Abstract
Background
In three-dimensional echocardiography (3DE), individual endocardial trabeculae are not clearly visible necessitating left ventricular (LV) volumes to be measured by tracing the innermost endocardial contour. Ultrasound contrast agents aim to improve endocardial definition, but may delineate the outermost endocardial contour by filling up intertrabecular space. Although measurement reproducibility may benefit, there may be a significant influence on absolute LV volume measurements.
Methods
Twenty patients with a recent myocardial infarction and good ultrasound image quality underwent 3DE using the TomTec Freehand method before and during continuous intravenous contrast infusion. LV volumes were measured offline using TomTec Echo-Scan software.
Results
The use of contrast enhancement increased end-diastolic (110±35 vs. 144±53 ml; p<0.01) and end-systolic volume measurements (68±31 vs. 87±45 ml; p<0.01) significantly compared with non-contrast; the ejection fraction remained unchanged (40±13 vs. 41±14%, p=NS). Measurement reproducibility did not improve significantly, however.
Conclusion
Volumes measured by 3DE are significantly larger when ultrasound contrast is used. Possibly, intertrabecular space comprises a substantial part of the LV cavity. In the presence of an adequate apical acoustic window, ultrasound contrast does not improve LV volume measurement reproducibility. (Neth Heart J 2008;16:47-52.)
Keywords: echocardiography, ultrasound, contrast, ventricle
In clinical cardiology, left ventricular (LV) volume and ejection fraction are widely used parameters, as they carry important diagnostic and prognostic information, particularly when evaluated quantitatively rather than qualitatively. Traditionally, LV volume and ejection fraction are evaluated using quantitative twodimensional echocardiography (2DE), radionuclide angiography or LV angiography. These modalities have been extensively validated and show a reasonable correlation with each other,1 yet it should be noted that they are not simply interchangeable due to the fundamentally different principles they are based on.2 Although magnetic resonance imaging (MRI) has progressed to the reference method for LV volume measurements over the last few years, its limited availability, longer examination times and higher costs preclude routine clinical use.3 Currently, 2DE is the most frequently applied method to assess LV volumes and ejection fraction in daily clinical practice. 2DE, like LV angiography, relies on geometric assumptions of LV shape, however, and is therefore not ideal. On the other hand, three-dimensional echocardiography (3DE) does not rely on geometric assumptions and promises to provide more narrow limits of confidence, while a better correlation between 3DE and MRI has been reported.4-10
In quantitative echocardiographic studies, it is customary to consider the endocardial wall to be a reasonably smooth surface that may be traced manually with a computer mouse. It was not appreciated fully that the endocardium consists of trabeculae because these are usually too small to be seen by echocardiography, and therefore the innermost endocardial contour has been called the endocardial wall. To improve LV endocardial definition in the substantial amount of patients with limited echogenicity, administration of intravenous ultrasound contrast agents is increasingly advocated, with a reported success rate of >95%.11-17 Besides improved endocardial border definition, several studies observed larger LV volumes following ultrasound contrast administration.11-22 This may be explained by the fact that the LV contrast fills up the intertrabecular space, thereby delineating the outermost rather than the innermost endocardial contour. Although ultrasound contrast agents have the potential to improve LV volume measurement reproducibility, the question arises where the LV cavity boundaries should be drawn: i.e. either the innermost endocardial contour in the absence of contrast enhancement, or the outermost contour, when ultrasound contrast is present? The present study aimed to compare measurements of end-systolic and enddiastolic LV volume and ejection fraction using 3DE without and with ultrasound contrast enhancement, and to evaluate the effect of contrast enhancement on measurement reproducibility.
Patients and Methods
Twenty clinically stable patients with recent myocardial infarction with a good acoustic apical window and absence of known or suspected contraindications to the ultrasound contrast agent (as specified in the package insert), underwent 3DE using the TomTec Freehand method.23 The study was approved by the medical ethics committee of our institution and all patients provided prior written informed consent. One 3DE acquisition was performed immediately before and one during continuous intravenous ultrasound contrast infusion. LV volumes, ejection fraction, and intra- and inter-observer variation were evaluated.
Three-dimensional echocardiography
The echocardiographic examinations were performed using an ATL HDI 5000 (Philips Medical Systems, Eindhoven, the Netherlands) or an HP 5500 ultrasound platform (Hewlett Packard, Andover, Massachusetts, USA). Imaging was continuous using the gray-scale second harmonic imaging (harmonic penetration) mode. During ultrasound contrast infusion, second harmonic imaging with lower mechanical indices (between 0.2 and 0.4) was used, and gain and compression settings were adjusted for optimal endocardial visualisation. The ultrasound platform was interfaced with a TomTec Compact 3D Cardiac Imaging system (TomTec GmbH, Munich, Germany) as described earlier.23 Briefly, a pyramidal 3DE dataset was acquired from the apical acoustic window by making a fan-like 120° sweep with the 2DE transducer from the epicardial anterior wall to the epicardial posterior wall or vice-versa, with a spatial locator mounted on top of the transducer. Using ECG triggering, consecutive imaging planes encompassing a full cardiac cycle were thus acquired at known spatial orientations along the 120° sweep. To circumvent artifacts caused by cardiac motion during respiration, image acquisition was performed during six to ten repeated periods (of 10 to 15 seconds each) of breath holding. Thus, a dynamic pyramidal 3DE dataset consisting of 60 to 80 ‘slices’ was generated within a total acquisition time of approximately three minutes at a temporal resolution of 40 ms. The acquired images were stored digitally for subsequent off-line analysis.
Ultrasound contrast infusion
After non-contrast 3DE, patients were cannulated in the right antecubital vein and a three-way stopcock was attached to the cannula. Using the main port, a 0.9% saline infusion running at 200 ml/h was given as a carrier liquid. As intravenous ultrasound contrast agent we used Optison (Mallinckrodt Medical, St. Louis, MO, USA), a suspension of perflutren-filled albumin microbubbles with a mean diameter of 2.0 to 4.5 μm at a concentration of 5 to 8⋅108 microspheres/ ml. At the side port, a bolus of 0.2 to 0.3 ml of Optison was given, followed by continuous infusion at approximately 25 ml/h, with infusion speed continuously adjusted to maintain steady-state LV opacification with no attenuation. The close proximity of the stopcock to the entry point prevented trapping of contrast microbubbles in the lines.24 During infusion, the pump was slowly and continuously agitated manually to keep microbubbles in suspension. Steady-state LV opacification could be maintained for four to six minutes, allowing 3DE acquisitions to be repeated once or twice. Total volume administration was approximately 20 ml.
LV volume measurement
For 3DE analysis, the TomTec workstation in conjunction with TomTec EchoScan 4.1 software was used to calculate LV volumes and ejection fraction. Data werepost-processed off-line and based on visual assessment of acquisition quality, e.g. presence of movement artifacts or attenuation, it was decided which acquisitions would be used. The end-diastolic and endsystolic frames were determined by the moments of closure of the mitral and aortic valves, respectively. LV volumes were measured by manual tracing of the blackand- white or the white-and-black endocardial contours for non-contrast and contrast-enhanced acquisitions, respectively, using nine equidistant long axes. Papillary muscles were included into the LV cavity. See also figure 1.
Figure 1.
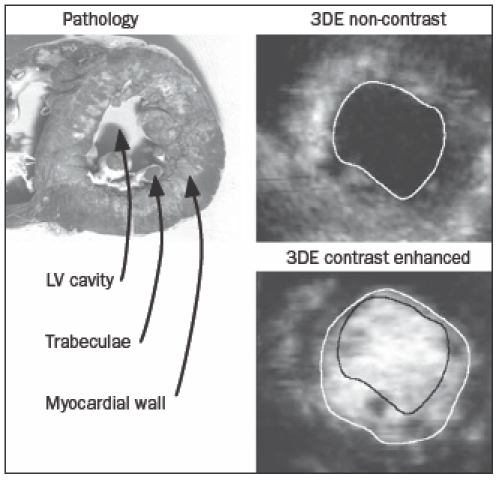
The LV is depicted as a pathology specimen and as seen by 3DE operators. Trabeculae may be appreciated clearly in the pathology specimen. The endocardial contours as drawn by the different operators are depicted as well. Because trabeculae are more easily appreciated on a short axis cut, short axis images are shown. For 3DE analysis however, long axis images were used.
For reproducibility analyses, the volume measurements were performed twice by one experienced observer (JvdH) at a four-week interval, and were repeated once by a second, blinded observer (LY).
Biostatistical analysis
For LV volumes and ejection fraction measured by 3DE with and without ultrasound contrast infusion, values were expressed in ml or % ± standard deviation. A two-sided paired T-test was performed for comparison. Intra- and inter-observer variability was bias ± 2 ⋅ 2 standard deviation, with the percentual intra- and inter-observer variability expressed relative to the mean volume. All statistical calculations were performed using Microsoft Excel 2003 software (MicroSoft Corporation, Seattle, Washington, USA). Differences with a p value of <0.05 were considered statistically significant.
Results
Study population
The study population consisted of 20 patients (18 male / 2 female), with an average age of 56±13 years (range 25-82). Infarct location was anterior in 15 patients, inferior in one and inferoposterior in four. At the time of the study, the patients were at a mean of 7.1±4.9 months after their index myocardial infarction; all were in a clinically stable condition.
Volume measurements
In two patients, contrast 3DE image quality was insufficient for analysis because of strong attenuation, resulting in 18 patients available for analysis. When compared with non-contrast 3DE measurements, the use of ultrasound contrast caused a significant increase in end-diastolic (110±35 vs. 144±53 ml, p<0.01) and end-systolic volumes (68±31 vs. 87±45 ml, p<0.01), but not in ejection fraction (40±13 vs. 41±14%, p=0.42). Figure 2 shows a Bland-Altman plot comparing non-contrast with contrast-enhanced measurements. A gradual increase in difference with increasing volumes can be clearly appreciated.
Figure 2.
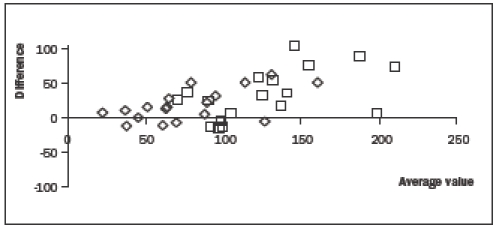
Bland-Altman plot comparing contrast with non-contrast enhanced measurements of end-diastolic volumes (squares) and end-systolic volumes (diamonds). On the horizontal axis, the average of the two measurements and on the vertical axis, the difference between the two measurements is shown. A gradual increase in difference with increasing volumes can be clearly appreciated.
Reproducibility
The use of contrast enhancement did not improve either intra- or inter-observer variation of volume measurements, as depicted in table 1. The magnitude of the reproducibility as observed in this study was comparable to earlier unenhanced studies performed by our group.23
Table 1.
Reproducibility of non-contrast and contrast-enhanced 3DE.
| Non-contrast | Contrast-enhanced | P value | |
|---|---|---|---|
| Intra-observer variation | |||
| End-diastolic volume (%) | -1.3±13.0 | -2.7±13.3 | 0.56 |
| End-systolic volume (%) | 1.8±16.2 | 1.7±17.2 | 0.84 |
| Ejection fraction (%) | - 1.8±7.1 | -3.2±15.8 | 0.67 |
| Inter-observer variation | |||
| End-diastolic volume (%) | -4.4±10.1 | -8.9±9.3 | 0.2 |
| End-systolic volume (%) | -9.1±14.7 | -8.7±15.0 | 0.86 |
| Ejection fraction (%) | 3.3±5.0 | 0.4±8.4 | 0.11 |
Discussion
The principal finding of the present study is that the use of ultrasound contrast enhancement results in a sizable and significant increase in both end-diastolic and end-systolic 3DE LV volumes, compared with non-contrast measurements. As the relative increases in end-diastolic and end-systolic volume were equal, ejection fraction measurements were not affected. Contrast enhancement did not significantly improve measurement reproducibility. To the best of our knowledge, the present study is the first ultrasound contrast 3DE study on LV volume measurement in patients.
The concept of the endocardial wall as a smooth surface, as is common to the echocardiographic mind, is challenged by the results from this study. Although it has been known since the earliest anatomic studies that the endocardium consists of sponge-like trabeculae with blood flowing in between them, the consequence of this anatomical fact appears to be largely underappreciated in quantitative 3DE analysis. As indicated by the striking increase in both LV end-systolic and end-diastolic volume measurements following contrast enhancement, the intertrabecular space may actually comprise a large part of the true LV cavity volume – a volume that traditionally remains undetected, as the LV trabeculae would be indistinguishable from the LV wall if contrast were not used. Although studies comparing ultrasound contrast 3DE and MRI have yet to be performed, Hundley et al. have reported a significantly better correlation of 2DE volume measurements with MRI measurements following contrast enhancement.19
Several earlier studies investigated the influence of contrast enhancement on LV endocardial wall visibility and volume measurements using both 2DE and 3DE, as described schematically in table 2.11-22,25 Among these, the results of two 2D studies using the ultrasound contrast agent Levovist are in line with the present study (a significant increase in LV volumes), effects that were ascribed to ultrasound contrast filling up intertrabecular space.17,19 A similar study with EchoGen showed a small but significant decrease in LV end-diastolic volume, and no change in LV endsystolic volume and ejection fraction measurements.23 Conceivably, the difference in LV end-systolic volume measurements between the studies may represent relatively more destruction of ultrasound contrast agent in the intertrabecular zone in the EchoGen study.
Table 2.
Previous studies on volume measurements by ultrasound contrast echocardiography.
| Author | Year | Contrastagent | N | Endocardialvisibility | EDV | ESV | EF | Reproducibility | Correlation withreference method |
|---|---|---|---|---|---|---|---|---|---|
| Hoffmann | 200522 | SonoVue | 120 | + | + | + | + | + (MRI and LV angiography) | |
| Ota | 200118 | Levovist | 12 | + | |||||
| Hirooka | 200119 | Levovist | 42 | + | + | = | + | + (LV angiography) | |
| Daniel | 200125 | Optison | 50 | + | |||||
| De Castro | 200011 | Levovist | 15 | + | |||||
| Lafitte | 200017 | Levovist | 25 | + | + | + | + | + (LV angiography) | |
| Kasprzak | 199916 | Levovist | 42 | + | |||||
| Hundley | 199820 | Echogen | 40 | – | = | = | = | + (MRI) | |
| Cohen | 199815 | Optison | 203 | + | |||||
| Grayburn | 199814 | Echogen | 254 | + | |||||
| Lindner | 199713 | Albunex | 42 | + | |||||
| Zotz | 199621 | Albunex | + | + | + | = (LV angiography) | |||
| Crouse | 199312 | Albunex | 175 | + |
+ indicates improvement/increase, = indicates no change, - indicates decrease. N=number of patients, EDV=end-diastolic volume, ESV=end-systolic volume, EF=ejection fraction.
The present study demonstrates an increase in LV volumes after administration of an ultrasound contrast agent. As the administered volume load during the contrast-enhanced studies was quite small (20 to 25 ml), this is highly unlikely to have caused the reported increase in LV volumes as obtained during contrastenhanced imaging. Ejection fraction, however, remained unchanged, as the relative increase in enddiastolic and end-systolic volumes was equal. The effect of ultrasound contrast filling up intertrabecular space seems to be equal both in end-diastole and in endsystole. It appears that in end-systole, intertrabecular space may not be completely obliterated, as is supported by both contrast echocardiography and MRI studies.19,26 In one contrast echocardiography study, LV end-diastolic volume showed a larger increase than LV end-systolic volume, with a resulting increase in ejection fraction.17 In this study, however, the amount of ultrasound contrast material that was injected was very small, which might have aggravated the effects of ultrasound contrast destruction.
In previous studies, patients were either selected for suboptimal acoustic windows, or consecutive patients were included. Consistently, ultrasound contrast infusion led to improved endocardial visibility11-16,18,21,25 and (when assessed) to improved volume measurement reproducibility.17,19,21,22 In our patient group acoustic windows were good even without ultrasound contrast enhancement, but with the 3DE method we used, image reconstruction inherently causes a slight deterioration in image quality. We therefore sought to improve image quality by the use of contrast infusion, but we were not able to demonstrate significant improvement in LV volume measurement reproducibility. Conceivably, ‘blurring’ after reconstruction may affect non-contrast and contrast-enhanced acquisitions equally. The advent of real-time 3DE may obviate reconstruction and its deteriorating effects on image quality, but whether ultrasound contrast infusion may indeed increase volume measurement reproducibility remains to be determined.27
Another reason for the failure of ultrasound contrast to improve reproducibility in our study may be related to a decrease in valve visibility during ultrasound contrast infusion. As noted by Kasprzak et al., visibility tends to be better at the LV apex than at the base due to attenuation.16 We observed significant basal attenuation in two patients and subsequently excluded them from further analysis, because we felt that technically satisfying LV opacification had not been accomplished. In most other patients, however, valve visibility, especially of the aortic valve, was decreased by ultrasound contrast. We did, however, feel that the endocardial contour was easier to trace with contrast enhancement, which is in accordance with virtually all studies on endocardial visibility using ultrasound contrast enhancement.11-15,18,25 Conceivably, the advent of real-time 3D echocardiography, in which blurring inherent to reconstruction algorithms is circumvented, holds new promises for increased measurement reproducibility by contrast enhancement.
Conclusion
In 3DE, the use of the ultrasound contrast agent Optison for LV cavity opacification significantly increases both end-diastolic and end-systolic LV volumes, but has no significant influence on the determination of the ejection fraction. In the presence of an adequate apical acoustic window, contrast enhancement does not further improve measurement reproducibility. Conceivably, measurement reproducibility may benefit from the advent of real-time 3D echocardiography.
References
- 1.Starling MR, Crawford MH, Sorensen SG, Levi B, Richards KL, O’Rourke RA. Comparative accuracy of apical biplane crosssectional echocardiography and gated equilibrium radionuclide angiography for estimating left ventricular size and performance. Circulation 1981;63:1075-84. [DOI] [PubMed] [Google Scholar]
- 2.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000;21:1387-96. [DOI] [PubMed] [Google Scholar]
- 3.Pattynama PM, De Roos A, Van der Wall EE, Van Voorthuisen AE. Evaluation of cardiac function with magnetic resonance imaging. Am Heart J 1994;128:595-607. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Fuisz AR, Fan PH, Hsu TL, Liu CP, Chiang HT. Realtime 3-dimensional echocardiographic evaluation of left ventricular volume: correlation with magnetic resonance imaging–a validation study. J Am Soc Echocardiogr 2001;14:1001-9. [DOI] [PubMed] [Google Scholar]
- 5.Kim WY, Sogaard P, Kristensen BO, Egeblad H. Measurement of left ventricular volumes by 3-dimensional echocardiography with tissue harmonic imaging: a comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2001;14:169-79. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt MA, Ohazama CJ, Agyeman KO, Freidlin RZ, Jones M, Laurienzo JM, et al. Real-time three-dimensional echocardiography for measurement of left ventricular volumes. Am J Cardiol 1999;84:1434-9. [DOI] [PubMed] [Google Scholar]
- 7.Shiota T, McCarthy PM, White RD, Qin JX, Greenberg NL, Flamm SD, et al. Initial clinical experience of real-time threedimensional echocardiography in patients with ischemic and idiopathic dilated cardiomyopathy. Am J Cardiol 1999;84:1068-73. [DOI] [PubMed] [Google Scholar]
- 8.Nosir YF, Stoker J, Kasprzak JD, Lequin MH, Dall’Agata A, Ten Cate FJ, et al. Paraplane analysis from precordial three-dimensional echocardiographic data sets for rapid and accurate quantification of left ventricular volume and function: a comparison with magnetic resonance imaging. Am.Heart J 1999;137:134-43. [DOI] [PubMed] [Google Scholar]
- 9.Buck T, Hunold P, Wentz KU, Tkalec W, Nesser HJ, Erbel R. Tomographic three-dimensional echocardiographic determination of chamber size and systolic function in patients with left ventricular aneurysm: comparison to magnetic resonance imaging, cineventriculography, and two-dimensional echocardiography. Circulation 1997;96:4286-97. [DOI] [PubMed] [Google Scholar]
- 10.Gopal AS, Schnellbaecher MJ, Shen Z, Boxt LM, Katz J, King DL. Freehand three-dimensional echocardiography for determination of left ventricular volume and mass in patients with abnormal ventricles: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 1997;10:853-61. [DOI] [PubMed] [Google Scholar]
- 11.De Castro S, Agati L, Cartoni D, Papetti F, Beni S, Adorisio R, et al. Harmonic imaging with Levovist for transthoracic echocardiographic reconstruction of left ventricle in patients with postischemic left ventricular dysfunction and suboptimal acoustic windows. J Am Soc Echocardiogr 2000;13:139-45. [DOI] [PubMed] [Google Scholar]
- 12.Crouse LJ, Cheirif J, Hanly DE, Kisslo JA, Labovitz AJ, Raichlen JS, et al. Opacification and border delineation improvement in patients with suboptimal endocardial border definition in routine echocardiography: results of the Phase III Albunex Multicenter Trial. J Am Coll Cardiol 1993;22:1494-500. [DOI] [PubMed] [Google Scholar]
- 13.Lindner JR, Dent JM, Moos SP, Jayaweera AR, Kaul S. Enhancement of left ventricular cavity opacification by harmonic imaging after venous injection of Albunex. Am J Cardiol 1997;79:1657-62. [DOI] [PubMed] [Google Scholar]
- 14.Grayburn PA, Weiss JL, Hack TC, Klodas E, Raichlen JS, Vannan MA, et al. Phase III multicenter trial comparing the efficacy of 2% dodecafluoropentane emulsion (EchoGen) and sonicated 5% human albumin (Albunex) as ultrasound contrast agents in patients with suboptimal echocardiograms. J Am Coll Cardiol 1998;32:230-6. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JL, Cheirif J, Segar DS, Gillam LD, Gottdiener JS, Hausnerova E, et al. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent. Results of a phase III Multicenter Trial. J Am Coll Cardiol 1998;32:746-52. [DOI] [PubMed] [Google Scholar]
- 16.Kasprzak JD, Paelinck B, Ten Cate FJ, Vletter WB, de Jong N, Poldermans D, et al. Comparison of native and contrast-enhanced harmonic echocardiography for visualization of left ventricular endocardial border. Am J Cardiol 1999;83:211-7. [DOI] [PubMed] [Google Scholar]
- 17.Lafitte S, Dos SP, Kerouani A, Robhan T, Roudaut R. Improved reliability for echocardiographic measurement of left ventricular volume using harmonic power imaging mode combined with contrast agent. Am J Cardiol 2000;85:1234-8. [DOI] [PubMed] [Google Scholar]
- 18.Ota T, Kisslo J, von Ramm OT, Yoshikawa J. Real-time, volumetric echocardiography: usefulness of volumetric scanning for the assessment of cardiac volume and function. J Cardiol 2001;37(Suppl 1):93-101. [PubMed] [Google Scholar]
- 19.Hirooka K, Yasumura Y, Tsujita Y, Hanatani A, Nakatani S, Miyatake K, et al. An enhanced method for left ventricular volume and ejection fraction by triggered harmonic contrast echocardiography. Int J Card Imaging 2001;17:253-61. [DOI] [PubMed] [Google Scholar]
- 20.Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Grayburn PA. Administration of an intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: comparison with cine magnetic resonance imaging. J Am Coll Cardiol 1998;32:1426-32. [DOI] [PubMed] [Google Scholar]
- 21.Zotz RJ, Genth S, Waaler A, Erbel R, Meyer J. Left ventricular volume determination using Albunex. J Am Soc Echocardiogr 1996;9:1-8. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann R, von Bardeleben S, Ten Cate F, et al. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J 2005;26:607-16. [DOI] [PubMed] [Google Scholar]
- 23.Mannaerts HF, Van Der Heide JA, Kamp O, Papavassiliu T, Marcus JT, Beek A, et al. Quantification of left ventricular volumes and ejection fraction using freehand transthoracic three-dimensional echocardiography: Comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2003;16:101-9. [DOI] [PubMed] [Google Scholar]
- 24.Miller JJ, Tiemann K, Podell S, Doerr Stevens JK, Kuvelas T, Greener Y, et al. In vitro, animal, and human characterization of OPTISON infusions for myocardial contrast echocardiography. J Am Soc Echocardiogr 1999;12:1027-34. [DOI] [PubMed] [Google Scholar]
- 25.Daniel GK, Chawla MK, Sawada SG, Gradus-Pizlo I, Feigenbaum H, Segar DS. Echocardiographic imaging of technically difficult patients in the intensive care unit: use of optison in combination with fundamental and harmonic imaging. J Am Soc Echocardiogr 2001;14:917-20. [DOI] [PubMed] [Google Scholar]
- 26.Papavassiliu T, Kuhl HP, Schroder M, Suselbeck T, Bondarenko O, Bohm CK, et al. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology 2005;236:57-64. [DOI] [PubMed] [Google Scholar]
- 27.Kuhl HP, Schreckenberg M, Rulands D, Katoh M, Schafer W, Schummers G, et al. High-resolution transthoracic real-time threedimensional echocardiography. Quantitation of cardiac volumes and function using semi-automated border detection and comparison with cardiac magnetic resonance. J Am Coll Cardiol 2004;43:2083-90. [DOI] [PubMed] [Google Scholar]


