Abstract
Background
A growing number of patients with end-stage heart failure undergo implantation of ventricular assist devices as a bridge to heart transplantation.
Objectives
In this study we investigated whether functional and haemodynamic recovery after implantation is sufficient to warrant the use of them as long-term alternative to heart transplantation.
Methods
We compared peak VO2 of a group of patients three months after implantation of a ventricular assist device and three months after heart transplantation. Furthermore, we analysed the degree of haemodynamic recovery, by comparing plasma levels of BNP and creatinine before and after implantation of the device.
Results
After implantation of a ventricular assist device, exercise capacity improved considerably; three months after implantation peak VO2 was 20.0±4.9 ml/kg/min (52% of predicted for age and gender). After heart transplantation exercise capacity improved even further; 24.0±3.9 ml/ kg/min (62% of predicted for age and gender) (p<0.001). In the three months after implantation, BNP plasma levels decreased from 570±307 pmol/l to 31±25 pmol/l and creatinine levels decreased from 191±82 μmol/l to 82±25 μmol/l, indicating significant unloading of the ventricles and haemodynamic recovery.
Conclusion
With regard to functional and haemodynamic recovery, the effect of implantation of a ventricular assist device is sufficient to justify its use as an alternative to heart transplantation. (Neth Heart J 2008;16:41-6.)
Keywords: ventricular assist device, heart transplantation, heart failure
Heart failure is currently one of the fastest growing problems in cardiovascular medicine. Although drug therapy for patients with chronic heart failure has improved over the years, mortality still remains high. The yearly mortality in patients with severe heart failure in the COPERNICUS trial was 11%, despite the use of ACE inhibitors as well as β-blockers.1
In patients with end-stage heart failure, heart transplantation (HTx) is the only therapy which improves functional capacity, quality of life and provides a better life expectancy.2,3 However, because of the limited numbers of donor organs available, resulting in a very long waiting time with potential haemodynamic deterioration, a growing number of patients are undergoing implantation of mechanical assist devices to bridge this period to transplantation.
Since 1993 we have been using implantable ventricular assist devices in Utrecht as a bridge to heart transplantation. Several types of ventricular assist devices (VADs) are now available, of which the HeartMate and the Thoratec (Thoratec Corporation, Pleasanton, California) and the Novacor (World Heart Corporation, Ottawa, Canada) are predominantly used as a bridge to transplantation in our centre. These are pulsatile devices with a maximum pump flow of 8 to 10 l/min depending on left ventricular filling. The HeartMate VE/XVE and Novacor are fully implantable, electrically driven pumps, whereas a previous HeartMate and the Thoratec PVAD and IVAD are pneumatically driven. Figure 1 shows a schematic example of the HeartMate XVE. With these assist devices, patients can be mobilised early and even discharged from hospital.4
Figure 1.
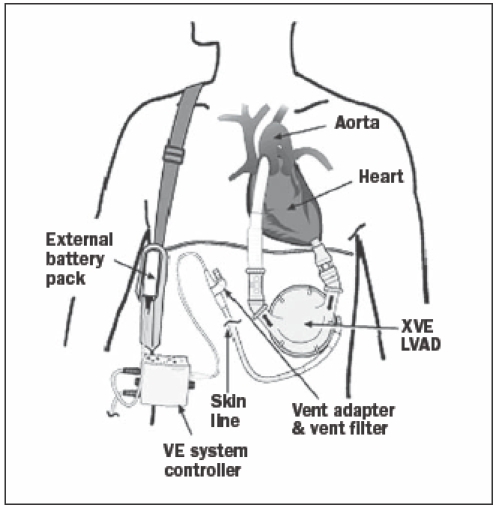
The HeartMate XVE left ventricular assist device (LVAD). The inflow cannula is implanted in the left ventricular apex, the outflow graft is connected to the ascending aorta
Until now, 84 patients with severely progressive haemodynamic deterioration refractory to combinations of intravenous inotropic drugs and often intra-aortic balloon pump (IABP) have received a ventricular assist device as a bridge to transplantation. Despite the severely compromised initial haemodynamic situation of these patients (cardiogenic shock), 76% survived to transplantation, with a one-year survival rate after transplantation of 78%. The duration of VAD support until transplantation was 206±129 days, with a maximum of 557 days.5
Worldwide, the experience with longer support times of patients on a VAD is growing, partly because of the long waiting time for heart transplantation. Today, a support time of more than one year is no longer an exception. This growing experience, in combination with the decreasing number of donor hearts, supports the use of these devices as an alternative to heart transplantation. In the REMATCH trial it was shown that in patients with end-stage heart failure who required HTx but did not qualify to receive a transplant, treatment with a left ventricular assist device showed a significantly better one-year survival than optimal medical therapy.6
Mechanical circulatory support, however, can only be a real alternative to HTx if it leads to normalisation of the decompensated state and enables the patient to perform exercise to a certain level, to allow a reasonably normal life. The latter can be deduced from exercise tests, measuring peak oxygen consumption; the former can be supported by the clinical condition of the patient and by the determination of serological markers of congestive heart failure, such as B-type natriuretic peptide (BNP). This cardiac neurohormone is synthesised in the cardiac ventricles, in case of volume overload and increased wall stress. Consequently it reflects the decompensated state of the ventricles.7-9 Plasma levels of BNP correlate with functional class according to the NYHA and can be used to differentiate cardiac from pulmonary dyspnoea.10,11 Plasma levels after VAD implantation are thought to give an indication of the extent of unloading of the ventricles. The aim of this study was to investigate whether functional and haemodynamic recovery after implantation of a ventricular assist device in patients with end-stage heart failure is sufficient to warrant the use of them as an alternative to transplantation.
Patients and methods
Patients
Of the 84 patients who received a ventricular assist device as bridge to transplantation, ten patients (12%) died in the first 30 days after implantation, due to multi-organ failure or right ventricular failure. There were six late deaths (7%), due to septic shock, cerebral embolus and in one case mechanical failure of the device, 164 days after implantation. In one patient with a peripartum cardiomyopathy, the LVAD could be removed after a support duration of 275 days, because of functional recovery of the native heart. Currently, five patients are still on a device. At the time of implantation of the VAD, all patients were critically ill, facing imminent death. The mean ejection fraction at the time of implantation was 16±5%, cardiac output 3.4±0.9 l/min and mean arterial pressure 62±9 mmHg, despite high doses of several inotropes and intra-aortic balloon counterpulsation. In 63 cases, a HeartMate LVAD (IP, VE or XVE) was used. A Thoratec BiVAD and a Thoratec LVAD were both used in eight cases. In five cases, a Novacor LVAD was used.
Exercise testing
In this study, 44 of the 84 patients who underwent VAD implantation performed an exercise test three months after implantation of the device. The reasons why 40 patients did not undergo an exercise test after implantation are summarised in table 1. Of the 44 patients, 29 performed a second exercise test three months after HTx. Fifteen patients were not able to undergo a second test, because they died during or shortly after heart transplantation (n=9), because of postoperative complications or because they are currently still on a VAD. In table 2, characteristics are shown of the patients who performed both exercise tests.
Table 1.
Reasons why an exercise test was not performed in all patients, three months after VAD implantation.
| Number of patients | |
|---|---|
| Patient died before testing | 13 |
| HTx before testing | 4 |
| CVA or TIA | 4 |
| Right ventricular failure | 1 |
| Critical illness polyneuropathy | 6 |
| Referral to another hospital | 3 |
| Prolonged recovery | 2 |
| Still too early | 4 |
| Unknown | 3 |
| Total | 40 (38%) |
HTx=heart transplantation, CVA=cerebrovascular accident, TIA=transient ischaemic attack.
Table 2.
Characteristics of patients with a ventricular assist device who performed an exercise test three months after VAD implantation as well as three months after HTx (n=29).
| DCM | 17 |
| IHD | 12 |
| LVEF | 16±5 |
| CO (l/min) | 3.4±0.9 |
| MAP (mmHg) | 62±9 |
| PVR (d.s.cm-5) | 192±77 |
| Assist time until HTx (days) | 232±111(range 71-557) |
CO=cardiac output, DCM=dilated cardiomyopathy, IHD=ischaemic heart disease, LVEF=left ventricular ejection fraction, MAP=mean arterial pressure, PVR=pulmonary vascular resistance, HTx=heart transplantation.
For exercise testing, a staged modified Naughton or modified Bruce treadmill protocol was used. Respiratory gas was analysed continuously (Oxycon, Jaeger, the Netherlands). Measurements included heart rate, blood pressure, oxygen consumption (VO2), carbon dioxide production (VCO2), minute ventilation (VE) and respiratory exchange ratio (RQ=VCO2/VO2).
Peak VO2 was defined as the average VO2 during the last minute of exercise and is expressed as ml/kg/min and as ml/min. The percentage of the predicted values was calculated according to Jones, to correct for age and gender.12 The anaerobic threshold (AT) was identified as the oxygen uptake before the systematic increase in the ventilatory equivalent for oxygen (VE/VO2), without an increase in the ventilatory equivalent for carbon dioxide (VE/VCO2), together with the V slope method. The ventilatory response to exercise was defined as VE/VCO2 (EqCO2) at peak exercise. All tests were performed in the automatic mode of the assist device, where VAD output follows an increase in venous return.
BNP determination and renal function
Blood samples for BNP determination were collected just before VAD implantation, and one week, one month and three months after VAD implantation. All samples were centrifuged within 30 minutes and the plasma was frozen until analysis. Analysis was performed on an ADVIA Centaur immunochemistry system (Bayer Diagnostics). Normal plasma levels in our laboratory are <30 pmol/l. Renal function was measured by serum creatinine levels, at the same time periods as BNP.
Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was performed using SSPS 10 for Windows software, with two-tailed paired and unpaired Student’s t tests. A p value <0.05 was considered statistically significant.
Results
Exercise testing
In the group of 29 patients who underwent exercise testing three months after VAD implantation, as well as three months after HTx, the subjective functional class was good: none of the patients experienced limitation of exercise capacity in daily life. To rule out selection of patients between the first and the second exercise test, an independent T test was done between the group of patients who only performed the first test and the group who performed both. Table 3 shows that exercise performance in both groups was similar (p=ns). Besides, there appeared to be no difference in exercise performance between patients with the pneumatic HeartMate LVAD and the electric VE/ XVE HeartMate LVAD, as well as between patients with the HeartMate LVAD and the Thoratec VAD (data not shown).
Table 3.
Comparison of exercise performance three months after VAD implantation, between patients who only performed this exercise test (Group 1; n=15) and the group of patients who performed the test three months after HTx as well (Group 2; n=29).
| Group 1 | Group 2 | P value | |
|---|---|---|---|
| Peak VO2 (ml/kg/min) | 20.1±4.5 | 20.0±4.9 | 0.99 |
| % Predicted | 55±9 | 52±12 | 0.30 |
| Weight (kg) | 74±8 | 73±12 | 0.79 |
| Peak VO2 (ml/min) | 1482±343 | 1454±343 | 0.80 |
| % Predicted | 55±12 | 50±12 | 0.20 |
| Anaerobic threshold (ml/kg/min) | 16.0±2.8 | 13.8±3.0 | 0.03 |
| VE/VCO2 (=EqCO2) | 35.8±4.4 | 35.9±6.4 | 0.94 |
| VCO2/VO2 (=RQ) | 1.21±0.11 | 1.23±0.12 | 0.53 |
VCO2= carbon dioxide production, VE=minute ventilation, VO2=oxygen consumption.
Despite the severely compromised haemodynamic situation at the time of VAD implantation, just three months later all patients demonstrated a peak VO2 fully compatible with activities of normal daily life, as can be seen in table 4 (peak VO2: 20.0±4.9 ml/kg/min). After heart transplantation a further increase was observed (peak VO2: 24.0±3.9 ml/kg/min). Expressed as percentage of the expected peak VO2 for age and gender the values increased from 52±12% while on the VAD to 62±11% after heart transplantation (p<0.001). The anaerobic threshold also improved after heart transplantation (15.7±2.5 ml/kg/min after transplantation vs. 13.8±3.0 ml/kg/min while on the VAD; p=0.009). In contrast EqCO2 in both groups was similar.
Table 4.
Comparison of exercise performance, three months after VAD implantation and three months after heart transplantation, in the same patients.
| 3 months after VAD implant | 3 months after HTx | P value | |
|---|---|---|---|
| Peak VO2 (ml/kg/min) | 20.0±4.9 | 24.0±3.9 | <0.001 |
| % Predicted | 52±12 | 62±11 | <0.001 |
| Weight (kg) | 72±11 | 75±13 | 0.03 |
| Peak VO2 (ml/min) | 1455±343 | 1819±354 | <0.001 |
| % Predicted | 50±12 | 62±11 | <0.001 |
| Anaerobic threshold (ml/kg/min) | 13.8±3.0 | 15.7±2.5 | 0.01 |
| VE/VCO2 (=EqCO2) | 35.9±6.4 | 34.7±4.9 | 0.33 |
| VCO2/VO2 (=RQ) | 1.23±0.12 | 1.17±0.10 | 0.07 |
VCO2=carbon dioxide production, VE=minute ventilation, VO2=oxygen consumption.
BNP determination
Before implantation of the VAD, BNP plasma levels were severely elevated in all patients (570±307 pmol/l), representing the seriously compromised haemodynamic situation at that time. However, just one week after implantation the values had decreased significantly (77±39 pmol/l), demonstrating nearly complete normalisation three months after implantation (31±25 pmol/l) (figure 2).
Figure 2.
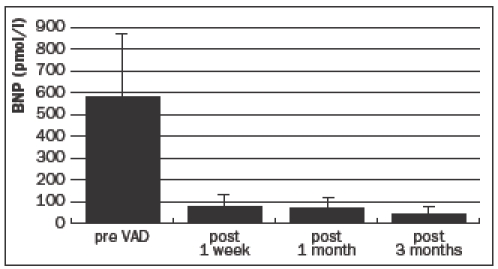
Mean plasma BNP levels, before and after ventricular assist device implantation.
Renal function
Renal function improved considerably after VAD implantation: in three months, creatinine levels decreased from 191±82 μmol/l to 82±25 μmol/l (figure 3).
Figure 3.
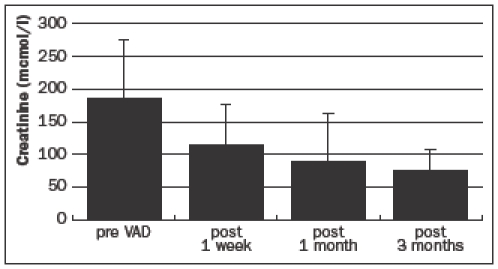
Mean serum creatinine levels, before and after ventricular assist device implantation.
Discussion
In this group of patients with refractory end-stage heart failure we demonstrated an impressive improvement in exercise capacity three months after VAD implantation. At that time peak VO2 was 20 ml/kg/min, or more than 50% of predicted for their age and gender. This means that, according to the Weber classification, they were at that time already in Class A, demonstrating little or no impairment in aerobic capacity (table 5).
Table 5.
Weber classification of functional limitation in heart failure.
| Class | Peak VO2(ml/min/kg) | Severity of functional impairment |
|---|---|---|
| A | >20 | Little or no impairment |
| B | 16-20 | Mild to moderate impairment |
| C | 10-16 | Moderate to severe impairment |
| D | <10 | Severe limitation |
VO2=oxygen consumption.
Three months after heart transplantation exercise capacity in the same patients improved even further, reaching values of 24 ml/kg/min, or more than 60% of predicted for age and gender. Although these are satisfactory figures and patients after heart transplantation generally do not suffer from functional limitation, exercise capacity does not normalise completely: most patients reach peak VO2 values in the range of 60 to 70% of predicted.13,14
It is remarkable that these patients, while on a VAD, experienced no functional limitations in daily life, while the objective assessment of functional capacity demonstrated that peak VO2 was just above 50% of predicted for their age and gender. Peak VO2 gives an indication of functional limitation, although most activities in daily life are performed at a submaximal level, also depending on endurance and training. It is generally accepted that peak VO2 after HTx does not recover to the same extent as functional capacity and exercise tolerance in daily life activities. Apparently, the lack of high filling pressures and activated compensatory mechanisms such as cytokines and sympathetic activation after HTx may play a crucial role in this discrepancy. Likewise, when maximal cardiac output on an LVAD is (technically) limited to 8 to 10 l/min, peak VO2 can be predicted to be approximately 50% of normal values, but not accompanied by major limitations in daily life activities, since the left ventricle is completely unloaded and compensatory mechanisms are no longer activated, as indicated by the near-normal BNP levels.
Probably a longer period of reconvalescence with intensive aerobic training can improve exercise capacity further in these patients, because it is well known that training can improve exercise capacity, independent of central haemodynamics.15-17
In our study the ventilatory response to exercise (VE/VCO2; EqCO2) was low while the patient was on the VAD and similar to that after heart transplantation. This variable is often increased in heart failure and is considered an important prognostic marker. It is evident that we do not have exercise data shortly before the implantation of the VAD. The low values of the EqCO2 three months after VAD implantation, however, suggest a complete normalisation of the decompensated state of the patients.18,19 This is further supported by the nearly complete normalisation of plasma BNP levels three months after VAD implantation, as well as the improvement of the severely compromised renal function.
The full haemodynamic recovery after implantation of an assist device combined with the almost unimpaired aerobic capacity suggests that these devices, with regard to these parameters, may be used as an alternative to heart transplantation in selected patients. In the United States destination therapy with an implantable left ventricular assist device in selected patients with refractory heart failure is already available. At this moment several shortcomings prevent the widespread use of this form of therapy, however. The devices used in the present study are relatively large and can not be used in patients with a body surface area <1.5 m2, excluding some female patients and adolescents. Also in bigger patients, the intra-abdominal position of the device sometimes results in gastrointestinal symptoms such as early satiety and decreased appetite and can even result in bowel obstruction.20
In the rematch study, one-year survival with an LVAD is about 50%. The two-year survival, however, appeared to be only 29%.6 Mortality in the second year after LVAD implantation in that study was mainly determined by mechanical failure of the device and infectious complications. Smaller, nonpulsatile devices, such as the Jarvik 2000 FlowMaker (Jarvik Heart Inc., New York, New York) and the HeartMate II (Thoratec Corporation, Pleasanton, California) were recently introduced.These devices show favourable results with regard to survival and quality of life, but long-term results are not yet available and thromboembolic complications and percutaneous lead infection remain a potential problem.21
Conclusion
Exercise performance in patients with severe end-stage heart failure, treated with a ventricular assist device, is compatible with activities of normal daily life. The normalisation of the ventilatory response to exercise after VAD implantation suggests full haemodynamic recovery. This is further supported by a rapid normalisation of BNP and creatinine levels. Therefore, regarding functional and haemodynamic recovery, VADs may be used as an alternative to heart transplantation. Due to the increasing shortage of donor hearts and the growing number of heart failure patients, this therapeutic option will have to be considered more and more in the coming years.
References
- 1.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-8. [DOI] [PubMed] [Google Scholar]
- 2.Jaski BE, Lingle RJ, Kim J, Branch KR, Goldsmith R, Johnson MR, et al. Comparison of functional capacity in patients with endstage heart failure following implantation of a left ventricular assist device versus heart transplantation: results of the experience with left ventricular assist device with exercise trial. J Heart Lung Transplant 1999;18:1031-40. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee K. Refractory heart failure - drugs and devices. Eur Heart J 2001;22:2227-30. [DOI] [PubMed] [Google Scholar]
- 4.Oosterom A, de Jonge N, Kirkels JH, Rodermans BF, Sukkel E, Klöpping C, et al. End-stage heart failure and mechanical circulatory support: feasibility of discharge from hospital. Neth Heart J 2007;15:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahpor JR, de Jonge N, van Swieten HA, Wesenhagen H, Klöpping C, Geertman JH, et al. Left ventricular assist device as bridge to transplantation in patients with end-stage heart failure. Neth Heart J 2002;10:267-71. [PMC free article] [PubMed] [Google Scholar]
- 6.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J 1998;135:825-32. [DOI] [PubMed] [Google Scholar]
- 8.Kemperman H, Van den Berg M, Kirkels H, De Jonge N. B-type natriuretic peptide (BNP) and N-terminal proBNP in patients with end-stage heart failure supported by a left ventricular assist device. Clin Chem 2004;50:1670-2. [DOI] [PubMed] [Google Scholar]
- 9.Sodian R, Loebe M, Schmitt C, Potapov EV, Siniawski H, Muller J, et al. Decreased plasma concentration of brain natriuretic peptide as a potential indicator of cardiac recovery in patients supported by mechanical circulatory assist systems. J Am Coll Cardiol 2001;38:1942-9. [DOI] [PubMed] [Google Scholar]
- 10.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161-7. [DOI] [PubMed] [Google Scholar]
- 11.Morrison LK, Harrison A, Krishnaswamy P, Kazanegra R, Clopton P, Maisel A. Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol 2002;39:202-9. [DOI] [PubMed] [Google Scholar]
- 12.Jones NL, Campbell EJM, Edwards RHT, Robertson DG. Clinical exercise testing. Philadelphia: WB Saunders, 1975:202. [Google Scholar]
- 13.De Jonge N, Kirkels JH, Lahpor JR, Klöpping C, Hulzebos EJ, de la Rivière AB, et al. Exercise performance in patients with endstage heart failure after implantation of a left ventricular assist device and after heart transplantation; an outlook for permanent assisting? J Am Coll Cardiol 2001;37:1794-9. [DOI] [PubMed] [Google Scholar]
- 14.Kao AC, Van Trigt P, Shaeffer-McCall GS, Shaw JP, Kuzil BB, Page RD, et al. Allograft diastolic dysfunction and chronotropic incompetence limit cardiac output response to exercise two to six years after heart transplantation. J Heart Lung Transplant 1995; 14:11-22. [PubMed] [Google Scholar]
- 15.Minotti JR, Johnson EC, Hudson TL, Zuroske G, Murata G, Fukushima E, et al. Skeletal muscle response to exercise training in congestive heart failure. J Clin Invest 1990;86:751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamopoulos S, Coats AJ, Brunotte F, Arnolda L, Meyer T, Thompson CH, et al. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J Am Coll Cardiol 1993;21:1101-6. [DOI] [PubMed] [Google Scholar]
- 17.Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol 1995;25:1239-49. [DOI] [PubMed] [Google Scholar]
- 18.Robbins M, Francis G, Pashkow FJ, Snader CE, Hoercher K, Young JB, et al. Ventilatory and heart rate responses to exercise: better predictors of heart failure mortality than peak oxygen consumption. Circulation 1999;100:2411-7. [DOI] [PubMed] [Google Scholar]
- 19.Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1997;29:1585-90. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson LW, Shekar P. Ventricular assist devices for durable support. Circulation 2005;112:e111-5. [DOI] [PubMed] [Google Scholar]
- 21.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [DOI] [PubMed] [Google Scholar]


