combination of both back-scattered (for gold nano-particle identification) and secondary electron signals, at magnifications up to 100,000X. Quantitative measurements were performed with the use of image-analysis software (JSMSCSI, JEOL Italia SpA, Milan, Italy).
Quantitative Evaluation
Fifteen micrographs with the same magnification (×20,000) were obtained for each sample. The labeling index was defined as the mean of the gold particle number/µm² (± SD) of the visible organic network on each image (Septier et al., 1998). We calculated the labeling index by utilizing the image analysis software after digitizing the mixed SEI-BEI images and 'thresholding' the grey level characteristic of both the gold particles and the background (i.e., showing the absence of any organic components). Since the data were not normally distributed (Kolmogorov-Smirnov tests), we used Mann-Whitney tests to compare the labeling indices for collagen fibrils and proteoglycans in normal hard vs. sclerotic dentin (Breschi et al., 2003a), with the level of statistical significance set at p = 0.05.
Controls
The controls consisted of dentin specimens processed as previously described for the pre-embedding immunohistochemical procedure and: (1) incubated overnight in 0.05 M TBS at pH 7.6 without the primary antibodies, then with the 2 secondary antibodies; (2) incubated with the anti-type I collagen primary antibody, and then with the anti-mouse IgM conjugated with 15-nm gold nanoparticles used for immunolabeling of proteoglycans; and (3) incubated with the anti-chondroitin 4/6 sulfate primary antibody, and then incubated with the anti-mouse IgG conjugated with 30-nm gold nanoparticles used for immunolabeling of type I collagen fibrils (Breschi et al., 2003b). (4) The immunohistochemical procedure was applied to the de-proteinated sound dentin substrates after removal of the organic matrix with 5% sodium hypochlorite applied for 10 min at room temperature; and (5) specimens of un-fixed predentin organic matrix were prepared, and the double-immunolabeling procedure was performed without surface chemical pre-treatment, so that a positive control could be obtained.
RESULTS
Type I collagen and proteoglycan labeling distributions in sclerotic dentin under caries lesions are shown in Fig. 1–Fig. 3, and those in normal hard dentin in Fig. 4. Low magnifications of the sclerotic dentin showed an inhomogeneous distribution of the 30-nm gold particles anchored to the collagen fibrillar network (Figs. 1A, 1B). Structural modifications of the collagen network, such as collapse, swelling, and branching, were evident at higher magnifications (Figs. 3A, 3B). Cross-banding was rarely identified from these collagen fibrils. Chondroitin sulfate labeling could be recognized as smaller, 15-nm gold particles, often appearing in clusters of 2 or 3 particles, along the branching points of the collagen fibrils (Fig. 3A). In the peritubular area, labeling of type I collagen was sparse, and the 15-nm gold particles were predominantly observed (Figs. 2A, 2B).
Figure 1.
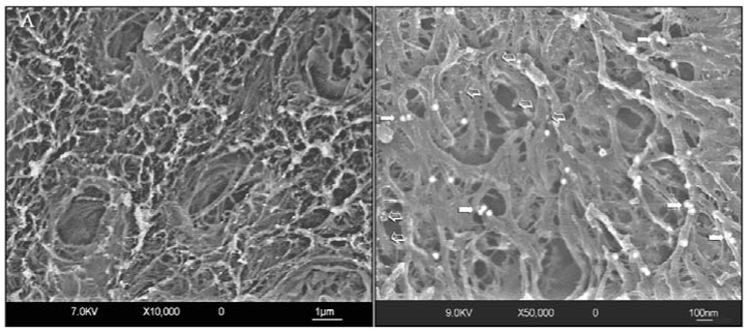
Field emission in-lens scanning electron micrographs (FEISEM) of sclerotic dentin after double-immunolabeling with monoclonal antibodies for type I collagen and proteoglycans. The images were obtained by a combination of secondary electron and back-scattered electron signals. (A) Low-magnification view of the surface of sclerotic dentin reveals partially patent tubular orifices surrounded by thick collars of peritubular fibrillar structures. Intertubular dentin is highly porous and is heterogeneously covered with the large (30 nm) gold particles used for labeling of antigenically intact collagen fibrils. Gold nanoparticles (15 nm) for labeling chondroitin sulfate cannot be discerned at this magnification. (B) A higher-magnification view of the sclerotic intertubular dentin, showing the labeling patterns for type I collagen (arrows) and chondroitin sulfate (open arrows). Only a few nanoparticles specific for chondroitin sulfate can be visualized at this level of magnification.
Figure 3.
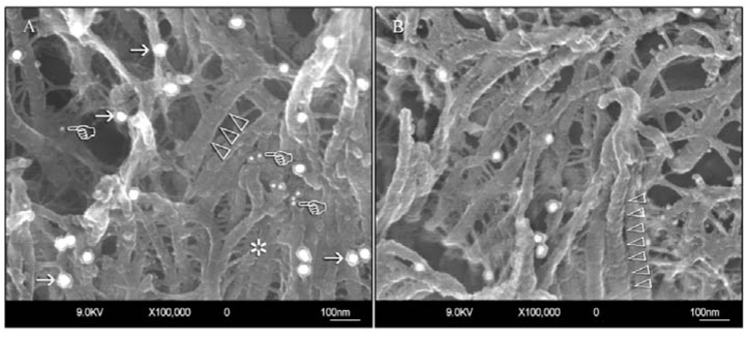
Very-high-magnification views of representative sclerotic intertubular dentin specimens after immunolabeling. (A) A specimen exhibiting moderately intense labeling for type I collagen and proteoglycans. Labeling of type I collagen is represented by the identification of larger (30 nm), discrete gold nanoparticles along the surfaces of the collagen fibrils (arrows). Labeling of proteoglycans is represented by smaller (15 nm) gold nanoparticles that appear either as discrete particles or in clusters of 2–3 particles (pointers). Collagen banding is infrequently seen on the collagen fibrils in sclerotic dentin and, when present, appears as very vague surface elevations (open arrowheads). The collagen fibrils appear collapsed and swollen, and exhibit extensive branching (asterisk) when compared with those observed in normal hard dentin (see Fig. 4). (B) A collapsed and swollen collagen network from another specimen of sclerotic dentin that exhibits less intense immunolabeling of both type I collagen and proteoglycans. A banded collagen fibril can be seen in the foreground (open arrowheads).
Figure 4.
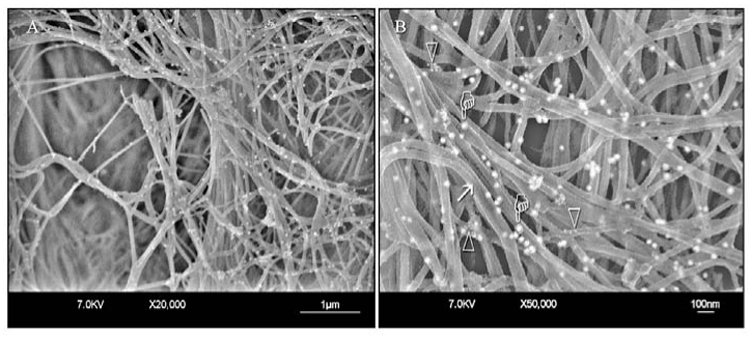
FEISEM micrographs of the collagen fibrillar network in normal hard dentin after immunolabeling. (A) Low magnification. (B) High magnification. Collagen fibrils appear unmodified, with surface cross-banding features (arrow) and gold nanoparticles (pointers) along the fibrils. Gold nanoparticles specific for proteoglycans appear as clusters of smaller electron-lucent particles around the collagen fibrils (open arrowheads). These clusters can be seen only at high magnification.
Figure 2.
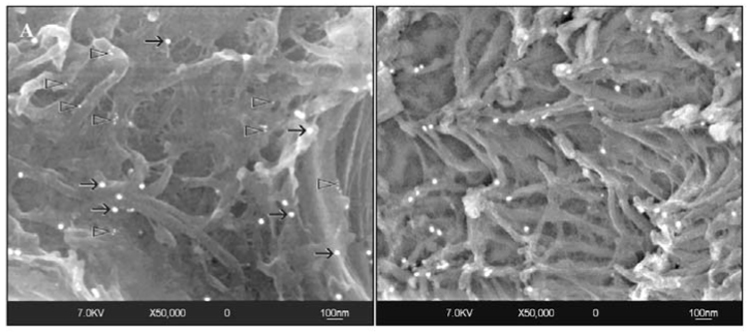
High-magnification views taken from the peritubular regions of sclerotic dentin after immunolabeling. (A) Labeling for collagen fibrils (black arrows) is sparse in this region. A few clusters of 15-nm nanoparticles (open arrowheads) that were bound to the anti-chondroitin sulfate monoclonal antibodies can also be identified. (B) Another specimen showing sparse labeling for antigenically intact type I collagen. Labeling for proteoglycans cannot be observed.
Specimens of normal hard dentin revealed intense labeling of type I collagen (Fig. 4A). Labeling of proteoglycans could be identified only at high magnifications and appeared as smaller scattered gold particles around the collagen fibrils (Fig. 4B). A periodic repetition of elevations and depressions could frequently be identified from the surfaces of the collagen fibrils (Fig. 4B).
Quantitative evaluation revealed labeling densities of 19.57 ± 3.01/µm² for type I collagen and 9.84 ± 2.62/µm² for chondroitin 4/6 sulfate in sclerotic dentin. In normal hard dentin, the values were 35.20 ± 2.73/µm² and 17.03 ± 1.98/µm², respectively. Significant differences (p < 0.05) were identified for both type I collagen fibrils and chondroitin 4/6 sulfate between sclerotic dentin and normal hard dentin.
Control specimens showed the complete absence of labeling (data not shown), confirming that there were no cross-reactions between the primary antibodies (incubation performed after protein removal [control #4]) or the secondary antibodies (incubation performed without the primary antibody [control #1]) and the mineral phase of dentin. Negative controls #2 and #3 confirmed the selective binding of the secondary antibodies with the correct primary antibodies, thus discriminating between IgG and IgM. Control #5 showed intense labeling for both type I collagen and proteoglycans on the predentin fibrillar organic network, confirming the effectiveness of the labeling procedure (data not shown).
DISCUSSION
Previous investigations of the sound dentin organic matrix revealed a three-dimensional network of fibrillar and globular structures (Marshall et al., 1997; Dahl et al., 1998). The main structural components of this network are type I collagen fibrils and proteoglycans (Breschi et al., 2003b), with minor contributions from non-collagenous proteins, such as dentin sialoproteins, phosphophoryns, bone morphogenic proteins, and insulin-like growth factors 1 and 2 (Dahl et al., 1998).
Carious dentin consists of a transparent zone of caries-affected dentin (deep and close to normal dentin) and a superficial opaque zone of caries-infected dentin (Frank, 1990). These two layers have previously been analyzed by confocal light microscopy (Banerjee et al., 1999), transmission electron microscopy (Ogawa et al., 1983), atomic force microscopy (Zheng et al., 2003), and scanning electron microscopy (Arends et al., 1989). These studies confirmed that changes such as tubular occlusion with mineral deposits occurred during the caries process. Identification of structurally or biochemically intact collagen fibrils from carious dentin has traditionally been based upon the detection of collagen banding or fibrillar cross-links (Marshall et al., 1997; Tjäderhane et al., 1998). Kuboki et al. (1977) demonstrated the presence of intermolecular cross-linking from collagen fibrils in the transparent zone. The authors suggested that caries-affected dentin is remineralizable and is a suitable substrate for dentin adhesion.
The antigenicity of a single protein, as revealed by its specific binding to a monoclonal antibody, provides definitive evidence of an optimal conservation of the epitope structure (Hall and Embery, 1997). Since monoclonal antibodies are highly sensitive (Willingham, 1999), the protocol for this study can be considered highly selective in identifying alterations in the protein epitopes. According to Lynn et al. (2004), epitopes for collagen type I monoclonal antibodies can be divided into helical, central, and terminal, depending on their ability to interact with the collagen peptides. The antibody anti-helical portion (such as the one used in this study) recognizes the substrate based on three-dimensional conformation that is related to the presence of an intact triple helix. Since the antibody recognizes the native form of collagen type I and does not react with the denatured molecule, we have to reject the null hypothesis that there are no differences in the distribution of antigenically intact collagen fibrils and proteoglycans between normal hard and sclerotic dentin under caries lesions. Indeed, the caries process induces modifications to both type I collagen fibrils and proteoglycans, as shown by the decreased labeling indices when sclerotic dentin was compared with sound dentin.
The decreased labeling indices associated with the sclerotic dentin under caries lesions may be caused either by the masking of the protein epitopes by the apatite mineral phase present in the hypermineralized peritubular areas of the transparent zone, or by the denaturing of the protein components (Breschi et al., 2003a). Marshall et al. (2001) demonstrated that intertubular dentin in the transparent zone is not hypermineralized compared with normal sound dentin. Thus, alteration in the antigenicity of the collagen fibrils and proteoglycans in sclerotic dentin under caries lesions appears to be the more logical explanation for the decreased labeling indices of the gold-conjugated antibodies.
To date, evidence of remineralization of enamel and dentinal caries is largely based upon the results obtained with densitometry (Arends et al., 1997). Although it has been shown that dentin remineralization is possible in the presence of low fluoride concentrations by ionic diffusion into porous caries-affected dentin (ten Cate, 2001; Mukai and ten Cate, 2002), evidence for remineralization within the gap zones of collagen fibrils (i.e., intrafibrillar remineralization) in caries-affected dentin is lacking. A recent study highlights the significance of intrafibrillar remineralization of dentin collagen, since intrafibrillar minerals are responsible for stiffening of the collagen fibrils, enabling them to function optimally under loading (Kinney et al., 2003). Although collagen fibrils in caries-affected dentin retain their structural identity as intact fibrillar entities (Yoshiyama et al., 2003), the reductions in antigenicity of both type I collagen and proteoglycans in this study raise concern regarding the possibility of intrafibrillar remineralization in sclerotic dentin. We hypothesize that extrafibrillar (i.e., surface deposition or interfibrillar) remineralization of the altered organic matrix alone is insufficient in re-establishing the mechanical properties of sclerotic dentin to those present in normal hard dentin. This hypothesis is testable, in principle, with the adjunctive use of atomic force microscopy and immunolabeling for transmission electron microscopy, and should be attempted in future work.
ACKNOWLEDGMENTS
We thank Gabriella Teti of the Electron Microscope Unit, University of Bologna, Italy, for technical assistance with the field-emission scanning electron microscope, and Mr. Aurelio Valmori for laboratory support. This study was supported by grant MIUR, Italy, grant 10204604/07840/08004/324/01, Faculty of Dentistry, the University of Hong Kong at Pokfulam, and by grants DE 014911 and DE 015306 from the NIDCR, NIH, USA (PI David Pashley). The authors are grateful to Michelle Barnes for secretarial support.
REFERENCES
- Arends J, Ruben J, Jongebloed WL. Dentine caries in vivo. Combined scanning electron microscopic and microradiographic investigation. Caries Res. 1989;23:36–41. doi: 10.1159/000261152. [DOI] [PubMed] [Google Scholar]
- Arends J, Ruben JL, Inaba D. Major topics in quantitative microradiography of enamel and dentin: R parameter, mineral distribution visualization, and hyper-remineralization. Adv Dent Res. 1997;11:403–414. doi: 10.1177/08959374970110040501. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Sherriff M, Kidd EA, Watson TF. A confocal microscopic study relating the autofluorescence of carious dentine to its microhardness. Br Dent J. 1999;187:206–210. doi: 10.1038/sj.bdj.4800241. [DOI] [PubMed] [Google Scholar]
- Bourdon MA, Oldberg A, Pierschbacher M, Ruoslahti E. Molecular cloning and sequence analysis of a chondroitin sulfate proteoglycan cDNA. Proc Natl Acad Sci USA. 1985;82:1321–1325. doi: 10.1073/pnas.82.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschi L, Perdigão J, Gobbi P, Mazzotti G, Falconi M, Lopes M. Immunocytochemical identification of type I collagen in acid-etched dentin. J Biomed Mater Res A. 2003a;66:764–769. doi: 10.1002/jbm.a.10073. [DOI] [PubMed] [Google Scholar]
- Breschi L, Gobbi P, Lopez S, Prati C, Falconi M, Teti G. Immunocytochemical analysis of dentin: a double-labeling technique. J Biomed Mater Res A. 2003b;67:11–17. doi: 10.1002/jbm.a.10048. [DOI] [PubMed] [Google Scholar]
- Dahl T, Sabsay B, Veis A. Type I collagen-phosphophoryn interactions: specificity of the monomer-monomer binding. J Struct Biol. 1998;123:162–168. doi: 10.1006/jsbi.1998.4025. [DOI] [PubMed] [Google Scholar]
- Frank RM. Structural events in the caries process in enamel, cementum, and dentin. J Dent Res. 1990;69(Spec Iss):559–566. doi: 10.1177/00220345900690S112. [DOI] [PubMed] [Google Scholar]
- Hall RC, Embery G. The use of immunohistochemistry in understanding the structure and function of the extracellular matrix of dental tissues. Adv Dent Res. 1997;11:478–486. doi: 10.1177/08959374970110041601. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Ruben J, Stokroos I, Takagi O, Arends J. The remineralization of EDTA-treated human dentine. Caries Res. 1999;33:275–280. doi: 10.1159/000016529. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res. 2003;82:957–961. doi: 10.1177/154405910308201204. [DOI] [PubMed] [Google Scholar]
- Kuboki Y, Ohgushi K, Fusayama T. Collagen biochemistry of the two layers of carious dentin. J Dent Res. 1977;56:1233–1237. doi: 10.1177/00220345770560102301. [DOI] [PubMed] [Google Scholar]
- Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Jr, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Jr, Chang YJ, Gansky SA, Marshall SJ. Demineralization of caries-affected transparent dentin by citric acid: an atomic force microscopy study. Dent Mater. 2001;17:45–52. doi: 10.1016/s0109-5641(00)00056-7. [DOI] [PubMed] [Google Scholar]
- Mukai Y, ten Cate JM. Remineralization of advanced root dentin lesions in vitro. Caries Res. 2002;36:275–280. doi: 10.1159/000063924. [DOI] [PubMed] [Google Scholar]
- Nakornchai S, Atsawasuwan P, Kitamura E, Surarit R, Yamauchi M. Partial biochemical characterisation of collagen in carious dentin of human primary teeth. Arch Oral Biol. 2004;49:267–273. doi: 10.1016/j.archoralbio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Yamashita Y, Ichijo T, Fusayama T. The ultrastructure and hardness of the transparent layer of human carious dentin. J Dent Res. 1983;62:7–10. doi: 10.1177/00220345830620011701. [DOI] [PubMed] [Google Scholar]
- Okuda M, Pereira PN, Nikaido T, Tagami J. Evaluation of in vitro secondary caries using confocal laser scanning microscope and x-ray analytical microscope. Am J Dent. 2003;16:191–196. [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Septier D, Hall RC, Lloyd D, Embery G, Goldberg M. Quantitative immunohistochemical evidence of a functional gradient of chondroitin 4-sulphate/dermatan sulphate, developmentally regulated in the predentine of rat incisor. Histochem J. 1998;30:275–284. doi: 10.1023/a:1003216024158. [DOI] [PubMed] [Google Scholar]
- ten Cate JM. Remineralization of caries lesions extending into dentin. J Dent Res. 2001;80:1407–1411. doi: 10.1177/00220345010800050401. [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77:1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- Van Strijp AJ, Jansen DC, DeGroot J, ten Cate JM, Everts V. Host-derived proteinases and degradation of dentine collagen in situ. Caries Res. 2003;37:58–65. doi: 10.1159/000068223. [DOI] [PubMed] [Google Scholar]
- Willingham MC. Conditional epitopes. Is your antibody always specific? J Histochem Cytochem. 1999;47:1233–1236. doi: 10.1177/002215549904701002. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Tay FR, Torii Y, Nishitani Y, Doi J, Itou K, et al. Resin adhesion to carious dentin. Am J Dent. 2003;16:47–52. [PubMed] [Google Scholar]
- Zheng L, Hilton JF, Habelitz S, Marshall SJ, Marshall GW. Dentin caries activity status related to hardness and elasticity. Eur J Oral Sci. 2003;111:243–252. doi: 10.1034/j.1600-0722.2003.00038.x. [DOI] [PubMed] [Google Scholar]


