Abstract
Vaccination with formalin-inactivated respiratory syncytial virus (FI-RSV) vaccine or RSV G glycoprotein results in enhanced pulmonary disease after live RSV infection. Enhanced pulmonary disease is characterized by pulmonary eosinophilia and is associated with a substantial inflammatory response. We show that the absence of the G glycoprotein or G glycoprotein CX3C motif during FI-RSV vaccination or RSV challenge of FI-RSV-vaccinated mice, or treatment with anti-substance P or anti-CX3CR1 antibodies, reduces or eliminates enhanced pulmonary disease, modifies T-cell receptor Vβ usage, and alters CC and CXC chemokine expression. These data suggest that the G glycoprotein, and in particular the G glycoprotein CX3C motif, is key in the enhanced inflammatory response to FI-RSV vaccination, possibly through the induction of substance P.
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections in infants and young children (1, 50, 54, 59). RSV can also cause serious lower respiratory tract disease in patients of any age with compromised immune, respiratory, or cardiac systems (7, 13, 14, 20, 26, 60, 62). Despite the importance of RSV as a respiratory pathogen, there is presently no safe and effective RSV vaccine available. The first RSV vaccine trial with a formalin-inactivated RSV (FI-RSV) vaccine yielded disastrous results in young vaccinees who were subsequently naturally infected with RSV, as many developed enhanced pulmonary disease leading to hospitalization, and even to death in a few vaccine recipients (5, 17, 73). This outcome prompted investigators to search for viral and/or host factors that may contribute to enhanced disease in the effort to ensure that RSV vaccines would be safe.
Studies with BALB/c mice have provided some indication of the mechanisms that may have contributed to FI-RSV-enhanced pulmonary disease. BALB/c mice vaccinated with vectors expressing G glycoprotein, purified G glycoprotein, or FI-RSV develop extensive enhanced pulmonary disease characterized by pulmonary eosinophilia, weight loss, exaggerated Th2-type cytokine responses, selective priming of Vβ14+ CD4+ T cells, and augmented substance P (SP) expression when challenged with RSV (23, 27, 51, 68, 70, 71). Interestingly, the soluble form of the G glycoprotein has been shown to be most effective at sensitizing for enhanced disease (34, 35). Studies from our laboratory comparing the immune responses to infection with wild-type RSV or an RSV mutant lacking the G and SH genes have shown that RSV G and/or SH glycoprotein expression alters pulmonary trafficking of innate immune cells (CD11b+ cells, polymorphonuclear cells [PMN], and NK cells), Th1- and Th2-type cytokine patterns, and CC and CXC chemokine mRNA expression by bronchoalveolar lavage (BAL) cells and is associated with increased pulmonary SP expression (64, 67, 68). In addition, recent studies from our laboratory have shown that the G glycoprotein contains a CX3C chemokine motif that interacts with the CX3C chemokine receptor CX3CR1, induces leukocyte chemotaxis, and facilitates virus infection (65). These studies also showed that G glycoprotein can compete with fractalkine for binding to CX3CR1, as well as inhibit fractalkine-mediated leukocyte chemotaxis (65), suggesting that RSV G glycoprotein has immune modulatory activities associated with the CX3C motif.
Chemokines are important factors that control leukocyte function and are essential in mediating leukocyte trafficking and orchestrating cell activation and cytokine expression. There are four structural groups of chemokines, i.e., C, CC, CXC, and CX3C; each category has multiple members, with the exception of the CX3C subgroup, of which fractalkine is the only known member (2, 31, 72, 75). Chemokines may interact with specific (i.e., single-ligand), shared (i.e., having multiple ligands of the same chemokine family), or promiscuous (i.e., having multiple ligands of different chemokine families) receptors (78, 79). CX3CR1 is a specific receptor for only fractalkine (6, 33). Fractalkine is important in Th1-type cell and NK cell responses, as these cell types express high levels of CX3CR1, while Th2-type cells express low levels of CX3CR1 and do not readily respond to fractalkine (15). Thus, inhibition of fractalkine-mediated immune responses by RSV G glycoprotein may alter Th1-type cell and NK cell responses and affect the pattern of cytokine or chemokine expression. Consistent with this possibility, BAL leukocytes from BALB/c mice infected with an RSV mutant lacking G and SH genes express increased Th1-type cytokines and increased CC and CXC chemokine mRNAs and have increased numbers of pulmonary NK cells compared to wild-type-infected mice (64, 67).
G glycoprotein CX3C-CX3CR1 interaction may also affect other host components that participate in the response to RSV infection, including expression of SP. Neurons and some immune cells, e.g., dendritic cells and eosinophils, express CX3CR1 and are capable of SP expression (18, 19, 28, 29, 32, 37, 46). CX3CR1 message is abundant in the lung (6), suggesting that CX3CR1 expression is important in signal transduction between the immune and nervous systems. Fractalkine, whose expression is enhanced by proinflammatory cytokines, including tumor necrosis factor alpha and gamma interferon (4, 15, 16, 44), may interact with neurons that innervate the lung or with CX3CR1+ cells inducing SP expression (47). G glycoprotein may act through a similar mechanism, as BALB/c mice infected with RSV have enhanced pulmonary levels of SP compared to mice infected with an RSV mutant lacking the G and SH genes (68). SP has diverse effects on the immune response that include exacerbating expression of proinflammatory mediators, mediating vascular extravasation of immune cells, and activating lymphocytes, macrophages, mast cells, and eosinophils (11, 36, 41, 55, 58, 69). In addition, SP has been shown to break Th1/Th2 cytokine polarization and directly induce secretion of interleukin-2 (IL-2), gamma interferon, IL-4, and IL-10 from T lymphocytes (42, 43). SP receptors are upregulated on CD4+ and CD8+ T lymphocytes and CD14+ cells during the primary immune response to RSV infection in BALB/c mice, and SP receptors are expressed predominantly on CD4+ T lymphocytes and CD43+ cells during the secondary immune response to RSV infection or following RSV challenge of FI-RSV-vaccinated mice (63). These results suggest that SP may affect the primary and secondary immune responses to RSV infection and the cellular response associated with enhanced pulmonary disease in FI-RSV-vaccinated mice. Consistent with this hypothesis, prophylactic or therapeutic treatment of RSV-infected BALB/c mice with anti-SP antibodies promptly reduces pulmonary cell trafficking and decreases the number of cells expressing proinflammatory cytokines (30), suggesting that SP is an important component contributing to the inflammatory response associated with RSV infection.
In this study, we examine features of the immune response to RSV infection or FI-RSV vaccination that are associated with enhanced pulmonary disease. We address the significance of RSV strain differences, G and/or SH glycoprotein expression, and G glycoprotein CX3C-CX3CR1 interaction in the context of FI-RSV-enhanced disease. In addition, we examine CC and CXC chemokine expression, SP expression, and pulmonary leukocyte trafficking that are associated with the development of FI-RSV-enhanced pulmonary disease.
MATERIALS AND METHODS
Viruses and vaccine.
RSV/A2 (A2) and RSV/B1 (B1) are wild-type strain A and B viruses, respectively. CP52, derived from B1, is an RSV mutant virus lacking the G and SH genes and was a kind gift of Brian Murphy, National Institutes of Health. Recombinant RSV derived from wild-type A2 (6340WT) and recombinant RSV lacking the G glycoprotein gene (6340ΔG), derived from 6340WT, were kind gifts of Peter Collins, National Institutes of Health. R10C7G, an RSV A2 point mutant having a Cys→Arg amino acid change at position 186 (56), was a kind gift of Jose Melero, Instituto de Salud Madrid, Madrid, Spain. All of these viruses and the JS strain of parainfluenza virus 3 (PIV-3) were propagated in Vero cells (African green monkey kidney fibroblasts; ATCC CCL 81) maintained in Dulbecco's modified Eagle's medium (GIBCO Laboratories, Grand Island, N.Y.) supplemented with 2% heat-inactivated (56°C) fetal bovine serum (HyClone Laboratories, Salt Lake City, Utah). The viruses were propagated until there was a detectable cytopathic effect, and the medium was decanted and replaced with a minimal volume of serum-free Dulbecco's modified Eagle's medium. The flasks were freeze-thawed twice at −70 and 4°C, and the contents were collected and centrifuged at 4,000 × g for 20 min at 4°C. The titer was determined by plaque assay on Vero cells by immunostaining (67).
Formalin-inactivated RSV vaccine was prepared as described previously (67). Briefly, 1 part formalin (Sigma, St. Louis, Mo.) was incubated with 4,000 parts clarified A2 or R10C7G lysate for 3 days at 37°C and pelleted by centrifugation for 1 h at 50,000 × g. The volume of virus was adjusted to a 1:25 dilution of the original volume in minimal essential medium (GIBCO) and subsequently precipitated with aluminum hydroxide (4 mg/ml) (Sigma), resuspended at a 1:100 dilution of the original volume in serum-free minimal essential medium, and stored at 4°C.
Vaccination, virus challenge, antibody treatment, and BAL collection.
Six-week-old, specific-pathogen free, female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, Maine). The mice were housed in microisolator cages and fed sterilized water and food ad libitum. Some of the mice were vaccinated with 0.1 ml of 106 PFU equivalents of formalin-inactivated RSV/A2 (FI-A2) or formalin-inactivated R10C7G (FI-R10C7G) in the superficial gluteal muscle and then rested for 4 weeks. On day −1 prior to challenge, a portion of the mice were treated intraperitoneally with 100 μg of rabbit anti-SP antibody (Accurate Chemical and Scientific Corp, Westbury, N.Y.), normal rabbit antibody (Accurate Chemical and Scientific Corp.), or rabbit anti-CX3CR1 antibody (Alexis Biochemicals, Lausen, Switzerland) diluted in phosphate-buffered saline (PBS). Naive and vaccinated mice were challenged intranasally with 106 PFU of live A2, B1, CP52, PIV-3, or R10C7G virus diluted in PBS (GIBCO). At various time points postinfection (p.i.), mice were anesthetized and exsanguinated by severing the right caudal artery, and the BAL leukocytes were collected by lavaging the lungs three times with 1 ml of PBS. The virus titers associated with A2, B1, CP52, or R10C7G infection were determined. Briefly, lungs were aseptically removed from three mice per group at 40 h p.i., and stored at −70°C until assay. Identical weights (0.1g of tissue) of individual lung samples were homogenized in 1 ml of Dulbecco's PBS (GIBCO), and 10-fold serial dilutions of the lung homogenates were subsequently added to confluent Vero cell monolayers. Plaques were enumerated by immunostaining as previously described (67). No significant differences in virus titers between the infection groups were observed at 40 h p.i. Virus titers ranged 5.0 to 5.6 log10 PFU/g of lung tissue.
Portions of the BAL cells were stained with hematoxylin and eosin (H&E), and the percentages of lymphocytes, PMN, eosinophils, and macrophages were determined. For H&E studies, between 200 and 500 cells/BAL sample were examined and scored as lymphocytes, PMN, eosinophils, or macrophages based on morphological and staining features of the cells.
For T-cell receptor (TCR) Vβ studies, 6-week-old, specific-pathogen free, female BALB/c mice purchased from Jackson Laboratories were vaccinated with 0.1 ml of 106 PFU equivalents of FI-A2 and rested for 4 weeks, and then age-matched naive or FI-A2-vaccinated mice were challenged intranasally with 106 PFU of live 6340WT, 6340ΔG, or R10C7G. The BAL cells were isolated at 7 days postchallenge for analysis.
RNA isolation and multiprobe RPA.
RNase protection analysis (RPA) of extracted BAL cell RNA was performed according to the methods described by BD PharMingen (San Diego, Calif.) and as previously described (64). Briefly, BAL cells were pelleted by centrifugation, and the supernatant was decanted. The cell pellet was resuspended in 300 μl of PBS (GIBCO) and used for RNA extraction. Total RNA was extracted by using RNA STAT-50 LS as described by the manufacturer (TEL-TEST Inc., Friendswood, Tex.) and precipitated with isopropanol. RNA-isopropanol pellets were resuspended in RNase-free water (TEL-TEST Inc.) and stored at −70°C.
Chemokine mRNA expression in BAL cells was detected by RPA with the RiboQuant MultiProbe RPA system (BD PharMingen). 32P-labeled antisense RNA probes were synthesized in an in vitro transcription reaction from DNA templates representing specific chemokine gene sequences of distinct length. Probes specific for macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene were synthesized in the same reaction. Five micrograms of RNA was mixed with 3.1 × 105 cpm of 32P-labeled antisense RNA probe set and allowed to hybridize in solution phase overnight at 56°C. After incubation, the free probe and single-stranded RNA were digested with RNase A. The remaining, RNase-protected probe fragments were purified by phenol-chloroform extraction and resolved on denaturing polyacrylamide gels by using the QuickPoint rapid nucleic acid separation system (NOVEX, San Diego, Calif.). Probe bands were identified and chemokine expression was quantified by Typhoon 9410 variable-mode imaging.
Quantitation of SP.
SP levels in cell-free BAL supernatant were analyzed by using a competitive enzyme-linked immunoassay kit (Cayman Chemical, Ann Arbor, Mich.) in accordance with the manufacturer's instructions and as previously described (68). The assay is based on the competition between free SP and an SP tracer for a limited number of SP-specific binding sites. The percent sample bound/maximum bound was calculated, and the SP concentration in each sample was determined based on the percent standard bound/maximum bound versus standard SP concentration. The intra- and interassay coefficients of variation were ≤ 10%.
Flow cytometry.
BAL cells were blocked with 10% normal mouse serum (Jackson Laboratories) in PBS and then stained with the appropriate combinations of fluorescein isothiocyanate- or phycoerythrin-labeled anti-CD3ɛ (145-2C11), anti-CD45R/B220 (RA3-6B2), anti-pan NK cell (DX5), anti-neutrophil (RB6-8C5), anti-CD11b (M1/70), and mouse isotype antibody controls (all from PharMingen) as previously described (66, 67). For TCR Vβ analysis, blocked BAL cells were costained with fluorescein isothiocyanate- or phycoerythrin-labeled anti-CD4 (RM4-5) and either anti-Vβ4 (KT4), anti-Vβ6 (RR4-7), anti-Vβ7 (TR310), anti-Vβ8 (MR5-2), anti-Vβ9 (MR10-2), anti-Vβ10 (B21.5), anti-Vβ13 (ΜΡ12-3), or anti-Vβ14 (14-2) monoclonal antibodies (all from PharMingen). The distribution of cell surface markers was determined in two-color mode on a FACScan with CellQUEST software (Becton-Dickinson, Mountain View, Calif.) from >10,000 lymphocyte-gated events.
Statistical analysis.
BAL cells were pooled from four to six animals per time point per experiment in three to six separate experiments, except for multiprobe RPA and TCR Vβ studies, in which BAL cells were pooled from four animals per time point per experiment in two separate experiments. Statistical significance was determined by Student's t test, where a P value of <0.05 was considered statistically significant.
RESULTS
Pulmonary cell numbers are affected by G and/or SH glycoprotein expression, SP expression, and G glycoprotein CX3C-CX3CR1 interaction.
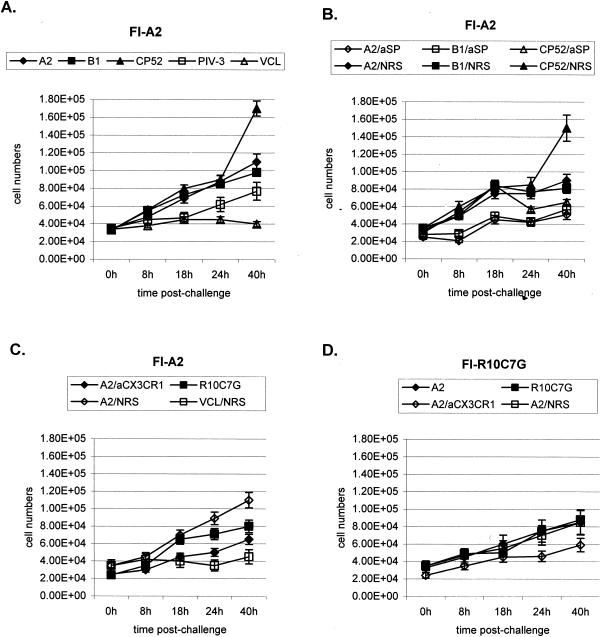
To determine if RSV strain differences, G and SH glycoprotein expression, or the G glycoprotein CX3C motif affected the total number of BAL cells that trafficked to the lungs, either untreated, normal rabbit serum (NRS)-treated, or anti-SP antibody-treated FI-A2- or FI-R10C7G-vaccinated mice were challenged with A2, B1, CP52, PIV-3, or R10C7G (Fig. 1). The A2 and B1 viruses are wild-type A and B strains of RSV, respectively. CP52, derived from B1, is an RSV mutant virus lacking the G and SH genes (39). R10C7G is an A2 point mutant lacking an intact CX3C motif; i.e., the Cys at amino acid position 186 has been changed to an Arg (56). All treatments of FI-A2-vaccinated mice gave similar results, with the exception of CP52 challenge, which gave a marked increase in BAL cell numbers at 40 h postchallenge, and the anti-SP and anti-CX3CR1 antibody treatments, which were associated with decreased BAL cell numbers at most time points postchallenge. Slightly lower levels of pulmonary cell infiltration occurred in FI-R10C7G-vaccinated mice challenged with A2 compared to similarly challenged FI-A2-vaccinated mice; however, the total cellular infiltration was similar in FI-R10C7G-vaccinated mice following A2 or R10C7G challenge. As expected, challenge with PIV-3 and virus-free cell lysate gave the lowest number of BAL cells.
FIG. 1.
The BAL cell numbers in the lungs of untreated FI-A2-vaccinated mice (A), anti-SP antibody (aSP)-treated or NRS-treated FI-A2-vaccinated mice (B), anti-CX3CR1 antibody (aCX3CR1)-treated or NRS-treated FI-A2-vaccinated mice (C), and anti-CX3CR1 antibody-treated or NRS-treated FI-R10C7G-vaccinated mice (D) were determined at 0, 8, 18, 24, and 40 h after A2, B1, CP52, PIV-3, or R10C7G challenge or following treatment with uninfected Vero cell lysate (VCL). The mean number of BAL cells (± standard error) from three independent experiments examining three mice per treatment is shown.
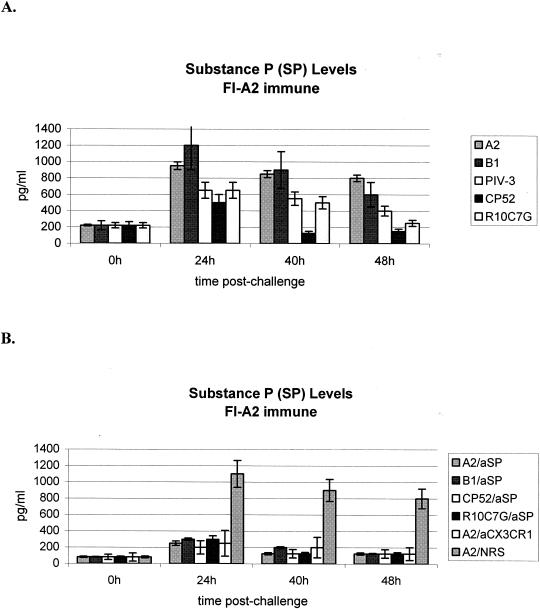
The level of pulmonary SP in cell-free BAL supernatant following treatment was determined (Fig. 2). Higher levels of pulmonary SP were detected in BAL supernatants from FI-A2-vaccinated mice challenged with RSV that expressed the G glycoprotein, e.g., A2 or B1, than in those from FI-A2-vaccinated mice challenged with viruses that lacked the G gene, e.g., CP52, PIV-3, or the G glycoprotein CX3C motif (e.g., R10C7G). As expected, anti-SP antibody treatment considerably reduced pulmonary SP levels. These results are consistent with our previous studies that indicated a role for SP in the inflammatory response in FI-RSV-immunized mice and a link between G glycoprotein and SP expression (68).
FIG. 2.
ELISA was used to determine the level of SP expression in cell-free BAL fluid from untreated FI-A2-vaccinated mice (A) or FI-A2-vaccinated mice treated with anti-SP antibody (aSP), anti-CX3CR1 antibody (aCX3CR1), or NRS (B) at 0, 8, 18, 24, and 40 h after A2, B1, CP52, PIV-3, or R10C7G challenge. The mean level of SP expression (± standard error) from three independent experiments examining three mice per treatment is shown.
Pulmonary eosinophilia is associated with G glycoprotein CX3C-CX3CR1 interaction and is reduced by anti-SP or anti-CX3CR1 antibody treatment.
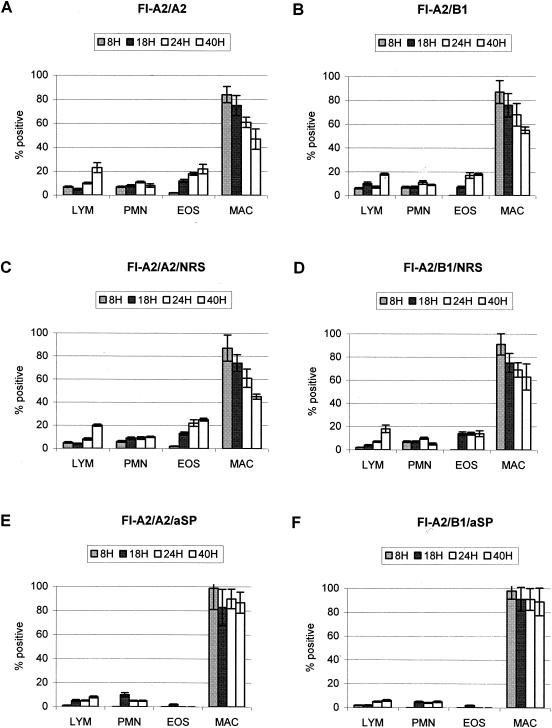
Pulmonary eosinophilia is a hallmark of FI-RSV vaccine-enhanced disease in BALB/c mice (22, 51), and thus we examined the BAL cell types that trafficked to the lungs of untreated, NRS-treated, or anti-SP antibody-treated FI-A2-vaccinated mice challenged with A2 or B1 (Fig. 3). Macrophages were the predominant BAL cell type with all treatments. In FI-A2-vaccinated mice challenged with A2 or B1, there was prominent pulmonary eosinophilia (Figs. 3A to D), as predicted from previous studies (67). FI-A2-vaccinated mice treated with anti-SP antibody had reduced pulmonary eosinophilia compared to untreated or NRS-treated mice and had reduced numbers of lymphocytes and PMN (Fig. 3E and F), also consistent with previous studies (68).
FIG. 3.
The BAL cell types in the lungs of untreated FI-A2-vaccinated mice challenged with A2 (A) or B1 (B), challenged with A2 or B1 and treated with NRS (C and D, respectively), or challenged with A2 or B1 and treated with anti-SP antibody (E and F, respectively), were determined at 8, 18, 24, and 40 h after A2 or B1 challenge. The mean percentage of each BAL cell type (± standard error) was determined by H&E staining and microscopic visualization from three independent experiments examining three mice per treatment. LYM, lymphocytes; EOS, eosinophils; MAC, macrophages.
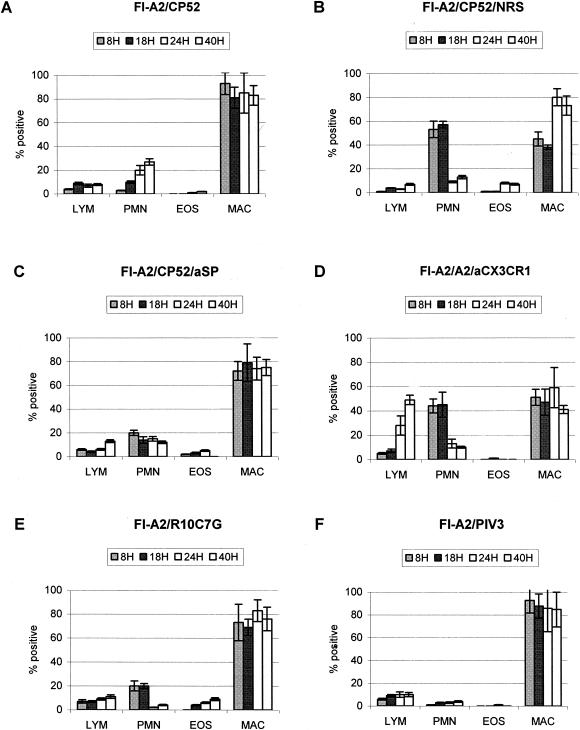
The role of G glycoprotein and G glycoprotein CX3C-CX3CR1 interaction in the development of enhanced disease was examined in untreated, NRS-treated, anti-SP antibody-treated, and anti-CX3CR1 antibody-treated FI-A2-vaccinated mice challenged with CP52, A2, or R10C7G (Fig. 4). As predicted from earlier studies (67), CP52 challenge of untreated, NRS-treated, or anti-SP antibody treated FI-A2-vaccinated mice did not induce pulmonary eosinophilia (Fig. 4A and B) compared to A2- or B1-challenged FI-A2-vaccinated mice (Fig. 3); however, PMN numbers in the lung were increased in NRS-treated mice. Similarly, R10C7G challenge of FI-A2-vaccinated mice did not induce pulmonary eosinophilia (Fig. 4E), suggesting an important role for the G glycoprotein CX3C motif in the induction of pulmonary eosinophilia. In addition, anti-CX3CR1 antibody treatment considerably reduced pulmonary eosinophilia in FI-A2-vaccinated mice challenged with A2 (Fig. 4D), and as expected, PIV-3 challenge of FI-A2-vaccinated mice did not induce pulmonary eosinophilia.
FIG. 4.
The BAL cell types in the lungs of untreated FI-A2-vaccinated mice challenged with CP52 (A), FI-A2-vaccinated mice challenged with CP52 and treated with NRS or anti-SP antibody (B and C, respectively), FI-A2-vaccinated mice challenged with A2 and treated with anti-CX3CR1 antibody (D), FI-A2-vaccinated mice challenged with R10C7G (E), or FI-A2-vaccinated mice challenged with PIV-3 (F) were determined at 8, 18, 24, and 40 h after CP52, R10C7G, or PIV-3 challenge. The mean percentage of each BAL cell type (± standard error) was determined by H&E staining and microscopic visualization from three independent experiments examining three mice per treatment. LYM, lymphocytes; EOS, eosinophils; MAC, macrophages.
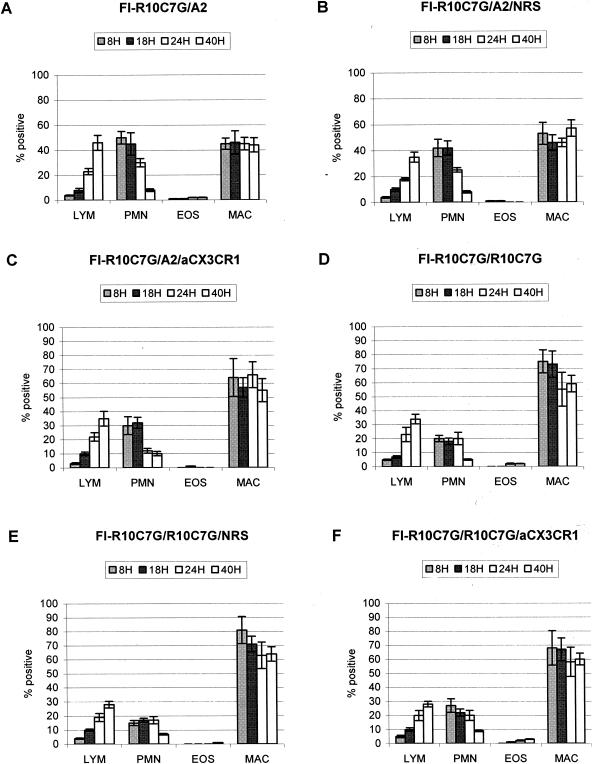
To determine if the G glycoprotein CX3C motif contributed to the memory response associated with FI-RSV-enhanced disease, untreated, NRS-treated, and anti-CX3CR1 antibody-treated FI-R10C7G-vaccinated mice were challenged with A2 or R10C7G (Fig. 5). No pulmonary eosinophilia was detected following any treatment, suggesting that the G glycoprotein CX3C motif is important in sensitizing for pulmonary eosinophilia.
FIG. 5.
The BAL cell types in the lungs of FI-R10C7G-vaccinated mice challenged with A2 (A), FI-R10C7G-vaccinated mice challenged with A2 and treated with NRS or anti-CX3CR1 antibody (B and C, respectively), FI-R10C7G-vaccinated mice challenged with R10C7G (D), FI-R10C7G-vaccinated mice challenged with R10C7G and treated with NRS (E) or FI-R10C7G-vaccinated mice challenged with R10C7G and treated with anti-CX3CR1 antibody (F) were determined at 8, 18, 24, and 40 h after A2 or R10C7G challenge. The mean percentage of each BAL cell type (± standard error) was determined by H&E staining and microscopic visualization from three independent experiments examining three mice per treatment. LYM, lymphocytes; EOS, eosinophils; MAC, macrophages.
As an indicator of severity of illness, body weights of FI-A2-vaccinated mice were measured before and at 40 h after A2, B1, CP52, or R10C7G challenge. There was a 15 to 22% decrease in body weight of FI-A2-vaccinated mice challenged with A2 or B1 but a considerably smaller decrease in body weight of FI-A2-vaccinated mice challenged with CP52 or R10C7G (8 to 16%). Similarly, A2-challenged FI-A2-vaccinated mice treated with anti-CX3CR1 antibody had less body weight loss (9 to 17%) than NRS-treated mice (12 to 24%). FI-R10C7G-vaccinated mice challenged with A2 or R10C7G also showed less body weight loss (8 to 17%), which was unchanged by the anti-CX3CR1 antibody treatment (10 to 18%). Collectively, these results suggest that disease severity may be affected by expression of the G and/or SH glycoprotein or by G glycoprotein CX3C-CX3CR1 interaction.
Pulmonary cell trafficking associated with G and/or SH glycoprotein expression and G glycoprotein CX3C-CX3CR1 interaction.
The cell types that trafficked to the lungs of untreated, NRS-treated, or anti-SP or anti-CX3CR1 antibody-treated FI-A2-vaccinated mice were examined following challenge with A2, B1, CP52, PIV-3, or R10C7G. The treatments associated with significant cell trafficking differences are shown in Table 1. The greatest difference occurred in FI-A2-vaccinated mice challenged with CP52, in which higher numbers of DX5+ cells, RB6-8C5+ cells, and CD11b+ cells trafficked to the lungs between 24 and 40 h postchallenge. These data are consistent with our previous findings (67), suggesting that G and/or SH glycoprotein expression modifies trafficking of DX5+, RB6-8C5+, and CD11b+ cells. Interestingly, increased numbers of DX5+ cells trafficked to the lungs of FI-A2-vaccinated mice challenged with R10C7G, suggesting that the G glycoprotein CX3C motif affects DX5+ NK cell trafficking. Similarly, anti-SP antibody treatment was also associated with increased pulmonary trafficking of DX5+ cells and is consistent with our previous findings (68). To address the association between CX3C-CX3CR1 interaction and pulmonary cell trafficking, FI-A2-vaccinated mice were treated with anti-CX3CR1 antibody. Anti-CX3CR1 antibody treatment was associated with decreased CD3+ cell trafficking at all time points examined and with decreased trafficking of DX5+ and RB6-8C5+ cells at various time points p.i., suggesting that CX3C-CX3CR1 interaction is important in pulmonary recruitment of these cell types.
TABLE 1.
Pulmonary leukocyte trafficking in untreated and anti-SP antibody- or anti-CX3CR1 antibody-treated FI-A2 vaccinated mice challenged with B1, CP52, or R10C7Ga
| Time (h) | Phenotype | Mean total BAL cells/lung ± SEMb in FI-A2-vaccinated mice challenged with:
|
|||||
|---|---|---|---|---|---|---|---|
| B1 | CP52 | R10C7G | A2 and treated with:
|
||||
| NRS | Anti-SP | Anti-CX3CR1 | |||||
| 0 | CD3+ | 1,600 ± 228 | 1,591 ± 246 | 1,575 ± 323 | 1,583 ± 310 | 1,575 ± 292 | 1,583 ± 312 |
| B220+ | 1,335 ± 113 | 1,283 ± 122 | 1,229 ± 230 | 1,291 ± 357 | 1,229 ± 446 | 1,291 ± 244 | |
| DX5+ | 2,507 ± 287 | 2,500 ± 216 | 2,455 ± 342 | 2,507 ± 400 | 2,457 ± 388 | 2,507 ± 360 | |
| RB6-8C5+ | 1,280 ± 112 | 1,309 ± 105 | 1,185 ± 206 | 1,285 ± 200 | 1,180 ± 195 | 1,185 ± 187 | |
| CD11b+ | 1,003 ± 60 | 1,005 ± 77 | 1,005 ± 205 | 991 ± 84 | 980 ± 65 | 991 ± 215 | |
| 8 | CD3+ | 3,585 ± 355 | 3,793 ± 405 | 3,586 ± 333 | 3,710 ± 405 | 1,955 ± 305d | 1,803 ± 225d |
| B220+ | 4,117 ± 288 | 4,075 ± 415 | 3,336 ± 317 | 3,995 ± 320 | 3,633 ± 313 | 2,863 ± 212 | |
| DX5+ | 2,693 ± 280 | 5,847 ± 732c | 6,058 ± 422c | 2,865 ± 415 | 1,531 ± 255d | 1,401 ± 194d | |
| RB6-8C5+ | 5,010 ± 361 | 11,103 ± 340c | 4,800 ± 442 | 4,610 ± 338 | 5,699 ± 445d | 4,585 ± 374 | |
| CD11b+ | 4,590 ± 372 | 10,820 ± 448c | 4,248 ± 420 | 4,801 ± 382 | 5,262 ± 367 | 4,399 ± 401 | |
| 18 | CD3+ | 20,965 ± 760 | 3,335 ± 418c | 23,645 ± 826 | 19,499 ± 756 | 21,310 ± 996 | 8,101 ± 485d |
| B220+ | 14,005 ± 441 | 5,117 ± 336c | 12,241 ± 1,010 | 15,897 ± 528 | 16,820 ± 800 | 15,810 ± 854 | |
| DX5+ | 4,355 ± 294 | 10,115 ± 505c | 5,178 ± 309c | 4,701 ± 480 | 9,240 ± 915d | 5,120 ± 300d | |
| RB6-8C5+ | 12,200 ± 585 | 11,951 ± 1,015 | 9,459 ± 509 | 12,133 ± 880 | 9,050 ± 605 | 4,055 ± 421d | |
| CD11b+ | 12,083 ± 921 | 18,770 ± 779 | 10,295 ± 1,066 | 12,805 ± 1,105 | 9,310 ± 719 | 5,333 ± 468d | |
| 24 | CD3+ | 16,830 ± 1,125 | 13,785 ± 1,002 | 14,650 ± 1,007 | 16,115 ± 1,264 | 20,952 ± 1,186 | 14,145 ± 995d |
| B220+ | 12,500 ± 741 | 11,055 ± 890 | 14,452 ± 945 | 11,231 ± 880 | 7,792 ± 543 | 12,773 ± 596 | |
| DX5+ | 3,850 ± 377 | 15,455 ± 863c | 8,317 ± 405c | 4,020 ± 411 | 9,074 ± 595d | 2,119 ± 240d | |
| RB6-8C5+ | 9,300 ± 580 | 18,737 ± 921c | 10,555 ± 990 | 10,197 ± 848 | 9,112 ± 525 | 9,855 ± 427 | |
| CD11b+ | 10,553 ± 608 | 18,743 ± 886c | 10,021 ± 573 | 10,011 ± 841 | 9,311 ± 625 | 9,965 ± 492 | |
| 40 | CD3+ | 84,500 ± 1,198 | 34,157 ± 978c | 77,054 ± 1,527 | 86,471 ± 2,322 | 61,952 ± 1,790d | 49,631 ± 1,465d |
| B220+ | 5,803 ± 650 | 16,655 ± 1,035c | 5,239 ± 291 | 5,623 ± 577 | 6,377 ± 452 | 6,227 ± 310 | |
| DX5+ | 3,225 ± 317 | 12,003 ± 674c | 4,933 ± 338c | 3,535 ± 317 | 5,142 ± 330d | 1,899 ± 220d | |
| RB6-8C5+ | 13,505 ± 1,004 | 193,200 ± 1,377c | 12,065 ± 805 | 13,005 ± 856 | 10,425 ± 799 | 10,975 ± 788 | |
| CD11b+ | 8,665 ± 627 | 39,300 ± 1,225c | 10,023 ± 719 | 8,735 ± 603 | 11,203 ± 880 | 9,055 ± 501 | |
FI-A2-vaccinated BALB/c mice were challenged with B1, CP52, or R10C7G. BAL cells were collected from four to six untreated, anti-SP antibody-treated, or anti-CX3CR1 antibody-treated mice per experiment at 0, 8, 18, and 40 h postchallenge.
Data represent the mean total BAL cells expressing CD3, B220, DX5, RB6-8C5, or CD11b per lung, from three separate experiments. The total number of BAL cells expressing a particular phenotype was determined by multiplying the mean total number of BAL cells by the mean percentage of cells expressing that phenotype. Boldface values indicate a significant difference (P < 0.05).
Comparison of virus infection to B1 infection.
Comparison of treatment to NRS treatment.
To determine if the G glycoprotein CX3C motif affects the memory response associated with FI-RSV vaccination, the cell types that trafficked to the lungs in untreated, NRS-treated, or anti-CX3CR1 antibody-treated FI-R10C7G-vaccinated mice challenged with A2 or R10C7G were examined (Table 2). Anti-CX3CR1 antibody treatment had the greatest effect on cell trafficking, principally reducing CD3+and DX5+ cell trafficking; however, DX5+ and RB6-8C5+ cell trafficking increased at 40 h postchallenge. Interestingly, increased numbers of DX5+ cells trafficked to the lungs of R10C7G-challenged mice between 18 and 40 h p.i., and increased RB6-8C5+ cells trafficked at 40 h p.i., suggesting that the G glycoprotein CX3C motif affects sensitization of the pulmonary cell response during FI-RSV vaccination.
TABLE 2.
Pulmonary leukocyte trafficking in untreated, NRS-treated, or anti-CX3CR1 antibody-treated FI-R10C7G-vaccinated mice challenged with A2 or R10C7Ga
| Time (h) | Phenotype | Mean total BAL cells/lung ± SEMb in FI-R10C7G-vaccinated mice challenged with:
|
|||
|---|---|---|---|---|---|
| A2 | R10C7G | A2 and treated with:
|
|||
| NRS | Anti-CX3CR1 | ||||
| 0 | CD3 | 1,600 ± 270 | 1,564 ± 234 | 1,612 ± 300 | 1,609 ± 261 |
| B220 | 1,335 ± 105 | 1,115 ± 94 | 1,132 ± 89 | 1,293 ± 144 | |
| DX5 | 2,007 ± 227 | 2,112 ± 186 | 2,020 ± 196 | 2,507 ± 227 | |
| RB6-8C5 | 1,280 ± 87 | 1,310 ± 118 | 1,291 ± 104 | 1,338 ± 125 | |
| CD11b | 1,003 ± 112 | 1,023 ± 115 | 1,013 ± 98 | 1,028 ± 124 | |
| 8 | CD3 | 3,663 ± 317 | 3,505 ± 339 | 3,700 ± 360 | 2,463 ± 322d |
| B220 | 3,665 ± 354 | 3,601 ± 320 | 3,275 ± 344 | 3,705 ± 371 | |
| DX5 | 2,671 ± 278 | 2,077 ± 249 | 2,865 ± 312 | 1,875 ± 300d | |
| RB6-8C5 | 8,447 ± 533 | 8,000 ± 500 | 8,610 ± 545 | 6,177 ± 509d | |
| CD11b | 11,100 ± 1,020 | 9,495 ± 602 | 9,887 ± 557 | 7,987 ± 518d | |
| 18 | CD3 | 9,559 ± 597 | 8,567 ± 488 | 10,499 ± 585 | 5,500 ± 501d |
| B220 | 8,600 ± 521 | 7,625 ± 436 | 8,709 ± 510 | 3,711 ± 371d | |
| DX5 | 3,003 ± 346 | 8,355 ± 515c | 4,001 ± 377 | 2,465 ± 583d | |
| RB6-8C5 | 8,695 ± 552 | 8,655 ± 520 | 8,633 ± 555 | 8,265 ± 914 | |
| CD11b | 5,900 ± 458 | 5,930 ± 500 | 6,005 ± 488 | 6,091 ± 603 | |
| 24 | CD3 | 18,115 ± 2,407 | 17,411 ± 1,249 | 17,031 ± 1,086 | 10,995 ± 1,164d |
| B220 | 10,073 ± 1,104 | 10,031 ± 1,008 | 10,099 ± 1,015 | 9,855 ± 647 | |
| DX5 | 4,435 ± 383 | 10,005 ± 935c | 4,520 ± 404 | 3,599 ± 644d | |
| RB6-8C5 | 7,275 ± 455 | 8,633 ± 580 | 7,377 ± 487 | 8,455 ± 561 | |
| CD11b | 6,003 ± 476 | 5,167 ± 230 | 5,909 ± 482 | 5,565 ± 263 | |
| 40 | CD3 | 63,655 ± 3,726 | 62,450 ± 3,139 | 62,867 ± 3,224 | 47,103 ± 2,166d |
| B220 | 9,099 ± 576 | 8,803 ± 515 | 7,985 ± 509 | 7,997 ± 528 | |
| DX5 | 900 ± 240 | 19,225 ± 2,472c | 977 ± 241 | 19,115 ± 2,601d | |
| RB6-8C5 | 9,750 ± 604 | 21,875 ± 2,844c | 9,805 ± 635 | 21,500 ± 2,773d | |
| CD11b | 14,401 ± 1,114 | 2,433 ± 190c | 14,773 ± 1,137 | 2,507 ± 229d | |
FI-R10C7G-vaccinated BALB/c mice were challenged with A2 or R10C7G. BAL cells were collected from four to six untreated, NRS-treated, or anti-CX3CR1 antibody-treated mice at 0, 8, 18, and 40 h postchallenge.
Data represent the mean total BAL cells expressing CD3, B220, DX5, RB6-8C5, or CD11b per lung from three separate experiments. The total number of BAL cells expressing a particular phenotype was determined by multiplying the mean total number of BAL cells by the mean percentage of cells expressing that phenotype. Boldface values indicate a significant difference (P < 0.05).
Comparison of R10C7G infection to A2 infection.
Comparison of anti-CX3CR1 antibody treatment to NRS treatment.
CD4+ TCR Vβ usage is affected by G glycoprotein expression or by the G glycoprotein CX3C motif.
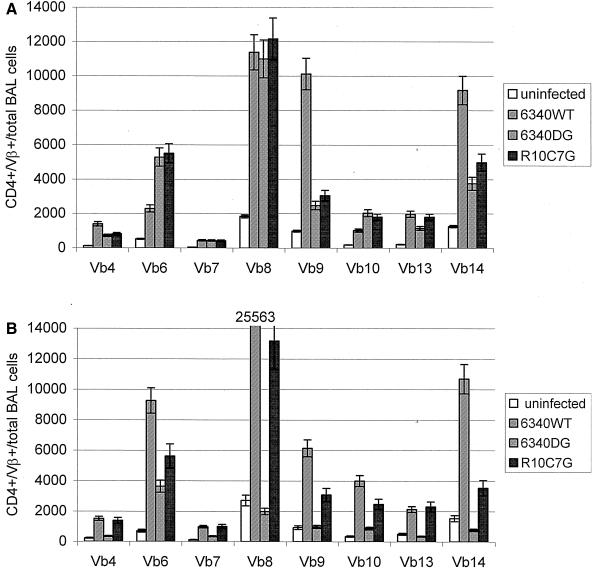
The T-cell epitope in G glycoprotein associated with enhanced pulmonary disease is dominated by TCR Vβ14+ usage by CD4+ T cells (71). To address whether the G glycoprotein or G glycoprotein CX3C motif affected CD4+ TCR Vβ expression in the primary immune response to RSV infection, naive mice were infected with RSV (6340WT), with an RSV mutant virus lacking the G gene (6340ΔG), or with RSV lacking the G glycoprotein CX3C motif, R10C7G, and TCR Vβ expression by BAL cells was examined at day 7 p.i. (Fig. 6A). 6340WT infection was associated with high TCR Vβ8, Vβ9, and Vβ14 expression and with lower TCR Vβ4, Vβ6, Vβ10, and Vβ13 expression (Fig. 6A). In contrast, high TCR Vβ6 expression and considerably lower TCR Vβ9 and Vβ14 expression followed 6340ΔG or R10C7G infection, suggesting that the G glycoprotein or G glycoprotein CX3C motif affects CD4+ TCR Vβ expression.
FIG. 6.
CD4+ TCR Vβ (Vb) usage by BAL cells in the lung following 6340WT, 6340ΔG (6340DG), or R10C7G infection of naive mice (A) or FI-A2-vaccinated mice (B) was determined at day 7 p.i. by flow cytometry. The results represent the mean (± standard error) of CD4+ TCR Vβ expression in total BAL cells collected and pooled from three mice in three individual experiments.
CD4+ TCR Vβ expression in FI-RSV-vaccinated mice challenged with 6340WT, 6340ΔG, or R10C7G was considerably different (Fig. 6B) from the primary immune response to RSV infection (Fig. 6A). Following 6340WT challenge, TCR Vβ8 expression was dominant, with high TCR Vβ6 and Vβ14 expression and some increase in TCR Vβ9 and Vβ10 expression. Challenge with R10C7G resulted in a similar pattern of increase in TCR Vβ expression, but to lower levels than with 6340WT. In contrast, there was minimal TCR Vβ expression, with the exception of some increase in Vβ6 expression, following 6340ΔG challenge. These data suggest that the CD4+ TCR Vβ response is affected by G glycoprotein expression and the G glycoprotein CX3C motif.
CC and CXC chemokine mRNA expression is modified by G glycoprotein expression, the G glycoprotein CX3C motif, and CX3C-CX3CR1 interaction.
The type of inflammatory cell infiltrate in the lungs is associated with the pattern of chemokines expressed, and RSV G glycoprotein expression has been linked to altered CC (MIP-1α and MIP-1β) and CXC (MIP-2) chemokine mRNA expression by BAL cells (64). CC and CXC chemokine mRNA expression by BAL cells was examined in untreated, virus-free Vero cell lysate-treated, NRS-treated, and anti-SP or anti-CX3CR1 antibody-treated FI-A2- or FI-RC10C7G-vaccinated mice between 8 and 40 h postchallenge with A2, B1, CP52, or R10C7G. The treatments associated with the greatest difference in chemokine mRNA expression are shown in Tables 3 and 4. Consistent with our previous studies (64), BAL cells expressed increased MIP-1α, MIP-1β, and MIP-2 mRNAs at 8 h after CP52 challenge of NRS-treated FI-A2-vaccinated mice, and MIP-2 mRNA expression was reduced by anti-SP antibody treatment (Table 3). These data suggest that G and/or SH glycoprotein expression during RSV challenge alters CC and CXC chemokine mRNA expression.
TABLE 3.
CC and CXC chemokine mRNA expression by BAL cells at 8 or 40 h postchallenge of FI-A2-vaccinated micea
| Time (h) | Chemokine | Increase in mRNA expressionb after the following virus challenge and treatment:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A2
|
B1
|
CP52
|
R10C7G
|
|||||||
| NRS | Anti-SP | Anti-CX3CR1 | NRS | Anti-SP | NRS | Anti-SP | NRS | Anti-SP | ||
| 8 | MIP-1α | 1.5 | 0.9 | 0.7 | 1.4 | 0.8 | 2.2c | 0.9 | 1.8 | 1.0 |
| MIP-1β | 1.1 | 0.9 | 0.8 | 0.9 | 1.0 | 2.6c | 1.3 | 0.8 | 0.8 | |
| MIP-2 | 1.2 | 1.0 | 1.1 | 1.1 | 0.9 | 5.2c | 2.6c | 1.4 | 0.8 | |
| 40 | MIP-1α | 1.0 | 1.0 | 9.5d | 1.0 | 0.9 | 0.9 | 0.9 | 3.9c | 2.2c |
| MIP-1β | 0.9 | 0.9 | 0.8 | 0.9 | 0.9 | 1.0 | 1.0 | 0.8 | 0.7 | |
| MIP-2 | 1.0 | 1.0 | 5.8d | 0.9 | 0.8 | 1.0 | 0.9 | 2.6c | 1.5c | |
Vaccinated BALB/c mice were challenged with A2, B1, CP52, or R10C7G and treated with NRS, anti-SP antibody, or anti-CX3CR1 antibody as indicated. BAL cells from three or four mice per experiment were collected at 8 or 40 h postchallenge or after virus-free Vero cell lysate control treatment, the mRNA was isolated, and chemokine expression was quantified by Typhoon 9410 variable-mode imaging. Mean band density ratios were determined for CC or CXC chemokine mRNA expression in three separate experiments by dividing the mean band density of GAPDH housekeeping gene expression by the mean band density of the chemokine.
Virus-specific increases in CC or CXC chemokine mRNA expression are shown and were determined by dividing the mean band density ratio for Vero cell lysate treatment by the mean band density for the virus treatment. Boldface values indicate a significant difference (P < 0.05).
Comparison of response to A2 infection and similar treatment.
Comparison of response to NRS control.
TABLE 4.
CC and CXC chemokine mRNA expression by BAL cells at 40 h postchallenge of FI-R10C7G-vaccinated micea
At 40 h postchallenge, anti-CX3CR1 antibody treatment of FI-A2-vaccinated mice was associated with increased MIP-1α and MIP-2 mRNA expression by BAL cells from A2-challenged mice (Table 3). Similarly, MIP-1α and MIP-2 mRNA expression was increased in NRS-treated FI-A2-vaccinated mice challenged with R10C7G, suggesting that G glycoprotein CX3C-CX3CR1 interaction modifies MIP-1α and MIP-2 mRNA expression. Interestingly, anti-SP antibody treatment reduced MIP-1α and MIP-2 mRNA expression by BAL cells from R10C7G-challenged mice, suggesting a role for SP modulation of the chemokine response.
To address whether absence of the G glycoprotein CX3C motif during vaccination affects CC or CXC chemokine expression, mice were vaccinated with FI-R10C7G, treated with NRS or anti-SP or anti-CX3CR1 antibody, and challenged with A2 or R10C7G (Table 4). At 40 h after A2 challenge, BAL cells from NRS-treated FI-R10C7G-vaccinated mice expressed higher levels of MIP mRNAs (Table 4) than BAL cells from similarly challenged FI-A2-vaccinated mice (Table 3), suggesting that the G glycoprotein CX3C motif affects expression of MIPs. Interestingly, anti-SP antibody treatment reduced MIP mRNA expression, and anti-CX3CR1 antibody treatment reduced MIP-1α and MIP-1β mRNA expression. Consistent with the findings for R10C7G challenge of NRS-treated FI-A2-vaccinated mice (Table 3), BAL cells from NRS-treated FI-R10C7G-vaccinated mice challenged with R10C7G expressed high levels of MIP-1α and MIP-2 mRNAs that were decreased in anti-SP antibody-treated mice.
DISCUSSION
FI-RSV vaccination or G glycoprotein sensitization is linked with enhanced inflammation in BALB/c mice challenged with live RSV (21, 22, 24, 49, 51). The mechanisms that contribute to this process are not fully defined. In this study, we present data that indicate that the G glycoprotein, and in particular the G glycoprotein CX3C motif, contributes to FI-RSV vaccine disease through a process that may be associated with the induction of SP. We have previously shown, and confirm in this study, that the absence of the G and SH glycoproteins in the challenge virus given to FI-A2-vaccinated mice reduces pulmonary expression of SP, enhances MIP mRNA expression in BAL cells, and does not induce pulmonary eosinophilia. In this report, we expand these observations to show that challenge viruses given to FI-A2-vaccinated mice that lack the G glycoprotein or an intact G glycoprotein CX3C motif similarly fail to induce pulmonary eosinophilia. The link to the G glycoprotein CX3C motif and FI-RSV vaccine disease is further substantiated by the results showing that antibodies that block G glycoprotein CX3C-CX3CR1 interaction, i.e., antibodies against CX3CR1, block pulmonary eosinophilia in FI-A2-vaccinated mice challenged with A2. Importantly, the CX3C site also appeared to affect the memory response that predisposed to eosinophilia; i.e., mice vaccinated with FI-RSV from a strain of RSV that lacks an intact CX3C site did not develop pulmonary eosinophilia when challenged with A2. The G glycoprotein CX3C site was also linked to weight loss, suppression of pulmonary MIP mRNA expression, elevated SP levels, and patterns of TCR Vβ usage.
Previously, several T-cell epitopes in the central conserved region of the G glycoprotein have been associated with sensitization for enhanced disease (61). Interestingly, these regions contain or are proximal to the CX3C motif, i.e., amino acids 182 to 186 in the G glycoprotein. We have previously shown that G glycoprotein CX3C binds to CX3CR1, and this interaction induces leukocyte chemotaxis (65). The mechanisms by which CX3C-CX3CR1 interaction contributes to FI-RSV-enhanced disease are yet to be fully understood, but they may be linked to induction of pulmonary SP expression. Our data suggest that G glycoprotein induction of SP is linked to G glycoprotein CX3C-CX3CR1 interaction, and treatment with anti-SP antibody reduces G glycoprotein-associated enhanced pulmonary eosinophilia. It is possible that the G glycoprotein CX3C motif interacts with CX3CR1 expressed on neurons and some immune cells in the lung (18, 19, 28, 29, 32, 37, 46), inducing excitatory effects that mediate the release of SP. Pulmonary leukocytes have been shown to express SP receptors, and SP receptors have been shown to increase following RSV infection, particularly on CD4+ T lymphocytes (63). G glycoprotein CX3C-CX3CR1 interaction may also modify the activities of CX3CR1+ cells. We have previously shown that G glycoprotein and 12-mer G glycoprotein CX3C peptides can compete with fractalkine for CX3CR1 binding and alter fractalkine-mediated responses (65). Fractalkine has been shown to be important in chemoattraction and activation of dendritic cells (8, 25, 38, 52). Thus, G glycoprotein CX3C-CX3CR1 interaction may modify dendritic cell recruitment or activation and affect the associated molecular or chemical signals important in T-cell activation, including MIPs and SP (10, 41, 53). Consistent with this hypothesis, we show that MIP mRNA expression by BAL cells is altered by the G glycoprotein CX3C motif or CX3C-CX3CR1 interaction. A potential effect that may be linked to modified MIP-1α or MIP-1β mRNA expression is altered eosinophil trafficking, as these chemokines have been shown to facilitate eosinophil trafficking (3, 9, 40, 45, 48, 57, 76). Importantly, we show that the absence of the G glycoprotein CX3C motif or CX3C-CX3CR1 interaction during FI-RSV vaccination or RSV challenge reduces or eliminates pulmonary eosinophilia and decreases pulmonary SP expression. MIP expression also appears to be important in the development of FI-RSV-enhanced disease, as MIP-1α has been shown to activate STAT1 (74) and STAT1 has been implicated in enhancement of illness associated with RSV infection (12).
The results of this study underscore the importance of understanding the host response to viral proteins being considered as vaccine candidates. The results show that enhanced disease associated with FI-RSV vaccination may be reduced by eliminating G glycoprotein expression, the G glycoprotein CX3C motif, or CX3C-CX3CR1 interaction. This study suggests new approaches for development of inactivated RSV vaccines and for treatments for RSV disease.
Acknowledgments
We thank Peter Collins and Brian Murphy (LID, National Institute of Allergy and Infectious Diseases, Bethesda, Md.) and Jose A. Melero and Blanca García-Barreno (Centro Nacional de Biología Fundamental, Instituto de Salud Carlos III, Madrid, Spain) for providing RSV mutant and recombinant viruses used in this study.
REFERENCES
- 1.Anonymous. 2002. Respiratory syncytial virus activity—United States, 2000-01 season. Morb. Mortal. Wkly. Rep. 51:26-28. [PubMed] [Google Scholar]
- 2.Bazan, J. F., K. B. Bacon, G. Hardiman, W. Wang, K. Soo, D. Rossi, D. R. Greaves, A. Zlotnik, and T. J. Schall. 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature 385:640-644. [DOI] [PubMed] [Google Scholar]
- 3.Borchers, M. T., T. Ansay, R. DeSalle, B. L. Daugherty, H. Shen, M. Metzger, N. A. Lee, and J. J. Lee. 2002. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J. Leukoc. Biol. 71:1033-1041. [PubMed] [Google Scholar]
- 4.Chakravorty, S. J., P. Cockwell, J. Girdlestone, C. J. Brooks, and C. O. Savage. 2002. Fractalkine expression on human renal tubular epithelial cells: potential role in mononuclear cell adhesion. Clin. Exp. Immunol. 129:150-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin, J., R. L. Magoffin, L. A. Shearer, J. H. Schieble, and E. H. Lennette. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 89:449-463. [DOI] [PubMed] [Google Scholar]
- 6.Combadiere, C., J. Gao, H. L. Tiffany, and P. M. Murphy. 1998. Gene cloning, RNA distribution, and functional expression of mCX3CR1, a mouse chemotactic receptor for the CX3C chemokine fractalkine. Biochem. Biophys. Res. Commun. 253:728-732. [DOI] [PubMed] [Google Scholar]
- 7.Couch, R. B., J. A. Englund, and E. Whimbey. 1997. Respiratory viral infections in immunocompetent and immunocompromised persons. Am. J. Med. 102:2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dichmann, S., Y. Herouy, D. Purlis, H. Rheinen, P. Gebicke-Harter, and J. Norgauer. 2001. Fractalkine induces chemotaxis and actin polymerization in human dendritic cells. Inflamm. Res. 50:529-533. [DOI] [PubMed] [Google Scholar]
- 9.Domachowske, J. B., C. A. Bonville, J. L. Gao, P. M. Murphy, A. J. Easton, and H. F. Rosenberg. 2000. The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J. Immunol. 165:2677-2682. [DOI] [PubMed] [Google Scholar]
- 10.Drakes, M. L., A. F. Zahorchak, T. Takayama, L. Lu, and A. W. Thomson. 2000. Chemokine and chemokine receptor expression by liver-derived dendritic cells: MIP-1alpha production is induced by bacterial lipopolysaccharide and interaction with allogeneic T cells. Transpl. Immunol. 8:17-29. [DOI] [PubMed] [Google Scholar]
- 11.Dunzendorfer, S., C. Meierhofer, and C. J. Wiedermann. 1998. Signaling in neuropeptide-induced migration of human eosinophils. J. Leukoc. Biol. 64:828-834. [DOI] [PubMed] [Google Scholar]
- 12.Durbin, J. E., T. R. Johnson, R. K. Durbin, S. E. Mertz, R. A. Morotti, R. S. Peebles, and B. S. Graham. 2002. The role of IFN in respiratory syncytial virus pathogenesis. J. Immunol. 168:2944-2952. [DOI] [PubMed] [Google Scholar]
- 13.Falsey, A. R. 1998. Respiratory syncytial virus infection in older persons. Vaccine 16:1775-1778. [DOI] [PubMed] [Google Scholar]
- 14.Falsey, A. R., and E. E. Walsh. 2000. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13:371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraticelli, P., M. Sironi, G. Bianchi, D. D'Ambrosio, C. Albanesi, A. Stoppacciaro, M. Chieppa, P. Allavena, L. Ruco, G. Girolomoni, F. Sinigaglia, A. Vecchi, and A. Mantovani. 2001. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J. Clin. Invest. 107:1173-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto, K., T. Imaizumi, H. Yoshida, S. Takanashi, K. Okumura, and K. Satoh. 2001. Interferon-gamma stimulates fractalkine expression in human bronchial epithelial cells and regulates mononuclear cell adherence. Am. J. Respir. Cell. Mol. Biol. 25:233-238. [DOI] [PubMed] [Google Scholar]
- 17.Fulginiti, V. A., J. J. Eller, O. F. Sieber, J. W. Joyner, M. Minamitani, and G. Meiklejohn. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 89:435-448. [DOI] [PubMed] [Google Scholar]
- 18.Gabuzda, D., and J. Wang. 1999. Chemokine receptors and virus entry in the central nervous system. J. Neurovirol. 5:643-658. [DOI] [PubMed] [Google Scholar]
- 19.Garland, A., J. Necheles, S. R. White, S. P. Neeley, A. R. Leff, S. S. Carson, L. E. Alger, K. McAllister, and J. Solway. 1997. Activated eosinophils elicit substance P release from cultured dorsal root ganglion neurons. Am. J. Physiol. 273:L1096-L1102. [DOI] [PubMed]
- 20.Glezen, W. P., S. B. Greenberg, R. L. Atmar, P. A. Piedra, and R. B. Couch. 2000. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 283:499-505. [DOI] [PubMed] [Google Scholar]
- 21.Graham, B. S. 1996. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 4:290-293. [DOI] [PubMed] [Google Scholar]
- 22.Graham, B. S. 1995. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am. J. Respir. Crit. Care Med. 152:S63-S66. [DOI] [PubMed]
- 23.Graham, B. S., T. R. Johnson, and R. S. Peebles. 2000. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology 48:237-247. [DOI] [PubMed] [Google Scholar]
- 24.Graham, B. S., J. A. Rutigliano, and T. R. Johnson. 2002. Respiratory syncytial virus immunobiology and pathogenesis. Virology 297:1-7. [DOI] [PubMed] [Google Scholar]
- 25.Guo, J., M. Zhang, B. Wang, Z. Yuan, Z. Guo, T. Chen, Y. Yu, Z. Qin, and X. Cao. 2003. Fractalkine transgene induces T-cell-dependent antitumor immunity through chemoattraction and activation of dendritic cells. Int. J. Cancer 103:212-220. [DOI] [PubMed] [Google Scholar]
- 26.Hall, C. B. 1999. Respiratory syncytial virus: a continuing culprit and conundrum. J. Pediatr. 135:2-7. [PubMed] [Google Scholar]
- 27.Hancock, G. E., D. J. Speelman, K. Heers, E. Bortell, J. Smith, and C. Cosco. 1996. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J. Virol. 70:7783-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison, J. K., Y. Jiang, S. Chen, Y. Xia, D. Maciejewski, R. K. McNamara, W. J. Streit, M. N. Salafranca, S. Adhikari, D. A. Thompson, P. Botti, K. B. Bacon, and L. Feng. 1998. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 95:10896-10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatori, K., A. Nagai, R. Heisel, J. K. Ryu, and S. U. Kim. 2002. Fractalkine and fractalkine receptors in human neurons and glial cells. J. Neurosci. Res. 69:418-426. [DOI] [PubMed] [Google Scholar]
- 30.Haynes, L. M., J. Tonkin, L. J. Anderson, and R. A. Tripp. 2002. Neutralizing anti-F glycoprotein and anti-substance P antibody treatment effectively reduces infection and inflammation associated with respiratory syncytial virus infection. J. Virol. 76:6873-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesselgesser, J., and R. Horuk. 1999. Chemokine and chemokine receptor expression in the central nervous system. J. Neurovirol. 5:13-26. [DOI] [PubMed] [Google Scholar]
- 32.Hughes, P. M., M. S. Botham, S. Frentzel, A. Mir, and V. H. Perry. 2002. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia 37:314-327. [PubMed] [Google Scholar]
- 33.Imai, T., K. Hieshima, C. Haskell, M. Baba, M. Nagira, M. Nishimura, M. Kakizaki, S. Takagi, H. Nomiyama, T. J. Schall, and O. Yoshie. 1997. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91:521-530. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, T. R., and B. S. Graham. 1999. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J. Virol. 73:8485-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and B. S. Graham. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 72:2871-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joos, G. F., K. O. De Swert, and R. A. Pauwels. 2001. Airway inflammation and tachykinins: prospects for the development of tachykinin receptor antagonists. Eur. J. Pharmacol. 429:239-250. [DOI] [PubMed] [Google Scholar]
- 37.Joos, G. F., P. R. Germonpre, and R. A. Pauwels. 2000. Neural mechanisms in asthma. Clin. Exp. Allergy 30(Suppl. 1):60-65. [DOI] [PubMed] [Google Scholar]
- 38.Kanazawa, N., T. Nakamura, K. Tashiro, M. Muramatsu, K. Morita, K. Yoneda, K. Inaba, S. Imamura, and T. Honjo. 1999. Fractalkine and macrophage-derived chemokine: T cell-attracting chemokines expressed in T cell area dendritic cells. Eur. J. Immunol. 29:1925-1932. [DOI] [PubMed] [Google Scholar]
- 39.Karron, R. A., D. A. Buonagurio, A. F. Georgiu, S. S. Whitehead, J. E. Adamus, M. L. Clements-Mann, D. O. Harris, V. B. Randolph, S. A. Udem, B. R. Murphy, and M. S. Sidhu. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA 94:13961-13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudlacz, E., C. Whitney, C. Andresenl, and M. Conklyn. 2002. Functional effects of eotaxin are selectively upregulated on IL-5 transgenic mouse eosinophils. Inflammation 26:111-119. [DOI] [PubMed] [Google Scholar]
- 41.Lambrecht, B. N. 2001. Immunologists getting nervous: neuropeptides, dendritic cells and T cell activation. Respir. Res. 2:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levite, M. 2000. Nerve-driven immunity. The direct effects of neurotransmitters on T-cell function. Ann. N.Y. Acad. Sci. 917:307-321. [DOI] [PubMed] [Google Scholar]
- 43.Levite, M. 1998. Neuropeptides, by direct interaction with T cells, induce cytokine secretion and break the commitment to a distinct T helper phenotype. Proc. Natl. Acad. Sci. USA 95:12544-12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludwig, A., T. Berkhout, K. Moores, P. Groot, and G. Chapman. 2002. Fractalkine is expressed by smooth muscle cells in response to IFN-gamma and TNF-alpha and is modulated by metalloproteinase activity. J. Immunol. 168:604-612. [DOI] [PubMed] [Google Scholar]
- 45.Lukacs, N. W., T. J. Standiford, S. W. Chensue, R. G. Kunkel, R. M. Strieter, and S. L. Kunkel. 1996. C-C chemokine-induced eosinophil chemotaxis during allergic airway inflammation. J. Leukoc. Biol. 60:573-578. [DOI] [PubMed] [Google Scholar]
- 46.Meucci, O., A. Fatatis, A. A. Simen, and R. J. Miller. 2000. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc. Natl. Acad. Sci. USA 97:8075-8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh, S. B., P. B. Tran, S. E. Gillard, R. W. Hurley, D. L. Hammond, and R. J. Miller. 2001. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 21:5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira, S. H., S. Lira, A. C. Martinez, M. Wiekowski, L. Sullivan, and N. W. Lukacs. 2002. Increased responsiveness of murine eosinophils to MIP-1beta (CCL4) and TCA-3 (CCL1) is mediated by their specific receptors, CCR5 and CCR8. J. Leukoc. Biol. 71:1019-1025. [PubMed] [Google Scholar]
- 49.Openshaw, P. J. 1995. Immunopathological mechanisms in respiratory syncytial virus disease. Springer Semin. Immunopathol. 17:187-201. [DOI] [PubMed] [Google Scholar]
- 50.Openshaw, P. J. 2002. Potential therapeutic implications of new insights into respiratory syncytial virus disease. Respir. Res. 3:S15-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Openshaw, P. J., F. J. Culley, and W. Olszewska. 2001. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 20(Suppl. 1):S27-S31. [DOI] [PubMed] [Google Scholar]
- 52.Papadopoulos, E. J., C. Sassetti, H. Saeki, N. Yamada, T. Kawamura, D. J. Fitzhugh, M. A. Saraf, T. Schall, A. Blauvelt, S. D. Rosen, and S. T. Hwang. 1999. Fractalkine, a CX3C chemokine, is expressed by dendritic cells and is up-regulated upon dendritic cell maturation. Eur. J. Immunol. 29:2551-2559. [DOI] [PubMed] [Google Scholar]
- 53.Penna, G., M. Vulcano, S. Sozzani, and L. Adorini. 2002. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum. Immunol. 63:1164-1171. [DOI] [PubMed] [Google Scholar]
- 54.Prince, G. A. 2001. An update on respiratory syncytial virus antiviral agents. Expert Opin. Investig. Drugs 10:297-308. [DOI] [PubMed] [Google Scholar]
- 55.Rameshwar, P. 1997. Substance P: a regulatory neuropeptide for hematopoiesis and immune functions. Clin. Immunol. Immunopathol. 85:129-133. [DOI] [PubMed] [Google Scholar]
- 56.Rueda, P., B. Garcia-Barreno, and J. A. Melero. 1994. Loss of conserved cysteine residues in the attachment (G) glycoprotein of two human respiratory syncytial virus escape mutants that contain multiple A-G substitutions (hypermutations). Virology 198:653-662. [DOI] [PubMed] [Google Scholar]
- 57.Sabroe, I., A. Hartnell, L. A. Jopling, S. Bel, P. D. Ponath, J. E. Pease, P. D. Collins, and T. J. Williams. 1999. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J. Immunol. 162:2946-2955. [PubMed] [Google Scholar]
- 58.Schaffer, M., T. Beiter, H. D. Becker, and T. K. Hunt. 1998. Neuropeptides: mediators of inflammation and tissue repair? Arch. Surg. 133:1107-1116. [DOI] [PubMed] [Google Scholar]
- 59.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1999. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282:1440-1446. [DOI] [PubMed] [Google Scholar]
- 60.Simoes, E. A. 1999. Respiratory syncytial virus infection. Lancet 354:847-852. [DOI] [PubMed] [Google Scholar]
- 61.Sparer, T. E., S. Matthews, T. Hussell, A. J. Rae, B. Garcia-Barreno, J. A. Melero, and P. J. Openshaw. 1998. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J. Exp Med. 187:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staat, M. A. 2002. Respiratory syncytial virus infections in children. Semin. Respir. Infect. 17:15-20. [DOI] [PubMed] [Google Scholar]
- 63.Tripp, R. A., A. Barskey, L. Goss, and L. J. Anderson. 2002. Substance P receptor expression on lymphocytes is associated with the immune response to respiratory syncytial virus infection. J. Neuroimmunol. 129:141-153. [DOI] [PubMed] [Google Scholar]
- 64.Tripp, R. A., L. Jones, and L. J. Anderson. 2000. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J. Virol. 74:6227-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tripp, R. A., L. P. Jones, L. M. Haynes, H. Zheng, P. M. Murphy, and L. J. Anderson. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2:732-738. [DOI] [PubMed] [Google Scholar]
- 66.Tripp, R. A., D. Moore, and L. J. Anderson. 2000. TH(1)- and TH(2)-type cytokine expression by activated T lymphocytes from the lung and spleen during the inflammatory response to respiratory syncytial virus. Cytokine 12:801-807. [DOI] [PubMed] [Google Scholar]
- 67.Tripp, R. A., D. Moore, L. Jones, W. Sullender, J. Winter, and L. J. Anderson. 1999. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J. Virol. 73:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tripp, R. A., D. Moore, J. Winter, and L. J. Anderson. 2000. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. J. Virol. 74:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Hagen, P. M., L. J. Hofland, A. M. ten Bokum, E. G. Lichtenauer-Kaligis, D. J. Kwekkeboom, D. Ferone, and S. W. Lamberts. 1999. Neuropeptides and their receptors in the immune system. Ann. Med. 31(Suppl. 2):15-22. [PubMed] [Google Scholar]
- 70.Varga, S. M., and T. J. Braciale. 2002. RSV-induced immunopathology: dynamic interplay between the virus and host immune response. Virology 295:203-207. [DOI] [PubMed] [Google Scholar]
- 71.Varga, S. M., X. Wang, R. M. Welsh, and T. J. Braciale. 2001. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity 15:637-646. [DOI] [PubMed] [Google Scholar]
- 72.Ward, S. G., and J. Westwick. 1998. Chemokines: understanding their role in T-lymphocyte biology. Biochem. J. 333:457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weibel, R. E., J. Stokes, Jr., M. B. Leagus, C. C. Mascoli, A. A. Tytell, A. F. Woodhour, P. P. Vella, and M. R. Hilleman. 1967. Respiratory virus vaccines. VII. Field evaluation of respiratory syncytial, parainfluenza 1, 2, 3, and Mycoplasma pneumoniae vaccines, 1965 to 1966. Am. Rev. Respir. Dis. 96:724-739. [DOI] [PubMed] [Google Scholar]
- 74.Wong, M., and E. N. Fish. 1998. RANTES and MIP-1alpha activate stats in T cells. J. Biol. Chem. 273:309-314. [DOI] [PubMed] [Google Scholar]
- 75.Wong, M. M., and E. N. Fish. 2003. Chemokines: attractive mediators of the immune response. Semin. Immunol. 15:5-14. [DOI] [PubMed] [Google Scholar]
- 76.Ying, S., Q. Meng, L. T. Barata, and A. B. Kay. 2001. Macrophage inflammatory protein-1alpha and C-C chemokine receptor-1 in allergen-induced skin late-phase reactions: relationship to macrophages, neutrophils, basophils, eosinophils and T lymphocytes. Clin. Exp. Allergy 31:1724-1731. [DOI] [PubMed] [Google Scholar]