Abstract
Two distinct mitochondrial energy dissipating systems, alternative oxidase (AOX) and uncoupling protein (UCP), have been implicated as crucial components of thermogenesis in plants and animals, respectively. To further clarify the physiological roles of AOX and UCP during homeothermic heat production in the thermogenic skunk cabbage (Symplocarpus renifolius), we identified the thermogenic cells and performed expression and functional analyses of these genes in this organism. Thermographic analysis combined with in situ hybridization revealed that the putative thermogenic cells surround the stamens in the florets of skunk cabbage and coexpress transcripts for SrAOX, encoding Symplocarpus AOX, and SrUCPb, encoding a novel UCP that lacks a fifth transmembrane segment. Mitochondria isolated from the thermogenic florets exhibited substantial linoleic acid (LA)-inducible uncoupling activities. Moreover, our results demonstrate that LA is capable of inhibiting the mitochondrial AOX pathway, whereas the proportion of pyruvate-stimulated AOX capacity was not significantly affected by LA. Intriguingly, the protein expression levels for SrAOX and SrUCPb were unaffected even when the ambient air temperatures increased from 10.3°C to 23.1°C or from 8.3°C to 24.9°C. Thus, our results suggest that functional coexpression of AOX and UCP underlies the molecular basis of heat production, and that posttranslational modifications of these proteins play a crucial role in regulating homeothermic heat production under conditions of natural ambient temperature fluctuations in skunk cabbage.
Thermogenesis is a phenomenon in which the temperature of a specific floral tissue increases due to endogenous heat production. To date, thermogenesis has been reported in several species of the arum lily family (Philodendron spp.; Nagy et al., 1972; Gottsberger and Amaral, 1984; Symplocarpus spp.; Seymour and Blaylock, 1999; Ito et al., 2004; Seymour, 2004; Helicodiceros muscivorus; Seymour et al., 2003), and also in palms (Bactris gasipaes; Schroeder, 1978; Carludovica palmata; Gottsberger, 1990; Bactris spp.; Listabarth, 1996), lotus (Nelumbo spp.; Miyake, 1898; Seymour and Schultze-Motel, 1996, 1998; Seymour et al., 1998; Watling et al., 2006), cycads (Ceratozamia miqueliana and Zamia furfuracea; Skubatz et al., 1993; Macrozamia machinii; Seymour et al., 2004), and water lilies (Victria spp.; Prance and Arias, 1975; Skubatz et al., 1990).
Heat production in thermogenic plants is thought to be associated with a large increase in the activity of the cyanide-resistant, nonphosphorylating electron transport pathway in mitochondria. This pathway is mediated by alternative oxidase (AOX) and is shared among plants, fungi, and nematodes (Berthold and Siedow, 1993; McIntosh, 1994; Day and Wiskich, 1995; Wagner and Krab, 1995; Moore et al., 2002; McDonald and Vanlerberghe, 2004; Clifton et al., 2006; McDonald and Vanlerberghe, 2006; Onda et al., 2007). The AOX of plant mitochondria accepts electrons from the ubiquinone pool and uses them to reduce oxygen to water, with no conservation of energy through proton gradient formation (Siedow and Umbach, 1995). The free energy generated by the flow of electrons from ubiquinol to AOX is generally believed not to result in the generation of ATP but to be instead lost as heat (Moore and Siedow, 1991).
In mammals, the mitochondrial uncoupling proteins (UCPs) have been shown to play a crucial role in thermogenesis (Nicholls and Locke, 1984; Boss et al., 1997; Fleury et al., 1997). UCPs reside in the mitochondrial inner membrane, across which they dissipate energy from the proton gradient that is built up by the respiratory chain, and this results in heat production (Ricquier and Bouillaud, 2000). Moreover, this heat production does not require muscular contraction (Janský, 1973) and is thus referred to as “nonshivering” thermogenesis. It is also well known that nonshivering thermogenesis in brown adipose tissue is the principal mechanism underlying homeothermic heat production in small animals during cold acclimation (Nedergaard et al., 2001). Although the functions of UCPs in nonthermogenic plants have been extensively studied (Vercesi et al., 2006), the roles of these UCPs in thermogenic plants remain poorly understood.
Among the thermogenic plants that have been so far characterized, the sacred lotus (Nelumbo nucifera; Seymour and Schultze-Motel, 1996; Seymour et al., 1998; Watling et al., 2006) and skunk cabbage (Symplocarpus foetidus; Knutson, 1974; Seymour and Blaylock, 1999; Symplocarpus renifolius; Uemura et al., 1993; Ito et al., 2003b, 2004; Ito and Ito, 2005) are known as homeothermic plants and are capable of increasing and maintaining their inflorescence temperatures for several days. For instance, the temperature of the lotus receptacle can be maintained at between 30°C and 36°C during a 2- to 4-d period, despite changes in environmental temperatures of between 10°C and 45°C (Seymour and Schultze-Motel, 1996, 1998; Seymour et al., 1998). In addition, the spadix of skunk cabbage, which belongs to the aroid family, can generate and maintain an internal temperature of about 20°C even when the ambient air temperature drops below 0°C (Knutson, 1974; Seymour and Blaylock, 1999; Ito et al., 2004).
Thus far, in relation to genes that mediate heat production in skunk cabbage, the SrAOX gene that encodes a pyruvate-sensitive AOX (Onda et al., 2007) and two cDNAs that encode UCPs, designated SrUCPa and SrUCPb (Ito, 1999), have been isolated from the thermogenic spadix. In addition, whereas the SrUCPa protein harbors six transmembrane segments that are commonly observed in mammals and nonthermogenic plants, SrUCPb lacks the fifth transmembrane segment. More recently, our functional analyses of SrUCPb have shown that this gene product acts as a UCP in yeast cells (Ito et al., 2006). These findings suggest that the UCP plays an important role in thermogenesis not only in mammals, but also in plants in conjunction with AOX.
In this study, we wished to further clarify the molecular mechanisms underlying thermoregulation in skunk cabbage. It has been shown that the homeothermic skunk cabbage found in Japan is S. renifolius (Uemura et al., 1993; Nie et al., 2006) and we have therefore performed expression and functional analyses of AOX and UCP genes in this species. Our results suggest that both AOX and UCP molecules are involved in tissue-specific thermoregulation in this plant.
RESULTS
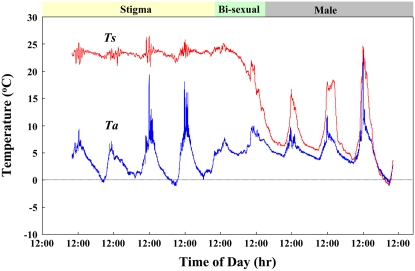
Stigma-Stage-Specific Homeothermic Control in the Spadix of Skunk Cabbage
It has been shown previously that heat production in skunk cabbage occurs during the stigma, bisexual, and early male stages of the spadices (Seymour and Blaylock, 1999). To confirm the developmental stages of homeothermic control in the skunk cabbage in this study, continuous recordings of the spadix and air temperatures were conducted during flowering (Fig. 1). Our data show that the protogynous spadices exhibit stigma-stage-specific thermoregulation and maintain their temperature at around 23°C, even when the ambient air temperatures fluctuate between −1.1°C and 19.4°C. This homeothermic control gradually dissipated as the spadices differentiated into the bisexual and early male stages, at which time pollen had appeared on their surface.
Figure 1.
Stigma-stage-specific homeothermic control in the spadix of skunk cabbage. Changes in both the spadix (Ts) and air temperatures (Ta) during the stigma, bisexual, and male stages are shown.
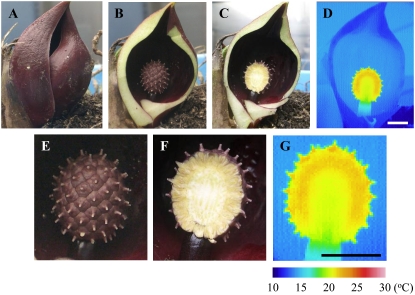
Localization of Heat Production in the Skunk Cabbage Spadix
Thermogenic tissues in the skunk cabbage spadices were identified using a high-resolution infrared thermal camera (Fig. 2). The thermogenic spadix is surrounded by a spathe (Fig. 2A), which was removed (Fig. 2, B and E). The analyzed spadix was at the stigma stage and had a temperature of 23.4°C before cutting when the ambient temperature was 8.5°C. The spadix was cut to generate a longitudinal section (Fig. 2, C and F; Supplemental Movie S1), and the surface temperatures of this longitudinal section revealed that the florets surrounding the spadix displayed a higher temperature than the rest of the spadix (Fig. 2, D and G; Supplemental Movies S2 and S3). Even at 2 d after the preparation of the longitudinal sections, the temperature of the florets was found to be 20.7°C ± 0.1°C (n = 3), whereas the temperature at the center of the spadix was measured as 19.5°C ± 0.2°C (Supplemental Movie S4).
Figure 2.
Thermographic analysis of longitudinal sections of skunk cabbage spadices. A to C, Structural features of skunk cabbage used for thermographic analysis. The cylindrical organ is the thermogenic spadix. A, An intact skunk cabbage. B, The spathe has been cut to reveal the developmental stage and level of thermogenesis. C, Longitudinal sectioning of the spadix. D, Thermal imaging of the plant shown in C using a high-resolution infrared thermal camera. E to G, High-magnification images of B to D, respectively. The temperature scale of the thermographic analysis is shown on the bottom right. Bars, 1 cm.
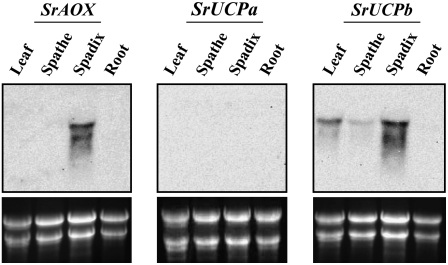
Expression Patterns of the SrAOX and SrUCP Genes
We speculated that if SrAOX directs stigma spadix-specific homeothermic control in conjunction with SrUCP, the thermogenic tissues, identified in our thermographic analyses, would express both of these genes. Hence, we first examined the expression pattern of SrAOX (Onda et al., 2007) together with SrUCPa and SrUCPb (Ito, 1999) at the organ level in the leaf, spathe, spadix, and root in the thermogenic plants. The expression of SrAOX transcripts was specifically detected in the spadix (Fig. 3). However, there were no detectable transcripts of SrUCPa in any of the plant tissues analyzed. The expression of SrUCPb, which encodes a novel five-transmembrane UCP, was found to be abundantly expressed in the thermogenic spadix (Fig. 3).
Figure 3.
Expression analysis of SrAOX, SrUCPa, and SrUCPb transcripts among various tissues from stigma-stage (thermogenic) skunk cabbage samples. Ten-microgram aliquots of total RNA extracted from the leaf, spathe, spadix, and root were resolved on formaldehyde gels, blotted onto a nylon filter, and hybridized with a labeled cDNA probe for SrAOX, SrUCPa, or SrUCPb. The rRNA bands in the ethidium bromide-stained gels are shown in the bottom panel as a loading control.
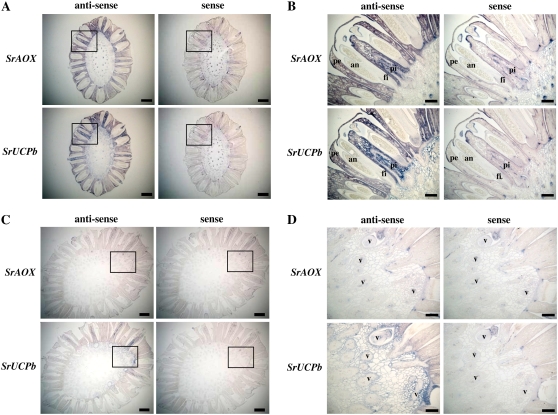
To further clarify the expression patterns of the SrAOX and SrUCPb gene transcripts in the spadix, in situ hybridization analysis was next carried out (Fig. 4). Both SrAOX and SrUCPb were found to be expressed in the thermogenic florets around the stigma-stage spadices (Fig. 4A). The putative thermogenic cells that coexpress both SrAOX and SrUCPb transcripts were found in the pistil and the petals surrounding the stamens in the florets (Fig. 4B). In contrast, although the expression of SrUCPb remained at low levels around the vascular bundle in the interior of the spadix, the expression levels of SrAOX and SrUCPb were dramatically decreased in the male stages (Fig. 4, C and D).
Figure 4.
In situ hybridization analysis of SrAOX and SrUCPb transcripts in the spadix of skunk cabbage. A, Transverse sections of the stigma-stage (thermogenic) spadix were hybridized with digoxigenin-labeled antisense (left) and sense (right) RNA probes for SrAOX (top) and SrUCPb (bottom). B, Magnification of the boxed area in A. C, Transverse sections of a male-stage (postthermogenic) spadix were hybridized with digoxigenin-labeled antisense (left) and sense (right) RNA probes for SrAOX (top) and SrUCPb (bottom). D, Magnification of the boxed area in C. an, Anther; pe, petal; pi, pistil; fi, filament; v, vascular bundle. Bars, 2 mm in A and C; 500 μm in B and D.
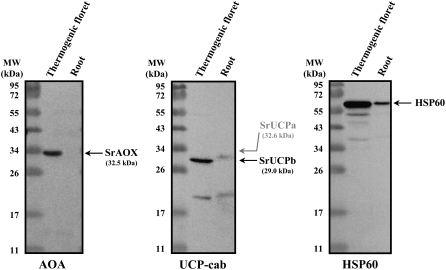
Both SrAOX and SrUCPb Proteins Are Expressed in Purified Mitochondria from Thermogenic Florets
To investigate the mitochondrial localization of the SrAOX and SrUCP proteins, mitochondria were isolated from thermogenic florets of the stigma-stage spadices and nonthermogenic roots by Percoll gradients. The expression of SrAOX (32.5 kD) was detected only in the mitochondrial isolates from thermogenic florets (Fig. 5). The UCP-cab antibody, which was raised against a conserved 12-amino-acid C-terminal peptide of SrUCPa and SrUCPb (see “Materials and Methods”), recognized a single band that corresponds to the estimated molecular mass of SrUCPb (29.0 kD) in mitochondria from thermogenic florets, whereas no signals for SrUCPa (32.6 kD) were obtained in either of the mitochondrial isolates (Fig. 5). Moreover, Hsp60 (mitochondrial matrix marker) was found to be expressed in both mitochondrial fractions.
Figure 5.
Western-blot analysis of mitochondria purified from the thermogenic florets and nonthermogenic roots of a skunk cabbage. Ten micrograms of purified mitochondrial proteins were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and incubated with antibodies directed against the proteins indicated below the panels. The predicted position of each protein species is indicated by an arrow on the right. Molecular mass standards are shown on the left of each panel.
Uncoupling Activity of Mitochondrial Isolates
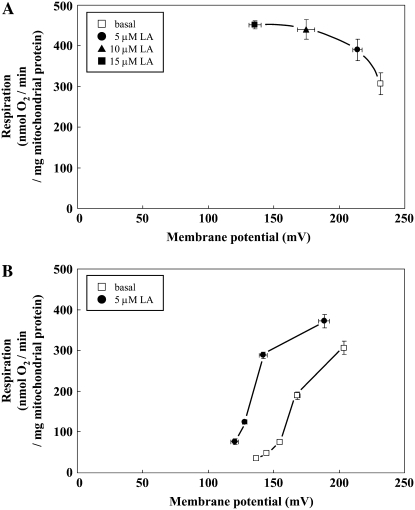
To further clarify the uncoupling activity in purified thermogenic floret mitochondria, we investigated the effects of linoleic acid (LA) on UCP-mediated proton conductance (Fig. 6). The kinetics of proton conductance were determined by generating plots of the respiration rate versus membrane potential, both of which were obtained by titration with LA (Fig. 6A) or KCN (Fig. 6B). These measurements were carried out in the presence of 1 μm oligomycin, 0.1 μm nigericin, 6 μm carboxyatractyloside, and 100 μm n-propyl gallate to inhibit ATP synthase, to clamp ΔpH, to inhibit adenine nucleotide translocase, and to inhibit AOX, respectively. Under these conditions, it was found that LA increased the respiratory rate but decreased the membrane potential (Fig. 6A). Moreover, a kinetic plot of the proton conductance as the respiratory rate and membrane potential showed that the addition of 5 μm concentrations of LA resulted in a substantial increase in the proton conductance rate in comparison with the basal proton leak (Fig. 6B).
Figure 6.
Stimulation of proton conductance by LA in isolated mitochondria from stigma-stage thermogenic florets. Purified mitochondria were incubated with 1 mm NADH substrate in assay medium containing 1 μm oligomycin (to inhibit ATP synthase), 0.1 μm nigericin (to clamp the ΔpH), 6 μm carboxyatractyloside (to inhibit adenine nucleotide translocase), and 100 μm n-propyl gallate (to inhibit SrAOX). The kinetics of proton conductance were then examined by simultaneous measurement of respiration and membrane potential. A, LA-induced dose-dependent increase in proton conductance. Increasing concentrations of LA (0–15 μm) were obtained by successive additions when the steady-state respiration rate and membrane potential were attained. B, The effects of 5 μm LA on the kinetics of proton conductance were examined with a KCN titration. The results shown are the mean ± sd of three measurements using mitochondria from the same preparation.
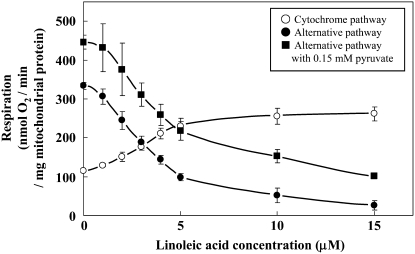
Effects of LA on Both Cytochrome and Alternative Pathway Respiration
To determine the relationship between the regulatory mechanisms for the SrAOX and SrUCPb proteins, the alternative and cytochrome pathways of respiration were measured at various concentrations of LA in purified mitochondria from thermogenic florets (Fig. 7). Alternative pathway respiration in the presence (black square) or absence (black circle) of pyruvate decreased with increasing concentrations of LA. In contrast, cytochrome pathway respiration increased with increasing concentrations of LA.
Figure 7.
The effects of LA on both the alternative and cytochrome pathway respirations in mitochondria purified from thermogenic florets of skunk cabbage. Purified mitochondria were incubated in the reaction medium without bovine serum albumin (containing 16.5 μm pyruvate) in the presence of 100 μm n-propyl gallate or 0.5 mm KCN for measurement of the cytochrome or alternative pathway respirations, respectively. Increasing concentrations of LA (0–15 μm) were obtained by successive additions when the steady-state respiration rate was attained. The results shown are the mean ± sd of three measurements using mitochondria from the same preparation.
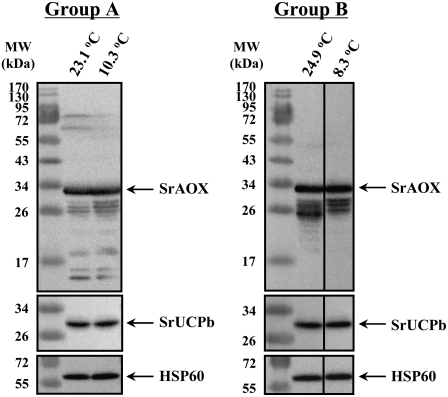
Expression of the SrAOX and SrUCPb Proteins under Different Ambient Temperatures
To further investigate the involvement of SrAOX and SrUCPb in homeothermic heat production in the spadix of skunk cabbage, we carried out expression analyses of these proteins under different ambient temperatures (Table I). When the ambient air temperature increased from either 10.3°C to 23.1°C or from 8.3°C to 24.9°C, the SrAOX and SrUCPb protein expression levels did not significantly differ compared with the controls (Fig. 8). Although samples in group A showed a slight oxidized signal for SrAOX, there was no oxidized form evident in group B. Because no reducing reagents such as dithiothreitol were used in these analyses, the SrAOX proteins were slightly oxidized during the purification procedures.
Table I.
Characteristics of the skunk cabbage spadices used in the current protein expression analysis under different thermogenic levels
Plants with stigma-stage spadices were placed in a growth chamber where the ambient air temperature (Ta) was kept at the indicated lower temperatures. When the spadix temperatures (Ts) became stable, half of the spadices were sectioned off and subjected to mitochondrial purification. After initial sampling, the Ta was subsequently increased to the indicated higher temperatures. The remaining spadices were collected for mitochondrial purification when their temperatures had stabilized. Experiments were conducted using two independent plant groups (group A and group B). The data are the mean ± sd (group A, n = 8; group B, n = 11) of the measurements. The rates of CO2 production were calculated from the equation 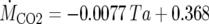 (Seymour, 2004).
(Seymour, 2004).
| Variable | Group A | Group B | ||
|---|---|---|---|---|
| Ta (°C) | 10.3 | 23.1 | 8.3 | 24.9 |
| Ts (°C) | 21.8 ± 1.8 | 25.2 ± 0.9 | 22.1 ± 1.3 | 26.8 ± 1.2 |
| Spadix mass (g) | 4.0 ± 1.4 | 3.8 ± 1.6 | 4.7 ± 1.4 | 5.2 ± 1.7 |
| CO2 production (mmol s−1 g−0.67) | 0.29 | 0.19 | 0.30 | 0.18 |
Figure 8.
Expression profiles of the SrAOX and SrUCPb proteins under different ambient temperatures. Mitochondria were purified immediately after sampling of the spadices (see Table I). Ten micrograms of purified mitochondrial proteins, prepared without reducing agents, were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and incubated with AOA, UCP-cab, or Hsp60 antibodies. The ambient temperatures are indicated above the panels. The position of each protein species is indicated by an arrow on the right. Molecular mass standards are shown on the left.
DISCUSSION
Thermogenic Tissue-Specific Expression of SrAOX and SrUCPb Transcripts
Over three decades ago, Knutson reported heat production and temperature regulation in the spadix of skunk cabbage (Knutson, 1974). However, the site of heat production in the spadix has remained elusive in the intervening period. In this study, a high-resolution infrared thermal camera was used to analyze the thermogenic tissues in stigma-stage spadices. Our results clearly demonstrate that the florets display higher temperatures than surrounding tissues (Fig. 2, D and G). Because the interior of an intact spadix cannot be cooler than the florets in the steady state, our results strongly suggest that the florets are the thermogenic tissues in skunk cabbage.
Our results also showed that both the SrAOX and SrUCPb transcripts are abundantly expressed in the spadix of the stigma-stage skunk cabbage (Fig. 3). No detectable signals for SrUCPa transcripts were found in any of the tissues examined in this study, which confirms our previous finding that UCP, which harbors six transmembrane segments like HmUCPa, a thermogenic Helicodiceros UCP, is not primarily involved in organ-specific heat production (Ito et al., 2003a).
Moreover, our data show that the expression of SrAOX and SrUCPb mRNAs colocalize in the thermogenic florets (Fig. 4, A and B), and that these transcripts were dramatically decreased upon development to the male stage (Fig. 4, C and D). Interestingly, in this study, an obvious localization of both SrAOX and SrUCPb transcripts was evident in the region surrounding the stamens in the thermogenic florets. Protogynous spadices harbor immature pollen during the homeothermic stigma stages, and mature pollen appears across the entire surface of the spadix during the male stage. Thus, it seems probable that one of the biologically significant aspects of homeothermic control during the stigma stages in skunk cabbage is the protection of pollen maturation against low temperature damage. Similarly, it has been shown in rice (Oryza sativa) that male sterility is induced by low temperatures (12°C–18°C) at the young microspore stage (boot stage), when it is most sensitive to cool temperatures during the reproductive period, and microscopic observations of developing rice anthers suggest that male sterility damage is attributable to dysfunctional pollen development under low temperatures (Hayase et al., 1969; Satake and Hayase, 1970). Thus far, common interpretations of the significance of thermogenesis in plants include the evaporation of scent compounds to attract pollinators (Meeuse and Raskin, 1988), protection against low temperature damage (Knutson, 1974), and the provision of a warm environment to insect pollinators (Seymour, 1997; Seymour et al., 2003). However, our data suggest that the functional significance of homeothermic control in skunk cabbage could be the prevention of low temperature damage during pollen maturation.
Mitochondrial Isolates Possess Uncoupling Activity Induced by LA
We have shown that both the SrAOX and SrUCPb proteins reside in the mitochondria of thermogenic florets (Fig. 5) and that the addition of exogenous LA stimulates proton leakage in purified mitochondria from thermogenic florets (Fig. 6). These results strongly suggest that the stimulation of proton conductance by LA is primarily mediated by the activation of SrUCPb.
Sluse and coworkers have previously demonstrated that AOX and UCP do not appear to function simultaneously at their maximal activity levels in vitro because an increase in LA concentration inhibits AOX by undetermined mechanisms (Sluse et al., 1998; Jarmuszkiewicz et al., 2001). In contrast, it has also been shown that expression of AOX and UCP during fruit ripening in mango (Mangifera indica) is not controlled in a reciprocal manner, and can occur simultaneously (Considine et al., 2001). Our data in this study are primarily in good accordance with Sluse's observations (Fig. 7). Interestingly, however, our data show that the activities of SrAOX and SrUCPb remain relatively high at lower concentrations of LA (Fig. 7; less than 5 μm), and SrAOX was found to be activated by pyruvate in the presence of any concentration of LA (0–15 μm). Therefore, our results further suggest that the inhibitory effects of LA on alternative pathway respiration are not related to the processes governing the activation of SrAOX by pyruvate, and that the simultaneous activation of SrAOX and SrUCPb can occur at lower concentrations of LA.
The Regulation of Homeothermic Heat Production in the Spadix of Skunk Cabbage
To maintain the skunk cabbage spadix temperature within a particular range under outdoor conditions (Fig. 1), mitochondrial respiration would need to be actively controlled, even when the ambient temperature fluctuates. Therefore, an important question arises as to whether the level of heat production is regulated by SrAOX and SrUCPb in the thermogenic spadix. To answer this question, the expression levels of these proteins were compared in spadices undergoing different levels of heat production. We have previously proposed a model for a time-dependent thermogenic oscillatory mechanism that acts as a precise thermal regulator in a dynamic environment (Ito et al., 2004). According to our working hypothesis, if heat production is influenced by SrAOX and SrUCPb proteins, the expression levels should be decreased when exposed to higher ambient temperature. Therefore, we determined the expression levels of SrAOX and SrUCPb when the ambient air temperature increased from either 10.3°C to 23.1°C or from 8.3°C to 24.9°C (Table I; Fig. 8). These increases in the ambient temperature lead to a decrease in the rate of respiration by 34% or 40%, respectively (Seymour, 2004). However, the expression of these two proteins did not dramatically change. Hence, our data suggest that the regulation of gene expression for SrAOX and SrUCPb is not crucial during the maintenance of homeothermic heat production in skunk cabbage, but that posttranslational modifications of the proteins are likely to play an important role in homeothermic control.
In skunk cabbage, the major respiratory substrates have been shown to be carbohydrates (Seymour and Blaylock, 1999). Moreover, we have previously reported that concentrations of Suc, Glc, and Fru in the xylem sap of thermogenic skunk cabbages were in the 2 to 5 mm range for each spadix fresh weight, and that a remarkable decrease in these sugars occurs in postthermogenic plants (Onda and Ito, 2005). It is thus probable that pyruvate, which is produced by glycolysis and is transported into mitochondria, would stimulate not only the SrAOX protein, which exists as a reduced monomer (Fig. 8; Onda et al., 2007), but also the tricarboxylic acid cycle that consequently leads to increased proton gradients (Laloi, 1999). Furthermore, because UCP-mediated reduced membrane potential has been shown to accelerate the tricarboxylic acid cycle flux (Smith et al., 2004), LA-inducible UCP activity detected in this study (Fig. 6) would have a significant role in maintaining an increased level of respiration, which leads to successive heat production in the spadix of skunk cabbage. Therefore, as shown in the previously proposed “'physical plus biochemical lag” model (Seymour, 2004), which introduces two biochemical factors that mimic a less than instantaneous change in heat production, any biochemical process that regulates the mitochondrial SrAOX and/or SrUCPb would be a candidate “biochemical thermostat” during thermoregulation. Nevertheless, considering that skunk cabbage utilizes carbohydrates as a respiratory substrate and that the simultaneous activation of SrAOX and SrUCPb preferentially occurs at lower concentrations of LA, glycolytic enzymes that associate dynamically with mitochondria in response to respiratory demand and support substrate channeling (Graham et al., 2007) would play a significant role in controlling the temperature-dependent respiration by increasing or decreasing the levels of pyruvate, a primal activator of SrAOX (Onda et al., 2007), during homeothermic heat production in skunk cabbage.
Recently, Watling et al. (2006) reported in vivo measurements of fluxes that operate through both the alternative and cytochrome respiratory pathways in thermogenic floral receptacles of the sacred lotus. A significant increase in the alternative pathway flux in these thermogenic receptacles was evident, whereas the flux of the cytochrome pathway did not change significantly. These results suggest that AOX activity is responsible for homeothermic heat production in the receptacle. Thus, it will be of great interest to determine whether it is gene transcription/translation or the posttranslational control of AOX that underlies this homeothermic control in the thermogenic stage of the lotus receptacle.
Skunk cabbage generates both heat and blooms in a cold environment where the ambient temperature often drops below freezing. Because no thermogenic plants apart from skunk cabbage bloom under such freezing conditions, it is possible that functional coexpression of AOX and UCP would significantly contribute to the explosive, continuous, and controlled respiration that appears to be unique to this plant. In any case, in addition to AOX, which is a classical thermogenic protein in plants, the UCP molecule should now be fully evaluated in additional studies of thermogenic plants.
MATERIALS AND METHODS
Plant Materials and Temperature Measurements
Skunk cabbage (Symplocarpus renifolius) plants were collected during March and April from 2000 to 2007 from fields situated on the Iwate University campus (39°43′N, 141°08′E), the Shizukuishi factory of Taishi Food (39°45′N, 141°00′E), Hakuba, Nagano prefecture (36°39′N, 137°50′E), and Omori, Akita prefecture (39°19′N, 141°20′E) on the main island of Japan. Temperatures of skunk cabbage spadices and the air were measured at 1-min intervals using an automatic recording thermometer connected to an electronic thermocouple (TR-5106; T & D). Measurements of spadix temperatures were made by inserting the thermocouple to a depth of 5 to 10 mm into spadix tissue (Ito et al., 2003b).
Thermal Imaging
Thermal images were obtained using an infrared color light-sound-dimension camera (TVS-8502; Nippon Avionics). The specified temperature resolution was below 0.025°C at room temperature and images were saved either as IRI files or as MPEG movies from the camera (see supplemental data). The IRI images were subsequently analyzed for temperature determination using the image analysis software provided by the manufacturer (Thermal Video Systems; Nippon Avionics).
Northern-Blot Analysis
Total RNA was isolated from the leaves, spathes, spadices, and roots of skunk cabbage plants according to our previously reported method (Onda et al., 2007). Ten micrograms of each RNA sample was then fractionated on a 1.0% agarose gel, transferred to a Hybond N+ membrane (Amersham Biosciences), and hybridized with a full-length SrAOX, SrUCPa, or SrUCPb cDNA as a probe.
In Situ Hybridization
Fresh thermogenic and postthermogenic spadices were fixed in 10% (v/v) formaldehyde in 50% (v/v) ethanol containing 5% (v/v) acetic acid for 5 h at 20°C. The fixed tissues were then dehydrated through an ethanol series and embedded in paraffin as described previously (Cox et al., 1984). Sections of 7-μm thickness were cut and mounted on amino-silanized glass. Antisense and sense RNA probes labeled with digoxigenin-11-rUTP were transcribed from the full-length SrAOX and SrUCPb cDNAs with T7 or T3 RNA polymerase (Amersham Biosciences). The sections were then dehydrated and hybridized at 58°C in 50% (v/v) formamide containing 2× SSC for 16 h. After hybridization, the slides were washed, and the probes were visualized as described previously (Steel et al., 1998).
Antibody Production and Purification
Antibodies were generated against a conserved 12-amino-acid C-terminal peptide (SrUCP-cab; [Cys]-CQVKKFFIKVPN) of SrUCPa (accession no. AB024733–1) and SrUCPb (accession no. AB024734–1). Before the injection of rabbits, this antigenic peptide was coupled to the carrier protein keyhole limpet hemocyanin. The resulting antibody preparation was affinity purified using an AF-Tresyl TOYOPEARL 650M column (Toshoh) according to the manufacturer's instructions. The affinity-purified antiserum is referred to as UCP-cab for SrUCPs.
Isolation of Mitochondria
Mitochondria were isolated from either the thermogenic florets or nonthermogenic roots of skunk cabbages as described previously (Onda et al., 2007).
Western Blotting of SrAOX and SrUCPs
Western-blotting analysis of SrAOX and SrUCPs was performed by equal loading of the samples onto 12.5% acrylamide gels and transfer onto polyvinylidene difluoride membranes. Prestained protein standards (11–170 kD; Fermentas) were used for molecular mass estimation. The filters were incubated for 1 h at room temperature in 5% (w/v) milk powder in Tris-buffered saline (137 mm NaCl, 2.68 mm KCl, and 25 mm Tris, pH adjusted to 7.4 with HCl) that contained 0.1% (v/v) Tween 20 (TBS-T) and then for an additional 1 h at room temperature with 5% (w/v) milk powder in TBS-T containing antibodies. Polyclonal antibodies against UCP-cab peptide and monoclonal antibodies to Sauromatum guttatum AOX (Elthon et al., 1989) were diluted to 1:1,000 and 1:500, respectively. Hsp60 was detected using anti-Hsp60 monoclonal antibodies (Stressgen Biotechnology), according to the manufacturer's instructions.
Measurement of Proton Conductance
Proton conductance was determined by the simultaneous measurement of oxygen consumption (Vigeolas et al., 2003; Nowaczyk et al., 2006) and membrane potential using an oxygen probe and the fluorescence of safranine O (Åkerman and Wikström, 1976; Moore and Bonner, 1982; Hourton-Cabassa et al., 2002). For the measurement of oxygen consumption, an oxygen probe (oxygen dipping probe POF-PSt3; PreSens) was used. This oxygen sensor consists of a polymer optical fiber with a polished distal tip (diameter, 4 mm) that is coated with a foil that contains a fluorescing dye. The fluorescence excited in the dye by the sensor's light source is quenched by collision with O2 molecules and represents a measure for the ambient O2 concentration. The oxygen meter FIBOX3 (PreSens) was used to convert the fluorescence signal to O2 units. For membrane potential measurements, safranine O fluorescence at 570 nm, when excited at 495 nm, was monitored by means of a temperature-controlled spectrofluorometer (RF-5300PC; Shimadzu). Mitochondria (100 μg) were resuspended in 2 mL of assay medium (0.3 m mannitol, 1 mm MgCl2, 100 mm KCl, 10 mm KH2PO4, and 10 mm HEPES/KOH [pH 7.0]) containing 1 μm oligomycin (to inhibit ATP synthase), 0.1 μm nigericin (to clamp ΔpH), 6 μm carboxyatractyloside (to inhibit adenine nucleotide translocase), and 100 μm n-propyl gallate (to inhibit SrAOX). NADH was added to a concentration of 1 mm to start the reaction. Membrane potential was progressively inhibited by the addition of KCN to a final concentration of between 3 and 25 μm. At the end of each run, 2 μm p-trifluoromethoxy-carbonylcyanide phenylhydrazone was added to dissipate the membrane potential completely, releasing all of the safranine O into the medium and allowing a correction for fluorometric measurements. The calibration of safranine fluorescence as a K+ diffusion potential was performed according to previous descriptions (Zottini et al., 1993) by using rat liver mitochondria as also described previously (Hogeboom, 1955). The K+ diffusion potential in rat liver mitochondria was induced by adding 0.05 mg/mL valinomycin.
Mitochondrial Respiration Measurements
Oxygen uptake by mitochondria was measured as described in our previous report (Onda et al., 2007).
Preparation of Skunk Cabbage Plants with a Different Thermogenic Status
Stigma-stage plants with two spadices (referred to as “twins”) that had been growing outdoors were transferred to a temperature-controlled growth chamber. These plants were then divided into groups A and B and subjected to different temperature treatments (Table I). One of the two spadices was collected for each temperature condition, and the purification of mitochondria was immediately started at 4°C. Mitochondria were purified as described above without ascorbic acid, Cys, or pyruvate to omit reducing reagents. The purified mitochondria were then frozen at −80°C until analysis. The expression levels of SrAOX, SrUCPs, and Hsp60 were determined by immunoblotting as described above following nonreducing SDS-PAGE (Umbach and Siedow, 1993).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AB024733 (SrUCPa), AB024734 (SrUCPb), and AB183695 (SrAOX).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Movie S1. Confirmation of the thermogenic status and generation of longitudinal sections of the spadix of a skunk cabbage.
Supplemental Movie S2. Assessment of the surface temperatures of the longitudinal section of a skunk cabbage spadix at 23 h after Supplemental Movie S1.
Supplemental Movie S3. Thermoscopic analysis of the surface temperature of longitudinal sections of the skunk cabbage spadix at 2 d after Supplemental Movie S1.
Supplemental Movie S4. Surface temperature measurements of the longitudinal section of the spadix shown in Supplemental Movie S3.
Supplementary Material
Acknowledgments
We thank Drs. Roger Seymour and Jennifer Watling for critical reading of the manuscript. We are grateful to Atsuo Shirakawa and Tsuyoshi Segawa for generously allowing us to sample skunk cabbages grown on their properties. We also thank Minoru Umemura for his permission to sample the skunk cabbage plants in the field near the Shizukuishi factory of Taishi Food Inc. We further thank Dr. Kazuei Matsubara for his generous gift of rat liver, and Drs. Naoki Kamo, Ryuzo Shingai, Hiroshi Osada, and Tetsuro Yamashita for helpful discussions.
This work was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS; grant no. 18380196 to K.I.), the Program for the Promotion of Basic Research Activities for Innovative Biosciences in Japan (to K.I.), the JSPS 21st Century Centers of Excellence Program, and a JSPS Research Fellowship for Young Scientists (to Y.O.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kikukatsu Ito (kikuito@iwate-u.ac.jp).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Åkerman KE, Wikström MK (1976) Safranine as a probe of the mitochondrial membrane potential. FEBS Lett 68 191–197 [DOI] [PubMed] [Google Scholar]
- Berthold DA, Siedow JN (1993) Partial purification of the cyanide-resistant alternative oxidase of skunk cabbage (Symplocarpus foetidus) mitochondria. Plant Physiol 101 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP (1997) Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett 408 39–42 [DOI] [PubMed] [Google Scholar]
- Clifton R, Millar AH, Whelan J (2006) Alternative oxidases in Arabidopsis: a comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochim Biophys Acta 1757 730–741 [DOI] [PubMed] [Google Scholar]
- Considine MJ, Daley DO, Whelan J (2001) The expression of alternative oxidase and uncoupling protein during fruit ripening in mango. Plant Physiol 126 1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, DeLeon DV, Angerer LM, Angerer RC (1984) Detection of mRNAs in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol 101 485–502 [DOI] [PubMed] [Google Scholar]
- Day DA, Wiskich JT (1995) Regulation of alternative oxidase activity in higher plants. J Bioenerg Biomembr 27 379–385 [DOI] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L (1989) Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol 89 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, et al (1997) Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 15 269–272 [DOI] [PubMed] [Google Scholar]
- Gottsberger G (1990) Flowers and beetles in the South American tropics. Bot Acta 103 360–365 [Google Scholar]
- Gottsberger G, Amaral A (1984) Pollination strategies in Brazilian Philodendron species. Ber Dtsch Bot Ges 97 391–410 [Google Scholar]
- Graham JWA, Williams TCR, Morgan M, Fernie AR, Ratcliffe RG, Sweetlove LJ (2007) Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell 19 3723–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase H, Satake T, Nishiyama I, Ito N (1969) Male sterility caused by cooling treatment at the meiotic stage in rice plants. II. The most sensitive stage to cooling and the fertilizing ability of pistils. Proc Crop Sci Soc Japan 38 706–711 [Google Scholar]
- Hogeboom GH (1955) Fractionation of cell components of animal tissues. Methods Enzymol 1 16–19 [Google Scholar]
- Hourton-Cabassa C, Mesneau A, Miroux B, Roussaux J, Ricquier D, Zachowski A, Moreau F (2002) Alteration of plant mitochondrial proton conductance by free fatty acids. J Biol Chem 277 41533–41538 [DOI] [PubMed] [Google Scholar]
- Ito K (1999) Isolation of two distinct cold-inducible cDNAs encoding plant uncoupling proteins from the spadix of skunk cabbage (Symplocarpus foetidus). Plant Sci 149 167–173 [Google Scholar]
- Ito K, Abe Y, Johnston SD, Seymour RS (2003. a) Ubiquitous expression of a gene encoding for uncoupling protein isolated from the thermogenic inflorescence of the dead horse arum Helicodiceros muscivorus. J Exp Bot 54 1113–1114 [DOI] [PubMed] [Google Scholar]
- Ito K, Ito T, Onda Y, Uemura M (2004) Temperature-triggered periodical thermogenic oscillations in skunk cabbage (Symplocarpus foetidus). Plant Cell Physiol 45 257–264 [DOI] [PubMed] [Google Scholar]
- Ito K, Matsukawa K, Kato Y (2006) Functional analysis of skunk cabbage SfUCPB, a unique uncoupling protein lacking the fifth transmembrane domain, in yeast cells. Biochem Biophys Res Commun 349 383–390 [DOI] [PubMed] [Google Scholar]
- Ito K, Onda Y, Sato T, Abe Y, Uemura M (2003. b) Structural requirements for the perception of ambient temperature signals in homeothermic heat production of skunk cabbage (Symplocarpus foetidus). Plant Cell Environ 26 783–788 [DOI] [PubMed] [Google Scholar]
- Ito T, Ito K (2005) Nonlinear dynamics of homeothermic temperature control in skunk cabbage, Symplocarpus foetidus. Phys Rev E Stat Nonlin Soft Matter Phys 72 051909. [DOI] [PubMed] [Google Scholar]
- Janský L (1973) Non-shivering thermogenesis and its thermoregulatory significance. Biol Rev Camb Philos Soc 48 85–132 [DOI] [PubMed] [Google Scholar]
- Jarmuszkiewicz W, Sluse-Goffart CM, Vercesi AE, Sluse FE (2001) Alternative oxidase and uncoupling protein: thermogenesis versus cell energy balance. Biosci Rep 21 213–222 [DOI] [PubMed] [Google Scholar]
- Knutson RM (1974) Heat production and temperature regulation in eastern skunk cabbage. Science 186 746–747 [DOI] [PubMed] [Google Scholar]
- Laloi M (1999) Plant mitochondrial carriers: an overview. Cell Mol Life Sci 56 918–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listabarth C (1996) Pollination of Bactris by Phyllotrox and Epurea. Implications of the palm breeding beetles on pollination at the community level. Biotropica 28 69–81 [Google Scholar]
- McDonald AE, Vanlerberghe GC (2004) Branched mitochondrial electron transport in the animalia: presence of alternative oxidase in several animal phyla. IUBMB Life 56 333–341 [DOI] [PubMed] [Google Scholar]
- McDonald AE, Vanlerberghe GC (2006) Origins, evolutionary history, and taxonomic distribution of alternative oxidase and plastoquinol terminal oxidase. Comp Biochem Physiol Part D Genomics Proteomics 1 357–364 [DOI] [PubMed] [Google Scholar]
- McIntosh L (1994) Molecular biology of the alternative oxidase. Plant Physiol 105 781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuse BJD, Raskin I (1988) Sexual reproduction in the arum lily family, with emphasis on thermogenicity. Sex Plant Reprod 1 3–15 [Google Scholar]
- Miyake K (1898) Some physiological observations on Nelumbo nucifera, Gaertn. Bot Mag Tokyo 12 112–117 [Google Scholar]
- Moore AL, Albury MS, Crichton PG, Affourtit C (2002) Function of the alternative oxidase: Is it still a scavenger? Trends Plant Sci 7 478–481 [DOI] [PubMed] [Google Scholar]
- Moore AL, Bonner WD Jr (1982) Measurements of membrane potentials in plant mitochondria with the safranine method. Plant Physiol 70 1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AL, Siedow JN (1991) The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta 1059 121–140 [DOI] [PubMed] [Google Scholar]
- Nagy KA, Odell DK, Seymour RS (1972) Temperature regulation by the inflorescence of Philodendron. Science 178 1195–1197 [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B (2001) UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504 82–106 [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Locke RM (1984) Thermogenic mechanisms in brown fat. Physiol Rev 64 1–64 [DOI] [PubMed] [Google Scholar]
- Nie Z-L, Sun H, Li H, Wen J (2006) Intercontinental biogeography of subfamily Orontioideae (Symplocarpus, Lysichiton, and Orontium) of Araceae in Eastern Asia and North America. Mol Phylogenet Evol 40 155–165 [DOI] [PubMed] [Google Scholar]
- Nowaczyk MM, Hebeler R, Schlodder E, Meyer HE, Warscheid B, Rögner M (2006) Psb27, a cyanobacterial lipoprotein, is involved in the repair cycle of photosystem II. Plant Cell 18 3121–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda Y, Ito K (2005) Changes in the composition of xylem sap during development of the spadix of skunk cabbage (Symplocarpus foetidus). Biosci Biotechnol Biochem 69 1156–1161 [DOI] [PubMed] [Google Scholar]
- Onda Y, Kato Y, Abe Y, Ito T, Ito-Inaba Y, Morohashi M, Ito Y, Ichikawa M, Matsukawa K, Otsuka M, et al (2007) Pyruvate-sensitive AOX exists as non-covalently associated dimer in the homeothermic spadix of the skunk cabbage, Symplocarpus renifolius. FEBS Lett 581 5852–5858 [DOI] [PubMed] [Google Scholar]
- Prance GT, Arias JR (1975) A study of the floral biology of Victoria amazonica (Poepp.) Sowerby (Nymphaeaceae). Acta Amazon 5 109–139 [Google Scholar]
- Ricquier D, Bouillaud F (2000) The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J 345 161–179 [PMC free article] [PubMed] [Google Scholar]
- Satake T, Hayase H (1970) Male sterility caused by cooling treatment at the young microspore stage in rice plants. V. Estimation of pollen developmental stage and the most sensitive stage to coolness. Proc Crop Sci Soc Japan 39 468–473 [Google Scholar]
- Schroeder CA (1978) Temperature elevation in palm inflorescences. Principes 22 26–29 [Google Scholar]
- Seymour RS (1997) Plants that warm themselves. Sci Am 276 90–95 [Google Scholar]
- Seymour RS (2004) Dynamics and precision of thermoregulatory responses of eastern skunk cabbage Symplocarpus foetidus. Plant Cell Environ 27 1014–1022 [Google Scholar]
- Seymour RS, Blaylock AJ (1999) Switching off the heater: influence of ambient temperature on thermoregulation by eastern skunk cabbage Symplocarpus foetidus. J Exp Bot 50 1525–1532 [Google Scholar]
- Seymour RS, Gibernau M, Ito K (2003) Thermogenesis and respiration of inflorescences of the dead horse arum Helicodiceros muscivorus, a pseudo-thermoregulatory aroid associated with fly pollination. Funct Ecol 17 886–894 [Google Scholar]
- Seymour RS, Schultze-Motel P (1996) Thermoregulating lotus flowers. Nature 383 305 [Google Scholar]
- Seymour RS, Schultze-Motel P (1998) Physiological temperature regulation by flowers of the sacred lotus. Philos Trans R Soc Lond B Biol Sci 353 935–943 [Google Scholar]
- Seymour RS, Schultze-Motel P, Lamprecht I (1998) Heat production by sacred lotus flowers depends on ambient temperature, not light cycle. J Exp Bot 49 1213–1217 [Google Scholar]
- Seymour RS, Terry I, Roemer RB (2004) Respiration and thermogenesis by cones of the Australian cycad Macrozamia machinii. Funct Ecol 18 925–930 [Google Scholar]
- Siedow JN, Umbach AL (1995) Plant mitochondrial electron transfer and molecular biology. Plant Cell 7 821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubatz H, Tang W, Meeuse BJD (1993) Oscillatory heat-production in the male cones of cycads. J Exp Bot 44 489–492 [Google Scholar]
- Skubatz H, Williamson PS, Schneider EL, Meeuse BJD (1990) Cyanide-insensitive respiration in thermogenic flowers of Victoria and Nelumbo. J Exp Bot 41 1335–1339 [Google Scholar]
- Sluse FE, Almeida AM, Jarmuszkiewicz W, Vercesi AE (1998) Free fatty acids regulate the uncoupling protein and alternative oxidase activities in plant mitochondria. FEBS Lett 433 237–240 [DOI] [PubMed] [Google Scholar]
- Smith AMO, Ratcliffe RG, Sweetlove LJ (2004) Activation and function of mitochondrial uncoupling protein in plants. J Biol Chem 279 51944–51952 [DOI] [PubMed] [Google Scholar]
- Steel JH, Gordon L, Polak JM (1998) Principles and applications of complementary RNA probes. In J Polak, JOD McGee, eds, In Situ Hybridization: Principles and Practice, Ed 2. Oxford University Press, Oxford, pp 49–69
- Uemura S, Ohkawara K, Kudo G, Wada N, Higashi S (1993) Heat-production and cross-pollination of the Asian skunk cabbage Symplocarpus renifolius (Araceae). Am J Bot 80 635–640 [Google Scholar]
- Umbach AL, Siedow JN (1993) Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol 103 845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi AE, Borecký J, Maia IG, Arruda P, Cuccovia IM, Chaimovich H (2006) Plant uncoupling mitochondrial proteins. Annu Rev Plant Biol 57 383–404 [DOI] [PubMed] [Google Scholar]
- Vigeolas H, van Dongen JT, Waldeck P, Hühn D, Geigenberger P (2003) Lipid storage metabolism is limited by the prevailing low oxygen concentrations within developing seeds of oilseed rape. Plant Physiol 133 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Krab K (1995) The alternative respiration pathway in plants: role and regulation. Physiol Plant 95 318–325 [Google Scholar]
- Watling JR, Robinson SA, Seymour RS (2006) Contribution of the alternative pathway to respiration during thermogenesis in flowers of the sacred lotus. Plant Physiol 140 1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottini M, Mandolino G, Zannoni D (1993) Oxidation of external NAD(P)H by mitochondria from taproots and tissue cultures of sugar beet (Beta vulgaris). Plant Physiol 102 579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.