Abstract
Sucrose (Suc) transporters belong to a large gene family. The physiological role of SUT1 proteins has been intensively investigated in higher plants, whereas that of SUT4 proteins is so far unknown. All three known Suc transporters from potato (Solanum tuberosum), SUT1, SUT2, and SUT4, are colocalized and their RNA levels not only follow a diurnal rhythm, but also oscillate in constant light. Here, we examined the physiological effects of transgenic potato plants on RNA interference (RNAi)-inactivated StSUT4 expression. The phenotype of StSUT4-RNAi plants includes early flowering, higher tuber production, and reduced sensitivity toward light enriched in far-red wavelength (i.e. in canopy shade). Inhibition of StSUT4 led to tuber production of the strict photoperiodic potato subsp. andigena even under noninductive long-day conditions. Accumulation of soluble sugars and Suc efflux from leaves of transgenic plants are modified in StSUT4-RNAi plants, leading to modified Suc levels in sink organs. StSUT4 expression of wild-type plants is induced by gibberellins and ethephon, and external supply of gibberellic acid leads to even more pronounced differences between wild-type and StSUT4-RNAi plants regarding tuber yield and internode elongation, indicating a reciprocal regulation of StSUT4 and gibberellins.
Phylogenetic analysis of the Suc transporter gene family shows redundancies in the SUT1 clade representing transporters involved in phloem loading and long-distance transport of Suc (Riesmeier et al., 1993; Kühn, 2003). The well-characterized members of the SUT1 family are highly expressed and essential in phloem loading. In contrast, the SUT2 and SUT4 families are represented with only one member each per species and expressed at a very low level, suggesting a function different from SUT1 (Kühn, 2003). For example, LeSUT2 plays an important role in pollen tube growth and pollen germination, thereby affecting fruit yield in tomato (Lycopersicon esculentum) plants (Hackel et al., 2006).
The function of the SUT4 protein remains to be elucidated. The activity of SUT4 proteins has been shown by Suc uptake experiments and yeast (Saccharomyces cerevisiae) complementation with AtSUT4 from Arabidopsis (Arabidopsis thaliana), StSUT4 from potato (Solanum tuberosum; Weise et al., 2000; Weschke et al., 2000), and the orthologous HvSUT2 from barley (Hordeum vulgare; Weise et al., 2000; Weschke et al., 2000). StSUT4 and LeSUT4 have been immunolocalized to the plasma membrane of phloem sieve elements in potato and tomato, respectively (Weise et al., 2000; Weschke et al., 2000), as was previously demonstrated for StSUT1 and LeSUT1 (Kühn et al., 1997). The yeast two-hybrid split ubiquitin system revealed interaction of the LeSUT4 protein with the colocalized LeSUT1 in yeast (Reinders et al., 2002). In comparison to StSUT1, the expression level of StSUT4 is very low (Weise et al., 2000). Therefore, the expression pattern and the function of SUT4 genes have not been analyzed in detail.
The aim of our work is the elucidation of the undercharacterized Suc transporter StSUT4 by help of transgenic plants. We addressed the putative role of StSUT4 in the regulation of flower induction and tuberization in potato plants. Potato is a short-day (SD) plant regarding tuberization and a long-day (LD) plant regarding flowering (Schittenhelm et al., 2004). Flower induction is a very well-investigated developmental process in higher plants and is mediated by different signal transduction pathways, including the photoperiod-dependent phytochrome signal transduction pathway, the Suc pathway, and the GA3 pathway (for review, see Searle and Coupland, 2004; Thomas, 2006). It is still unclear whether these flower-inducing pathways act independently or synergistically via a common signaling pathway.
Photoperiodic regulation is not only important to determine flowering time in many plants, but promotes tuberization in potato as well. Flowering tobacco (Nicotiana tabacum) shoots grafted onto potato stocks promote tuberization, indicating that the floral and tuber-inducing signals might be similar. Thus, common regulatory pathways were assumed to be involved in both flowering and tuberization responses (Rodriguez-Falcon et al., 2006). Overexpression of the Arabidopsis flowering-time gene CONSTANS induced tuberization in potato plants (Martinez-Garcia et al., 2002), and it is suggested that the function of the potato orthologs of CONSTANS and FLOWERING LOCUS T (FT) is conserved for tuberization control.
Inhibition of StSUT4 expression in transgenic potato plants by RNA interference (RNAi) leads to early flowering and increased tuber yield. The interrelation between the observed phenomena with photoperiodic control was tested by using the strictly photoperiodic potato subsp. andigena instead of subsp. tuberosum. Graft experiments showed that the flower and tuber-inducing stimulus is graft transmissible and requires the presence of source leaves, arguing for a phloem-mobile leaf-derived signal. In addition, StSUT4-RNAi plants do not alter the elongation of internodes, leaf angle, flowering, or apical dominance in response to shading by neighboring plants or in response to far-red light enrichment. They do not display what is summarized by the shade avoidance syndrome (SAS), suggesting that photoreceptor signaling is deregulated. It will be discussed whether there is convergence of the signal transduction mechanisms triggering flowering, tuberization, and shade avoidance response.
RESULTS
StSUT4 Is a Plasma Membrane Protein
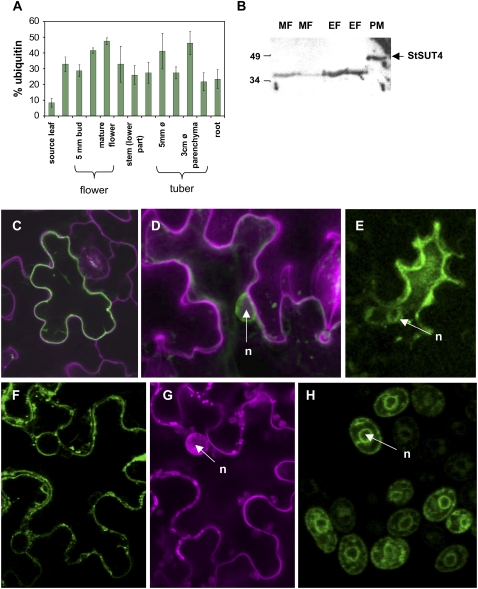
Whereas StSUT1 is mainly expressed in exporting source leaves (Riesmeier et al., 1993), the expression pattern of StSUT4 is highest in sink organs. The StSUT4 protein has previously been localized in phloem sieve elements (Weise et al., 2000), and our expression studies indicate that StSUT4 transcripts accumulate during flower and tuber development (Fig. 1A).
Figure 1.
A, Expression pattern of StSUT4 in sink and source organs as determined by real-time PCR. StSUT4 expression increases during flower development and strongest expression is detected in young developing tubers and mature flowers. B, Western-blot analysis of StSUT4 in leaves of potato. The microsomal fraction (MF) has been loaded in the first two lanes. Plasma membranes (PM) and endosomal membranes (EF) have been separated by two-phase partitioning and loaded on SDS-PAGE. In each lane, 15 μg of membrane proteins are loaded. StSUT4-specific peptide antibodies (Weise et al., 2000) detected the StSUT4 protein in the correct size of 47 kD only in the plasma membrane fraction. C, D, F, and G, Expression of StSUT4-GFP fusion expressed under the cauliflower mosaic virus 35S promoter in a pCF203 derivative in A. tumefaciens-infiltrated tobacco leaves. E, The same StSUT4-GFP construct expressed in infiltrated potato leaves. C, F, and G, Single scans. D and E, Overlay projections of confocal z stacks. GFP is not only detectable at the plasma membrane, but also in a perinuclear ring as shown by propidium iodide staining. F, StSUT4-GFP fluorescence is detectable at the plasma membrane of tobacco cells as well as in perinuclear rings. G, Same cell shown in F with propidium iodide-specific filter settings. H, Yeast cells expressing a LeSUT4-GFP construct under control of the Adh1 promoter in the yeast expression vector 112A1NE. GFP fluorescence is detected at the plasma membrane and in ER stacks surrounding the nucleus. n, Nucleus.
StSUT4 is functional in Suc uptake in yeast cells (Weise et al., 2000). Thus, plasma membrane-specific localization is a prerequisite for this Suc uptake. Two-phase partitioning of plant microsomal membranes separating the plasma membrane and endosomal membrane fraction revealed preferential localization of StSUT4 in the plasma membrane fraction (Fig. 1B) as shown by the use of a StSUT4-specific peptide antibody (Weise et al., 2000). In the microsomal and endosomal fraction, a smaller band of 38 kD is recognized by the StSUT4-specific antibody, which might represent a degradation product of the StSUT4 protein. Western-blot analysis was confirmed by localization studies with a StSUT4-GFP fusion protein in infiltrated tobacco and potato leaves. The protein was localized at the plasma membrane (Fig. 1C) and, in addition, in a perinuclear ring (Fig. 1, D–G). A similar phenomenon is observed when LeSUT4 from tomato fused to GFP is expressed in yeast cells (Fig. 1H). Expression of GFP fusion constructs in yeast and plant cells is interpreted as dual targeting of SUT4 to both the plasma membrane and endomembranes surrounding the nucleus.
Suc Transporters Show Oscillation in Constant Light
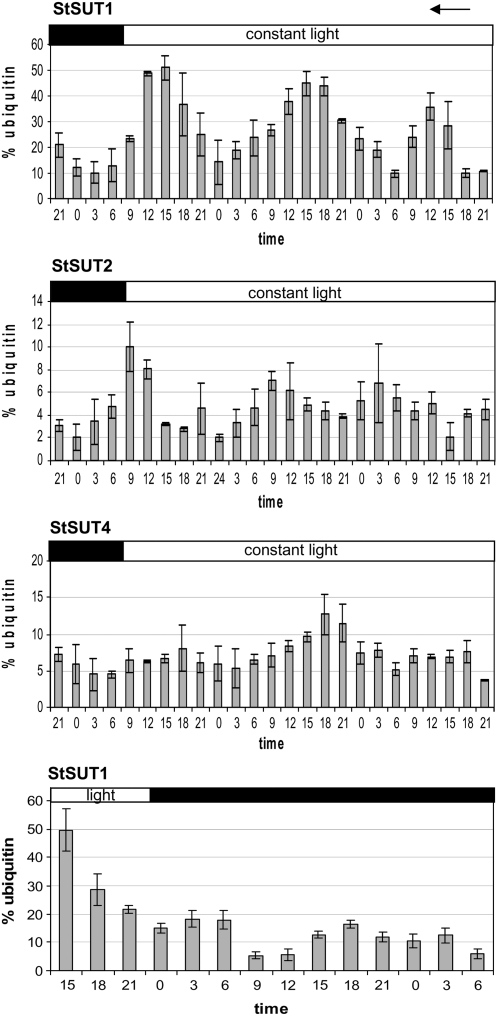
SUT1 transcript levels from tomato show diurnal oscillation with the highest expression at the end of the light period (Kühn et al., 1997). We performed real-time PCR analysis of all known Suc transporter genes in wild-type potato plants. Figure 2 represents oscillating Suc transporter transcript levels in potato leaves under constant light conditions. StSUT1 and StSUT4 show a similar expression pattern with maximal transcript levels in the middle of the light period, whereas StSUT2 mRNA peaks at the beginning of the light period (Fig. 2B). RNA levels continue oscillation within 64 h of continuous illumination, arguing for circadian regulation of Suc transporter gene expression. Moreover, elements for circadian regulation of transcription were found in the promoter sequences of Suc transporters when analyzed with the Web Signal Scan Program (http://www.dna.affrc.go.jp/sigscan/signal1.pl). According to Harmer and Kay, an imperfect evening element with the consensus AAAATATCT is present in the LeSUT1 promoter sequence (Harmer and Kay, 2005).
Figure 2.
Quantification of Suc transporter mRNA accumulation by real-time PCR analysis in constant light and StSUT1 transcript quantification in constant darkness. All three known Suc transporters from potato are expressed diurnally with distinct maxima. StSUT2 shows peak levels at the beginning of the light period, whereas StSUT1 and StSUT4 show maximal transcript accumulation at the end of the light period. Oscillation of transcript amounts is continuous even under 72 h of constant light. The amplitude of StSUT1 oscillation strongly decreases in constant darkness. Relative quantification was performed with ubiquitin as internal standard. sd is given.
Inhibition of SUT4 Leads to Early Flowering and Tuberization
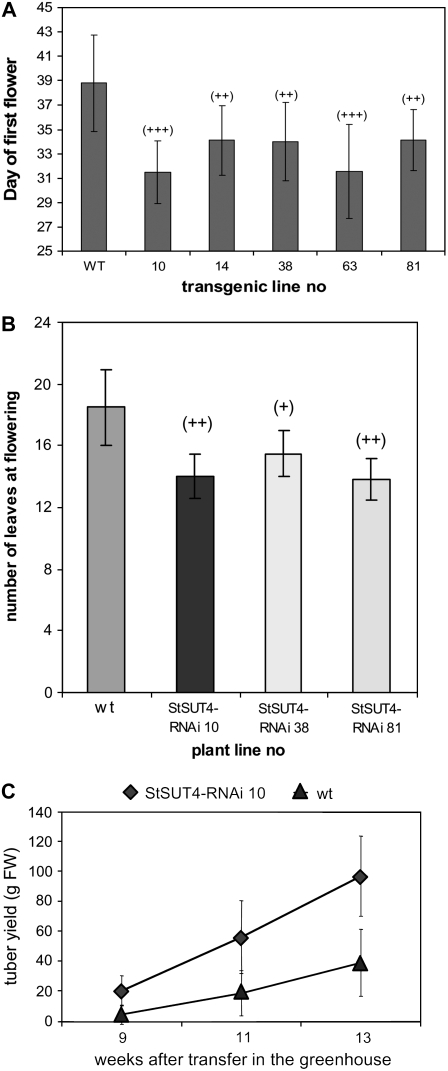
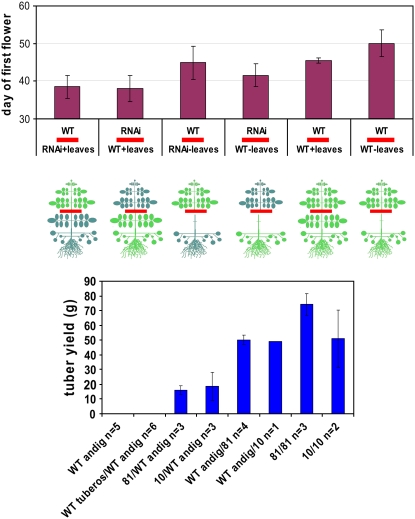
As many as nine independent transformant lines have been identified with efficiently down-regulated expression of the StSUT4 gene (Supplemental Fig. S1). PCR analysis confirmed the presence of the StSUT4-RNAi construct and real-time PCR revealed reduction of StSUT4 expression in the tissue of highest endogenous StSUT4 RNA content. StSUT4 expression level is reduced up to 67% in flowers compared to wild-type flowers. StSUT4 inactivation is specific and StSUT1 expression was not significantly altered in StSUT4-RNAi plants (Supplemental Fig. S2). Seven transformant lines with significant reduction of StSUT4 expression in flowers were selected for further analysis. After 4 weeks of growth under LD conditions, potato plants with reduced StSUT4 expression started to flower at least 6 d before wild-type flowering (Figs. 3A and 4A). StSUT4-RNAi plants had significantly fewer leaves at flowering time than wild-type plants (Fig. 4A). Neither potato ‘Désirée’ wild-type plants nor StSUT4-RNAi plants were able to flower under SD conditions. The selected transformants showed reduced internode elongation regardless of the day length or light quality (Figs. 3B and 8B; Supplemental Fig. S3). Moreover, they tuberized earlier than control plants and showed significantly increased tuber yields (with P < 0.05) when grown under LD conditions (Fig. 4B; Table I) and only slightly increased yield when grown under SD conditions compared to wild-type plants (data not shown).
Figure 3.
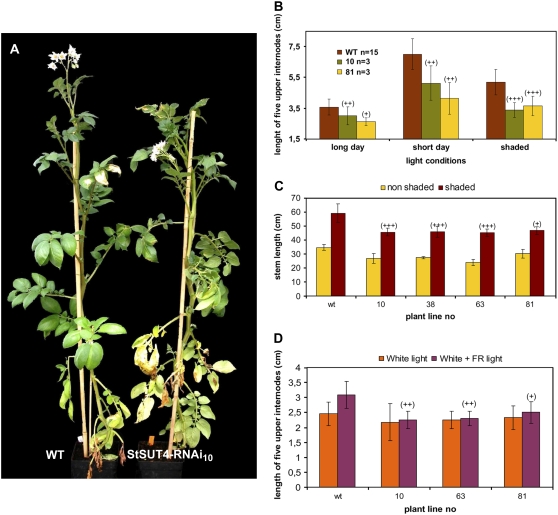
Phenotype of StSUT4-RNAi plants. A, Plants with reduced StSUT4 expression show early flowering under LD conditions. ‘Désirée’ wild-type and StSUT4-RNAi10 plants after 5 weeks in the greenhouse. B, Internode elongation of potato ‘Désirée’ wild-type and StSUT4-RNAi plants. Quantification of internode elongation is shown in Figure 9C. C, Internodes of transformed potato andigena plants are reduced in length in comparison with andigena wild-type plants. andigena plants transformed with a StSUT4-RNAi construct showing reduced StSUT4 transcript levels are able to produce tubers even under LD conditions (D), whereas andigena wild type does not (E). StSUT4-RNAi2/5 shows early flowering compared to potato andigena wild-type plants grown under LD conditions (F).
Figure 4.
A, StSUT4-RNAi ‘Désirée’ plants flower on average 6 d earlier than wild-type plants when grown under LD conditions. Flowering was observed with StSUT4-RNAi lines 10 (n = 8), 81 (n = 9), and 38 (n = 9) and potato subsp. tuberosum plants (n = 23) grown in the greenhouse under LD conditions. B, StSUT4-RNAi ‘Désirée’ plants have significantly fewer leaves at flowering if grown under LD conditions in the greenhouse (n = 6 for each plant line). C, The tuber yield of StSUT4-RNAi potato plants is significantly increased under noninductive LD. All experiments are reproduced at least three times. One representative example is given (n = 5–6 for each line and each time point). sd is given.
Figure 8.
A, To reduce the red to far-red ratio for greenhouse-grown plants, they were planted at a density of 21 plants m−2. Wild-type plants show shade avoidance response under canopy shade, showing elongated internodes and hyponastic leaf movement to capture light under crowded conditions (left side). StSUT4-RNAi plants do not show shade avoidance under canopy shade. Neither internode elongation nor leaf angle adaptation was observed (right side). B, Internode elongation of wild-type and StSUT4-RNAi potato plants grown under LD and SD conditions or in high-density populations under LD conditions. The experiment was reproduced in the greenhouse and in the growth chamber under LD and SD conditions showing the same results in each case. The length of the five upper internodes was measured as described by Martinez-Garcia et al. (2002). C, Internode elongation of potato plants grown under canopy shade in the greenhouse. Shaded plants were grown at high plant density (21 plants m−2), whereas control plants were grown at low density (7 plants m−2). D, Internode elongation of potato plants grown under artificial light conditions in the phytochamber. Internode length was measured after 3 weeks of growth under white light or under white light with additional far-red light. sd is given.
Table I.
Tuber yield of potato wild-type and StSUT4-RNAi plants (potato ‘Désirée’) in grams fresh weight after 3 months of growth in the greenhouse under LD conditions
Mean values of four independent experiments are represented (with n = 3–11 plants/line and experiment); sd is given. Tuber yield of StSUT4-RNAi plants is significantly increased (with P < 0.05).
| Plant Line No. | Tuber Yield | sd | Significance |
|---|---|---|---|
| g fresh wt | |||
| Wild type | 144.6 | 49.5 | |
| StSUT4-RNAi10 | 183.4 | 57.5 | (+) |
| StSUT4-RNAi14 | 178.1 | 46.8 | (+) |
| StSUT4-RNAi38 | 181.2 | 59.3 | (+) |
| StSUT4-RNAi81 | 192.6 | 61.7 | (++) |
| StSUT4-RNAi10 | 181.7 | 59.1 | (+) |
Whereas potato subsp. tuberosum is able to tuberize under LD conditions, potato subsp. andigena requires an obligatory SD period for tuberization and does not tuberize under LD conditions (Jackson et al., 1998). We tested whether the effect of StSUT4 on tuberization depends on the day length. Transformation of the strictly photoperiodic potato subsp. andigena was performed with the same StSUT4-RNAi construct as used for transformation of potato ‘Désirée’. Six independent transformant lines with reduced StSUT4 expression were selected (Supplemental Fig. S1; up to 50% reduction of the StSUT4 expression level in source leaves of transgenic potato plants). All six transformant lines (1) were able to produce tubers in at least two out of three independent experiments when grown in the greenhouse under noninductive LD conditions (Fig. 3, D and E); (2) had shorter internodes (Fig. 3C); and (3) showed early flowering compared to andigena wild-type plants (Fig. 3F). The ability to produce tubers even under LD conditions is correlated with a reduction of the StSUT4 expression in these plants (Supplemental Fig. S1). Thus, nine independent transgenic lines of potato subsp. tuberosum and six independent transgenic lines of the photoperiodic potato subsp. andigena show similar effects on flowering and tuberization upon inhibition of StSUT4 expression.
SUT4-Mediated Flower and Tuber Induction Is Graft Transmissible
To analyze whether the flower and tuber-inducing signal is graft transmissible, reciprocal grafts were performed with transgenic StSUT4-RNAi and wild-type potato plants (subsp. tuberosum and subsp. andigena) after the plants had developed four to five leaves, and with or without removal of the source leaves of the corresponding graft rootstock. Flowering time was recorded and tubers of grafted plants were harvested 3 months after transfer of the plants into the greenhouse.
Wild-type potato plants from both species, which were grafted with StSUT4-RNAi plants that included their leaves, showed a similar phenotype as transgenic StSUT4-RNAi plants: They displayed early flowering (Fig. 5A) and produced higher amounts of tubers as compared to grafts with control plants (Fig. 5C). Therefore, it is suggested that a phloem-mobile information molecule is involved in triggering flowering time and tuberization in a SUT4-dependent manner. Both early flowering as well as higher tuber yield strongly depend on the presence of source leaves of the rootstock regardless of its genotype. It cannot be excluded that the reduced total leaf area available for assimilation has an impact on the tuber yield.
Figure 5.
A, Flowering behavior of grafted potato wild-type plants under LD conditions when grafted with StSUT4-RNAi plants. Early flowering is also observed in wild-type plants if grafted with StSUT4-RNAi plants, depending on the presence of source leaves at the rootstock. B, Schematic representation of graft experiments. Plants were regenerated from tubers and grafted after development of the first six leaves. Graft experiments were repeated twice with potato ‘Désirée’ with six reciprocal grafts from each transgenic line per experiment. C, Tuber yield of grafted StSUT4-RNAi potato plants (subsp. tuberosum) grafted on potato wild-type plants (subsp. andigena), which do not tuberize under LD conditions. Plants were grafted when they have four to five leaves and were kept under LD conditions. As a control, transgenic plants were grafted on their own rootstock (right, StSUT4-RNAi81 grafted on StSUT4-RNAi81 and StSUT4-RNAi10 grafted on StSUT4-RNAi10) and potato subsp. tuberosum wild type was grafted on potato subsp. andigena, which did not lead to tuber production (left, wild-type tuberosum grafted on wild-type andigena). sd is given. andig, andigena; tuberos, tuberosum.
SUT4 Inhibition Induces Increased Suc Efflux and Changes in Sugar Accumulation
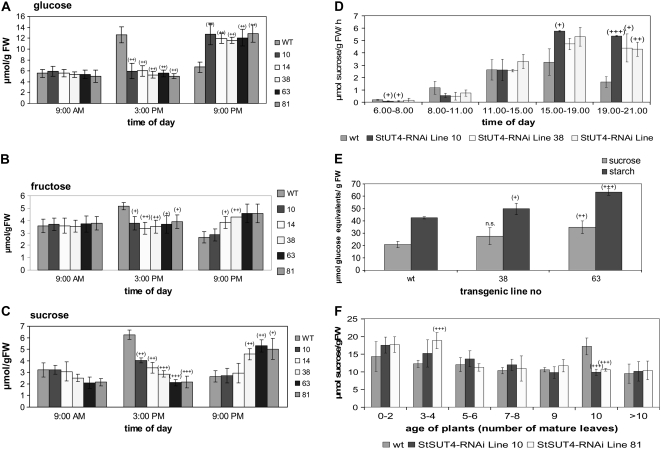
Detailed analysis of the content of soluble sugars at different time points over the day revealed significant differences between wild-type and RNAi plants, depending on the time of day (Fig. 6, A–C). Whereas soluble sugars are present in lower concentration in the source leaves of transgenic plants than in those of wild-type plants at the beginning and in the middle of the light period, they accumulate to much higher amounts at the end of the light period. This increase in the content of soluble sugars in StSUT4-RNAi plants is accompanied by an increase in Suc efflux. Suc export from petioles of transgenic plants as measured by exudation in the presence of EDTA was twice as much as in wild-type plants at the end of the light period (Fig. 6D).
Figure 6.
A to C, Content of soluble sugars in source leaves of StSUT4-RNAi plants compared to potato wild-type plants determined enzymatically. At the end of the light period, transgenic plants show significantly increased Glc (A), Fru (B), and Suc (C) content per gram fresh weight. D, Efflux of Suc from leaves of wild-type and StSUT4-RNAi plants was determined by exudation in the presence of EDTA. Suc exudation was determined enzymatically in intervals of 3 h during the light period from plants kept under LD conditions in the greenhouse. Suc efflux from wild-type leaves shows maxima at the end of the light period, whereas in StSUT4-RNAi plants Suc efflux remains high even in darkness. E, Suc and starch content of in vitro-grown microtubers (n = 4 for each plant line). Tubers were harvested 20 d after tuber induction in darkness. sd is given. F, Suc content in the shoot apical meristem of potato wild-type and StSUT4-RNAi plants. Samples were taken at the end of the light period (9 pm). Fresh weight of samples was between 20 and 60 mg. Error bars indicate sd. Experiments were performed under LD conditions. Note that floral buds of StSUT4-RNAi plants were first detected when plants had five to six mature leaves, whereas wild-type potato plants started transition from the vegetative to the generative phase when they had >10 leaves.
As expected, due to the strong increase of Suc efflux rates at the end of the light period, Suc content in sink organs was consequently affected as well. Suc as well as starch content are significantly increased in in vitro-induced microtubers of StSUT4-RNAi plants compared to wild-type tubers (Fig. 6E). Suc content in the shoot apical meristems was measured at different developmental stages (Fig. 6F). Whereas the content of Glc and Fru was not significantly changed between wild-type and transgenic plants, the level of Suc differs conspicuously. As already described in Arabidopsis (Eriksson et al., 2006), a Suc peak can be observed in the shoot apical meristem shortly before flower onset when the wild type undergoes transition from the vegetative to the generative phase. In contrast, Suc levels in StSUT4-RNAi apical meristems show peak levels much earlier. This correlates with early flower induction in transgenic plants. Floral buds are detectable when the transgenics developed five to six mature leaves, whereas wild-type plants had more than 10 mature leaves when first buds are visible (Fig. 6F). Thus, modified Suc efflux from leaves is accompanied by changes of Suc levels in terminal sinks.
StSUT4-RNAi Plants Do Not Show Shade Avoidance
Transgenic plants have shorter stems due to reduced internode elongation, and show early flowering and higher tuber yield. Tuberization in potato is negatively controlled by GAs and phytochrome B (phyB; Jackson and Prat, 1996). PhyB is involved in the photoperiodic control of tuberization in potato subsp. andigena (Jackson and Prat, 1996). Plants with reduced levels of phyB tuberize in LD as well as in SD conditions, whereas wild-type plants will only tuberize under SD conditions (Jackson et al., 1996). StSUT4-RNAi plants are comparable to phyB antisense plants regarding tuberization and flowering (Jackson et al., 1998). They are also comparable to transgenic plants with reduced biosynthesis of GAs because these also show shorter stems, reduced internode elongation, and tuberized earlier (Carrera et al., 2000). The phenotype of StSUT4-RNAi plants would therefore be consistent with a reduced amount of phyB or a reduced biosynthetic capacity for GA3s. Transcript levels of phyA and phyB in StSUT4-RNAi plants were determined by quantitative real-time PCR. Neither phyB nor phyA expression was significantly affected in StSUT4-RNAi plants (data not shown).
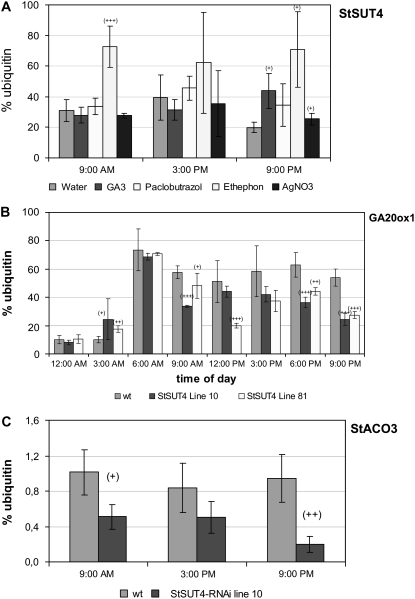
At least for tobacco plants it was shown that ethylene and GAs interact in phy-mediated SAS (Pierik et al., 2004a). To test whether StSUT4 is under phytohormonal control, wild-type potato plants were treated with GA3 and paclobutrazol; with ethephon, a soluble ethylene precursor; and with silver nitrate, an efficient inhibitor of the ethylene receptor. Here, we observed that StSUT4 expression in wild-type potato plants was induced by GA3 treatment at the end of the light period, and a significant increase of the SUT4 expression was also observed in ethephon-treated wild-type leaves at all time points (Fig. 7A).
Figure 7.
A, Transcript levels of StSUT4, the GA biosynthetic enzyme GA20ox1, and the ethylene biosynthetic enzyme StACO3 in StSUT4-RNAi plants as determined by quantitative real-time PCR. A, StSUT4 expression in potato leaves treated with phytohormones and phytohormone inhibitors paclobutrazol (inhibitor of GA biosynthesis) and silver nitrate (inhibitor of the ethylene receptor). StSUT4 expression is inducible by GA3 at the end of the light period and by ethephon treatment over the whole light period. Potato wild-type plants were treated with 20 μm GA3, 350 μm paclobutrazol, 350 μm ethephon, or 1 mm silver nitrate, and StSUT4 mRNA was determined by real-time PCR analysis relative to the level of ubiquitin transcripts. B, Quantification of transcripts of the GA biosynthetic enzyme GA20ox1 in wild-type and StSUT4-RNAi plants showing reduced levels of GA20ox1 in source leaves of StSUT4-RNAi plants at the end of the light period if compared to wild-type levels. C, Quantification of the transcripts of the ethylene biosynthetic enzyme StACO3 in wild-type and StSUT4-RNAi plants showing significantly reduced levels of StACO3 mRNA levels in StSUT4-RNAi plants at any time. Ubiquitin transcript levels were used as internal standard for relative quantification in all experiments. Experiments were performed with greenhouse plants grown under LD conditions. sd is given.
Transcript levels of both ethylene and GA biosynthetic key enzymes were determined by quantitative real-time PCR. Quantification of the transcript level of the GA biosynthetic enzyme GA20ox1 as well as 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase StACO3 showed significant changes in StSUT4-RNAi plants compared to potato wild-type plants (Fig. 7, B and C). The level of GA20ox1 is significantly decreased in StSUT4-RNAi plants at dawn, which might explain the increase in tuber yield and the reduction of internode elongation (Fig. 7B).
StSUT4 expression is not only increased by GAs, but also by ethephon treatment, a precursor of ethylene (Fig. 7A). Quantification of the transcript level of the ethylene biosynthetic enzyme ACC oxidase StACO3 was found to be significantly decreased in StSUT4-RNAi plants at any time of day (Fig. 7C). Ethylene as well as GA biosynthesis might therefore be affected in StSUT4-RNAi plants.
Stem elongation and early flowering belong to SAS. The shade avoidance response is phyB-mediated and antagonized by phyA (Vandenbussche et al., 2005). To test the ability of transgenic plants to display the phyB-mediated shade avoidance response, plants were grown at high density to shade the source leaves by the canopy of neighboring plants. Although the phyB transcript level is not decreased in StSUT4-RNAi plants, the typical shade avoidance response cannot be observed. Stem elongation under decreased red to far-red light ratio by canopy shade increased to a much lower extent in StSUT4-RNAi plants than in wild-type plants (Fig. 8).
The experiment was repeated under artificial shade conditions in the phytochamber, where plants were grown under white light or under white light with an additional source of far-red light (>730 nm). After 3 weeks of growth under these artificial shade conditions, internode elongation of StSUT4-RNAi plants was not significantly increased if compared to identical plants grown under white light, whereas wild-type plants showed significantly increased internode elongation and stem length, as expected (Fig. 8, C and D). Thus, StSUT4-RNAi plants behave similarly under canopy shade as under far-red light enrichment.
GA Signaling Is Affected in StSUT4-RNAi Plants
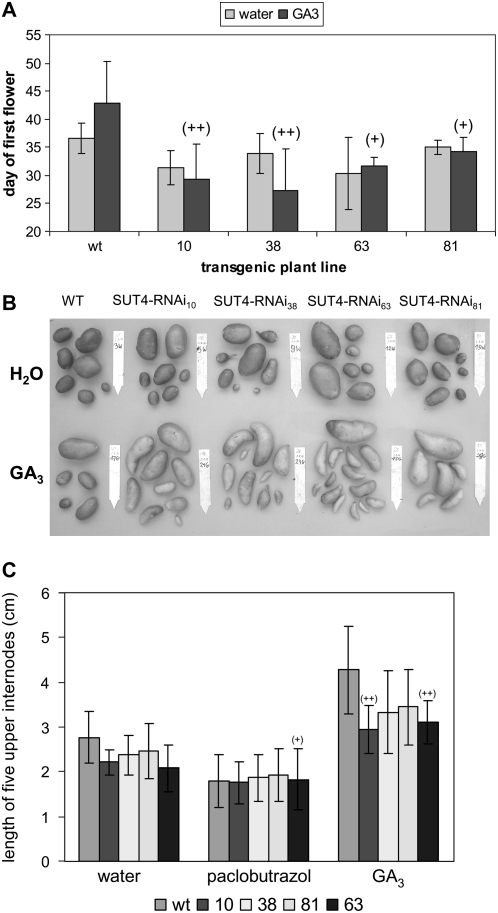
Because StSUT4-RNAi plants behave similar to GA20ox1 antisense potato plants regarding internode elongation, stem length, and tuberization (Carrera et al., 2000), we aimed to rescue the wild-type phenotype of our RNAi plants by application of external GA3. GA3 was sprayed directly on source leaves over a 2-week period in a concentration of 20 μm supplemented with Triton X-100, whereas control plants were treated with water with Triton X-100. The exogenous GA3 supplied was not able to rescue the wild-type phenotype regarding stem elongation, tuber yield, or flowering (Fig. 9). The differences between wild-type and transgenic plants were even more pronounced than in water-treated plants.
Figure 9.
GA3 treatment of potato wild-type and StSUT4-RNAi plants grown under LD conditions in the greenhouse. A, Source leaves were treated with 20 μm GA3 solution every 2 d over a period of 2 weeks. Flowering was analyzed after the indicated period of time. B, Tubers were harvested after 2 months of growth in the greenhouse. Water-treated StSUT4-RNAi plants show higher tuber yield than water-treated wild-type plants due to increased tuber size, whereas GA3-treated StSUT4-RNAi plants show higher tuber number and tuber size than the wild-type control. C, Internode elongation of potato wild-type and StSUT4 plants treated with GA3 or paclobutrazol. Error bars indicate sd.
Regarding tuber yield, not only the tuber size was increased in StSUT4-RNAi plants, but also the tuber number (Fig. 9B), indicating that not only the starch accumulation and tuber development is disturbed but also the induction of tuberization. However, paclobutrazol, a specific inhibitor of GA biosynthesis, was able to mimic the StSUT4-RNAi phenotype in wild-type plants. Paclobutrazol treatment resulted in the same internode length in wild-type and StSUT4-RNAi plants (Fig. 9C), indicating that the GA-induced response is already impaired in StSUT4-RNAi plants.
DISCUSSION
Localization of SUT4-GFP in Plant Cells
Members of the SUT4 subfamily, namely, AtSUT4 from Arabidopsis, StSUT4 from potato, and HvSUT2, the SUT4 ortholog in barley, are able to contribute to the 14C-Suc uptake into yeast cells at the plasma membrane (Weise et al., 2000; Weschke et al., 2000). StSUT4 and LeSUT4 have been immunolocalized at the plasma membrane of sieve elements, and AtSUT4 promoter∷GUS studies revealed the highest AtSUT4 expression in minor veins (Weise et al., 2000). Our localization experiments using GFP constructs are consistent with these previous results (Fig. 1). The StSUT4-GFP fusion protein was found at the plasma membrane of infiltrated tobacco and potato leaves, and a LeSUT4-GFP fusion protein was localized at the plasma membrane of yeast cells (Fig. 1). Nevertheless, members of the SUT4 family have been identified by proteomic approaches either in the chloroplastic fraction in Arabidopsis (Rolland et al., 2003) or in the vacuolar fraction in Arabidopsis and barley (Endler et al., 2006). SUT4-mediated Suc uptake in yeast cells was explained by mistargeting of the proteins in yeast, where it is detectable at the plasma membrane as well as in internal membrane structures. Our localization data with StSUT4-GFP in plant cells revealed a very similar distribution of the fusion protein both at the plasma membrane and in internal membranes surrounding the nucleus (Fig. 1). We suggest that SUT4 from Solanaceae is located in both the plasma membrane and the endomembrane system and undergoes dual targeting. It cannot be excluded that localization of the SUT4 protein underlies dynamic changes leading to localization in different compartments. Species-specific differences might be the reason for different localization of AtSUT4 and StSUT4.
StSUT4 Affects Suc Efflux from Leaves and Suc Levels in Sinks
SUT1 is the most important Suc transporter for the efflux of Suc from mature leaves because it is highly expressed in source leaves. SUT2 and SUT4 expression is more prominent in sink tissues (Fig. 1A). Our transcript analysis of StSUT4 confirms its low expression and revealed a significant reduction of StSUT4 in flowers of StSUT4-RNAi plants (Supplemental Fig. S1). StSUT1 mRNA levels are unaffected in StSUT4-RNAi plants (Supplemental Fig. S2). Thus, transcriptional control of SUT1 via SUT4 is unlikely. Nevertheless, Suc efflux from leaves is significantly increased at the end of the light period in StSUT4-RNAi plants and Suc content is increased in in vitro-grown tubers and at earlier stages in the shoot apical meristem of StSUT4-RNAi plants (Fig. 6). Changes in source-to-sink allocation in transgenic plants might be one reason for the early onset of flowering and tuberization.
Overexpression of a SUT1 gene in transgenic tobacco plants leads to a similar early-flowering phenotype as observed for StSUT4 inhibition in potato plants (Riesmeier and Frommer, 1994). StSUT4 might play a role as an inhibitor of StSUT1, and increased Suc efflux from leaves of StSUT4-RNAi plants is then explained by the missing StSUT4-mediated StSUT1 inhibition.
It is known from yeast two-hybrid studies that the LeSUT4 protein is able to interact with LeSUT1 protein in yeast (Reinders et al., 2002). Therefore, posttranslational regulation by heterodimerization of StSUT1, the main phloem loader in potato, and StSUT4 protein cannot be excluded.
SUT4 Is Involved in Shade Avoidance
SAS is a very complex reaction of plants toward canopy shade of neighboring plants involving photoperiodic control and the interaction of phytochromes and blue-light receptors. SAS is not only triggered by the red to far-red light ratio via phytochromes, but also by a reduction of blue light under canopy shade (Pierik et al., 2004b). Circadian gating also plays an important role in the shade avoidance response (Vandenbussche et al., 2005).
PhyB is known to inhibit flowering in LD plants like Arabidopsis because phyB mutants show early flowering (Guo et al., 1998). Antisense potato plants, in which phyB expression is down-regulated, tuberize earlier under LD conditions (Jackson et al., 1996, 1998) as found for StSUT4-RNAi plants. It was shown that phyB, at least in LD plants, affects flowering negatively via inhibition of CONSTANS and FT (Cerdan and Chory, 2003; Endo et al., 2005).
Thus, phyB inhibits tuberization and flowering in LD conditions and is relevant for the induction of a shade avoidance response under a far-red-enriched light regime. StSUT4-RNAi plants flower and tuberize earlier and do not show shade avoidance response, suggesting that mediation of the photoreceptor signal transduction correlates with sufficient SUT4 levels. Because StSUT4-RNAi plants have a lower phyB-mediated shade avoidance response, phy-mediated inhibition of flowering parallels with StSUT4 expression in potato plants. Thus, in StSUT4-RNAi plants, the phyB transcript level is unaffected, but StSUT4 seems to be required to transfer the phyB-emitted signal further downstream.
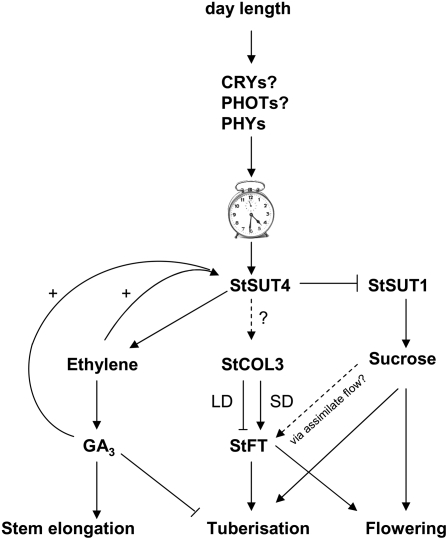
StSUT4 might act downstream of photoreceptors detecting the light quality in source leaves, and upstream of ethylene and GAs (as summarized in Fig. 10). So far, it is known that both photoreceptors, sugar, and phytohormones such as ethylene and GAs are involved in shade avoidance (Pierik et al., 2004a; Kozuka et al., 2005), but it is still unclear how these two signaling pathways are interconnected to each other.
Figure 10.
Hypothetical model of StSUT4-mediated interconnection of the photoreceptor and the GA3 signaling pathway triggering tuberization, flowering, and shade avoidance response. The model is partially adapted from Rodriguez-Falcon et al. (2006).
SUT4 Is Involved in GA Signaling
PhyB action negatively affects flowering in LD plants and inhibits tuberization in potato plants (Jackson and Prat, 1996; Endo et al., 2005). Graft experiments between phyB antisense and wild-type potato plants revealed that a graft-transmissible inhibitor of tuberization is responsible for inhibition of potato tuber induction under noninductive LD conditions (Jackson et al., 1998). It is also known that phytochromes act by transferring a leaf-derived signal toward the shoot apical meristem to induce flowering (Valverde et al., 2004).
In tobacco plants, the phy-mediated shade avoidance response involves ethylene action by modulating GA action (Pierik et al., 2004a). It is also known that phyB and light regulate GA3 biosynthesis (Reed et al., 1996).
The phenotype of StSUT4-RNAi plants including decreased length of internodes and early tuberization leading to higher tuber yields was exactly described for plants with reduced expression of GA20ox1 (Carrera et al., 2000). In addition, StSUT4-RNAi plants show early flowering. The overall phenotype of StSUT4-RNAi plants also includes a reduced level of GA20ox1 at the end of the day and is in accordance with reduced biosynthesis of GAs. Thus, reciprocal regulation of StSUT4 and GAs is assumed.
Feedback control of GA3 biosynthetic enzymes by GA3 and diurnal oscillation in potato under SD conditions has already been described (Carrera et al., 1999). External application of GAs to StSUT4-RNAi leaves was not able to rescue the wild-type phenotype. The reason might be the negative feedback regulation of GA20ox1 by external GA3 application (Carrera et al., 1999), leading to even more severe effects in StSUT4-RNAi plants where GA biosynthesis is already down-regulated. Involvement of StSUT4 in GA signaling is strongly supported by the fact that inhibition of GA biosynthesis by paclobutrazol affects stem elongation of wild-type potato plants, mimicking the phenotype of StSUT4 inhibition and leading to the same internode length in both sets of plants.
Suc as a Signaling Molecule
Strong expression of StSUT4 in flowers and tubers argues for an important role of this membrane protein in sink organs. Nevertheless, the observed effects regarding photoperiodically regulated developmental processes in StSUT4-RNAi plants like early flowering and tuberization under LD conditions are graft transmissible and depend on the presence or absence of source leaves, indicating an important role of SUT4 not only in sink tissues, but also in source leaves where photoperception occurs. Therefore, a long-distance component is needed to transmit the information from photoreceptors in leaves to GA biosynthesis in tubers and flower induction in the shoot apical meristem. Several phloem-mobile signaling molecules are discussed and Suc itself might play a role as a phloem-mobile signaling molecule (Smeekens, 2000).
It is also discussed that assimilates act as a part of a complex flowering signal (Bernier and Perilleux, 2005) because photosynthesis and photoperiodism were shown to interact in flower induction (Friend, 1984). The Arabidopsis flowering time in noninductive SD conditions is determined by sharp increases of GA4 and Suc in the apical meristem shortly before flower initiation (Eriksson et al., 2006), and both GAs and Suc are discussed to be part of the florigenic signal. Alternatively, phloem mobility of FT might be dependent upon a sufficient mass flow of assimilates (Thomas, 2006). It is known that tuberization in potato depends on StCOL3 and StFT interplay (Rodriguez-Falcon et al., 2006), and we showed that accumulation of Suc transporter mRNAs follows circadian oscillation. Thus, it cannot be excluded that StSUT4 affects the photoperiodic pathway via the level of florigenic and tuberigenic proteins StCOL3 and StFT (as postulated in the model in Fig. 10).
We were able to show that peak Suc levels are detectable earlier in the apical meristem of StSUT4-RNAi plants, which is a strong argument for the Suc molecule to be necessary to build up a flower-inducing component in potato plants. Temporal and spatial fine tuning of Suc concentrations as well as GA levels seems to be extremely important to integrate flower and tuber-inducing mechanisms.
Therefore, we conclusively suggest that StSUT4 seems to play an important role in the interconnection of carbon availability with flower-inducing mechanisms, thereby linking light quality with light quantity effects on flowering and tuberization.
MATERIALS AND METHODS
Recombinant DNA
Isolation of StSUT4 cDNA was described previously (Weise et al., 2000). For GFP fusion, the multiple cloning site of the vector pCF203 was modified and additional restriction sites were inserted via synthetic oligo linker (SacI, KpnI, SpeI, XbaI, XhoI, BamHI cloned into the SacI and BamHI restrictions sites of pCF203). pCF203 carries GFP under the control of the cauliflower mosaic virus 35S promoter. StSUT4 cDNA was amplified with primers with restriction sites for KpnI and EcoRV (forward, TATGGTACCATGCCGGAGATATAGAAAGG and reverse, GATGAATATCTGTGCAAAGATCTTGGGTTTC) and cloned in the modified pCF203 linearized with BamHI, blunted, and redigested with KpnI. For LeSUT4 fusion to GFP, LeSUT4 was amplified from cDNA using proofreading DNA polymerase and cloned via PstI and NotI restriction sites together with the NotI-EcoRI fragment of GFP into the yeast (Saccharomyces cerevisiae) expression vector 112A1NE (Riesmeier et al., 1992) linearized with PstI and EcoRI.
The RNAi construct was cloned into the pRT 100 derivative (Töpfer et al., 1987), pRT-RNAi (Hirner et al., 2006) kindly provided by Axel Hirner, and transferred into pJH212. For construction of the RNAi construct, a 989-bp fragment of StSUT4 cDNA was amplified with primers forward, TATGGTACCATGCCGGAGATATAGAAAGG and reverse, GAGACTCGAGTGCAAAGATCTTGGGTTTCTC, digested with XhoI and SmaI, and cloned into the SalI and SmaI sites of pRT-RNAi. A second StSUT4 fragment (XhoI-SmaI digested) was inserted via the XhoI and Ecl136I sites into pRT-RNAi. A 3.5-kb PstI fragment containing both StSUT4 fragments was afterward transferred into the PstI site of pJH212, a pPZP212 derivative.
Plant Transformation
Gene transfer into plants was performed with Agrobacterium tumefaciens (strain C58C1, pGV2260; Deblaere et al., 1985). Potato (Solanum tuberosum) was transformed according to the method described (Rocha-Sosa et al., 1989) with small modifications. Regenerated plants were screened by PCR for integration of the construct using NPTII and StSUT4 primers (primer sequences: NPTIIa, ACCGGATCTGGATCGTTTCG; NPTIIb, TTGGTCCCTCATTTCGAACC; StSUT4-RNAi, GAGACTCGAGTGCAAGATCTTGGGTTTCTC; intron out reverse, GATGATTTATGTATATAACAACG). Plants containing integrated DNA were amplified in tissue culture and placed in the greenhouse for further analysis. Experiments were carried out with either in vitro-propagated clones or tuber-regenerated plants.
Plant Growth Conditions and Tissue Culture
Potato plants in sterile culture were grown on 2× Murashige and Skoog medium (Murashige and Skoog, 1962) with 2% Suc in tissue culture chambers at 24°C, at 50% humidity, and 1,000 μmol photons m−2 s−1 with a 16-h-light/8-h-dark cycle. Following transformation, leaf discs were put on 2× Murashige and Skoog medium with 1 μg/L naphthylacetic acid and 0.1 μg/L benzylaminopurine. For selection of transformant tissue, 3× Murashige and Skoog medium with 2 μg/L zeatin and 35 μg/L kanamycin was used. Root induction of plantlets was performed on 2× Murashige and Skoog medium with 2 μg/L indol butyric acid and 50 μg/L kanamycin. After 2 weeks, plantlets were placed on 2× Murashige and Skoog medium containing 50 μg/L kanamycin.
In Vitro Tuberization Assay
Stem segments including at least one node of 6-week-old sterile potato plants were prepared under sterile conditions and planted on Murashige and Skoog medium containing 10% Suc. After 1 week under LD conditions in the growth chamber (16 h light/8 h dark, 24°C), scions were transferred into darkness to induce tubers. In vitro tubers were harvested after 20 d.
Greenhouse
Transgenic plants were amplified in tissue culture and 60 plants were transferred to soil and grown in a cycle of 16 h light (22°C)/8 h dark (15°C) in 60% humidity. The mean photosynthetic photon flux density (PPFD; 400–700 nm) was about 150 μmol photons m−2 s−1 and additional illumination was provided by high-pressure sodium lamps SON-T Green Power and metal halide lamps MASTER LPI-T Plus (Philips Belgium). Emitted light from Philips SON-T Green Power has a red to far-red ratio (660/730 nm) of 2.63 and from Philips HPI-T Plus of 1.25. Both lamps are distributed equally in the greenhouse.
Experiments were repeated independently using either in vitro-propagated clones of the transformants or potato tubers. Determination of internode elongation was performed as described elsewhere (Martinez-Garcia et al., 2001, 2002). Shading experiments were performed at a plant density of 21 plants m−2 (shaded plants) and compared to plants grown at a density of 7 plants m−2 (nonshaded plants). PPFDs were determined with a LI-189 (LI-COR) at the level of the investigated leaves and amounted 380 ± 54 (upper leaves of shaded plants), 11 ± 4 (lower leaves of shaded plants), 430 ± 145 (upper leaves of nonshaded plants), and 150 ± 28 μmol photons m−2 s−1 (lower leaves of nonshaded plants). The red to far-red ratio was determined with a Spectroradiometer FieldSpec Pro II FR (with integrated remote cosine receptor; Analytical Spectral Devices). The ratio 660/730 nm was 0.3 ± 0.1 (lower leaves of shaded plants), 1.8 ± 0.1 (upper leaves of shaded plants), 1.5 ± 0.2 (lower leaves of nonshaded plants), and 2.1 ± 0.2 (upper leaves of nonshaded plants). Thus, far-red light exceeded red light at least 3-fold during shading experiments. Dark samples were taken under a green light source in the phytochamber.
Phytohormone treatment was performed over a 2-week period by spraying plants with 20 μm GA3 solution supplied with two drops of Triton X-100 per liter. Control plants were sprayed with water containing two drops of Triton X-100. Ethephon and paclobutrazol were supplied in a concentration of 350 μm, AgNO3 as a 1 mm solution. All chemicals were purchased from Sigma-Aldrich.
Artificial Shade Experiment
Plants were grown for 3 weeks in a phytochamber (Heraeus) at 24°C under LD conditions with a white-light source (Osram L36W-31) and an additional far-red light source (Chopper light type 730 supplied with a Hama 730-nm filter; Chopper Light GmbH). Control plants were exposed to white light alone. PPFD was 290 μmol photons m−2 s−1 as determined with a LI-189 (LI-COR). The experiment was performed with the wild type and three different transgenic lines (n = 3 per plant line).
Grafting
Plants had four to five leaves in total when grafted. The experiment was performed as described elsewhere (Martinez-Garcia et al., 2001, 2002).
Analysis of Enzyme Activity and Determination of Soluble Sugars
Soluble sugars and starch were quantified in potato leaf samples extracted with 80% ethanol and 20 mm HEPES-KOH, pH 7.5, as described previously (Hackel et al., 2006).
RNA Quantification by Real-Time PCR
RNA was isolated from different organs of greenhouse-grown potato ‘Désirée’ and andigena or from leaf discs of potato plants grown in the phytochamber. RNA extraction was performed with Trisure (Bioline) or peqGold Trifast (Peqlab) according to the manufacturer's protocol. Reverse transcription (RT) was performed with the Qiagen Omniscript RT kit according to the manual. Optimized conditions included using oligo(dT) primers for the initial RT reaction on approximately 1 μg of total RNA after digestion with RNase-free DNase (Qiagen).
Aliquots of 0.2 μL of the 10-μL RT reaction were used for the subsequent PCR reaction in the presence of SYBR Green with HotGoldStar DNA polymerase (Eurogentec) in a Rotor Gene 3000 cycler (LTF Labortechnik) using Rotor Gene software (version 4.6.94). The best products were obtained with the following program: denaturation at 95°C for 30 s, annealing for 30 s at 61°C, and elongation for 30 s at 72°C, in a program of 45 cycles in a 20-μL reaction volume. Relative quantification of transcript amounts was always calculated in relation to the respective ubiquitin transcript level and given as percentage of ubiquitin. Primers were designed to obtain a 50- to 150-bp amplicon using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi).
Primer sequences used for real-time PCR analysis were: ubiquitin forward, CACCAAGCCAAAGAAGATCA and ubiquitin reverse, TCAGCATTAGGGCACTCCTT; LC-SUT1 forward, TTCCATAGCTGCTGGTGTTC and LC-SUT1 reverse, TACCAGAAATGGGTCCACAA; StSUT2 forward, GGCATTCCTCTTGCTGTAACC and StSUT2 reverse, GCGATACAACCATCTGAGGGTAC; StSUT4 forward, GCTCTTGGGCTTGGACAAGGC and StSUT4 reverse, GGCTGGTGAATTGCCTCCACC; PhyB forward, TTTGCCTGATGCTGGGTATC and PhyB reverse, CTTTGCACCACCCCACTTA; GA20ox1 forward, CAAGATTGTGTTGGCGGACT and Ga20ox1 reverse, ACTGCTCTGTGCAGGCAACT; PhyA forward, TGCTCACTCTCGTGGAGGAT and PhyA reverse, CCCTGCAATGCTAATTCCAA; and StACO3 forward, GTGAGGCCATCATTTCTCCA and StACO3 reverse, CTTGAAAGCGGAGGTGACAG. Real-time PCR data were corrected by calculation of the PCR efficiency individually using LinReg PCR software. Statistical analysis was performed with Student's t test with 0.05 > P > 0.01 (+), 0.01 > P > 0.001 (++), and 0.001 > P (+++).
Western-Blot Analysis
Isolation of the microsomal fraction from plant material as well as two-phase partitioning and western blotting were performed as previously described (Lemoine et al., 1996). The StSUT4-specific peptide antibody is raised against a central loop peptide of SUT4 (NH2-CGSSHTGEEIDESSHGQEEAFLW-CONH2). The specificity of the affinity-purified antibody has been tested previously and the purified antibody was used for immunolocalization as well as western-blot analysis (Weise et al., 2000).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AF237780.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. StSUT4 expression level in StSUT4 RNAi plants.
Supplemental Figure S2. StSUT1 expression level in StSUT4 RNAi plants.
Supplemental Figure S3. Internode length and weight of StSUT4 RNAi plants.
Supplementary Material
Acknowledgments
We gratefully acknowledge Hanjo Hellmann for helpful discussion and Sutton Mooney for English corrections. We thank Yvonne Pörs for setting the light conditions for shading experiments and Dieter Oellerich for setting the artificial shade experiment; Karin Schumacher for providing material; Aleksandra Hackel for excellent technical assistance; and Angelika Pötter for excellent care of greenhouse plants.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 429 to C.K., P.G., and J.M.) and Nachwuchsförderungsgesetz (stipend to I.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christina Kühn (christina.kuehn@biologie.hu-berlin.de).
The online version of this article contains Web-only data.
References
- Bernier G, Perilleux C (2005) A physiological overview of the genetics of flowering time control. Plant Biotechnol J 3 3–16 [DOI] [PubMed] [Google Scholar]
- Carrera E, Bou J, Garcia-Martinez JL, Prat S (2000) Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J 22 247–256 [DOI] [PubMed] [Google Scholar]
- Carrera E, Jackson SD, Prat S (1999) Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato. Plant Physiol 119 765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdan PD, Chory J (2003) Regulation of flowering time by light quality. Nature 423 881–885 [DOI] [PubMed] [Google Scholar]
- Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J (1985) Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res 13 4777–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG (2006) Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Nakamura S, Araki T, Mochizuki N, Nagatani A (2005) Phytochrome B in the mesophyll delays flowering by suppressing FLOWERING LOCUS T expression in Arabidopsis vascular bundles. Plant Cell 17 1941–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Bohlenius H, Moritz T, Nilsson O (2006) GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 18 2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D (1984) The interaction of photosynthesis and photoperiodism in induction. In D Vince-Prue, B Thomas, E Cockchull, eds, Light and Flowering Process. Academic Press, London
- Guo H, Yang H, Mockler TC, Lin C (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279 1360–1363 [DOI] [PubMed] [Google Scholar]
- Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C (2006) Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J 45 180–192 [DOI] [PubMed] [Google Scholar]
- Harmer SL, Kay SA (2005) Positive and negative factors confer phase-specific circadian regulation of transcription in Arabidopsis. Plant Cell 17 1926–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirner A, Ladwig F, Stransky H, Okumoto S, Keinath M, Harms A, Frommer WB, Koch W (2006) Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 18 1931–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SD, Heyer A, Dietze J, Prat S (1996) Phytochrome B mediated the photoperiodic control of tuber formation in potato. Plant J 9 159–166 [Google Scholar]
- Jackson SD, James P, Prat S, Thomas B (1998) Phytochrome B affects the levels of a graft-transmissible signal involved in tuberization. Plant Physiol 117 29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SD, Prat S (1996) Control of tuberization in potato by gibberellins and phytochrome B. Physiol Plant 98 407–412 [Google Scholar]
- Kozuka T, Horiguchi G, Kim GT, Ohgishi M, Sakai T, Tsukaya H (2005) The different growth responses of the Arabidopsis thaliana leaf blade and the petiole during shade avoidance are regulated by photoreceptors and sugar. Plant Cell Physiol 46 213–223 [DOI] [PubMed] [Google Scholar]
- Kühn C (2003) Comparison of the sucrose transporter systems of different plant species. Plant Biol 5 215–232 [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275 1298–1300 [DOI] [PubMed] [Google Scholar]
- Lemoine R, Kühn C, Thiele N, Delrot S, Frommer WB (1996) Antisense inhibition of the sucrose transporter: effects on amount of carrier and sucrose transport activity. Plant Cell Environ 19 1124–1131 [Google Scholar]
- Martinez-Garcia JF, Garcia-Martinez JL, Bou J, Prat S (2001) The interaction of gibberellins and photoperiod in the control of potato tuberization. J Plant Growth Regul 20 377–386 [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia JF, Virgos-Soler A, Prat S (2002) Control of photoperiod-regulated tuberization in potato by the Arabidopsis flowering-time gene CONSTANS. Proc Natl Acad Sci USA 99 15211–15216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Pierik R, Cuppens ML, Voesenek LA, Visser EJ (2004. a) Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol 136 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LA, de Kroon H, Visser EJ (2004. b) Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J 38 310–319 [DOI] [PubMed] [Google Scholar]
- Reed JW, Foster KR, Morgan PW, Chory J (1996) Phytochrome B affects responsiveness to gibberellins in Arabidopsis. Plant Physiol 112 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A, Schulze W, Kühn C, Barker L, Schulz A, Ward JM, Frommer WB (2002) Protein-protein interactions between sucrose transporters of different affinities colocalized in the same enucleate sieve element. Plant Cell 14 1567–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Frommer WB (1994) Einfluß der Überexpression von Saccharosetransportern auf das Blühverhalten von Pflanzen. German Patent No. P 44 39 748.8
- Riesmeier JW, Hirner B, Frommer WB (1993) Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell 5 1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11 4705–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L (1989) Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J 8 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Falcon M, Bou J, Prat S (2006) Seasonal control of tuberization in potato: conserved elements with the flowering response. Annu Rev Plant Biol 57 151–180 [DOI] [PubMed] [Google Scholar]
- Rolland N, Ferro M, Seigneurin-Berny D, Garin J, Douce R, Joyard J (2003) Proteomics of chloroplast envelope membranes. Photosynth Res 78 205–230 [DOI] [PubMed] [Google Scholar]
- Schittenhelm S, Menge-Hartmann U, Oldenburg E (2004) Photosynthesis, carbohydrate metabolism, and yield of phytochrome-B-overexpressing potatoes under different light regimes. Crop Sci 44 131–143 [Google Scholar]
- Searle I, Coupland G (2004) Induction of flowering by seasonal changes in photoperiod. EMBO J 23 1217–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51 49–81 [DOI] [PubMed] [Google Scholar]
- Thomas B (2006) Light signals and flowering. J Exp Bot 57 3387–3393 [DOI] [PubMed] [Google Scholar]
- Töpfer R, Matzeit V, Gronenborn B, Schell J, Steinbiss HH (1987) A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res 15 5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303 1003–1006 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D (2005) Reaching out of the shade. Curr Opin Plant Biol 8 462–468 [DOI] [PubMed] [Google Scholar]
- Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer WB, Ward JM (2000) A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H, Wobus U (2000) Sucrose transport into barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J 21 455–467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.