Abstract
Rice (Oryza sativa) is the most aluminum (Al)-resistant crop species among the small-grain cereals, but the mechanisms responsible for this trait are still unclear. Using two rice cultivars differing in Al resistance, rice sp. japonica ‘Nipponbare’ (an Al-resistant cultivar) and rice sp. indica ‘Zhefu802’ (an Al-sensitive cultivar), it was found that Al content in the root apex (0–10 mm) was significantly lower in Al-resistant ‘Nipponbare’ than in sensitive ‘Zhefu802’, with more of the Al localized to cell walls in ‘Zhefu802’, indicating that an Al exclusion mechanism is operating in ‘Nipponbare’. However, neither organic acid efflux nor changes in rhizosphere pH appear to be responsible for the Al exclusion. Interestingly, cell wall polysaccharides (pectin, hemicellulose 1, and hemicellulose 2) in the root apex were found to be significantly higher in ‘Zhefu802’ than in ‘Nipponbare’ in the absence of Al, and Al exposure increased root apex hemicellulose content more significantly in ‘Zhefu802’. Root tip cell wall pectin methylesterase (PME) activity was constitutively higher in ‘Zhefu802’ than in ‘Nipponbare’, although Al treatment resulted in increased PME activity in both cultivars. Immunolocalization of pectins showed a higher proportion of demethylated pectins in ‘Zhefu802’, indicating a higher proportion of free pectic acid residues in the cell walls of ‘Zhefu802’ root tips. Al adsorption and desorption kinetics of root tip cell walls also indicated that more Al was adsorbed and bound Al was retained more tightly in ‘Zhefu802’, which was consistent with Al content, PME activity, and pectin demethylesterification results. These responses were specific to Al compared with other metals (CdCl2, LaCl3, and CuCl2), and the ability of the cell wall to adsorb these metals was also not related to levels of cell wall pectins. All of these results suggest that cell wall polysaccharides may play an important role in excluding Al specifically from the rice root apex.
It has been estimated that approximately 50% of the world's potentially arable lands are acidic soils, where the rhizotoxic species of aluminum (Al), Al3+, is solubilized into the soil solution to levels that inhibit root tip growth and function (von Uexkull and Mutert, 1995). Although extensive efforts have been directed to uncover the mechanisms of Al-induced root growth inhibition, little is known concerning the primary causes of toxicity. Furthermore, it is still a matter of debate whether Al toxicity results from symplastic or apoplastic lesions (Horst, 1995; Kochian, 1995; Zheng and Yang, 2005). However, some plant species or cultivars within the same species have evolved sophisticated mechanisms to cope with Al toxicity (Kochian, 1995; Matsumoto, 2000; Barcelo and Poschenrieder, 2002; Kochian et al., 2004). Two strategies for plant Al resistance have been suggested (Taylor, 1991; Kochian, 1995). One is based on exclusion of Al from the root symplasm, whereas the other relies on the ability to tolerate symplastic Al. Several mechanisms have been proposed to account for the exclusion of Al, including exudation of Al-chelating ligands, increase in rhizosphere pH, immobilization of Al at the cell wall, selective permeability of the plasma membrane, and Al efflux. On the other hand, chelation of Al and subsequent compartmentation into vacuoles has been suggested for the internal tolerance mechanism. Among the likely exclusion mechanisms, a role for organic acid efflux has been well documented in a number of plant species (for review, see Ma et al., 2001; Ryan et al., 2001; Kochian et al., 2004). However, most of the other proposed mechanisms are still highly speculative.
Wenzl et al. (2001) reported that the high Al resistance in signalgrass (Brachiaria decumbens Stapf) is not associated with these proposed exclusion mechanisms. Piñeros et al. (2005) also showed that, whereas maize (Zea mays) Al resistance was correlated with reduced root tip Al accumulation, the observed Al tolerance differences between six genotypes were not adequately explained by differences in root citrate exudation. Zheng et al. (2005) recently demonstrated in buckwheat (Fagopyrum esculentum) that Al-tolerant and Al-sensitive cultivars had similar patterns of oxalate release. However, evidence was presented showing that higher levels of Al-phosphate complexes might be presented in the apoplast of the Al-tolerant cultivar, suggesting a novel mechanism for Al exclusion from the cytoplasm. In soybean (Glycine max) plants, it was shown that phosphorous (P) efficiency is involved in Al resistance through both direct Al and P interactions and indirect effects of P nutrition on organic acid efflux (Liao et al., 2006). Therefore, it is likely that multiple mechanisms of resistance to Al are functioning in many plant species.
Rice (Oryza sativa) is not only a worldwide staple crop, but also an important monocot model plant. It is the most Al-resistant species among small-grain cereal crops (Foy, 1988). However, little is known about rice Al resistance mechanisms. Ma et al. (2002) reported that root organic acid secretion does not contribute to differential Al resistance, although root tip Al exclusion does occur in rice. Therefore, it is likely that mechanisms other than those already identified are operating in rice. Using two rice cultivars differing in Al tolerance, we identified some interesting correlations between cell wall polysaccharide content and Al exclusion that could be the basis for a novel Al resistance mechanism.
RESULTS
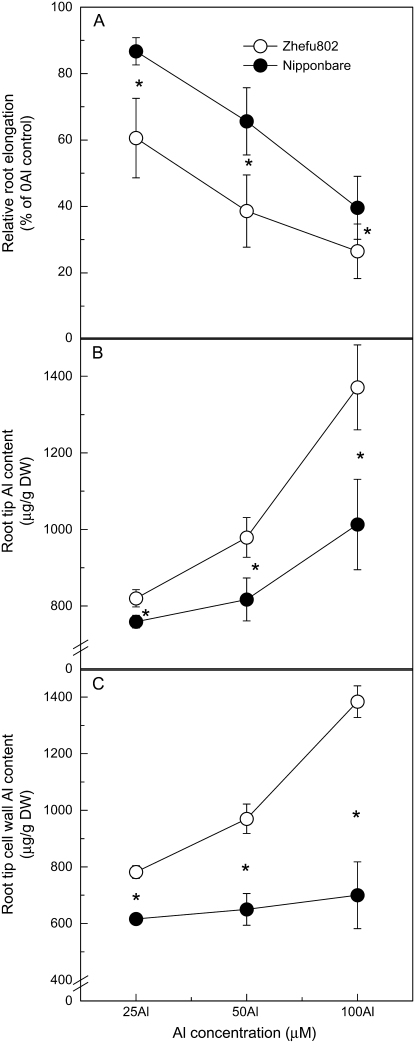
Inhibition of root elongation increased with increasing external Al concentrations in both cultivars; however, Al resistance as determined by relative root elongation (RRE) was always greater in ‘Nipponbare’ compared with ‘Zhefu802’, with 25 μm Al treatment resulting in the greatest difference (Fig. 1A). For example, Al inhibited root elongation by about 40% in ‘Zhefu802’, but only 14% in ‘Nipponbare’ after a 24-h treatment with 25 μm Al (Fig. 1A). Also, Al content of the root apex was significantly higher in ‘Zhefu802’ compared to ‘Nipponbare’ (Fig. 1B). Over the whole range of Al concentrations, root tip Al content was negatively correlated with RRE in both cultivars, indicating that differences in Al resistance correlate with differences in their potential to exclude Al from the root apex. Subsequently, the root apex cell wall fraction was isolated and analyzed for Al content. As seen in Figure 1C, the difference between the two cultivars was greater than that seen for total Al content in the root apex. Almost all the Al (95%–100%) was present in the cell wall fraction in the Al-sensitive ‘Zhefu802’, whereas 60% to 80% of the Al was in the cell wall in Al-resistant ‘Nipponbare’. This result suggests that Al mainly binds to the root cell wall and Al content in the cell wall might significantly relate to differential Al resistance.
Figure 1.
Different Al resistance and Al content in rice ‘Nipponbare’ and ‘Zhufu802’. Three-day-old seedlings were exposed for 24 h to 0.5 mm CaCl2 solution (pH 4.5) containing 0, 25, 50, or 100 μm Al.Al/root elongation without Al × 100 and the root length was measured before and after treatment. Error bars represent ±sd (n = 10). After treatments, root apices were cut for Al extraction or for cell wall extraction. Al in root apices (B) or cell wall fraction (C) was extracted by 2 m HCl for 24 h and determined by ICP-AES. Error bars represent ±sd (n = 3). *, Differences between the cultivars at P < 0.05.
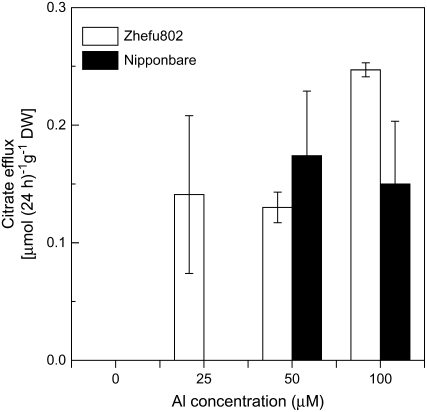
To determine whether the differences in root apex Al exclusion are due to Al-induced secretion of organic acids, we quantified organic acids in the root exudate. Thus, root exudates were collected after exposure to different concentrations of Al for 24 h. No organic acid anions were detected in the root exudates without Al treatment using HPLC. In 25 μm Al, only a small amount of citrate was detected in the root exudates of Al-sensitive ‘Zhefu802’, and no citrate exudation was seen in Al-resistant ‘Nipponbare’ (Fig. 2). Although at higher Al concentrations a small amount of citrate was found in the root exudates of ‘Nipponbare’, there was apparently no correlation between root organic acid anion secretion and differences in Al resistance (Fig. 2).
Figure 2.
Al-induced secretion of citrate in rice ‘Zhefu802’ and ‘Nipponbare’. Six-week-old seedlings were exposed to a 0.5 mm CaCl2 solution (pH 4.5) containing 0 or 25 μm Al. Root exudates were collected after 24-h exposure and citrate was analyzed by HPLC. Error bars represent ±sd (n = 3).
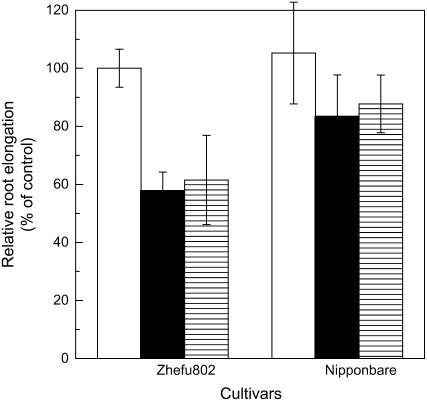
Al-induced increase in the root surface pH has been reported as an alternative Al-resistance mechanism associated with Al exclusion (Degenhardt et al., 1998). We investigated the effects of the pH buffer Homo-PIPES to pH clamp the rhizosphere, while not chelating Al, on Al-induced root growth inhibition. We found that this buffer did not affect root growth in the control treatment, and there was also no significant difference in relative root elongation whether the rhizosphere pH was buffered at pH 4.5 with Homo-PIPES or unbuffered for both cultivars (Fig. 3).
Figure 3.
Effect of Al on root elongation in pH-buffered solution. Three-day-old seedlings were exposed to 0.5 mm CaCl2 solution containing 0 or 25 μm Al with or without 2.5 mm Homo-PIPES buffer (pH 4.5). Root length was measured in both buffered and unbuffered conditions with a ruler before and after treatments (24 h). White bars represent the RRE between buffered and unbuffered control conditions; black bars represent the RRE between control and Al treatment both under unbuffered conditions; hatched bars represent RRE between control and Al treatment both under buffered conditions. Error bars represent ±sd (n = 10).
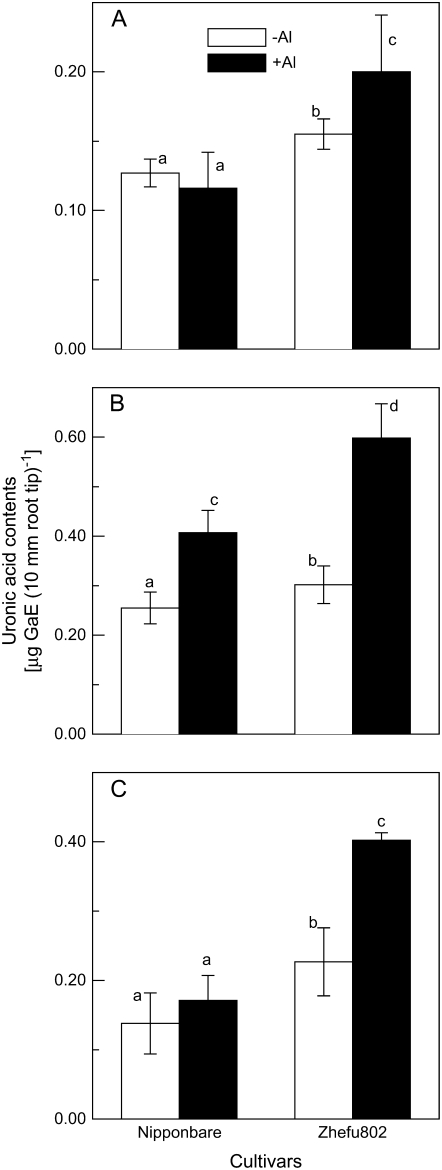
A growing body of evidence indicates that the root apoplast is a key site for Al toxicity and resistance (for review, see Horst 1995; Blamey, 2001; Zheng and Yang, 2005). Hence, we measured the cell wall composition of the root apex and found that cell wall polysaccharides, including pectin (Fig. 4A), hemicellulose 1 (HC1; Fig. 4B), and hemicellulose 2 (HC2; Fig. 4C), were significantly higher in Al-sensitive ‘Zhefu802’. Al treatment resulted in an increase in polysaccharide content, except for pectin in the cell walls of ‘Nipponbare’, and this Al-induced increase was more prominent in ‘Zhefu802’ (Fig. 4).
Figure 4.
Uronic acid content of cell wall fractions extracted from the rice root apex of ‘Zhefu802’ and ‘Nipponbare’. Three-day-old seedlings were exposed to 0.5 mm CaCl2 solution containing 0 or 25 μm Al for 24 h. Root apices were cut and cell wall polysaccharides were fractionated into pectin (A), HC1 (B), and HC2 (C) for uronic acid content measurement. Error bars represent ±sd (n = 3). Bars with different letters are significantly different at P < 0.05.
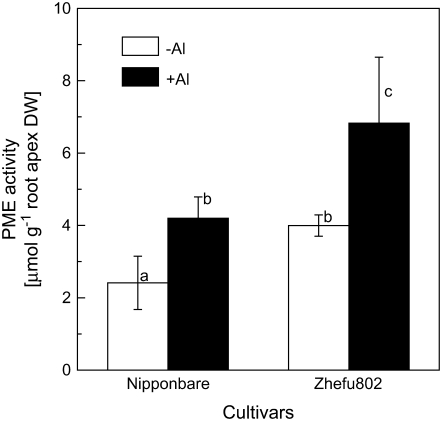
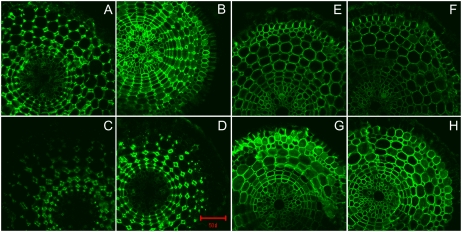
The degree of pectin methylation is an important factor affecting the properties of the cell wall. We employed two methods to determine the degree of pectin methylation. First, we analyzed pectin methylesterase (PME) activity using a sensitive colorimetric assay method based on the amount of methanol released from cell wall pectin extracted from the rice root apex (0–10 mm). As shown in Figure 5, PME activity was significantly higher in Al-sensitive rice ‘Zhefu802’ in the absence of Al, and Al treatment resulted in an increase in PME activity in both cultivars. Second, monoclonal antibodies (JIM5 and JIM7), which are specific for cell wall pectin differing in the degree of methylation, were used for immunofluorescence localization of cell wall pectins. JIM5 stains low-methyl-ester pectins (Willats et al., 2000, 2001), and it was clear that JIM5 fluorescence was brighter in Al-sensitive ‘Zhefu802’ and the spatial distribution was also different in the two cultivars. The quantity of low-methyl-ester pectin was higher in the epidermis and cortex, and quite low in the cell walls of vascular tissues in ‘Zhefu802’, but higher in cortical cells in ‘Nipponbare’ in the absence of Al. Fluorescence intensity was enhanced by Al treatment in both cultivars, but more evident in ‘Zhefu802’, especially in the epidermis and vascular tissues (Fig. 6, A–D). JIM7 is a pectin epitope specifically staining high-methyl-ester pectins (Knox et al., 1990; Willats et al., 2000; Clausen et al., 2003). Contrary to JIM5 staining, JIM7 fluorescence intensity was higher in ‘Nipponbare’, especially in the epidermis and the cortex in the absence of Al; Al treatment decreased the fluorescence intensity, especially in the epidermis, in both cultivars; however, the intensity was still higher in ‘Nipponbare’ (Fig. 6, E–H).
Figure 5.
PME activity in the root apex of rice. Three-day-old seedlings were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 0 or 25 μm Al for 24 h. Root apices were cut for cell wall and PME extraction. PME activity was determined colorimetrically. Data are means ±sd (n = 3). Bars with different letters are significantly different at P < 0.05.
Figure 6.
Immunolocalization of low-methyl-ester pectin (JIM5 epitope; A–D) and high-methyl-ester pectin (JIM7 epitope; E–H) in root cross-sections of two rice cultivars. Three-day-old seedlings of Al-sensitive ‘Zhefu802’ (A, B, E, F) or Al-resistant ‘Nipponbare’ (C, D, G, H) were subjected to 0.5 mm CaCl2 solution with (B, D, F, H) or without (A, C, E, G) 25 μm Al for 24 h. Root sections were taken from 1 to 3 mm behind the apex. Scale bar = 50 μm for all images.
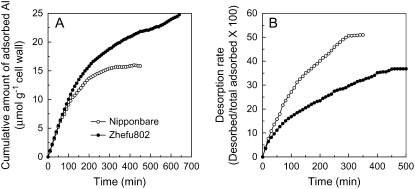
Cell wall pectins are the main Al-binding component of the cell wall because the trivalent Al cation is attracted to the negatively charged carboxyl groups of unmethylated pectins (Horst, 1995; Chang et al., 1999). The difference in cell wall polysaccharide composition (Fig. 4) and demethylesterification (Figs. 5 and 6) should result in the different ability to bind Al. Thus, a time-dependent kinetic study of Al adsorption and desorption was conducted and showed that the cell walls of Al-sensitive ‘Zhefu802’ adsorbed more Al and the adsorbed Al was retained more tightly in ‘Zhefu802’ (lower desorption rate; Fig. 7).
Figure 7.
Adsorption (A) and desorption (B) kinetics of Al in the root cell wall of rice cultivars ‘Zhefu802’ and ‘Nipponbare’. Cell wall materials were extracted as described in “Materials and Methods.” Cell wall materials (10 mg) were placed in a 2-mL column and kinetics were conducted as previously described (Zheng et al., 2004).
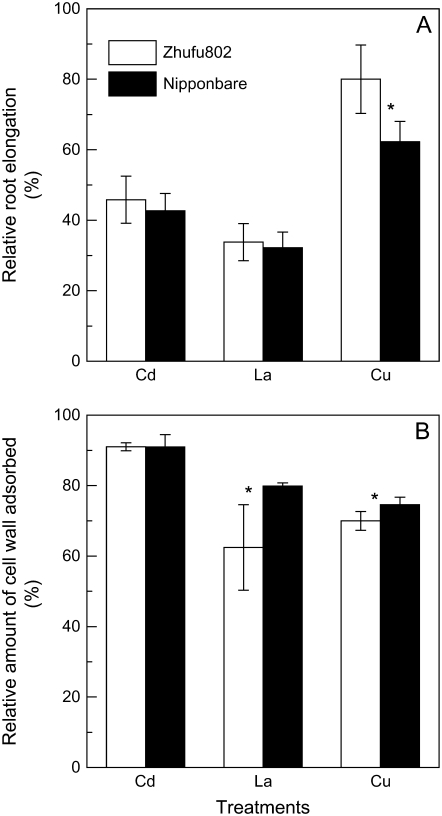
To verify whether the differences in cell wall polysaccharides are also associated with rice resistance to other toxic metals in these two cultivars, we examined the effect of cadmium (Cd), lanthanum (La), and copper (Cu) on root growth and the ability of the cell wall to adsorb these metals. Contrary to the response to Al, there was no genotypic difference in response to 25 μm Cd or 10 μm La treatment, and Al-resistant ‘Nipponbare’ was even more sensitive to Cu treatment (Fig. 8A). Because the adsorption of Cd, La, and Cu to cell wall materials was quickly saturated, it was not possible to conduct a kinetic study of metal absorption/desorption. Instead, we directly measured the metal levels retained in the cell wall after equilibration with solutions containing specific metal concentrations. As seen in Figure 8B, there were no differences in Cd adsorption between the two cultivars, whereas the root tip cell wall of Al-resistant ‘Nipponbare’ adsorbed more La and Cu than did ‘Zhefu802’.
Figure 8.
Effect of other cations on root elongation of rice ‘Zhefu802’ and ‘Nipponbare’, and the adsorption ability of cell wall materials extracted from the root apex of rice ‘Zhefu802’ and ‘Nipponbare’ to metals. A, Three-day-old seedlings were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 0, 25 μm CdCl2, 10 μm LaCl2, or 0.5 μm CuCl2 for 24 h. Root length was measured before and after treatments. Error bars represent ±sd (n = 10). B, Cell wall materials (3 mg) were suspended with 1.5 mL of 2 μm CdCl2, 2 μm LaCl2, or 2 μm CuCl2 for 1 h. Metal content in suspension solution before or after adsorption was determined by ICP-AES. Error bars represent ±sd (n = 3). *, Differences between cultivars at P < 0.05.
DISCUSSION
Al Resistance in Rice Is Achieved by Exclusion of Al from the Root Apex
Whereas the relative importance of symplastic versus apoplastic damage as a basis for Al toxicity remains a matter of debate, Horst (1995) proposed that the apoplast of the root apex is the primary site of Al toxicity, especially during the early stage of Al stress. Although binding of Al in the cell wall has been proposed as a potential exclusion mechanism (Taylor, 1991), it depends on the components that can bind Al. For example, Zheng et al. (2005) reported that immobilization of Al with excessive phosphate in the apoplast is related to Al resistance in buckwheat. Silicon-ameliorated Al toxicity is associated with the formation of hydroxyaluminum silicates in the apoplast of the maize root apex (Wang et al., 2004). However, binding of Al to cell wall pectins is correlated with Al toxicity in maize (Horst et al., 1999). In this study, a significantly higher percentage of root apex Al was found to be bound to the cell wall of Al-sensitive ‘Zhefu802’ (Fig. 1, B and C), suggesting that greater root growth inhibition could result from apoplastic damage induced by Al bound to the wall.
Relative root elongation has proved to be a suitable index to assess Al resistance in plants grown in simple salt (calcium [Ca]) solutions. For example, we successfully screened six wheat (Triticum aestivum) cultivars, four rye (Secale cereale) cultivars, 16 triticale (× Triticosecale ‘Wittmark’) cultivars, 36 triticale breeding lines, and nine buckwheat cultivars based on this index (Yang et al., 2005). Ma et al. (2005) isolated an Al-hypersensitivity mutant from 560 candidate rice lines using RRE as parameter. Here, we found that exposure of rice ‘Nipponbare’ to different concentrations of Al for 24 h resulted in a 14% to 60% reduction of RRE, whereas inhibiting root growth by 40% to 73% in rice ‘Zhefu802’ (Fig. 1A), indicating ‘Nipponbare’ is more Al resistant than ‘Zhefu802’. The degree of Al resistance in rice seems to be associated with physiological parameters that facilitate Al exclusion from the root apex (Fig. 1, B and C).
Al Resistance in Rice Is Not Associated with Known External Detoxification Mechanisms
It is well documented that Al-activated organic acid anion efflux plays a very important role in excluding Al from the root apex (Ma et al., 2001; Ryan et al., 2001; Kochian et al., 2004). However, this mechanism does not appear to be involved in the greater ability of ‘Nipponbare’ to exclude Al from the root apex due to the following reasons. First, at an Al concentration of 25 μm, which results in the greatest genotypic Al resistance differences between the two cultivars, no organic acid anions were detected in the root exudates of ‘Nipponbare’, whereas a trace amount of citrate was detected in the root exudates of ‘Zhefu802’ (Fig. 2). Second, there was no correlation between root citrate efflux and Al resistance between the two cultivars. Third, the amount of secreted citrate was significantly lower than in other species, such as rye (Li et al., 2000), sorghum (Sorghum bicolor; Magalhaes et al., 2007), Cassia tora (Yang et al., 2006b), and rice bean (Vigna umbellate; Yang et al., 2006a), which showed lower or comparable resistance than rice. In a previous study, Ma et al. (2002) also found that citrate efflux is not the major mechanism of resistance to Al in rice.
We also evaluated the hypothesis of Al exclusion from the root apex via a root-mediated increase in rhizosphere pH (Taylor, 1991). The hypothesis was based upon the theory that alkalinization of the root surface would decrease the activity of the rhizotoxic Al3+ species. The only experimental evidence for this mechanism has been presented by Degenhardt et al. (1998), who showed that Al resistance in an Al-resistant Arabidopsis (Arabidopsis thaliana) mutant, alr-104, was achieved by Al-induced net H+ influx of the root apex. In this study, we investigated the effects of pH-buffered or unbuffered conditions on Al-induced root growth inhibition to examine whether Al-induced pH changes are involved in exclusion of Al from the root apex. In the absence of Al, there was no difference in root elongation between buffered and unbuffered conditions in both cultivars, indicating that the concentration of non-Al-chelating buffer, Homo-PIPES, used here is suitable. In the presence of Al, there were no differences in RRE between buffered and unbuffered conditions, and the RRE was higher in ‘Nipponbare’ than in ‘Zhefu802’ (Fig. 3). This result suggests that Al-induced changes in root surface pH are also not involved in the greater ability of the ‘Nipponbare’ root apex to exclude Al.
Is Cell Wall Polysaccharide Composition a Novel Al-Resistance Mechanism?
The plant cell wall is mainly composed of cellulose and matrix polysaccharides, which are divided into two classes: pectins and hemicelluloses (for review, see Cosgrove, 2005). Because the primary structure of cellulose is an unbranched (1,4)-linked β-d-glucan, it theoretically should not bind Al. However, it is likely that certain matrix polysaccharides may play an important role in Al resistance and toxicity (see Horst, 1995). In this study, we found that the ‘Zhefu802’ root apex is more abundant in cell wall polysaccharides (both pectins and hemicelluloses; Fig. 4) and this is consistent with the higher measured Al content in ‘Zhefu802’, especially in the root apex cell wall (Fig. 1, B and C). It is noteworthy that we classified cell wall polysaccharides into pectin and hemicellulose fractions in this study; however, some previous studies have defined all the cell wall polysaccharides as pectin, which will depend on the experimental protocols used to extract pectin. For example, Zhong and Läuchli (1993) extracted pectin form cotton (Gossypium hirsutum) seedlings using ammonium oxalate buffer. However, Horst et al. (1999) and Eticha et al. (2005) extracted pectin from maize roots using concentrated H2SO4, which can release all the polysaccharides into solution.
There is ample experimental evidence that Al strongly binds to cell wall pectins (Chang et al., 1999; Horst et al., 1999; Blamey, 2001). Current evidence indicates that pectins are synthesized and methylesterified in the Golgi and thereafter secreted into the wall in a highly methylesterified state (Micheli, 2001). Schmohl et al. (2000) reported that the Al sensitivity of maize cell suspension cultures was negatively related to the degree of pectin methylesterification and they concluded that the degree of pectin methylesterification and the activity of PME can be important in the expression of Al toxicity and resistance. Using immunofluorescence techniques to localize pectins, Eticha et al. (2005) also found that an Al-sensitive maize cultivar had a higher proportion of low-methylated pectin than did an Al-resistant maize cultivar, which could result in higher Al binding in the Al-sensitive cultivar. However, PME activity was not determined nor did those authors measure the changes in pectin composition after Al treatment, which we think might be very important to explain the different behaviors of the cultivars studied in that paper in response to Al stress. Here, using N-methylbenzothiazolinone-2-hydrazone (MBTH) as a formaldehyde trap, we observed that PME activity was constitutively higher in Al-sensitive ‘Zhefu802’, although Al treatment induced the increase in PME activity in both cultivars (Fig. 5). Using immunofluorescence localization of pectin with two kinds of antibodies to detect both low- and high-methyl-ester pectins, we found that Al-resistant ‘Nipponbare’ had more high-methyl-ester pectin and consequently less low-methyl-ester pectin than Al-sensitive ‘Zhefu802’ (Fig. 6). These results suggest that pectins in the ‘Zhefu802’ root apex have a higher degree of demethylesterification, which might result in a higher capability to bind Al. Blamey et al. (1990) reported that an Al-resistant Lotus cultivar, ‘Maku’, had lower cation exchange capacity and accumulated less Al than the Al-sensitive cultivar ‘Maitland’. To directly link cell wall properties to the wall's Al-binding capacity, we measured the Al adsorption and desorption kinetics of root tip cell walls and found that cell walls of Al-sensitive ‘Zhefu802’ could bind more Al, and the bound Al was retained more tightly than in Al-resistant ‘Nipponbare’ (Fig. 7).
To further clarify whether the differences of cell wall polysaccharides are specifically involved in the exclusion of Al from the root apex, we examined the responses of the two cultivars to other metals. The results presented here indicate that the resistance of ‘Nipponbare’ to Al is specific because the root growth responses of the two cultivars to other toxic metals differed from the response to Al (Figs. 1 and 8A). Furthermore, the ability of cell wall polysaccharides to adsorb these metals was also different from their ability to adsorb Al (Figs. 7 and 8B). These results suggest that lower cell wall polysaccharide content and higher pectin methylesterification in ‘Nipponbare’ is involved in specific Al exclusion from the root apex.
Hypothesis for Al Toxicity
The earliest and most dramatic visual symptom of Al toxicity is inhibition of root elongation. It appears that PME plays an important role in cell wall extension through deesterification of pectin, which favors pectin gelation and stiffening through Ca2+ cross-bridges between free carboxylic groups of adjacent pectin chains (Catoire et al., 1998). It is assumed that cell wall extension is under the precise control of the loosening and stiffening of polysaccharide polymers (Cosgrove, 2005). Al3+ has a higher binding affinity to carboxylic groups than does Ca2+, and Al stress often results in the deficiency of Ca (for review, see Rengel and Zhang, 2003). Binding of Al3+ to pectin carboxylic groups may result in stiffening but not loosening of the cell wall, which consequently could result in distortion of cell wall extension. Under such conditions, theoretically, plant cells will synthesize more pectin and PME will be secreted into cell walls for wall extension; this will result in the accumulation of pectin polysaccharides in the cell wall under Al stress and enhancement of PME activity. In this study, Al-sensitive ‘Zhefu802’ had more pectin (Fig. 4) and higher PME activity (Figs. 5 and 6) in the root tip cell wall, which will set up conditions of greater accumulation of pectin and hemicellulose in the cell wall (Fig. 4) and greater Al binding than in Al-resistant ‘Nipponbare’. This is in accordance with previous reports that Al stress can modify the metabolism of cell wall components and result in the typical thick and rigid cell walls associated with Al toxicity due to the accumulation of polysaccharides (Eleftherios et al., 1993; Tabuchi and Matsumoto, 2001) and lignin (Sasaki et al., 1996). Mao et al. (2004) also identified several Al-regulated genes from rice roots that are involved in cell wall metabolism, and these include genes involved in the synthesis of lignin, hemicellulose, glycoprotein, and other secondary metabolites. Thus, more accumulation but less catabolism of cell wall metabolites should be associated with the extent of Al toxicity.
CONCLUSION
The differential Al resistance observed in the two rice cultivars studied here is associated with Al exclusion from the root apex. We present circumstantial evidence that lower cell wall polysaccharide content with a higher degree of methylesterification results in less carboxylic groups, which can serve as Al-binding sites; this could result in the greater Al exclusion observed in Al-resistant rice ‘Nipponbare’. This could be a novel mechanism of Al resistance in rice, which is one of the most Al-resistant cereal species.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Rice (Oryza sativa) sp. japonica ‘Nipponbare’ and rice sp. indica ‘Zhefu802’ were used in this study. Seeds were surface sterilized for 20 min in a 1% (v/v) sodium hypochlorite solution, washed three times with deionized water, and soaked in deionized water overnight. Then they were transferred to an incubator at 25°C for germination. Germinated seeds were transferred to a net tray floated on a container filled with 5 L of 0.5 mm CaCl2 solution at pH 4.5. The solution was renewed daily. After 3 d of culture, seedlings were subjected to a compartmental hydroponic system for various treatments according to Yang et al. (2005). All experiments were conducted in a growth chamber with a 14-h/26°C d and a 10-h/23°C night regime, a light intensity of 400 μmol m−2 s−1, and a relative humidity of 60%.
Evaluation of Al Resistance in Rice
Al resistance in rice was examined by measuring the root elongation of primary roots of 3-d-old seedlings grown in 0.5 mm CaCl2 solution, pH 4.5, containing 0, 10, 25, or 100 μm AlCl3. Root length was measured with a ruler before and after treatments (24 h). Relative root elongation was defined as the percentage of the root elongated by Al treatment compared to the Al-free control.
Al Content in Root Apices and Root Apical Cell Walls
The content of Al bound to root cell walls was estimated by homogenizing the frozen root apices (10 tips for each sample) with 0.5 mL of ice-cold distilled water in an Eppendorf tube using a plastic grinder according to Ma et al. (2004). The homogenate was centrifuged at 13,000g for 10 min and the precipitate was then washed three times with 10 volumes of 80% ethanol and one with 10 volumes of a methanol:chloroform mixture (1:1 [v/v]), followed by 10 volumes of acetone. After drying, the precipitate was resuspended in 1 mL of 2 m HCl at room temperature for 24 h with occasional shaking. For total Al determination of the root apices, the excised root apices (10 tips/sample) were placed in a 1.5-mL Eppendorf tube containing 1 mL of 2 m HCl. The tubes stood for at least 24 h with occasional shaking. Al concentrations in the extracts were determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES; IRIS/AP optical emission spectrometer).
Measurement of Citrate Efflux
To analyze organic acids secreted from rice roots, root exudates from both ‘Zhefu802’ and ‘Nipponbare’ were collected. Three-day-old seedlings were transplanted into a 1.1-L plastic pot (eight seedlings per pot) filled with 1 L of one-half-strength Kimura B nutrient solution. The nutrient solution contained the macronutrients (mm) (NH4)2SO4 (0.18), MgSO4×7H2O (0.27), KNO3 (0.09), Ca(NO3)2×4H2O (0.18), and KH2PO4 (0.09), and the micronutrients (μm) NaEDTA-Fe×3H2O (30), MnCl2×4H2O (0.5), H3BO3 (3), (NH4)6Mo7O24×4H2O (1), ZnSO4×7H2O (0.4), and CuSO4×5H2O (0.2). The pH of nutrient solution was adjusted to 4.5 with 1 m HCl and renewed every 3 d. After 6 weeks of culture, roots were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 0, 25, 50, or 100 μm AlCl3. Root exudates were collected after a 24-h treatment. Organic acids in root exudates were purified and analyzed according to Zheng et al. (2005).
Sensitivity to Al in pH-Buffered Solution
The experiment was performed in a pH-buffered CaCl2 solution containing 0 or 25 μm Al at pH 4.5. The pH-buffered solution contained 2.5 mm Homo-PIPES. Root length of 10 seedlings each was measured before and after treatments (24 h).
Cell Wall Extraction and Measurement of Polysaccharide Content
Cell wall materials were extracted according to Zhong and Läuchli (1993). Afterward, freeze-dried, cell wall materials were stored at 4°C for further use or immediately used for the extraction of PME. Cell wall materials were fractionated into three fractions: pectin, HC1, and HC2. The pectin fraction was extracted twice by 0.5% ammonium oxalate buffer containing 0.1% NaBH4 (pH 4) in a boiling water bath for 1 h each and pooling the supernatants. Pellets were subsequently subjected to triple extractions with 4% KOH containing 0.1 NaBH4 at root temperature for a total time of 24 h, followed by similar extraction with 24% KOH containing 0.1% NaBH4. The pooled supernatants from 4% and 24% KOH extraction thus yielded the HC1 and HC2 fractions, respectively.
Uronic acid content in each cell wall fraction was assayed according to the method of Blumenkrantz and Asboe-Hansen (1973). GalA was used as a calibration standard; thus, the root pectin content was expressed as GalA equivalents.
Al Adsorption and Desorption Kinetics
To determine whether cell walls are involved in differential Al resistance of the two cultivars, an Al adsorption and desorption kinetics study was used (Zheng et al., 2004).
PME Activity Assay
For extraction of PME, cell wall materials (50 apices for each sample) were suspended in 1 m NaCl solution (pH 6.0) at 4°C for 1 h with repeated vortexing (20 s for 10 min each). Extracts were centrifuged (14,000g, 10 min) and the supernatant was collected. A highly sensitive colorimetric assay method based on the condensation of an aldehyde with a molecule of MBTH under neutral conditions was used to analyze cell wall PME activity according to Anthon and Barrett (2004). Incubation contained 100 μL of 100 mm Tris-HCl (pH 7.5) containing 0.4 mg mL−1 of pectin, 10 μL of alcohol oxidase (AO) at 0.01 units μL−1, 40 μL of MBTH (3 mg/mL, dissolved in water), and 100 μL of sample or 1 m NaCl (as blank). After addition of AO, samples were incubated for 20 min at 30°C and then 200 μL of a solution containing 5 mg mL−1 each of ferric ammonium sulfate and sulfamic acid was added. After 20 min at root temperature, 550 μL of water was added to give a final volume of 1.0 mL and A620 determined.
Immunofluorescence
Three-day-old seedlings were subjected to a compartmental hydroponic system (Yang et al., 2005) with or without 25 μm AlCl3 in treatment solutions. After 24-h treatment, fresh roots were hand sectioned from the root zone 1 to 3 mm behind the apex and directly collected into a fixative solution containing 4% paraformaldehyde in 50 mm PIPES, 5 mm MgSO4, and 5 mm EGTA, pH 6.9. After 1 h of fixation at room temperature, samples were washed repeatedly with phosphate-buffered saline (PBS) and blocked with 0.2% bovine serum albumin in PBS for 30 min. Then the samples were incubated with the monoclonal antibodies JIM5 and JIM7, diluted 1:10 in PBS containing 0.2% bovine serum albumin for 2 h. Subsequently, samples were washed three times in PBS and incubated with goat anti-rat IgG (whole molecule) fluorescein isothiocyanate conjugate, diluted 1:50 in PBS containing 0.2% bovine serum albumin for 2 h at 37°C. Samples were washed briefly with PBS three times and mounted on glass slides and examined under a laser-scanning confocal microscope (LSM 510; Zeiss). Control samples (not treated with the primary antibody but only with secondary antibody) were examined.
Other Metal Treatments
To verify whether the genotypic Al differences between the two cultivars are specific, we examined the responses of the two cultivars to other metals (CdCl2, LaCl3, and CuCl2). Three-day-old seedlings were exposed to a 0.5 mm CaCl2 solution, pH 4.5, containing 25 μm CdCl2, 10 μm LaCl3, or 0.5 μm CuCl2 for 24 h. The 0.5 mm CaCl2 solution was used as a basal treatment. Root length was measured with a ruler before and after treatment. After treatment, root apices were cut and extracted by 2 m HCl for 24 h.
To examine the adsorption ability of cell wall polysaccharides to these metals, cell wall material (3 mg) was suspended in 1.5 mL of 2 μm CdCl2, LaCl3, or CuCl2 solution and was shaken on a rotary shaker for 1 h. After treatment, samples were centrifuged at 23,000g and the supernatant was collected for determination of concentration. The adsorption ability of cell wall material to metals was expressed as a percentage of the amount adsorbed by cell wall materials to the amount in suspension solution before adsorption.
Statistical Analyses
Experiments were arranged in a randomized complete design and data were statistically evaluated by sd and Student's t-test methods.
Acknowledgments
We are grateful to Dr. J. Paul Knox (Centre for Plant Science, University of Leeds, UK) for kindly donating the monoclonal antibodies specific for pectins (JIM5 and JIM7) and Dr. Gordon E. Anthon (Department of Food Science and Technology, University of California, Davis, CA) for his valuable suggestions on the determination of PME activity. We thank the anonymous reviewers for their valuable comments and suggestions.
This work was supported by the Natural Science Foundation of China (grant nos. 30625026, 30570324, 30571113) and the Program for New Century Excellent Talents in University (grant no. NCET–04–0554) from the Education Ministry of China.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Shao Jian Zheng (sjzheng@zju.edu.cn).
References
- Anthon GE, Barrett DM (2004) Comparison of three colorimetric reagents in the determination of methanol with alcohol oxidase. Application to the assay of pectin methylesterase. J Agric Food Chem 52 3749–3753 [DOI] [PubMed] [Google Scholar]
- Barcelo J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48 75–92 [Google Scholar]
- Blamey FPC (2001) The role of the cell wall in aluminum toxicity. In N Ae, J Arihara, K Okada, A Srinivasan, eds, Plant Nutrient Acquisition: New Perspectives. Springer-Verlag, Tokyo, pp 201–226
- Blamey FPC, Edmeades DC, Wheeler DM (1990) Role of root cation-exchange capacity in differential aluminium tolerance of Lotus species. J Plant Nutr 13 729–744 [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54 484–489 [DOI] [PubMed] [Google Scholar]
- Catoire L, Pierron M, Morvan C, du Penhoat CH, Goldberg R (1998) Investigation of the action patterns of pectin methylesterase isoforms through kinetic analyses and NMR spectroscopy. Implications in cell wall expansion. J Biol Chem 273 33150–33156 [DOI] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22 1009–1017 [Google Scholar]
- Clausen MH, Willats WGT, Knox JP (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr Res 338 1797–1800 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6 850–861 [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV (1998) Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol 117 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftherios E, Moustakas M, Fragiskos N (1993) Aluminate-induced changes in morphology and ultrastructure of Thinopyrum roots. J Exp Bot 44 427–436 [Google Scholar]
- Eticha D, Stass A, Horst WJ (2005) Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ 28 1410–1420 [Google Scholar]
- Foy CD (1988) Plant adaptation to acid aluminum-toxic soils. Commun Soil Sci Plant Anal 19 959–987 [Google Scholar]
- Horst WJ (1995) The role of the apoplast in aluminum toxicity and resistance of higher plants: a review. Z Pflanzenernaehr Bodenkd 158 419–428 [Google Scholar]
- Horst WJ, Schmohl N, Kollmeier M, Baluska F, Sivaguru M (1999) Does aluminium affect root growth of maize through interaction with the cell wall-plasma membrane-cytoskeleton continuum? Plant Soil 215 163–174 [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181 512–521 [DOI] [PubMed] [Google Scholar]
- Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46 237–260 [Google Scholar]
- Kochian LV, Hoekenga AO, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55 459–493 [DOI] [PubMed] [Google Scholar]
- Li XF, Ma JF, Matsumoto H (2000) Pattern of aluminum-induced secretion of organic acids differs between rye and wheat. Plant Physiol 123 1537–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV (2006) Phosphorous and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiol 141 674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Nagao S, Huang CF, Nishimura M (2005) Isolation and characterization of a rice mutant hypersensitive to Al. Plant Cell Physiol 46 1054–1061 [DOI] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E (2001) Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6 273–278 [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen R, Nagao S, Tanimoto E (2004) Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol 45 583–589 [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen R, Zhao Z, Wissuwa M, Takeuchi Y, Ebitani T, Yano M (2002) Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant Cell Physiol 43 652–659 [DOI] [PubMed] [Google Scholar]
- Magalhaes JV, Liu J, Guimaraes CT, Lana UGP, Alves VM, Wang YH, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE, et al (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39 1156–1161 [DOI] [PubMed] [Google Scholar]
- Mao C, Yi K, Yang L, Zheng B, Wu Y, Liu F, Wu P (2004) Identification of aluminium-regulated genes by cDNA-AFLP in rice (Oryza sativa L.): aluminium-regulated genes for the metabolism of cell wall components. J Exp Bot 55 137–143 [DOI] [PubMed] [Google Scholar]
- Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200 1–45 [DOI] [PubMed] [Google Scholar]
- Micheli F (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6 414–419 [DOI] [PubMed] [Google Scholar]
- Piñeros MA, Shaff JE, Manslank HS, Alves VMC, Kochian LV (2005) Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiological study. Plant Physiol 137 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z, Zhang WH (2003) Role of dynamics of intracellular calcium in aluminium toxicity syndrome. New Phytol 159 295–314 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52 527–560 [DOI] [PubMed] [Google Scholar]
- Sasaki M, Yamamoto Y, Matsumoto H (1996) Lignin deposition induced by aluminium in wheat (Triticum aestivum) roots. Physiol Plant 96 193–198 [Google Scholar]
- Schmohl N, Pilling J, Fisahn J, Horst WJ (2000) Pectin methylesterase modulates aluminium sensitivity in Zea mays and Solanum tuberosum. Physiol Plant 109 419–427 [Google Scholar]
- Tabuchi A, Matsumoto H (2001) Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminium-induced growth inhibition. Physiol Plant 112 353–358 [DOI] [PubMed] [Google Scholar]
- Taylor GJ (1991) Current views of the aluminum stress response: the physiological basis of tolerance. In DD Randall, DG Blevins, CD Miles, eds, Current Topics in Plant Biochemistry and Physiology, Vo1 10. University of Missouri, Columbia, MO, pp 57–93
- von Uexkull HR, Mutert E (1995) Global extent, development and economic impact of acid soils. In RA Date, NJ Grundon, GE Raymet, ME Probert, eds, Plant-Soil Interactions at Low pH: Principles and Management. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 5–19
- Wang Y, Stass A, Horst WJ (2004) Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol 136 3762–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzl P, Patiño GR, Chaves AL, Mayer JE, Rao IM (2001) The high levels of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiol 122 1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TMIE, Visser J, Voragen A, Mikkelsen JD, Knox JP (2000) Analysis of pectin epitopes recognized by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res 327 309–320 [DOI] [PubMed] [Google Scholar]
- Willats WGT, Orfila C, Limberg G, Buchholt HC, van Alebeek GWN, Voragen AGJ, Marcus SE, Christensen TMIE, Mikkelsen JD, Murray BS, et al (2001) Modulation of the degree and pattern of methyl-esterification of pectin homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J Biol Chem 276 19404–19413 [DOI] [PubMed] [Google Scholar]
- Yang JL, Zhang L, Li YY, You JF, Wu P, Zheng SJ (2006. a) Citrate transporters play a critical role in aluminium-stimulated citrate efflux in rice bean (Vigna umbellata) roots. Ann Bot (Lond) 97 579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zheng SJ, He YF, Tang C, Zhou GD (2005) Genotypic differences among plant species in response to aluminum stress. J Plant Nutr 28 949–961 [Google Scholar]
- Yang JL, Zheng SJ, He YF, You JF, Zhang L, Yu XH (2006. b) Comparative studies on the effect of a protein-synthesis inhibitor on aluminum-induced secretion of organic acids from Fagopyrum esculentum Moench and Cassia tora L. roots. Plant Cell Environ 29 240–246 [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Lin XY, Yang JL, Liu Q, Tang C (2004) The kinetics of aluminum adsorption and desorption by root cell walls of an aluminum resistant wheat (Triticum aestivum L.) cultivar. Plant Soil 261 85–90 [Google Scholar]
- Zheng SJ, Yang JL (2005) Target sites of aluminum phytotoxicity. Biol Plant 49 321–331 [Google Scholar]
- Zheng SJ, Yang JL, He YF, Yu XH, Zhang L, You JF, Shen RF, Matsumoto H (2005) Immobilization of aluminum with phosphorous in roots is associated with high aluminum resistance in buckwheat. Plant Physiol 138 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Läuchli A (1993) Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J Exp Bot 44 773–778 [Google Scholar]