Abstract
Self-etching primers/adhesives that combine acidic methacrylate monomers with water in a single bottle are hydrolytically unstable and require refrigeration to extend their shelf-lives. This study tested the null hypothesis that one year of intermittent refrigeration of a 4-MET-containing simplified self-etching primer does not result in hydrolytic changes that are identifiable by transmission electron microscopy and 13C NMR spectroscopy. Human dentin was bonded with UniFil Bond immediately after being unpacked, or after one year of intermittent refrigeration at 4°C. Fresh and aged primers were analyzed by NMR for chemical changes. Ultrastructural observations indicated that there was an augmentation in etching capacity of the aged adhesive that was not accompanied by resin infiltration or effective polymerization. New NMR peaks detected from the aged ethanol-based primer confirmed that degradation occurred initially via esterification with ethanol, followed by hydrolysis of both ester groups in the 4-MET. Hydrolysis of functional methacrylate monomers occurs despite intermittent refrigeration.
Keywords: hydrolysis, single-bottle, 4-MET, self-etching, nuclear magnetic resonance
INTRODUCTION
Several compromises have to be made when self-etch adhesives containing conventional acidic methacrylate-based resin monomers are simplified into single-bottle formulations (Tay and Pashley, 2003; Moszner et al., 2005). Since these adhesives have pH values between 1 and 2, water must be sequestered from the acidic and hydrophilic methacrylate monomers, because acidified water attacks the ester bonds of these monomers, causing their hydrolysis during storage (Nishiyama et al., 2004). Thus, two- and one-step self-etch adhesives were mostly marketed as two-bottle sets, separating the 30–50% water that is required for dissociation of the functional monomers from the hydrolytically unstable acidic/hydrophilic methacrylate monomers. In the absence of the incorporation of more hydrolytically stable resin monomers, such as the phosphonates and acrylamides (Moszner et al., 1999, 2003; Nishiyama et al., 2004; Salz et al., 2005), this is where adhesive simplification procedures should have stopped. Instead, marketing forces overruled the objections of research chemists and led to the introduction of single-bottle-type self-etching primers and one-step self-etch adhesives that combine functional acidic methacrylate monomers with water in the same bottle.
There is accumulating evidence showing that these simplified adhesives have poor shelf-lives. Alterations in the composition of a single-bottle self-etching primer at increased storage temperature or prolonged storage time resulted in reductions of resin-dentin bond strengths (Okazaki, 2000). The rate of hydrolysis of 2-hydroxyethyl methacrylate (HEMA) in acidic solutions increases with temperature (Kazantsev et al., 2003). Conventional methacrylate monomers undergo rapid hydrolysis under acidic aqueous conditions, with up to 90% degradation after 16 wks at 42°C (Salz et al., 2005). 13C nuclear magnetic resonance (NMR) spectroscopy revealed that 80% of an aqueous solution of HEMA, acidified to pH 0.94 to simulate the environment of self-etch adhesives, was hydrolyzed into methacrylic acid and ethylene glycol after incubation at 37°C for 14 days. Conversely, control mixtures of HEMA/water incubated at pH 5.57 did not exhibit hydrolysis over the 14-day period (Nishiyama et al., 2004). Hydrolysis of the methacryloxy ester portion of functional monomers was also confirmed when commercial single-bottle-type self-etching primers, containing acidic methacrylate monomers with phosphate or carboxyl functional groups, were stored at 37°C for 10 wks (Fujita and Nishiyama, 2006b). The protonated water clusters generated during the dissociation of the ionic monomers in water function as auto-catalysts for the degradation of the ester linkages within these monomers (Fujita and Nishiyama, 2006a).
Accelerated aging is important in degradation studies. However, since most clinicians or assistants faithfully follow manufacturers’ instructions by refrigerating these adhesives after use, confirmation of previous results is required with the use of a longer storage period and a lower storage temperature. Moreover, the functional and morphologic correlates of hydrolysis of methacrylate ester bonds are unknown. Thus, the objective of this study was to determine whether hydrolytic degradation could occur when a 4-methacryloxyethyltrimellitic acid (4-MET)-containing, ethanol-based, single-bottle self-etching primer was stored intermittently at 4°C. A one-year storage period was selected, being the ‘half-life’ of the two-year shelf-life generally recommended for self-etch adhesives. The null hypothesis tested was that one year of intermittent refrigeration of a 4-MET-containing simplified self-etching primer does not result in hydrolytic changes that are identifiable with transmission electron microscopy and 13C NMR spectroscopy.
MATERIALS & METHODS
Special arrangements were made with the manufacturer to deliver the kits as soon as they were manufactured. To simulate clinical handling, we removed the primer and bonding agent bottles from the refrigerator on a weekly basis, and allowed them to stand at ambient temperature (20–25°C) for a day before returning them to the refrigerator. The single-bottle “Self-etching Primer” contains HEMA, 4-MET, camphorquinone, ethanol, and water; the “Bonding Agent” contains HEMA, urethane dimethacrylate, camphorquinone, and fumed silica fillers. We performed microtensile bond strength evaluation (Shono et al., 1999) as a screening procedure to ascertain if there was a significant drop in bond strength (18%; p < 0.05) in dentin bonded with the fresh (44.4 ± 7.2 MPa) and the aged adhesive (36.5 ± 7.1 MPa), before more labor-intensive investigations were subsequently performed.
For bonding purposes, non-carious human third molars that were stored in a 0.5% chloramine T solution at 4°C were used within one month following extraction. These teeth were collected after the patients’ informed consent had been obtained under a protocol approved by the Institutional Review Board of the Medical College of Georgia, Augusta. The occlusal enamel was removed by means of a slow-speed saw with a diamond-impregnated disk (Isomet, Buehler Ltd., Lake Bluff, IL, USA) under water cooling. A 180-grit silicon carbide paper was used under running water to create a smear layer on the dentin surface.
Transmission Electron Microscopy
Three teeth each were examined for both the fresh and aged adhesives. The “Self-etching Primer” was applied to dentin for 20 sec with agitation, then was air-dried for 5 sec. The “Bonding Agent” was left at room temperature for 5 min after removal from the refrigerator, applied to the primed dentin, and light-cured for 10 sec. This was followed by placement of a 2-mm-thick layer of light-cured, microfilled resin composite (Epic-TMPT, Parkell Inc., Farmingdale, NY, USA).
Undemineralized, 1-mm-thick slabs containing the bonded interfaces were exposed to 50 wt% ammoniacal silver nitrate (pH = 9.5) for 24 hrs according to a previously described protocol (Tay et al., 2002). The silver-impregnated slabs were then rinsed thoroughly and processed for TEM examination according to Tay et al.(1999). Demineralized, but not silver-impregnated, sections were also prepared by being double-stained with 2% uranyl acetate and Reynolds’ lead citrate, for comparison of the conditions of the stained resin-dentin interfaces. The sections were examined under a TEM (EM208S, Philips, Eindhoven, The Netherlands) operating at 80 kV. Since the TEM procedures were performed one year apart, digitized TEM images taken from the two periods were blindly evaluated by two experienced examiners who were unaware of the period in which the adhesive was applied.
13C NMR Spectroscopy
The single-bottle UniFil Bond “Self-etching Primer” was examined immediately after being opened (0 days) and after storage at 4°C for 12 mos (365 days). For each observation period, 0.2 g of deuterium oxide was added to 0.8 g of the UniFil Bond Primer. A 600-mg quantity of the deuterated primer solution was used for each recording. 13C NMR spectra were recorded at 298 K in an NMR spectrometer (EX-270; JEOL, Tokyo, Japan), operating at 67.8 MHz, according to the method previously reported by Fujita and Nishiyama (2006a). A 45° pulse angle was used for the NMR observations, with data accumulation and repetition times being 5000 sec and 3.8 sec, respectively. Hexamethyldisiloxane (HMDSO) was used as an external reference. The experiment was conducted twice for each of the two periods.
RESULTS
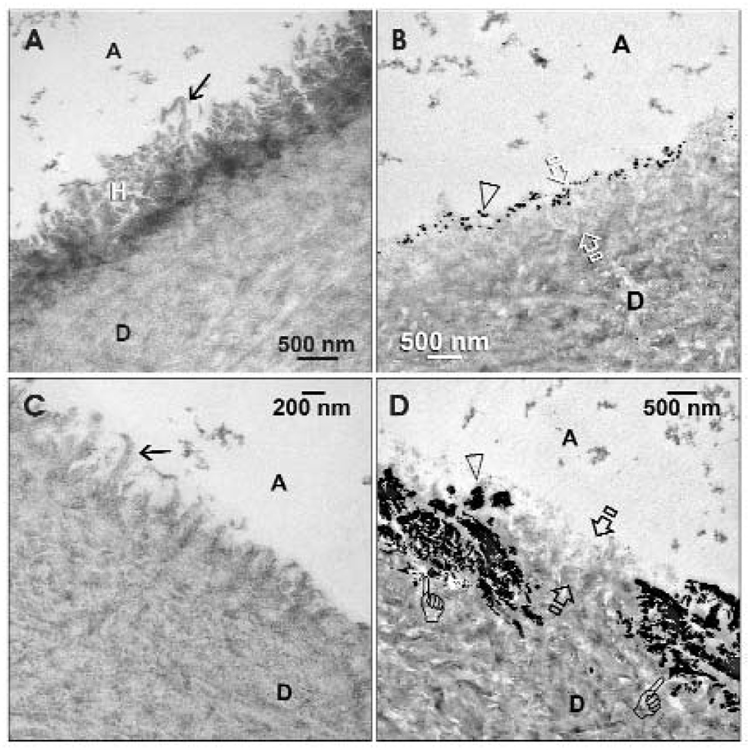
When the fresh self-etching primer adhesive was used, intensively stained hybrid layers were observed (Fig. 1A), with minimal silver deposits within the corresponding zone of partially demineralized dentin (ca. 1 µm thick; Fig. 1B). With the use of the aged adhesive, a stained hybrid layer could not be identified from stained sections (Fig. 1C). Extensive silver deposits were evident within and beneath the partially demineralized dentin (ca. 2 µm thick; Fig. 1D).
Figure 1.
Transmission electron micrographs illustrating the application of UniFil Bond to dentin when the adhesive was used (A–B) immediately after being opened and (C–D) at 12 mos after storage at ambient temperature. A, filled adhesive; D, dentin. (A) A stained, demineralized section showing the presence of a 1-µm-thick, electron-dense hybrid layer (H) when the 4-MET-containing self-etching primer was used immediately. Loosely arranged collagen fibrils (arrow) could be identified from the hybrid layer surface. (B) The corresponding undemineralized, silver-impregnated section revealed a 1-µm-thick, partially demineralized zone (between open arrows) that was consistent with the hybrid layer observed in stained, demineralized sections. Little nanoleakage was observed, and appeared as silver deposits (open arrowhead) along the surface of the partially demineralized zone. (C) A stained, demineralized section showing the absence of a stainable hybrid layer when the self-etching primer was used after 12 mos. However, evidence of etching by the primer solution could be identified by the absence of smear layer remnants and the presence of loosely oriented surface collagen fibrils (arrow). (D) The corresponding undemineralized, silver-impregnated section showed the presence of extensive silver deposits (open arrow) within the partially demineralized zone (between open arrows) and beyond (pointers). The depth of these silver deposits (ca. 2 µm) suggested that etching of the dentin was more aggressive, but there was poor resin infiltration or polymerization within the etched dentin.
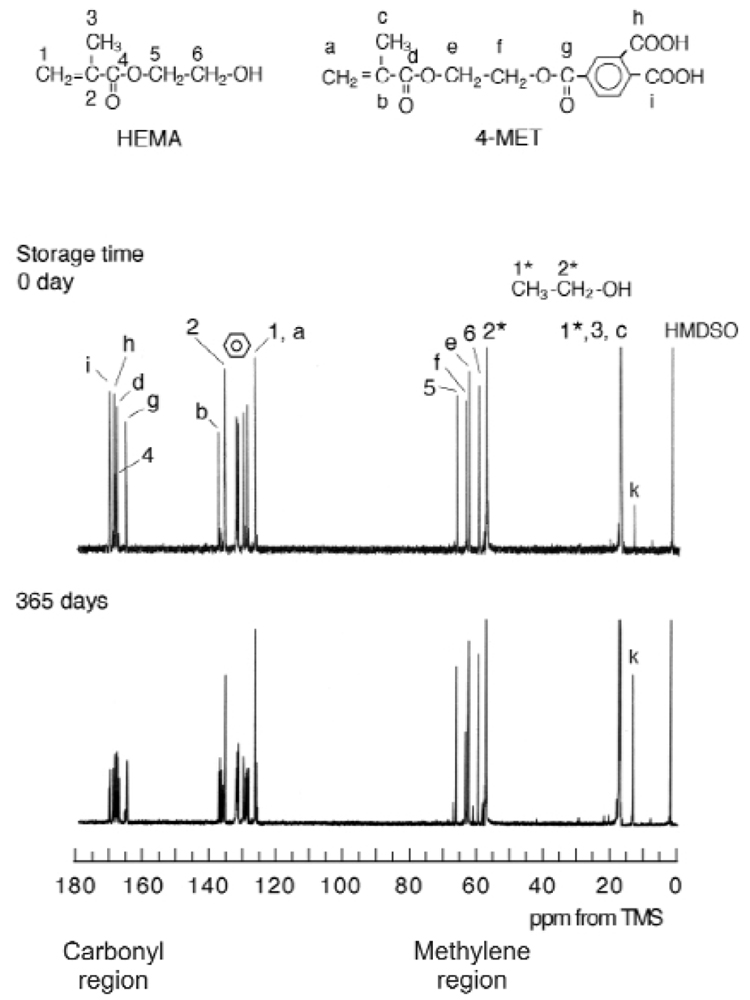
Fig. 2 represents the 13C NMR spectra of the UniFil Bond primer before and after storage. The 13C NMR peak assignments of HEMA and 4-MET were based upon previous reports (Fujisawa and Ito, 1999; Nishiyama et al., 2004). The spectral data confirmed that the primer was dissolved in ethanol, since peaks ‘1*’, assigned to the methyl carbon, and ‘2*’, assigned to the methylene carbon of ethanol, were detected.
Figure 2.
13C NMR spectra obtained from the same bottle of UniFil Bond Primer immediately after being opened and after intermittent storage at 4°C for 365 days.
After storage, there was an increase in the intensity of peak ‘k’ that was assigned to the methyl carbon of the ethyl ester (Fig. 4), indicating that the carboxyl group of the meta- or the para-portion in the 4-MET structure was esterified with ethanol. There was also an apparent increase in the intensity of the NMR peak ‘e’, assigned to the methylene carbon linked with the methacryloyl ester in the 4-MET. This apparent increase was the consequence of superimposition; during the same chemical shift, the NMR peak ‘j’, assigned to the methylene carbon from the ethyl ester group in the esterified 4-MET, was detected along with peak ‘e’.
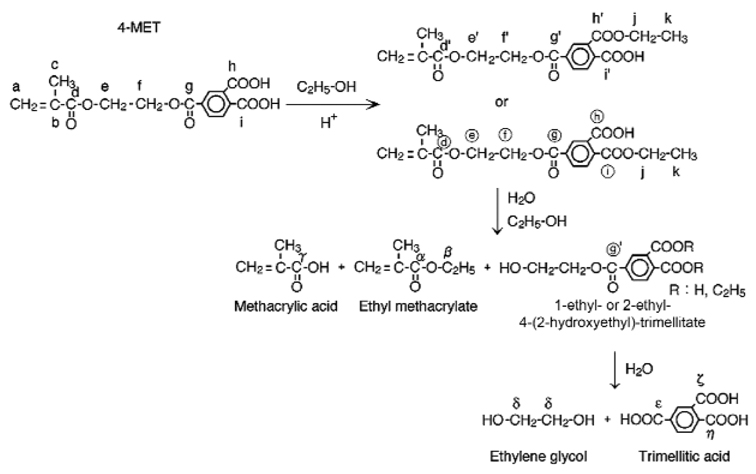
Figure 4.
Degradation mechanisms of 4-MET from the UniFil Bond Primer consist of initial esterification with ethanol and subsequent hydrolysis into methacrylic acid, ethyl methacrylate, and 1-ethyl- or 2-ethyl-4-(2-hydroxyethyl)-trimellitate, which is further hydrolyzed into ethylene glycol and trimellitic acid.
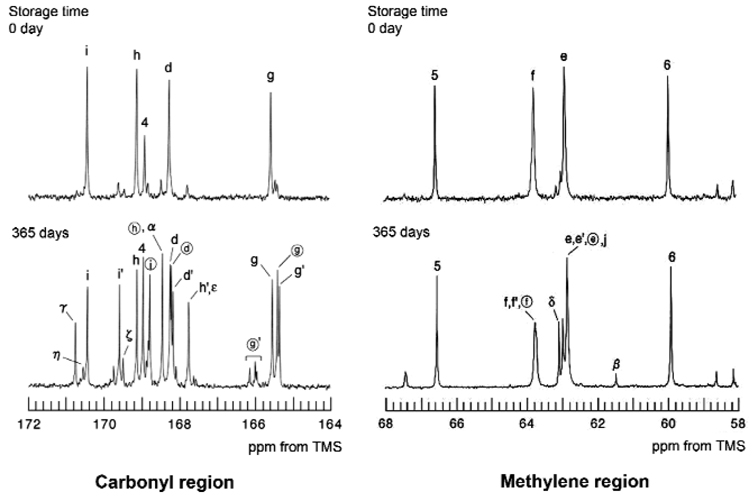
The expanded 13C NMR spectra of the carbonyl and methylene regions are shown in Fig. 3. After storage, additional peaks ‘g′’, ‘i′’, and ‘h′’ were detected at the carbonyl region. They were respectively assigned to the carbonyl carbons from the ester portion (g′) of the trimellitate, the carboxyl group of the para-portion (i′), and the carboxyl group of the meta-portion (h′) in the 4-MET structure that had been esterified with ethanol to form 2-ethyl-4-methacryloxyethyl trimellitate (Fig. 4). Similarly, new peaks ‘circle g’, ‘circle i’, and ‘circle h’ were respectively assigned to the carbonyl carbons from the ester portion of the trimellitate, the carboxyl group of the meta-portion, and the carboxyl group of the para-portion in the 4-MET structure that had been esterified with ethanol to form 1-ethyl-4-methacryloxyethyl trimellitate. New peaks ‘γ’, ‘α’, and ‘β’ were assigned respectively to the carbonyl carbon from the carboxyl group (γ) in methacrylic acid, the ester carbonyl carbon (α), and the methylene carbon (β) linked with the ester bond in ethyl methacrylate. The new peak ‘circle g′’—assigned to the carbonyl carbon (circle g′) linked between 2-hydroxyethyl ester and the benzene ring in 2-ethyl-4-(2-hydroxyethyl)-trimellitate or 1-ethyl-4-(2-hydroxyethyl)-trimellitate—was indicative of the hydrolysis of the methacryloxy ester bond in the 4-MET (Fig. 4). Continuing hydrolysis of the trimellitic ester portion in 1-ethyl- or 2-ethyl-4-(2-hydroxyethyl)-trimellitate resulted in the detection of new peaks—‘ε’ , ‘ζ’, and ‘η’—that were respectively designated to the carbonyl carbon from the three carboxylic acid groups in trimellitic acid. In addition, the new peaks ‘δ’ were attributed to the two methylene carbons in ethylene glycol.
Figure 3.
Expanded 13C NMR spectra from the carbonyl and methylene regions indicated in Fig. 2.
DISCUSSION
Since extensive ultrastructural changes and new NMR peaks were identified in the aged adhesive, we must reject the null hypothesis that one-year intermittent refrigeration does not result in hydrolytic changes that are identifiable by TEM and 13C NMR spectroscopy. Nanoleakage that occurs in self-etch adhesives should be confined within the hybrid layer (Li et al., 2003; Bedran-de-Castro et al., 2004), and not beyond the hybrid layer. The thickness of hybrid layer in these adhesives was around 1 µm in the fresh adhesive, whereas the zone of silver deposits increased to 2 µm thick in the aged adhesive. Thus, there appeared to be an increase in the etching capacity of the aged adhesive that was not accompanied by resin infiltration or effective polymerization. This permitted the aqueous ammoniacal silver nitrate to penetrate the interfibrillar spaces in mineralized dentin beneath the hybrid layer prior to the TEM specimen-processing.
A plausible explanation for this phenomenon is summarized in Fig. 4. 4-META, the acidic methacrylate anhydride, dissociates immediately in the presence of water to form 4-MET. Ester exchange of the carboxyl group of the meta- or para-portion in the 4-MET occurred initially in the presence of ethanol to form 1-ethyl- or 2-ethyl-4-(2-hydroxyethyl)-trimellitate. This was followed by hydrolysis of both ester groups in 4-MET. Hydrolysis of the methacryloxy ester portion of the 4-MET and the esterified 4-MET resulted in the formation of methacrylic acid, ethyl methacrylate, and 1-ethyl- or 2-ethyl-4-(2-hydroxyethyl)-trimellitate. Further hydrolysis of the ester portion (designated as ‘circle g′’), linked with the benzene ring of 1-ethyl- or 2-ethyl-4-(2-hydroxyethyl)-trimellitate, resulted in the formation of ethylene glycol and trimellitic acid. Whereas 4-MET is a polymerizable, moderately acidic dicarboxylic acid monomer, trimellitic acid (1,2,4 benzenetricarboxylic acid) lacks a polymerizable methacryloxy group and is a slightly stronger acid than 4-MET (Brown et al., 1955). The trimellitic acid may continue to etch beyond the original hybrid layer into the underlying mineralized dentin after polymerization of the adhesive (Carvalho et al., 2005). It should be mentioned that such a degradation mechanism was based upon NMR peak assignments of a mixture of degradation products only. Full-proof peak assignments require more detailed NMR analyses of each of the monomers present in the original product, as well as all potential degradation molecules. In the absence of these additional analyses, it is possible that alter native degradation mechanisms exist for 4-MET.
This study examined only the degradation of a commercial 4-MET-containing adhesive that was stored for 12 mos. Understandably, the impact of a 12-month storage period may not be clinically significant, since, in routine dental practices, adhesives are consumed at a very fast rate. However, in our more recent bond-strength analysis of an experimental 4-MET-containing single-bottle self-etch adhesive, we were able to control the commencement of the hydrolytic process by reconstituting the anhydrous adhesive components with water. Our results showed that decline in microtensile bond strength occurred as early as 3 mos when the adhesive was aged at 4°C, and as early as 1 mo when the adhesive was aged at 25°C and 37°C (Tay and Salz, unpublished results).
Since either ethanol or acetone may be used as a solvent in 4-MET-containing single-bottle self-etch adhesives, it is prudent to elaborate on the potential of degradation of 4-MET in acetone. The esterification reaction of the carboxylic acid in the 4-MET occurred in the present study because the UniFil Bond Primer was diluted by ethanol. This was necessary, since 4-MET is insoluble in water. Both the esterification reaction of the carboxylic acid and the hydrolysis reaction of the methacryloxy ester portion in the 4-MET may occur. However, the esterification reaction of the carboxylic acid takes precedence over the hydrolysis reaction of the methacryloxy ester portion in the 4-MET. Conversely, if acetone had been used in lieu of ethanol as the diluent solvent, the esterification reaction of the carboxylic acid would not occur. However, the methacryloxy ester portion in the 4-MET would be hydrolyzed because of the dissociation of the carboxylic acid in the 4-MET.
Apart from carboxylic acid esters, it is known that esters such as 2-hydroxyethyl methacrylate (HEMA), triethyleneglycol dimethacrylate (TEGDMA), methacryloyloxydecyl dihydrogen phosphate (MDP), and HEMA-phosphate are hydrolytically degradable under acidic conditions (Moszner et al., 2005; Salz et al., 2005). It appears that the degradation mechanism of phosphate-ester-containing resin monomers may be even more complex than those of the carboxylic-acid-containing monomers (Fujita and Nishiyama, 2006a). Similar to the carboxylic acid esters, hydrolysis of both the methacrylate and phosphate ester bonds may occur in phosphoric acid esters. However, the hydrolytic stability of the phosphoric ester bonds depends on the level of substitution of the phosphoric acid, with dialkyl hydrogen phosphates being less stable than trialkyl phosphates, and with the latter being less stable than monoalkyl dihydrogen phosphates (Moszner et al., 2005).
Regardless of the acidic resin monomers and the solvent types, it appears that hydrolytic degradation is inevitable as long as methacrylate-based functional monomers are utilized in single-bottle-type self-etching primers and one-step self-etch adhesives. Conversely, amide derivatives of methacrylates were found to be more stable and did not undergo hydrolysis initially at pH 0.94, and began to hydrolyze only after 70 days (Nishiyama et al., 2004). Further studies should be performed on these methacrylamide- or phosphonic-acid-containing self-etch adhesives by correlating chemo-analytical data with ultrastructural findings.
ACKNOWLEDGMENTS
The UniFil Bond kits used in this study were generous gifts from the GC Corp. This study was supported by a Grant from the Ministry of Education, Culture, Sports, Science, and Technology to promote 2001-multidisciplinary research projects (2001–2005) in Japan, grants DE 014911 and DE 015306 from the NIDCR, USA (PI, David Pashley), and by grant 10204604/07840/08004/324/01, Faculty of Dentistry, the University of Hong Kong. The authors are grateful to Michelle Barnes for secretarial support.
REFERENCES
- Bedran-de-Castro AK, Pereira PN, Pimenta LA, Thompson JY. Effect of thermal and mechanical load cycling on nanoleakage of Class II restorations. J Adhes Dent. 2004;6:221–226. [PubMed] [Google Scholar]
- Brown HC, McDaniel DH, Häfliger O. Dissociation constants. In: Braude EA, Nachod FC, editors. Determination of organic structures by physical methods. New York: Academic Press; 1955. pp. 567–589. [Google Scholar]
- Carvalho RM, Chersoni S, Frankenberger R, Pashley DH, Prati C, Tay FR. A challenge to the conventional wisdom that simultaneous etching and resin infiltration always occur in self-etch adhesives. Biomaterials. 2005;26:1035–1042. doi: 10.1016/j.biomaterials.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Ito S. 1H-NMR studies of the interaction of dental adhesive monomer, 4-META with calcium. Dent Mater. 1999;18:54–62. doi: 10.4012/dmj.18.54. [DOI] [PubMed] [Google Scholar]
- Fujita K, Nishiyama N. 13C NMR analysis of the etching efficacy of acidic monomers in self-etching primers. J Dent. 2006a;34:123–133. doi: 10.1016/j.jdent.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Fujita K, Nishiyama N. Alteration mechanisms and effects on self-etching primers effectuated by the primer's storage period. Am J Dent. 2006b (in press) [PubMed] [Google Scholar]
- Kazantsev OA, Shirshin KV, Sivokhin AP, Tel’nov SV, Zhiganov IV, Kuznetsov AE, et al. Hydrolysis of 2-hydroxyethyl methacrylate in concentrated aqueous solutions. Russian J Appl Chem. 2003;76:1296–1298. [Google Scholar]
- Li H, Burrow MF, Tyas MJ. The effect of concentration and pH of silver nitrate solution on nanoleakage. J Adhes Dent. 2003;5:19–25. [PubMed] [Google Scholar]
- Moszner N, Zeuner F, Fischer UK, Rheinberger V. Monomers for adhesive polymers, 2. Synthesis and radical polymerization of hydrolytically stable acrylic phosphonic acids. Macromol Chem Phy. 1999;200:1062–1067. [Google Scholar]
- Moszner N, Zeuner F, Angermann J, Fischer UK, Rheinberger V. Monomers for adhesive polymers, 4. Macromol Mater Eng. 2003;288:621–628. [Google Scholar]
- Moszner N, Salz U, Zimmermann J. Chemical aspects of selfetching enamel-dentin adhesives: a systematic review. Dent Mater. 2005;21:895–910. doi: 10.1016/j.dental.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Nishiyama N, Suzuki K, Yoshida H, Teshima H, Nemoto K. Hydrolytic stability of methacrylamide in acidic aqueous solution. Biomaterials. 2004;25:965–969. doi: 10.1016/s0142-9612(03)00616-1. [DOI] [PubMed] [Google Scholar]
- Okazaki K. A study of light-cured resins, influence of storage temperature of adhesive system on bond strength to bovine dentin. Jpn J Conserv Dent. 2000;43:1187–1196. (in Japanese) [Google Scholar]
- Salz U, Zimmermann J, Zeuner F, Moszner N. Hydrolytic stability of self-etching adhesive systems. J Adhes Dent. 2005;7:107–116. [PubMed] [Google Scholar]
- Shono Y, Ogawa T, Terashita M, Carvalho RM, Pashley EL, Pashley DH. Regional measurement of resin-dentin bonding as an array. J Dent Res. 1999;78:699–705. doi: 10.1177/00220345990780021001. [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003;69:726–731. [PubMed] [Google Scholar]
- Tay FR, Moulding KM, Pashley DH. Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent. 1999;1:103–117. [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002;81:472–476. doi: 10.1177/154405910208100708. [DOI] [PubMed] [Google Scholar]