Abstract
Replication-defective adenovirus (ADV) vectors represent a promising potential platform for the development of a vaccine for AIDS. Although this vector is typically administered intramuscularly, it would be desirable to induce mucosal immunity by delivery through alternative routes. In this study, the immune response and biodistribution of ADV vectors delivered by different routes were evaluated. ADV vectors expressing human immunodeficiency virus type 1 (HIV-1) Gag, Pol, and Env were delivered intramuscularly or intranasally into mice. Intranasal immunization induced greater HIV-specific immunoglobulin A (IgA) responses in mucosal secretions and sera than in animals with intramuscular injection, which showed stronger systemic cellular and IgG responses. Administration of the vaccine through an intranasal route failed to overcome prior ADV immunity. Animals exposed to ADV prior to vaccination displayed substantially reduced cellular and humoral immune responses to HIV antigens in both groups, though the reduction was greater in animals immunized intranasally. This inhibition was partially overcome by priming with a DNA expression vector expressing HIV-1 Gag, Pol, and Env before boosting with the viral vector. Biodistribution of recombinant adenovirus (rADV) vectors administered intranasally revealed infection of the central nervous system, specifically in the olfactory bulb, possibly via retrograde transport by olfactory neurons in the nasal epithelium, which may limit the utility of this route of delivery of ADV vector-based vaccines.
To respond to diverse infections in vivo, the immune system must respond to pathogens at different sites. The peripheral immune system patrols the body and maintains lymphoid homeostasis, while the mucosal immune system provides a barrier to microbes that enter through the airway, intestine, and urogenital tract. Because these systems face different challenges, they may require different routes of immunization for optimal activation of an antigen-specific immune response. The transmission of human immunodeficiency virus type 1 (HIV-1) occurs primarily at mucosal surfaces (18, 49), including the genital mucosa, in heterosexual transmission, or the oropharyngeal mucosa, in breast milk infection. Sexual transmission of HIV is likely to be initiated by virus-host cell interaction occurring in the genital mucosa or submucosa, followed by dissemination of HIV to draining lymph nodes (22). To prevent dissemination of the virus to the systemic lymphoid tissue, protective humoral and cell-mediated immunity must be generated in the genital mucosa, submucosa, and draining lymph node. Though a strong systemic vaccine may induce an immune response at mucosal sites (5), immunization targeted to mucosal surfaces may further enhance protective responses at the primary site of infection. If such a vaccine could effect a response at the mucosal site of entry at the same time that it confers systemic immunity, it might provide enhanced protection. In this study, alternative routes of administration of adenoviral vectors and their effects on humoral and cellular immunity were analyzed.
MATERIALS AND METHODS
Animals.
Female BALB/c (H-2d) mice 8 to 10 weeks of age were purchased from Charles River Laboratories (Wilmington, Mass.). They were housed at the Vaccine Research Center animal facility in filter-topped cages on standard rodent diet and allowed to acclimate for at least 1 week prior to the study. The animals were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
DNA vaccines.
The pVRC 2805, 2801, and 4306 plasmids expressing, respectively, the gp145ΔCFI, gp140ΔCFI, and Gag/Pol/Nef fusion proteins of HIV-1 have been described previously (12; W.-P. Kong et al., unpublished data). The plasmids pVRC 2805 and pVRC 2801, expressing HIV-1 envelope in the modified forms (with deletions in the cleavage site, fusogenic domain, and spacing of heptad repeats 1 and 2, termed ΔCFI), gp145ΔCFI and gp140ΔCFI, were shown previously to be able to induce both cytotoxic-T-lymphocyte and antibody responses (12).
For the Pol gene, in order to make sure the Pol protein does not have any undesirable function when introduced in an animal subject, three mutations, in the protease (PR), the reverse transcriptase (RT), and the integrase (IN), termed Pol(ΔPR ΔRT ΔIN), were made in the Pol constructs. The Nef construct from clade B was made in a modified form lacking the ability to down-regulate both major histocompatibility complex class I and CD4 molecules. We used the concept of a fusion protein with different antigens; a fusion form of Gag-Pol-Nef constructs was constructed based on Gag plus Pol(ΔPR ΔRT ΔIN) and with the modified Nef fused at the amino terminus.
Plasmids expressing the HIV genes were made synthetically with sequences designed to disrupt viral RNA structures that limited protein expression or to make the expression Rev independent by codon modification for expression in human cells (2, 33, 34, 36, 37, 39). The cDNAs were cloned in the expression vector pVR1012 (12, 52).
For the Gag-Pol-Nef fusion protein, the protein sequence of the Gag polyprotein (Pr55, amino acids 1 to 432) from HXB2 was used. The synthetic Gag gene makes all of the mature Gag proteins except for the last two, which are normally cleaved from the carboxy terminus of the Gag polyprotein, p1 and p6 (amino acids 433 to 500). The synthetic Gag gene was ligated in frame with sequences encoding the Pol polyprotein (amino acids 3 to 1003) from NL4-3. In order to avoid the translational frameshifting to express the Gag-Pol polyprotein, the synthetic coding region for the last four amino acids of the nucleocapsid protein through the rest of Gag plus an additional three amino acids from Pol were replaced with the corresponding viral sequences (nucleotides 2074 to 2302 on the HXB2 genome) from NL4-3. The deleted frameshifted (ΔFS) protein has five T nucleotides deleted between the Gag and Pol sequences. In addition, the PR was inactivated by mutating amino acid 553 (R to G), the RT was mutated at amino acid 771 (D to H), and the IN mutation is at Gag-Pol amino acid 1209 (D to A). The codon-optimized Nef gene without any mutation was fused downstream of the Pol gene of Gag(ΔFS)Pol(ΔPR ΔRT ΔIN).
Recombinant adenovirus (ADV) vaccines.
Efficient generation of recombinant adenoviral vectors has been described previously (4). The adenoviral shuttle vector pVRC 1290 used in the present study was derived from the AdApt vector (Crucell, Leiden, The Netherlands) by substituting the existing poly(A) with the bovine growth hormone poly(A) and a loxP site. To construct the adenoviral vector coding for gp140ΔCFI, the XbaI/BamHI gp140ΔCFI fragment from pVRC 2801 was inserted into XbaI/BamHI of pVRC 1290. To construct the adenoviral vector coding for HIV-1 Gag-Pol, the SalI/KpnI fragment from pVRC 4308 was inserted into pVRC 1356 (a modified version of pVRC1290) by deletion of the SalI site in position 4195. Viruses were propagated on 293 cells and purified by cesium chloride gradient.
Immunizations.
Mice were immunized intramuscularly (i.m.) with plasmid DNA and either i.m. or intranasally (i.n.) with adenoviral vectors. For i.m. injection of plasmid DNA (100 μg) or ADV vectors (1010 particles), mice (n = 5) were injected with 200 μl divided equally into both quadriceps. i.n. immunization was performed by lightly anesthetizing the mice (n = 5) with isofluorane and then applying 20 μl of ADV (1010 particles) into the nares via a micropipette.
The effect of the various routes of immunization was tested in the presence or absence of preexisting immunity to the ADV vector. To induce preexisting immunity, mice were immunized 9 weeks before the prime, using a control vector (delta E1 ADV) (5) at a dose of 1010 particles, administered i.m. or i.n. as indicated. Then, various combinations of immunization routes for priming and boosting were tested: ADV i.m. priming and ADV i.m. boosting (ADV i.m./ADV i.m.), ADV i.n./ADV i.n., DNA i.m./ADV i.m., and DNA i.m./ADV i.n. For the ADV alone protocol, mice were primed at week 9 and boosted at week 14. For the DNA/ADV protocol, mice received DNA three times, at weeks 9, 10, and 11, and were then boosted with ADV at week 13.
All samples (blood, feces, saliva, and vaginal secretions) were collected 1 week after each immunization and at the time of sacrifice, 13 weeks after the boost. Fecal extracts were prepared by collecting four fresh voided fecal pellets, mixing them in the fecal extract buffer (phosphate-buffered saline [PBS], 0.1% sodium azide), and vortexing for 15 min at room temperature. The vortexed mixture was centrifuged for 10 min at 13,000 rpm in an Eppendorf microcentrifuge, and the supernatant was collected and stored at −20°C until assayed. Salivary secretions were collected by injecting mice intraperitoneally with 50 μl of pilocapine (0.5% [wt/vol] in PBS) (Sigma, St. Louis, Mo.) to induce salivation. Approximate volumes of 100 μl of saliva were collected and stored at −20°C. Vaginal lavage was obtained by rinsing the vaginal cavity three times with 50 μl of PBS. All samples collected were stored at −20°C until assayed.
HIV-1-specific immunoglobulin G (IgG) and IgA analysis.
The anti-HIV-1 ELISA assay was performed using microwell plates coated with HIV-1 antigens (viral lysate and Escherichia coli recombinant, Genetic Systems rLAV EIA; Bio-Rad Laboratories, Redmond, Wash.). Mouse serum and secretions were diluted in PBS, 1% fetal bovine serum, and 0.02% Tween 20 (dilution buffer). Sera were serially diluted from 1:1,000 to 1:128,000. Secretions were diluted as follows: feces, 1:10; saliva, 1:2; vaginal secretions, 1:5. Sera and secretion dilutions were added to the HIV-1-coated plates and incubated overnight at 4°C. Plates were then washed five times with PBS-0.2% Tween 20 and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000; Chemicon International Inc., Temecula, Calif.) or with horseradish peroxidase-conjugated goat anti-mouse IgA (1:500; Zymed Laboratories Inc., San Francisco, Calif.) for 1 h at room temperature. Plates were washed five times, and 100 μl of OPD peroxidase substrate (Sigma) was added to each well. The reaction was stopped after 30 min by addition of 50 μl of 2N H2SO4. The plates were read on an enzyme-linked immunosorbent assay (ELISA) reader at 490 nm.
Cytokine secretion analysis.
Spleens from treated animals were removed aseptically, and unicellular suspensions were prepared by passing the spleens through a metal mesh. Red blood cells were lysed by suspending the splenocytes in Pharmlyse buffer (Pharmingen). Cells were then washed, and 106 cells were stimulated using 2.5-μg/ml HIV-1 peptide pools (15-mer peptides covering the whole gag (122 peptides), pol (248 peptides), and env (158 peptides) genes from HIV-1 subtype B; 11 residues overlap between each peptide). Stimulation was performed in the presence of anti-CD28 and anti-CD49D antibodies (BD Pharmingen) at 1 μg/ml. Positive control stimulation was performed by using phorbol myristate acetate at 25 ng/ml (Sigma) and ionomycin at 1 μg/ml (Sigma) as stimulants. Negative controls consisted of nonstimulated cells and cells stimulated with an irrelevant peptide pool (Ebola virus glycoprotein-specific peptide). Stimulation was first performed at 37°C for 1 h. Brefeldin A (10 μg/ml) (Sigma) was then added to inhibit the cytokine secretion, and stimulation was completed by incubation at 37°C for five more hours.
For the staining, stimulation medium was discarded and cells (106) were treated with anti-CD16/CD32 antibody (5 μg/ml) (BD Pharmingen) for 15 min at 4°C. Cells were centrifuged at 900 × g, 4°C, for 3 min, and cell pellets were resuspended in 150 μl of Cytofix/Cytoperm (BD Pharmingen) and incubated at room temperature for 20 min. Cells were washed in PBS-0.1% saponin (Sigma) and resuspended in 100 μl of PBS-0.1% saponin containing 0.15 μg of anti-CD3 phycoerythrin, 0.3 μg of anti-CD4 peridin chlorophyll-a protein (PerCP), 0.6 μg of anti-CD8 allophycocyanin, 0.375 μg of anti-gamma interferon (IFN-γ) fluorescein isothiocyanate, and 0.188 μg anti-tumor necrosis factor alpha (TNF-α) fluorescein isothiocyanate. Cells were incubated with these antibodies for 25 min at 4°C. Cells were then washed and analyzed by flow cytometry for intracellular IFN-γ and TNF-α staining.
Biodistribution of adenoviral vectors after i.n. administration.
A recombinant ADV encoding placental alkaline phosphatase (PlAP) used as a reporter gene was prepared as previously described (3). Eleven-week-old female BALB/c mice (three animals per group) were immunized i.n. with 1010 or 2 × 1010 particles of PlAP-ADV. Negative controls (three animals) received 20 μl of PBS in the nares. Biodistribution of the vector was monitored 2 and 7 days after adenoviral administration by sacrificing the animals for histological examination. The histology of various organs (middle ears, brain, inguinal lymph nodes, ovaries, liver, spleen, kidneys, heart, thyroid gland, thymus, bone marrow, lung, nasal turbinates, and olfactory bulb) was examined by microscopic observation of hematoxylin-eosin-stained slides and of alkaline phosphatase (AP)-stained slides (p-nitro blue tetrazolium chloride-5-bromo-4-chloro-3-indolyl-phosphate, toluidine-salt staining followed by methyl green counterstaining).
Statistical analysis.
Kruskal-Wallis tests were performed to determine overall differences among the various groups. If the P value from the Kruskal-Wallis test was less than 0.05, Wilcoxon rank tests were then performed.
RESULTS
Vaccine-induced cellular immune response.
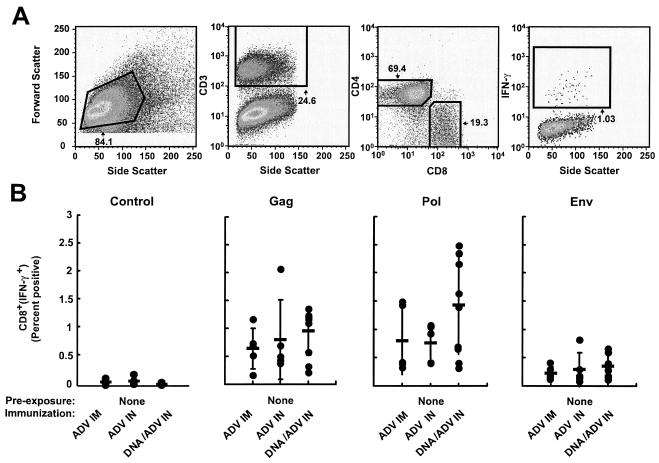
The effects of preexposure to wild-type ADV and the different routes of immunization were analyzed with mice using intracellular flow cytometry for IFN-γ with pools of peptides from HIV-1 Gag, Pol, and Env. Cellular immune responses induced in the absence of preexisting anti-ADV vector immunity were first compared after ADV immunization through an i.m. or i.n. route. ADV vectors introduced by these two routes induced similar CD8+ IFN-γ responses that were comparable to DNA priming and i.n. ADV boosting (Fig. 1), though the latter showed a slight non-statistically significant trend toward increased responsiveness. Similar results were obtained with each peptide pool (HIV-1 Gag, Pol, and Env).
FIG. 1.
In the absence of prior immunity, immunization with ADV vector alone or with DNA priming followed by viral vector boosting, by either route (i.m. or i.n.), induces similar cellular immune responses. (A) Representative gating strategy for flow cytometry. Splenocytes from a mouse immunized with DNA priming followed by i.n. viral vector boosting were stimulated in vitro with an HIV-1 Gag peptide pool as described in Materials and Methods and then analyzed by intracellular flow cytometry. Lymphocytes were selected using a forward scatter versus side scatter gate. CD8 cells were defined by first gating the CD3+ population, followed by selection of CD4+ and CD8+ stained cells. Cytokine-positive cells in the CD8+ population were defined as a percentage of the subset. (B) Percentages of CD8 T cells producing IFN-γ are shown for each animal in the various groups. Bars represent geometric means. The control graph represents splenocytes from each group stimulated with an irrelevant peptide pool. BALB/c mice were immunized using the various protocols (recombinant adenovirus [rADV] alone or DNA/ADV), using various routes (i.m. or i.n.). HIV-specific IFN-γ secretions were measured from spleen T cells at the time of sacrifice of the mice, 13 weeks after the last immunization. Splenocytes were stimulated in vitro with pools of Gag, Pol, and Env peptides, and IFN-γ-producing cells were counted by flow cytometry. Studies on unimmunized mice have shown that the frequency of IFN-γ-positive cells following stimulation with HIV peptide pools was essentially negative.
To evaluate whether the route of prior exposure to the adenoviral vector affected the immune response, CD8+ IFN-γ+ cells were measured in splenocytes from mice immunized with ADV i.m. or i.n. (Fig. 2A and B, respectively) after adenoviral preexposure i.m. or i.n.. Following ADV immunization i.m., no significant difference was observed in the Gag- and Pol-specific CD8 responses, measured by intracellular IFN-γ synthesis 13 weeks after the final immunization, between prior exposure to ADV by the i.m. or i.n. route. The Env-specific CD8+-T-cell response was significantly greater for mice that were not exposed to ADV than for those exposed i.n. or i.m., in which the cellular response to HIV-1 Env induced by ADV immunization through either route was completely abolished (Fig. 2A and B, last column).
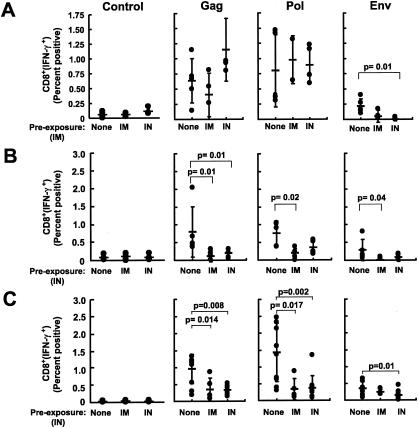
FIG. 2.
Suppression of cellular immune responses by prior immunity to ADV is greater with immunization by the i.n. route than with i.m. immunization. HIV-specific cellular immune responses were quantified in BALB/c mice immunized i.m. with rADV (A) or i.n. with rADV alone (B) or DNA/ADV (C). HIV-specific IFN-γ secretions in CD8 T cells were measured as described in the legend for Fig. 1.
Mice immunized i.n. with ADV were more sensitive to preexisting immunity to the ADV vector (Fig. 2B). To overcome the effect of antivector immunity, an alternative immunization approach, with DNA priming and adenoviral boosting, was analyzed and compared to ADV vector-only immunization. Similarly to the earlier experiment, the cellular response was significantly diminished in the presence of antivector immunity for DNA/ADV immunization (Fig. 2C).
We next analyzed this data set to determine whether the route of immunization could differentially affect cellular immune responses based on the route of prior exposure to the vector. Animals that had been immunized to the ADV vector by i.m. injection (Fig. 3A) or i.n. inoculation (Fig. 3B) were compared side by side for their response to injection with ADV or DNA/ADV through an i.m. or i.n. route. Intranasal vaccination in these mice induced a significantly lower CD8+ IFN-γ responses than i.m. immunization (Fig. 3A and B), further suggesting that i.n. immunization is more affected by vector immunity than i.m. vaccination. When mice were immunized with a DNA prime and ADV boost, the boost was more effective when given i.m. than i.n., though both responses were less attenuated by prior vector immunity. For all immunogens, responses obtained after DNA/ADV immunization was always equal to or better than those obtained after immunization with ADV alone (Fig. 3B, ADV versus DNA/ADV).
FIG. 3.
Suppression of cellular immune responses by prior immunity to ADV is greater with immunization by rADV for priming and boosting than with DNA priming and viral vector boosting. HIV-specific cellular immune responses were quantified for BALB/c mice immunized with the various protocols (rADV alone or DNA/ADV), using various routes (i.m. or i.n.) after preexposure to ADV using the i.m. (A) or i.n. (B) route. HIV-specific IFN-γ secretions in CD8 T cells were measured as described in the legend for Fig. 1.
Immunoglobulin production in systemically and mucosally immunized mice.
The systemic humoral response was analyzed by ELISA to characterize the HIV-1-specific IgG response. In all groups, IgG antibodies became detectable 10 days after the adenoviral boost (Fig. 4). Mice with preexisting ADV immunity produced a significantly lower level of IgG than mice with no anti-ADV vector immunity by the Wilcoxon rank test. In mice with no preexisting immunity, mice that were primed and boosted with ADV i.m. produced significantly lower levels of antibody than all other groups. The other three groups gave similar IgG responses, with better results for the DNA/ADV protocol than for ADV alone.
FIG. 4.
Prior immunity to ADV inhibits systemic humoral immune responses induced by immunization using either rADV or DNA priming and viral vector boosting. HIV-1-specific IgG antibodies were detected from serum of BALB/c mice immunized with the various protocols (rADV alone or DNA/ADV), using various routes (i.m. or i.n.), in the presence or absence of preexisting immunity to the adenoviral vector. Blood samples were collected before preexposure (closed bars) and postboost (open bars). Serum samples were diluted at 1:1,000 and analyzed by ELISA as previously described (see Materials and Methods). Data are represented as averages with standard deviations.
Combination of i.n. and i.m. immunization.
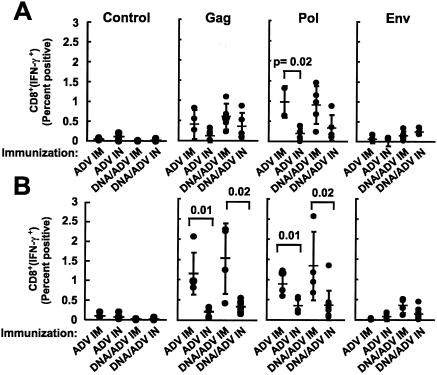
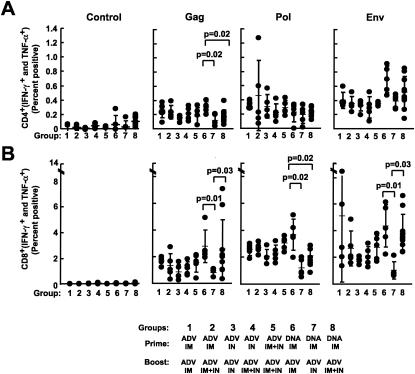
Because i.n. administration of ADV, alone or in combination with DNA priming, did not induce significantly higher production of anti-HIV-1 IgG or T-cell responses than i.m. injection, simultaneous i.n. and i.m. injection was tested to determine if it would further improve the immune response. Various combinations of ADV or DNA/ADV were analyzed (Table 1), and T-cell responses were analyzed 10 days after the ADV boost using intracellular flow cytometry for IFN-γ and TNF-α. All groups immunized with ADV alone showed similar CD4 responses (Fig. 5A). Mice immunized with DNA/ADV i.m. gave significantly higher responses (P = 0.02) than with any other vaccination protocols. A similar response was observed for DNA/ADV with Env peptides (Fig. 5A, P = 0.02). The combination of i.n. and i.m. immunizations yielded responses intermediate between those with either route alone. All DNA/ADV protocols, by any route, induced significantly greater responses than any ADV alone protocol (Fig. 5A, P = 0.01). All groups of mouse cells, when stimulated with Pol peptides, gave similar results. HIV-specific (Gag, Pol, and Env) CD4 responses from any group were determined to be significantly greater than in the corresponding groups stimulated with an irrelevant peptide.
TABLE 1.
Immunization schedule and routesa
| Group | Prime
|
Boost
|
Sample collection (wk) | ||||
|---|---|---|---|---|---|---|---|
| Immunogen | Route | Wk | Immunogen | Route | Wk | ||
| 1 | Ad | i.m. | 0 | Ad | i.m. | 6 | −2, 2, 7 |
| 2 | Ad | i.m. | 0 | Ad | i.m. + i.n. | 6 | −2, 2, 7 |
| 3 | Ad | i.n. | 0 | Ad | i.n. | 6 | −2, 2, 7 |
| 4 | Ad | i.n. | 0 | Ad | i.m. + i.n. | 6 | −2, 2, 7 |
| 5 | Ad | i.m. + i.n. | 0 | Ad | i.m. + i.n. | 6 | −2, 2, 7 |
| 6 | DNA | i.m. | 0, 2, 4 | Ad | i.m. | 6 | −2, 5, 7 |
| 7 | DNA | i.m. | 0, 2, 4 | Ad | i.n. | 6 | −2, 5, 7 |
| 8 | DNA | i.m. | 0, 2, 4 | Ad | i.m. + i.n. | 6 | −2, 5, 7 |
Ad, ADV vector.
FIG. 5.
Lower cellular immune responses are induced by immunization using the i.n. route than with i.m. immunization, but this response is partially rescued by the combination of i.n. and i.m. routes of immunization. HIV-specific cellular immune responses were quantified for BALB/c mice immunized with the various protocols (rADV alone or DNA/ADV), using various routes (i.m., i.n., or i.m. plus i.n.). HIV-specific IFN-γ plus TNF-α secretions were measured from spleen T cells at the time of sacrifice of the mice, 10 days after the last immunization. Splenocytes were in vitro stimulated with pools of Gag, Pol, and Env peptides, and IFN-γ- and TNF-α-producing cells were counted by flow cytometry. Shown are the percentages of CD4 (A) and CD8 (B) T cells producing IFN-γ and TNF-α for each animal in the various groups. Bars represent geometric means. The control graph represents splenocytes from each group stimulated with an irrelevant peptide pool.
HIV-specific CD8 responses confirmed the results described above for CD4 cells (Fig. 5B). For mice stimulated with Gag peptides, the i.n. route of immunization was less effective than i.m. injection (P = 0.01 for DNA/ADV); however, the combination of the two routes for the boost in the DNA/ADV protocol significantly rescued (P = 0.03) the low cellular response obtained with the i.n. route. This observation was confirmed with the two other stimulants (Pol and Env).
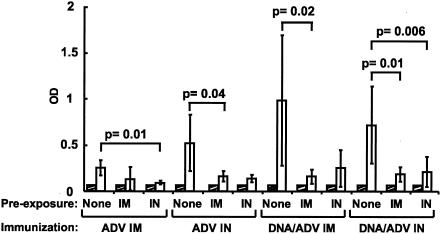
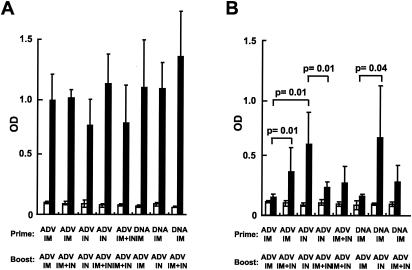
Analysis of humoral responses following immunization was performed using an HIV-specific ELISA (Fig. 6). Immunization of mice using various routes and vectors (ADV alone or DNA/ADV, i.m., i.n., or i.m. and i.n.) induced similar titers of IgG in the serum (Fig. 6A). In contrast, IgA responses were induced at a higher level in saliva by i.n. immunization (Fig. 6B). In mice immunized with ADV alone, HIV-specific IgA levels were highest in animals primed and boosted i.n., whereas i.m. immunization did not induce any IgA response. For the DNA/ADV protocol, i.n. administration of ADV also induced the highest IgA response. A combination of i.n. and i.m. vaccination induced IgA responses that were intermediate compared to each separately. The patterns of IgA antibody responses in sera, feces, and vaginal secretions were generally similar to those in saliva (data not shown), confirming the ability of the i.n. route to induce IgA antibodies at diverse mucosal sites. The values were not normalized for total IgA levels in these analyses as shown; however, samples were subsequently analyzed for total IgA in all groups and were comparable (data not shown), suggesting that HIV-specific IgA increases observed in i.n. immunized mice were not due to a dilution effect introduced by the collection method.
FIG. 6.
i.n. route of immunization induces greater humoral immune responses at the mucosal level than the i.m. route, and the combination of both routes induces intermediate responses. HIV-1-specific IgG and IgA antibodies were measured in sera and saliva from mice immunized with the various protocols. IgG present in serum (A) and IgA present in saliva (B) of preimmune animals (white bars) or 10 days postimmunization (black bars) were detected by ELISA as described in Materials and Methods.
Distribution of ADV vectors following i.n. administration.
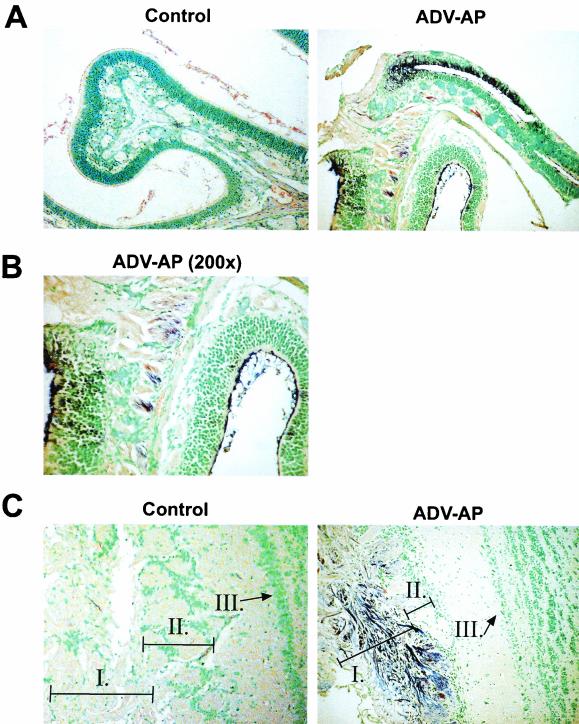
Since nasopharynx resides in close proximity to the brain, i.n. delivery of ADV could facilitate the migration of the recombinant vector into the central nervous system, where it might potentially cause local inflammation or toxicity. To investigate this possibility, the biodistribution of ADV vectors was studied after i.n. administration with a PlAP reporter gene. By 7 days after i.n. administration of 2 × 1010 particles, all animals showed substantial AP-specific staining in the olfactory bulb. Other tissues, including middle ears, brain, inguinal lymph nodes, ovaries, liver, spleen, kidneys, heart, thyroid gland, thymus, or bone marrow, were negative (data not shown). Positive animals showed PlAP expression in the olfactory neuron layer and in some glomeruli of the olfactory bulb (Fig. 7C). Confirmation of neurons was characterized by enhanced staining of their extensive axonal processes as well as neuronal body cytoplasm. Close analysis of the histological slides based on cell morphology and previous characterization of cell types found in this region of the brain revealed the absence of positively stained antigen-presenting cells. Positive animals also showed PlAP expression in olfactory cilia, olfactory receptor cells, and olfactory nerve fibers of the nasal turbinates (Fig. 7A and B). The corresponding sections stained using the hematoxylin and eosin coloration did not reveal inflammation in this tissue, nor was an inflammatory response observed in any tissue.
FIG. 7.
Infection of nasal epithelium and olfactory nerves by recombinant ADV following i.n. administration. Eleven-week-old BALB/c mice were immunized with 20 μl (2 × 1010 particles) of heat-resistant PlAP ADV vectors directly spotted in the nostrils using a micropipette. After 2 and 7 days, mice were euthanized, and tissues were harvested and prepared for histologic examination. Detection of PlAP-expressing cells was performed with nasal turbinates and olfactory bulb from mice immunized i.n. with PlAP-ADV. Tissue sections treated with Nitro Blue Tetrazolium chloride--5-bromo-4-chloro-3-indolyl-phosphate (Gibco BRL), chromagenic substrate for AP, and counterstained with methyl green are shown. The purple staining specific for AP makes it possible to follow the biodistribution of ADV through the expression of the reporter gene they carry, the AP. Sections ADV-AP correspond to a mouse administered PlAP-ADV i.n. The control sections correspond to a mouse administered 20 μl of PBS i.n. Nasal turbinate cavities, olfactory epithelium, and olfactory nerve fibers are shown in panels A (magnification, ×100) and B (magnification, ×200). Panel C corresponds to the olfactory bulb and allows the identification of the olfactory neuron layer (area I) at the periphery of the bulb, glomeruli (area II), and the mitral cell layer (area III) in the center of the olfactory bulb.
DISCUSSION
A vaccine that reduces the risk of sexual transmission across cervicovaginal and rectal mucosal surfaces might reduce the likelihood of HIV-1 infection and contribute to vaccine efficacy (17, 25). In the female genital tract and in the rectum, HIV-1 may enter via intraepithelial or subepithelial dendritic cells, respectively, and infect mucosal T cells (19, 30, 32, 43). Alternatively, the virus may traverse specialized antigen transporting epithelial cells, known as M cells (1, 7). Once within the mucosa, HIV-1 replicates in resident CD4+ T lymphocytes and/or macrophages and may be carried by these cells, as well as dendritic cells, to draining lymphoid organs within days after initial exposure (20, 27, 30, 43, 44). Therefore, an HIV vaccine that reduces or prevents initial virus entry into the mucosa could arrest infection in mucosal tissues before local or systemic reservoirs can be established. Since IgA is a key component for the protection against virus infection in the mucosa, its enhanced production may reduce or prevent sexual transmission of HIV (6, 8, 14, 21, 24, 26, 31). In this report, the i.n. route of immunization induced significantly higher levels of IgA in body secretions than the i.m. route.
Systemic immunization resulted in lower but measurable IgA production at mucosal surfaces, but systemic immunization, particularly by DNA priming and ADV boosting, caused activation of central immune responses, including HIV-specific IgG and T-cell responses. Accordingly, there appears to be substantial cross-communication between systemic and mucosal immune systems. The finding supports the hypothesis that DNA priming administered i.m. could, in some cases, increase the mucosal response obtained after i.n. administration of ADV vectors. These data suggest that prime-boost regimens using central and mucosal routes of immunization might enhance the immune response at both sites.
Adenoviral vectors have proven their potency in numerous previous studies (45, 47, 51). ADVs invade their host naturally via mucosal surfaces, notably through the airways, and replicate, at least initially, in mucosal sites of the respiratory or gastrointestinal tracts. Therefore, ADVs represent attractive vectors for the delivery of vaccines to mucosal surfaces. Nevertheless, preexisting immunity in humans, who frequently encounter these ubiquitous viruses and generally seroconvert within their first years of life, is expected to interfere with the efficacy of such vaccines (50, 51). Our data confirm the high sensitivity of adenoviral vectors to preexisting immunity. HIV-specific cellular immunity in immunized mice was in all cases greatly affected by prior antivector immunity. Furthermore, the decrease in systemic immunity in i.n. immunized mice showed that this route of administration is more sensitive to prior ADV immunity. The development of highly effective vaccines based on adenoviral vectors will benefit from the discovery of ways to circumvent the problem of preexisting ADV immunity. One solution to this problem is suggested by the data showing that DNA priming not only increases the systemic and humoral responses to HIV-1 antigens but also partially rescues the loss of immunity induced by preexisting immunity to the adenoviral vectors. This finding is consistent with a previous study using an Ebola virus glycoprotein vaccine model (53). Another report has shown enhanced immunogenicity of a DNA prime-poxvirus combination for herpes simplex virus administered mucosally (16). The present study suggests that the DNA priming step may overcome this immunity independently of the antigen introduced by the vector, but it also suggests that i.n. immunization is less effective than i.m. injection for this purpose.
DNA vaccines have been shown previously to prime the immune system for a subsequent booster immunization with a traditional vaccine (38, 40). The findings in this study suggest that DNA priming and ADV boosting might be highly suitable for use in humans with previous exposure to wild-type ADV. These data are also consistent with those of Xiang et al., showing that DNA vaccine priming reduced the antibody response to the adenoviral vector after i.n. booster immunization with ADV (50).
Although the use of DNA priming may not completely overcome preexisting adenoviral immunity in humans, it could probably be successfully associated with other strategies to evade this immune response, for example, the use of alternative serotypes of ADV. Although ADV vectors seem to be promising, especially when associated with a DNA prime and administered i.n., the localization of recombinant gene expression to the olfactory bulb of the central nervous system suggests that this route of administration must be evaluated for its safety before moving forward to human clinical trials. ADV comes into direct contact with the nasal epithelium after i.n. administration. This characteristic could be useful, since antigen uptake across nasal epithelium not associated with lymphoid follicles has been shown to be potentially important for stimulation of immunity (23); however, safety concerns can be raised if one considers the high innervation of this epithelium and its close anatomic proximity to the brain. This distribution likely arises from retrograde transport of these viruses through the olfactory nerves to the olfactory bulb. In the olfactory bulb, ADV was found in the olfactory neuron layer as well as in the glomeruli, where the olfactory neurons connect to second-order neurons targeting the brain cortex, which remained negative for the AP staining during the limited observation period. Previous studies performed with other models have shown retrograde transport of ADV in motor neurons following peripheral injection (35), and this study now suggests that a similar mechanism applies to olfactory nerve fibers. Although ADV vectors have been used in clinical trials for the treatment of brain tumors (48), reports of vector-associated toxicity and inflammation have raised safety concerns (10, 11). Significant toxicity both in vivo and in vitro has been reported when these vectors are used to infect cells at a high multiplicity of infection (9, 13, 15, 28, 29, 42). The mechanism of this toxicity may involve a combination of direct vector-mediated cytotoxicity and the elicitation of a chronic, but not acute, inflammatory response, induced by high vector doses (108 infectious units and above) (46). In the central nervous system, ADV titers must be carefully controlled, since excessive levels result in nuclear degeneration followed by a loss of viability (41).
Our results demonstrating the ability of ADV vectors to infect some parts of the central nervous system do not necessarily preclude their use through the i.n. route, but they emphasize the need to carefully test their possible toxicity to the central nervous system, notably by using vaccine doses in animal models consistent with those to be used in human trials. Because i.n. immunization with ADV induced IgA at a variety of mucosal surfaces, these findings also suggest that immunization at alternative mucosal sites, such as the rectal, vaginal, or intestinal mucosa, may afford the opportunity to induce similarly potent mucosal responses while avoiding the possibility of ADV delivery into the central nervous system.
Acknowledgments
We thank members of the Nabel laboratory for helpful comments and discussions, Ati Tislerics for manuscript preparation, Nancy Sullivan for critique of the manuscript, Mathew Starost for participation in histologic analysis, Mario Roederer for assistance in statistical analysis, and Karen Stroud and Toni Miller for preparation of figures.
REFERENCES
- 1.Amerongen, H. M., R. Weltzin, C. M. Farnet, P. Michetti, W. A. Haseltine, and M. R. Neutra. 1991. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J. Acquir. Immune Defic. Syndr. 4:760-765. [PubMed] [Google Scholar]
- 2.Andre, S., B. Seed, J. Eberle, W. Schraut, A. Bultmann, and J. Haas. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 72:1497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, K., L. M. Akyurek, H. San, K. Leung, M. S. Parmacek, E. G. Nabel, and G. J. Nabel. 2000. Restricted expression of an adenoviral vector encoding Fas ligand (CD95L) enhances safety for cancer gene therapy. Mol. Ther. 1:555-565. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, K., C. Barker, X. Danthinne, M. J. Imperiale, and G. J. Nabel. 1999. Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol. Med. 5:224-231. [PMC free article] [PubMed] [Google Scholar]
- 5.Baig, J., D. B. Levy, P. F. McKay, J. E. Schmitz, S. Santra, R. A. Subbramanian, M. J. Kuroda, M. A. Lifton, D. A. Gorgone, L. S. Wyatt, B. Moss, Y. Huang, B. K. Chakrabarti, L. Xu, W. P. Kong, Z. Y. Yang, J. R. Mascola, G. J. Nabel, A. Carville, A. A. Lackner, R. S. Veazey, and N. L. Letvin. 2002. Elicitation of simian immunodeficiency virus-specific cytotoxic T lymphocytes in mucosal compartments of rhesus monkeys by systemic vaccination. J. Virol. 76:11484-11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyrer, C., A. W. Artenstein, S. Rugpao, H. Stephens, T. C. VanCott, M. L. Robb, M. Rinkaew, D. L. Birx, C. Khamboonruang, P. A. Zimmerman, K. E. Nelson, C. Natpratan, et al. 1999. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. J. Infect. Dis. 179:59-67. [DOI] [PubMed] [Google Scholar]
- 7.Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 3:42-47. [DOI] [PubMed] [Google Scholar]
- 8.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. [DOI] [PubMed] [Google Scholar]
- 9.Brand, K., R. Klocke, A. Possling, D. Paul, and M. Strauss. 1999. Induction of apoptosis and G2/M arrest by infection with replication-deficient adenovirus at high multiplicity of infection. Gene Ther. 6:1054-1063. [DOI] [PubMed] [Google Scholar]
- 10.Byrnes, A. P., J. E. Rusby, M. J. Wood, and H. M. Charlton. 1995. Adenovirus gene transfer causes inflammation in the brain. Neuroscience 66:1015-1024. [DOI] [PubMed] [Google Scholar]
- 11.Cartmell, T., T. Southgate, G. S. Rees, M. G. Castro, P. R. Lowenstein, and G. N. Luheshi. 1999. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J. Neurosci. 19:1517-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti, B. K., W. P. Kong, B. Y. Wu, Z. Y. Yang, J. Friborg, X. Ling, S. R. King, D. C. Montefiori, and G. J. Nabel. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 76:5357-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowsill, C., T. D. Southgate, G. Morrissey, R. A. Dewey, A. E. Morelli, T. C. Maleniak, Z. Forrest, D. Klatzmann, G. W. Wilkinson, P. R. Lowenstein, and M. G. Castro. 2000. Central nervous system toxicity of two adenoviral vectors encoding variants of the herpes simplex virus type 1 thymidine kinase: reduced cytotoxicity of a truncated HSV1-TK. Gene Ther. 7:679-685. [DOI] [PubMed] [Google Scholar]
- 14.Devito, C., K. Broliden, R. Kaul, L. Svensson, K. Johansen, P. Kiama, J. Kimani, L. Lopalco, S. Piconi, J. J. Bwayo, F. Plummer, M. Clerici, and J. Hinkula. 2000. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165:5170-5176. [DOI] [PubMed] [Google Scholar]
- 15.Easton, R. M., E. M. Johnson, and D. J. Creedon. 1998. Analysis of events leading to neuronal death after infection with E1-deficient adenoviral vectors. Mol. Cell Neurosci. 11:334-347. [DOI] [PubMed] [Google Scholar]
- 16.Eo, S. K., M. Gierynska, A. A. Kamar, and B. T. Rouse. 2001. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J. Immunol. 166:5473-5479. [DOI] [PubMed] [Google Scholar]
- 17.Heilman, C. A., and D. Baltimore. 1998. HIV vaccines---where are we going? Nat. Med. 4:532-534. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg, S. D. 1997. Risk factors for sexual transmission of human immunodeficiency virus, p. 569-575. In V. DeVita, S. Hellman, and S. A. Rosenberg, (ed.), AIDS, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 19.Hussain, L. A., and T. Lehner. 1995. Comparative investigation of Langerhans' cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology 85:475-484. [PMC free article] [PubMed] [Google Scholar]
- 20.Ignatius, R., F. Isdell, U. O'Doherty, and M. Pope. 1998. Dendritic cells from skin and blood of macaques both promote SIV replication with T cells from different anatomical sites. J. Med. Primatol. 27:121-128. [DOI] [PubMed] [Google Scholar]
- 21.Kaul, R., D. Trabattoni, J. J. Bwayo, D. Arienti, A. Zagliani, F. M. Mwangi, C. Kariuki, E. N. Ngugi, K. S. MacDonald, T. B. Ball, M. Clerici, and F. A. Plummer. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13:23-29. [DOI] [PubMed] [Google Scholar]
- 22.Kiyono, H., C. J. Miller, Y. Lu, T. Lehner, M. Cranage, Y. T. Huang, S. Kawabata, M. Marthas, B. Roberts, J. G. Nedrud, M. E. Lamm, L. A. Bergmeier, R. Brookes, L. Tao, and J. R. McGhee. 1995. The common mucosal immune system for the reproductive tract: basic principles aplied toward an AIDS vaccine. Adv. Drug Deliv. Rev. 18:23-51. [Google Scholar]
- 23.Kuper, C. F., P. J. Koornstra, D. M. Hameleers, J. Biewenga, B. J. Spit, A. M. Duijvestijn, P. J. van Breda Vriesman, and T. Sminia. 1992. The role of nasopharyngeal lymphoid tissue. Immunol. Today 13:219-224. [DOI] [PubMed] [Google Scholar]
- 24.Lehner, T., Y. Wang, M. Cranage, L. A. Bergmeier, E. Mitchell, L. Tao, G. Hall, M. Dennis, N. Cook, R. Brookes, L. Klavinskis, I. Jones, C. Doyle, and R. Ward. 1996. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat. Med. 2:767-775. [DOI] [PubMed] [Google Scholar]
- 25.Mayer, K. H., and D. J. Anderson. 1995. Heterosexual HIV transmission. Infect. Agents Dis. 4:273-284. [PubMed] [Google Scholar]
- 26.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 27.Miller, C. J., N. J. Alexander, P. Vogel, J. Anderson, and P. A. Marx. 1992. Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. J. Med. Primatol. 21:64-68. [PubMed] [Google Scholar]
- 28.Morral, N., W. O'Neal, H. Zhou, C. Langston, and A. Beaudet. 1997. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum. Gene Ther. 8:1275-1286. [DOI] [PubMed] [Google Scholar]
- 29.O'Leary, M. T., and H. M. Charlton. 1999. A model for long-term transgene expression in spinal cord regeneration studies. Gene Ther. 6:1351-1359. [DOI] [PubMed] [Google Scholar]
- 30.Parr, M. B., L. Kepple, and E. L. Parr. 1991. Antigen recognition in the female reproductive tract. II. Endocytosis of horseradish peroxidase by Langerhans cells in murine vaginal epithelium. Biol. Reprod. 45:261-265. [DOI] [PubMed] [Google Scholar]
- 31.Pastori, C., C. Barassi, S. Piconi, R. Longhi, M. L. Villa, A. G. Siccardi, M. Clerici, and L. Lopalco. 2000. HIV neutralizing IgA in exposed seronegative subjects recognise an epitope within the gp41 coiled-coil pocket. J. Biol. Regul. Homeost. Agents 14:15-21. [PubMed] [Google Scholar]
- 32.Pope, M. 1999. Mucosal dendritic cells and immunodeficiency viruses. J. Infect. Dis. 179(Suppl. 3):S427-S430. [DOI] [PubMed] [Google Scholar]
- 33.Qiu, J. T., R. Song, M. Dettenhofer, C. Tian, T. August, B. K. Felber, G. N. Pavlakis, and X. F. Yu. 1999. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J. Virol. 73:9145-9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rana, T. M., and K. T. Jeang. 1999. Biochemical and functional interactions between HIV-1 Tat protein and TAR RNA. Arch. Biochem. Biophys. 365:175-185. [DOI] [PubMed] [Google Scholar]
- 35.Ridoux, V., J. J. Robert, X. Zhang, M. Perricaudet, J. Mallet, and G. Le Gal La Salle. 1994. Adenoviral vectors as functional retrograde neuronal tracers. Brain Res. 648:171-175. [DOI] [PubMed] [Google Scholar]
- 36.Roebuck, K. A., and M. Saifuddin. 1999. Regulation of HIV-1 transcription. Gene Expr. 8:67-84. [PMC free article] [PubMed] [Google Scholar]
- 37.Romano, G., M. Kasten, G. De Falco, P. Micheli, K. Khalili, and A. Giordano. 1999. Regulatory functions of Cdk9 and of cyclin T1 in HIV tat transactivation pathway gene expression. J. Cell Biochem. 75:357-368. [PubMed] [Google Scholar]
- 38.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 39.Schneider, R., M. Campbell, G. Nasioulas, B. K. Felber, and G. N. Pavlakis. 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 71:4892-4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slack, R. S., and F. D. Miller. 1996. Viral vectors for modulating gene expression in neurons. Curr. Opin. Neurobiol. 6:576-583. [DOI] [PubMed] [Google Scholar]
- 42.Smith, J. G., S. E. Raper, E. B. Wheeldon, D. Hackney, K. Judy, J. M. Wilson, and S. L. Eck. 1997. Intracranial administration of adenovirus expressing HSV-TK in combination with ganciclovir produces a dose-dependent, self-limiting inflammatory response. Hum. Gene Ther. 8:943-954. [DOI] [PubMed] [Google Scholar]
- 43.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stahl-Hennig, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261-1265. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 46.Thomas, C. E., D. Birkett, I. Anozie, M. G. Castro, and P. R. Lowenstein. 2001. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 3:36-46. [DOI] [PubMed] [Google Scholar]
- 47.Tims, T., D. J. Briggs, R. D. Davis, S. M. Moore, Z. Xiang, H. C. Ertl, and Z. F. Fu. 2000. Adult dogs receiving a rabies booster dose with a recombinant adenovirus expressing rabies virus glycoprotein develop high titers of neutralizing antibodies. Vaccine 18:2804-2807. [DOI] [PubMed] [Google Scholar]
- 48.Trask, T. W., R. P. Trask, E. Aguilar-Cordova, H. D. Shine, P. R. Wyde, J. C. Goodman, W. J. Hamilton, A. Rojas-Martinez, S. H. Chen, S. L. Woo, and R. G. Grossman. 2000. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol. Ther. 1:195-203. [DOI] [PubMed] [Google Scholar]
- 49.Vermund, S. H. 1997. Transmission of HIV, p. 147-165. In V. DeVita, S. Hellman, and S. A. Rosenberg (ed.), AIDS, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 50.Xiang, Z. Q., S. Pasquini, and H. C. Ertl. 1999. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J. Immunol. 162:6716-6723. [PubMed] [Google Scholar]
- 51.Xiang, Z. Q., Y. Yang, J. M. Wilson, and H. C. Ertl. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219:220-227. [DOI] [PubMed] [Google Scholar]
- 52.Yang, Z., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034-1037. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Z. Y., L. S. Wyatt, W. P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]