Abstract
We recently identified mutants of the human FKBP12 protein that are unstable and rapidly degraded when expressed in mammalian cells. We call these FKBP mutants destabilizing domains (DDs), because their instability is conferred to any protein fused to the DDs. A cell-permeable ligand binds tightly to the DDs and prevents their degradation, thus providing small molecule control over intracellular protein levels. We now report the synthesis and functional characterization of a stabilizing ligand called Shield-2. The synthesis of Shield-2 is efficient, and this ligand binds to the FKBP(F36V) protein with a dissociation constant of 29 nM.
The ability to regulate the functions of specific proteins using cell-permeable small molecules is a powerful method to interrogate biological systems.1 Studies using several natural products have proven the utility of this approach,2–5 and the desire for additional reagents has driven screening efforts to identify new, and hopefully specific, perturbants.6,7 The fruits of these searches may ultimately be a large collection of reagents that might be used to interrogate many different proteins.
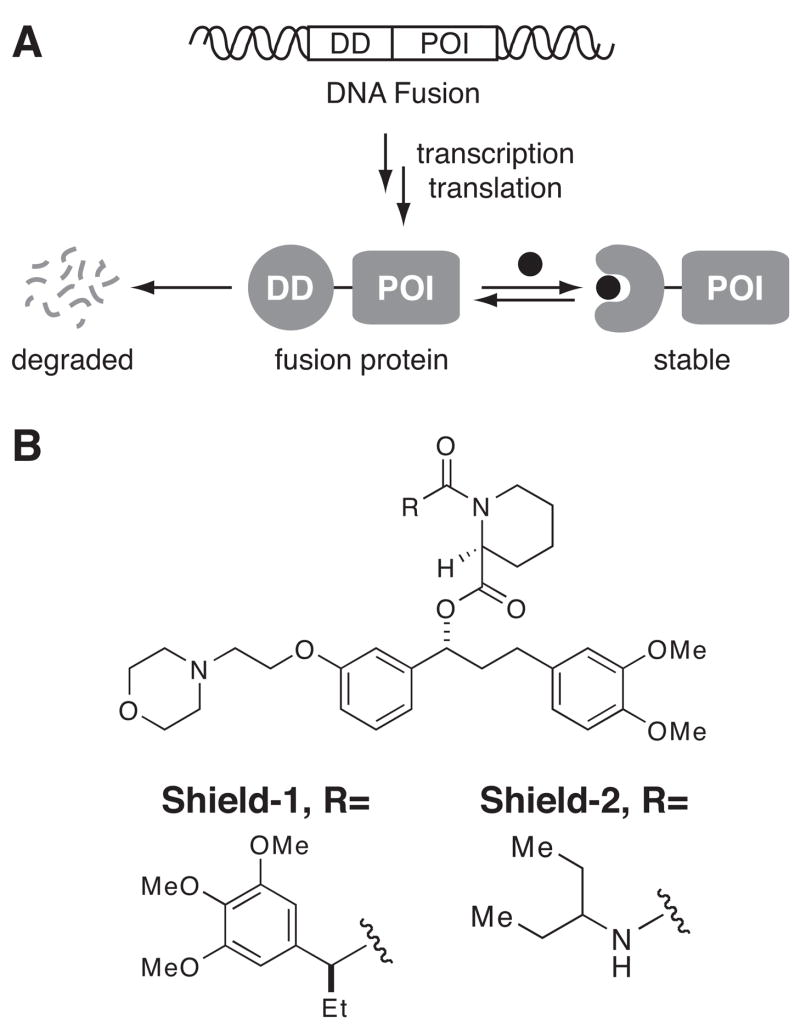
We have pursued an alternative strategy of creating a “one ligand-many proteins” strategy to manipulate protein function. We recently developed an experimental system in which the stability of a small protein domain depends upon the presence of a synthetic, cell-permeable molecule (Figure 1A).8 The specificity of this method comes from the genetic fusion of the destabilizing domain (DD) to any protein of interest, and the cell-permeable stabilizing small molecule provides the elements of speed, reversibility, and tunability to regulate protein levels.
Figure 1.
(A) Strategy for conferring ligand-dependent stability to any protein of interest (POI) by fusing the DNA sequence encoding a destabilizing domain (DD) to the gene encoding the POI. (B) Structures of the ligands for FKBP(F36V).
We started with a well-studied protein-ligand pair: the human FKBP12 protein and a high-affinity, synthetic ligand called Shield-1 (Figure 1B). Shield-1 possesses a structural “bump” and the FKBP partner harbors the F36V mutation, which provides a complementary cavity for the ligand.9–10 Because of these structural modifications, Shield-1 binds to the FKBP(F36V) mutant approximately 1600-fold more tightly than to the wild-type FKBP protein, and as expected, Shield-1 does not elicit any detectable response when administered to cultured mammalian cells.11
FKBP(F36V) can typically be fused to other proteins without affecting the stability of the resulting fusion protein, so we screened an library of FKBP sequences to identify mutants that are unstable in the absence of Shield-1. Additional screening enriched for FKBP mutants that are stabilized by Shield-1, and further characterization of these mutants revealed that the most destabilizing mutants cause a 50-fold to 100-fold reduction in the levels of the proteins to which they are fused.8 FKBP-derived destabilizing domains (DDs) confer instability to a variety of proteins, and these DDs destabilize proteins that are expressed in cultured mammalian cells as well as in living mice.
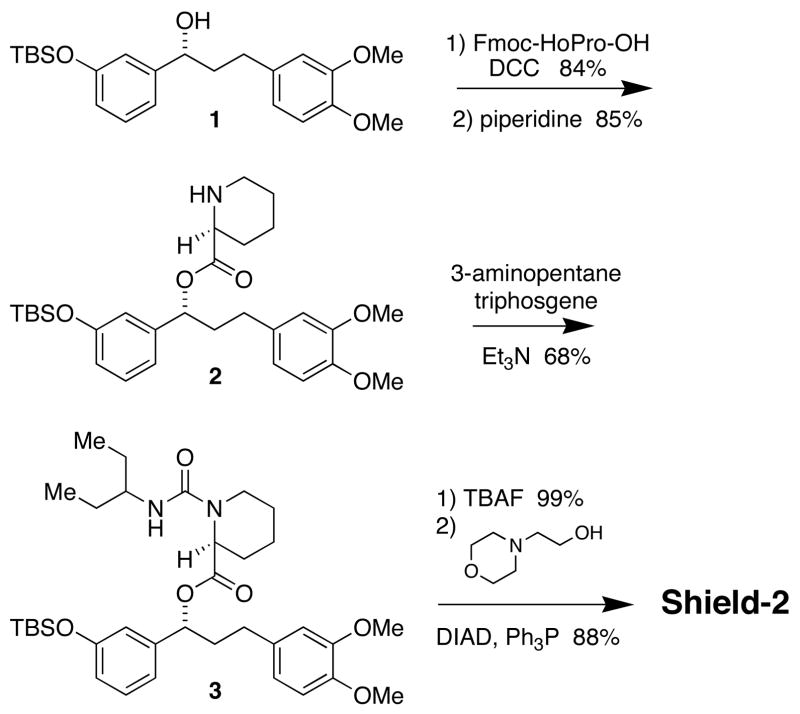
The stabilizing ligands are key elements to the success of this technology. Biophysical properties (e.g., affinity, kon, koff) as well as pharmacological properties will influence the potency and kinetic behavior of this system in both cultured cells and living animals. We have synthesized additional ligands for the FKBP(F36V) protein and evaluated these molecules as stabilizing ligands for the FKBP-derived DDs.11 Yang and colleagues recently reported an FKBP ligand in which the chiral 2-arylbutyric ester of Shield-1 is replaced with an achiral urea, significantly simplifying the synthesis.12 The synthesis of a stabilizing ligand called Shield-2 that incorporates this modification is shown in Scheme 1. Alcohol 1 was acylated with Fmoc-protected pipecolinic acid, and treatment with piperidine furnished amine 2. This intermediate was treated with triphosgene and 3-aminopentane, which furnished urea 3. The phenol was deprotected with TBAF, and alkylation of the phenol with 4-(2-hydroxyethyl)morpholine under Mitsunobu conditions provided Shield-2.
Scheme 1.
Synthesis of Shield-2.
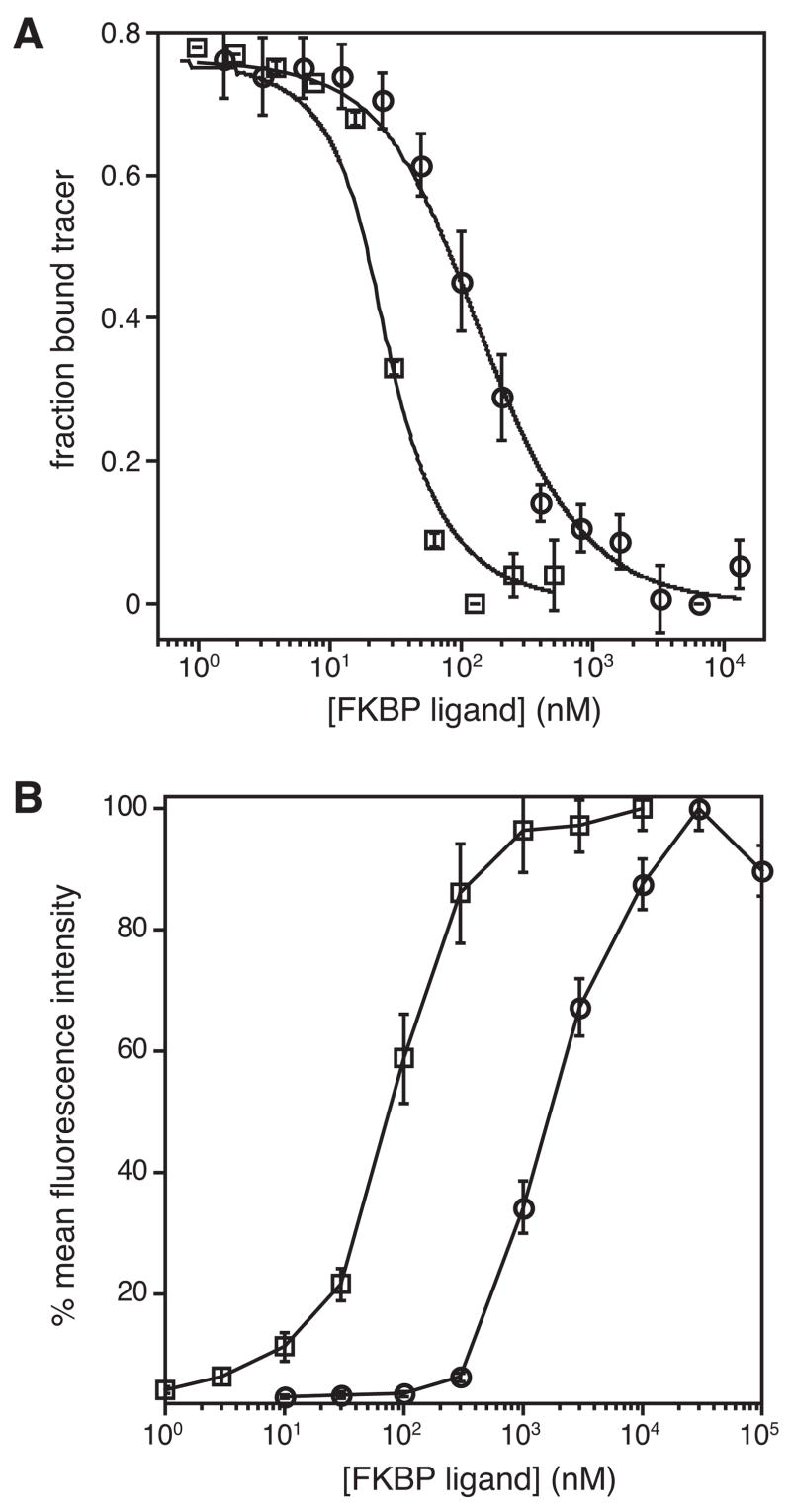
We used a fluorescence polarization-based competition binding assay to measure the affinity of each ligand for the FKBP(F36V) protein.13 A fluorescent FKBP ligand was incubated with various concentrations of purified FKBP(F36V), and this saturation binding experiment provided the dissociation constant for this interaction (KD=3.6 nM, Supplementary data, Fig S1). To measure the affinities of the ligands for FKBP(F36V), the protein and fluorescent tracer were incubated at fixed concentrations along with various concentrations of Shield-1 or Shield-2, and the fraction of bound tracer is plotted as a function of competitor concentration (Figure 2A). Fitting these competition binding curves reveals that Shield-1 and Shield-2 bind to the F36V protein with affinities of 2.4 nM and 29 nM, respectively.
Figure 2.
(A) Binding analysis of Shield-1 and Shield-2. FKBP(F36V) (20 nM) and the fluorescent tracer FL-SLF (1 nM) were incubated with Shield-1 (□, 500 nM to 1 nM) or Shield-2 (○, 12.8 μM to 1.6 nM). Data were fit as described in reference 12 to obtain dissociation constants of 2.4 nM (R2 = 0.98) and 29 nM (R2 = 0.99) for Shield-1 and Shield-2, respectively. Error bars represent the standard deviation of four repetitions. (B) NIH3T3 cells stably expressing the FKBP(F36V, L106P)-YFP fusion protein were treated with Shield-1 (□, 10 μM to 1 nM, EC50~100 nM) or Shield-2 (○, 100 μM to 10 nM, EC50~1.2 μM), and YFP fluorescence was measured at 24 hours using flow cytometry. Error bars represent the standard deviation of three repetitions.
To test the stabilizing ligands in cultured mammalian cells, we used an MMLV-derived retrovirus to stably transduce NIH3T3 cells with the FKBP(F36V/L106P) DD fused to yellow fluorescent protein (YFP). In the absence of stabilizing ligand the levels of the YFP-containing fusion protein levels are 2–3% of ligand-stabilized control. Populations of these cells were then treated with various concentrations of either Shield-1 or Shield-2, and the YFP levels were quantitated using analytical flow cytometry (Figure 2B). Both ligands stabilize the DD-YFP fusion protein, and Shield-1 is approximately 12-fold more potent than Shield-2.
We have developed a general small molecule-dependent strategy to regulate the stability, and thus function, of specific proteins when expressed in mammalian cells. The dose-dependence that is conferred by the stabilizing ligand makes this a particularly attractive experimental approach for studying biological processes in which the cellular concentration of a particular protein exerts a strong effect on biological function.14 Shield-2 is considerably easier to synthesize than Shield-1, and continued studies with these ligands as well as others will likely produce compounds that display a variety of biophysical and pharmacological properties. The choice of the appropriate ligand will be governed by the experimental strategy and biological context.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at XXX.
Acknowledgments
Financial support was provided by the NIH (GM073046) and the California Tobacco-Related Disease Research Program (15DT-0015) to J.S.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Banaszynski LA, Wandless TJ. Chem Biol. 2006;13:11. doi: 10.1016/j.chembiol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Cell. 1991;66:807. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 3.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Schreiber SL. Nature. 1994;369:756. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 4.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Science. 1995;268:726. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 5.Taunton J, Hassig CA, Schreiber SL. Science. 1996;272:408. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 6.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Science. 1999;286:971. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 7.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Science. 2003;299:1743. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 8.Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AGL, Wandless TJ. Cell. 2006;126:995. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clackson T, Yang W, Rozamus LW, Hatada M, Amara JF, Rollins CT, Stevenson LF, Magari SR, Wood SA, Courage NL, Lu XD, Cerasoli F, Gilman M, Holt DA. Proc Natl Acad Sci USA. 1998;95:10437. doi: 10.1073/pnas.95.18.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang W, Rozamus LW, Narula S, Rollins CT, Yuan R, Andrade LJ, Ram MK, Phillips TB, van Schravendijk MR, Dalgarno D, Clackson T, Holt DA. J Med Chem. 2000;43:1135. doi: 10.1021/jm9904396. [DOI] [PubMed] [Google Scholar]
- 11.Maynard-Smith LA, Chen LC, Banaszynski LA, Ooi AGL, Wandless TJ. J Biol Chem. 2007;282:24866. doi: 10.1074/jbc.M703902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W, Keenan TP, Rozamus LW, Wang XR, Rivera VM, Rollins CT, Clackson T, Holt DA. Bioorg Med Chem Lett. 2003;13:3181. doi: 10.1016/s0960-894x(03)00707-8. [DOI] [PubMed] [Google Scholar]
- 13.Braun PD, Wandless TJ. Biochemistry. 2004;43:5406. doi: 10.1021/bi035839g. [DOI] [PubMed] [Google Scholar]
- 14.Niwa H, Miyazaki J, Smith AG. Nat Gen. 2000;24:372. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at XXX.