Abstract
Plants respond to a large variety of environmental signals, including changes in the gravity vector (gravistimulation). In Arabidopsis (Arabidopsis thaliana) seedlings, gravistimulation is known to increase the cytoplasmic free calcium concentration ([Ca2+]c). However, organs responsible for the [Ca2+]c increase and the underlying cellular/molecular mechanisms remain to be solved. In this study, using Arabidopsis seedlings expressing apoaequorin, a Ca2+-sensitive luminescent protein in combination with an ultrasensitive photon counting camera, we clarified the organs where [Ca2+]c increases in response to gravistimulation and characterized the physiological and pharmacological properties of the [Ca2+]c increase. When the seedlings were gravistimulated by turning 180°, they showed a transient biphasic [Ca2+]c increase in their hypocotyls and petioles. The second peak of the [Ca2+]c increase depended on the angle but not the speed of rotation, whereas the initial peak showed diametrically opposite characters. This suggests that the second [Ca2+]c increase is specific for changes in the gravity vector. The potential mechanosensitive Ca2+-permeable channel (MSCC) inhibitors Gd3+ and La3+, the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and the endomembrane Ca2+-permeable channel inhibitor ruthenium red suppressed the second [Ca2+]c increase, suggesting that it arises from Ca2+ influx via putative MSCCs in the plasma membrane and Ca2+ release from intracellular Ca2+ stores. Moreover, the second [Ca2+]c increase was attenuated by actin-disrupting drugs cytochalasin B and latrunculin B but not by microtubule-disrupting drugs oryzalin and nocodazole, implying that actin filaments are partially involved in the hypothetical activation of Ca2+-permeable channels. These results suggest that the second [Ca2+]c increase via MSCCs is a gravity response in the hypocotyl and petiole of Arabidopsis seedlings.
Gravity is a ubiquitous force on the earth and affects the growth and morphogenesis in plants. Many higher plants sense gravity and orient their growth direction with respect to the gravity vector, a phenomenon known as gravitropism. Changes in the gravity vector (gravistimulation) are supposed to be transduced into certain intracellular signals in the early process of gravitropic response (Chen et al., 1999; Blancaflor and Masson, 2003; Morita and Tasaka, 2004). However, the signaling molecules and the underlying transduction mechanisms remain largely obscure.
It is widely accepted that Ca2+ plays a crucial role in the growth and development of plants (Trewavas and Malho, 1998; White and Broadley, 2003; Hepler, 2005). The relationship between Ca2+ and gravitropism has been closely investigated in the past decades. Gravitropism in maize (Zea mays) roots and oat (Avena sativa) coleoptiles was nearly completely eliminated by soaking them in Ca2+ chelators (e.g. EGTA) or distilled water (Lee et al., 1983a; Daye et al., 1984; Millet and Pickard, 1988), indicating the necessity of extracellular Ca2+ for gravitropic responses. An asymmetrical distribution of cytoplasmic, vacuolar, and apoplastic Ca2+ across oat coleoptiles was observed after gravistimulation, which was spatially correlated with the bending region (Slocum and Roux, 1983). Furthermore, gravistimulation caused a downward redistribution of apoplastic Ca2+ across the horizontally placed root tip in maize (Lee et al., 1983b; Björkman and Cleland, 1991). The gravistimulation-induced redistribution of Ca2+ took place in decapitated maize roots that regenerated gravitropic sensitivity but not in decapitated roots that lost it (Björkman and Cleland, 1991). These results suggest that Ca2+ plays a key role in gravitropism and is involved in the process of gravity sensing and/or following signal transduction.
Because cytoplasmic Ca2+ is considered as a ubiquitous intracellular second messenger, a possible involvement of changes in cytoplasmic free calcium concentration ([Ca2+]c) in gravity response has repeatedly been pointed out (Sinclair and Trewavas, 1997; Fasano et al., 2002; Blancaflor and Masson, 2003). Increases in [Ca2+]c induced by gravistimulation were observed in maize coleoptiles using the [Ca2+]c indicator, fluo-3 (Gehring et al., 1990). Several minutes after gravistimulation, [Ca2+]c increased in the lower side of the horizontally placed maize coleoptiles. This result raised the possibility that gravistimulation is transduced into increases in [Ca2+]c, although the underlying molecular mechanisms are still unclear. Recently, refined technologies of [Ca2+]c imaging revealed changes in the [Ca2+]c induced by gravistimulation in Arabidopsis (Arabidopsis thaliana) seedlings expressing the luminous Ca2+-reporting protein, apoaequorin (Plieth and Trewavas, 2002). Hundreds of Arabidopsis seedlings mounted on a wheel in front of a photomultiplier tube (PMT) showed a biphasic [Ca2+]c transient lasting for over 10 min in response to gravistimulation. Continuous gravistimulation using a clinorotation caused a sustained increase in [Ca2+]c for more than 30 min. On the other hand, when gravitational acceleration increased to 100g from 1g using a centrifuge, a monophasic [Ca2+]c transient lasting for several minutes was detected in Arabidopsis seedlings expressing apoaequorin (Toyota et al., 2007). The peak amplitude of the monophasic [Ca2+]c transient was dependent on the magnitude of the gravitational acceleration. These findings imply an involvement of [Ca2+]c increases in gravity response and/or signal transduction in Arabidopsis seedlings. However, the organs where [Ca2+]c increased in response to changes in the gravity vector (including direction and magnitude of gravitational acceleration) and the underlying cellular and molecular mechanisms remain to be solved.
We developed an imaging system to provide a spatial resolution of aequorin luminescence during gravistimulation, which clarified the organs that responded to gravistimulation in Arabidopsis seedlings. Furthermore, the properties of the biphasic [Ca2+]c increase were investigated in more detail by fine control of gravistimulation in combination with pharmacology. Our results provide a deeper insight into the cellular and molecular mechanisms of the [Ca2+]c increase, leading to a conclusion that the second [Ca2+]c increase is specific for changes in the gravity vector, while the initial one is related to rotational motion in the shoots of Arabidopsis seedlings.
RESULTS
[Ca2+]c Increases in Hypocotyls and Petioles in Response to Gravistimulation
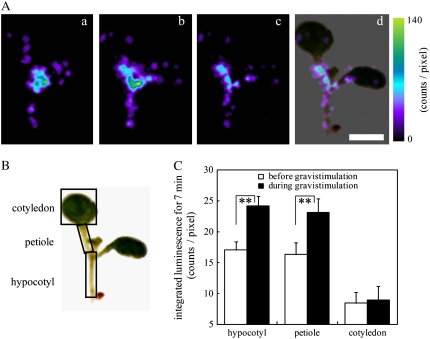
A plate of seedlings of Arabidopsis expressing apoaequorin was mounted under an ultrasensitive photon-counting camera (PCC) in a light-tight dark box (Fig. 1A) and subjected to gravistimulation. Aequorin luminescence from individual seedlings was integrated for 7 min before and 20 s after a rotation (Fig. 2A). When the seedlings were turned 180° at the speed of 6 rpm, increases in luminescence intensity were observed in a hypocotyl and its petioles (Fig. 2A). By subtracting the control image as background luminescence (Fig. 2A, a) from the image after the 180° rotation (Fig. 2A, b), gravistimulation-responsive organs were visualized more clearly (Fig. 2A, c and d). Quantitative analyses of changes in the integrated luminescence intensity showed that aequorin luminescence increased by 40% to 45% in hypocotyls and petioles but not in cotyledons to the gravistimulation (Fig. 2, B and C). Hypocotyls and petioles in general show gravitropic responses (Fukaki et al., 1998; Mano et al., 2006), which is spatially related to the [Ca2+]c increase. Thus, our observations raise the possibility that the [Ca2+]c increase is a gravity response in shoots of Arabidopsis seedlings. We did not detect aequorin luminescence from roots, probably due to an incomplete reconstitution of aequorin (see “Materials and Methods”). The maximum luminescence intensity (Lmax) induced by adding ethanol plus CaCl2 was detected in hypocotyls, petioles, and cotyledons but was extremely small in roots (data not shown), suggesting that aequorin luminescence from seedlings is mainly originated from shoots in our system. Therefore, changes in [Ca2+]c in roots were not examined.
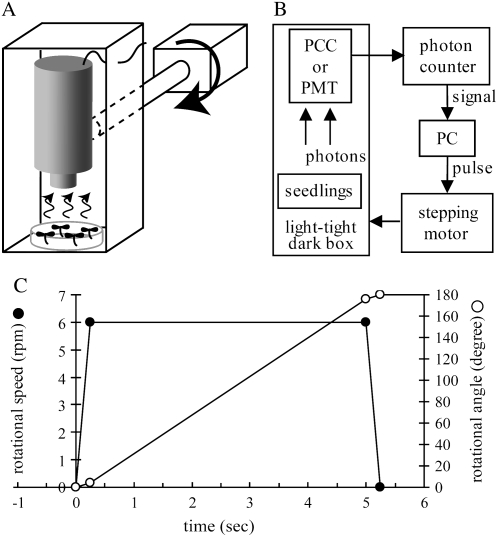
Figure 1.
Schematic diagrams of a device for gravistimulation and a photon-counting system. A, A plate of Arabidopsis seedlings was mounted under an ultrasensitive PCC or a PMT in a light-tight dark box connected to a stepping motor, enabling gravistimulation while monitoring the intensity of aequorin luminescence. B, The luminescence intensity was monitored with a photon counter and stored in a personal computer. The rotational speed and angle of the stepping motor was controlled by the same personal computer. C, Time course of changes in the rotational speed (black circle) and angle (white circle) while turning 180° at the speed of 6 rpm.
Figure 2.
Increases in [Ca2+]c induced by gravistimulation in hypocotyls and petioles. A, [Ca2+]c-dependent aequorin luminescence from a whole seedling was integrated for 7 min before and during gravistimulation (180° rotation) using an ultrasensitive PCC system. Typical images with pseudocolor before (a) and during (b) gravistimulation, the subtracted (c; b − a), and the overlaid image with a bright-field image (d) are shown. The color scale indicates photon counts per pixel. B and C, Quantitative analysis with averaging the integrated luminescence intensities at each pixel in three regions (hypocotyls, petioles, and cotyledons) before (white bar) and during (black bar) gravistimulation. Data represent means ± ses (n = 40). **, Statistically significant differences (P < 0.05, the two-tailed Student's t test). Scale bar = 2 mm.
Properties of Biphasic [Ca2+]c Transient Induced by Gravistimulation
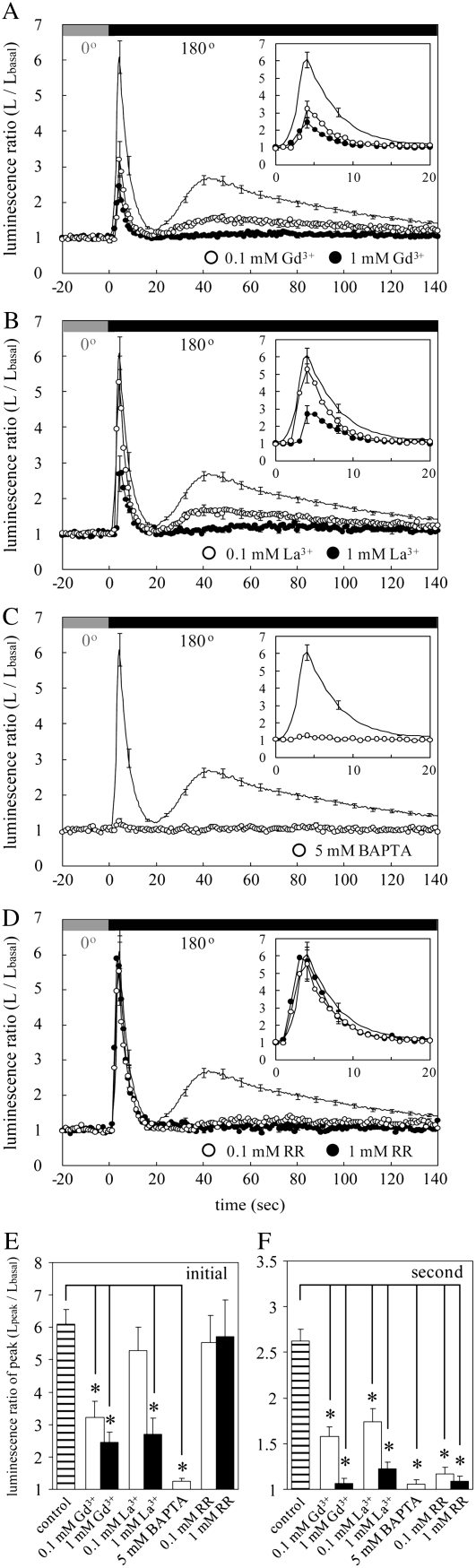
Aequorin luminescence was monitored with a PMT to examine the time course of the [Ca2+]c increase induced by gravistimulation. When a plate of seedlings was subjected to gravistimulation by turning 180° at the speed of 6 rpm, a biphasic [Ca2+]c transient was observed (Fig. 3A, white and black arrowheads), which was consistent with the previous report (Plieth and Trewavas, 2002). The initial [Ca2+]c transient peaked within 4 s when the gravistimulation was applied and decayed exponentially with a time constant of approximately 3 s (Fig. 3A, white arrowhead). The second [Ca2+]c transient peaked at around 40 s from the start of the turning and decayed exponentially with a time constant of approximately 60 s (Fig. 3A, black arrowhead). When the seedlings were turned back 180° at the same speed, the peak amplitude of the second [Ca2+]c transient was strongly attenuated (Fig. 3A, asterisk), whereas that of the initial [Ca2+]c transient remained almost at the same level. Subsequently, the same seedlings were subjected to the same series of gravistimuli (±180°) within 15 min after the cessation of the second gravistimulation. The initial [Ca2+]c transient was almost unchanged to the third and fourth gravistimuli, whereas the second [Ca2+]c transient was strongly attenuated (data not shown), indicating that the second [Ca2+]c transient showed a desensitization to repetitive gravistimulation. These results suggest that the initial and second [Ca2+]c transients arise from different cellular and/or molecular mechanisms.
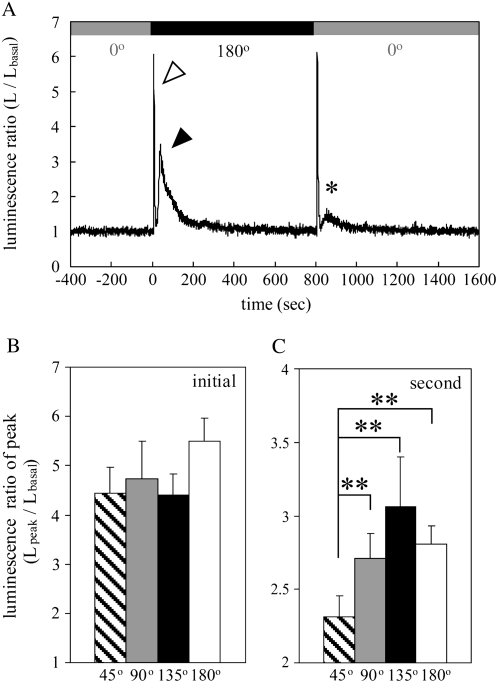
Figure 3.
Biphasic [Ca2+]c transient induced by gravistimulation in Arabidopsis seedlings. A, Typical time course of changes in luminescence ratio induced by gravistimulation (±180° rotation). Approximately 40 Arabidopsis seedlings grown in a plate were turned 180° at the speed of 6 rpm at 0 s on the abscissa and retained at 180° for 800 s, which induced a biphasic [Ca2+]c transient with an initial peak and a second one (white and black arrowheads, respectively). Subsequently, seedlings were turned back −180° at the same speed, which also induced the biphasic [Ca2+]c transient with a small second peak (asterisk). The shaded and black bars above the data represent the rotational angles (0° and 180°, respectively). B and C, The effect of rotational angles on the initial and second [Ca2+]c peaks. Plates of seedlings were turned through different angles at the speed of 6 rpm. The peak amplitudes of the initial (B) and second [Ca2+]c transients (C) in response to 45°, 90°, 135°, and 180° gravistimulation are presented as hatched (n = 18), shaded (n = 22), black (n = 16), and white bars (n = 32), respectively. Data represent means ± ses. **, P < 0.05, the two-tailed Student's t test.
To characterize the biphasic [Ca2+]c transient in more detail, we examined the effect of rotational angle and speed on the peak amplitudes of the initial and second [Ca2+]c transients. The normalized amplitudes of the initial peak induced by turning through different angles showed no significant difference (Fig. 3B), indicating that the amplitude of the initial [Ca2+]c transient is independent of the angle of rotation. On the other hand, the peak amplitudes of the second [Ca2+]c transients were dependent on the angle of rotation (Fig. 3C). The maximum peak of the second [Ca2+]c transient was detected when the seedlings were turned 135°, which was consistent with the previous report (Plieth and Trewavas, 2002).
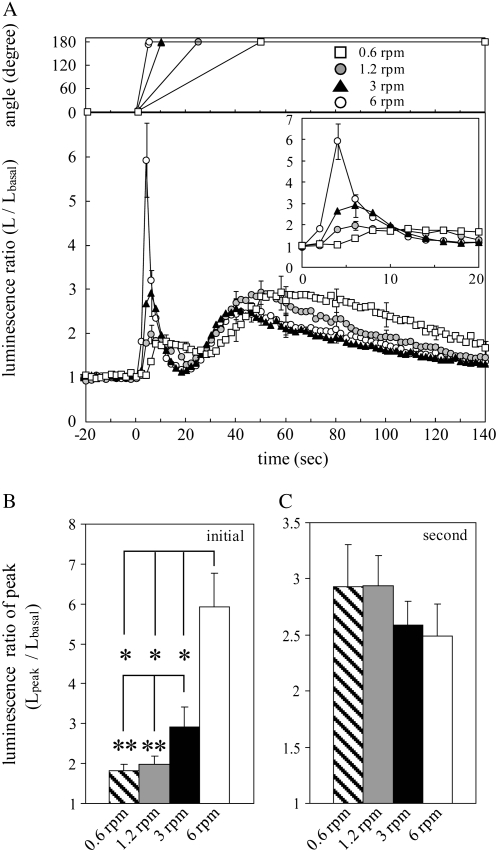
Next, the effect of rotational speed on the initial and second [Ca2+]c transients was examined. As the rotational speed was step-wisely decreased to 0.6 rpm, the initial peak was attenuated in a rotational speed-dependent manner (Fig. 4, A and B). In contrast, the second peak was not significantly affected by slower rotational speed than 6 rpm (Fig. 4, A and C), whereas the time to peak of the second [Ca2+]c transient was slightly delayed (Fig. 4A), probably due to a delay in reaching 180°. Taken together, the amplitude of the second [Ca2+]c transient was dependent on the angle of gravistimulation (Fig. 3C) but not on its speed (Fig. 4C), suggesting that the second [Ca2+]c transient is induced by changes in gravity vector. Therefore, we named the second [Ca2+]c transient the graviinduced [Ca2+]c transient and focused mainly on this response in this study. On the other hand, the amplitude of the initial [Ca2+]c transient was dependent on the speed of gravistimulation (Fig. 4B) but not on its angle (Fig. 3B), suggesting that the initial [Ca2+]c transient is induced by forces related to the rotational speed. No correlation of the peak amplitude between the initial and second [Ca2+]c transients was observed (Figs. 3, B and C, and 4, B and C), further supporting the idea that these [Ca2+]c transients have different origins.
Figure 4.
The effect of rotational speed on the initial and second [Ca2+]c transients. A, The averaged trace of changes in luminescence ratio in response to gravistimulation at the speeds of 0.6, 1.2, 3, and 6 rpm (white square, shaded circle, black triangle, and white circle, respectively). Plates of seedlings were turned 180° at the above speeds at 0 s on the abscissa and retained at 180°. Time course of changes in the rotational angle at each speed is shown in the top graph. The inset shows an enlargement of the initial [Ca2+]c transients. B and C, The peak amplitudes of the initial (B) and second [Ca2+]c transients (C) induced by 0.6, 1.2, 3, and 6 rpm gravistimulation are presented as hatched (n = 8), shaded (n = 13), black (n = 6), and white bars (n = 18), respectively. Data represent means ± ses. *, P < 0.01; **, P < 0.05, the two-tailed Student's t test.
Inhibitor Analyses on the Biphasic [Ca2+]c Transient
Gravistimulation is often regarded as a sort of mechanical stimulation (Trewavas and Knight, 1994). In general, mechanical stimulus is thought to be transduced into a certain intracellular signal thorough mechanosensitive (MS) channels (Hamill and Martinac, 2001). We examined a possible involvement of MS Ca2+-permeable channels (MSCCs) in the biphasic [Ca2+]c transient. Potential MSCC inhibitors, Gd3+ and La3+, suppressed the graviinduced [Ca2+]c transient in a concentration-dependent manner (Fig. 5, A, B, and F). The Ca2+ chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), also nearly completely inhibited the graviinduced [Ca2+]c transient (Fig. 5, C and F). After washing out these chemicals with growth medium, nearly the same amplitude of the graviinduced [Ca2+]c transient without drug treatments was recovered (data not shown). These results suggest that the graviinduced [Ca2+]c transient arises from Ca2+ influx via putative MSCCs in the plasma membrane. On the other hand, the potential endomembrane Ca2+-permeable channel inhibitor, ruthenium red (RR), also suppressed the graviinduced [Ca2+]c transient in a concentration-dependent manner (Fig. 5, D and F), suggesting the involvement of Ca2+ release from intracellular Ca2+ stores in the graviinduced [Ca2+]c transient. The slow vacuolar channels are hypothesized to play an important role in Ca2+-induced Ca2+ release in Beta vulgaris (Ward and Schroeder, 1994; Bewell et al., 1999). Because it is known that RR inhibits the Ca2+-perameable channels in the endoplasmic reticulum (Bauer et al., 1998) and the slow vacuolar channels in the vacuole (Pottosin et al., 1999), it is plausible to expect that gravistimulation causes the Ca2+ influx via the MSCCs followed by the Ca2+-induced Ca2+ release. In contrast, the initial [Ca2+]c transient was inhibited by Gd3+, La3+, and BAPTA but not by RR (Fig. 5, A–E). This result suggests that the initial [Ca2+]c transient originates mainly from Ca2+ influx through putative MSCCs in the plasma membrane and is mechanistically distinct from the graviinduced [Ca2+]c transient.
Figure 5.
The effect of chemical agents on the initial and second [Ca2+]c transients. A to D, Potential MSCC inhibitors (Gd3+ and La3+), a Ca2+ chelator (BAPTA), and an endomembrane Ca2+-permeable channel inhibitor (RR) were extracellularly applied to Arabidopsis seedlings for 1 h. Each averaged trace shows changes in luminescence ratio induced by gravistimulation at the speed of 6 rpm in control (solid line) and chemical agent-treated seedlings (white or black circles). The inset in each figure shows an enlargement of the initial [Ca2+]c transients. E and F, The peak amplitudes of the initial (E) and second (graviinduced) [Ca2+]c transients (F) in control (hatched bar) and chemical agent-treated seedlings (white or black bars) are shown. Symbols and concentrations of the chemicals are presented in each figure (A–D) and below the bar graphs (E and F). Data represent means ± ses, n = 19, 8, 12, 10, 8, 7, 8, and 9 from the left bar in E and F, respectively. *, P < 0.01, the two-tailed Student's t test.
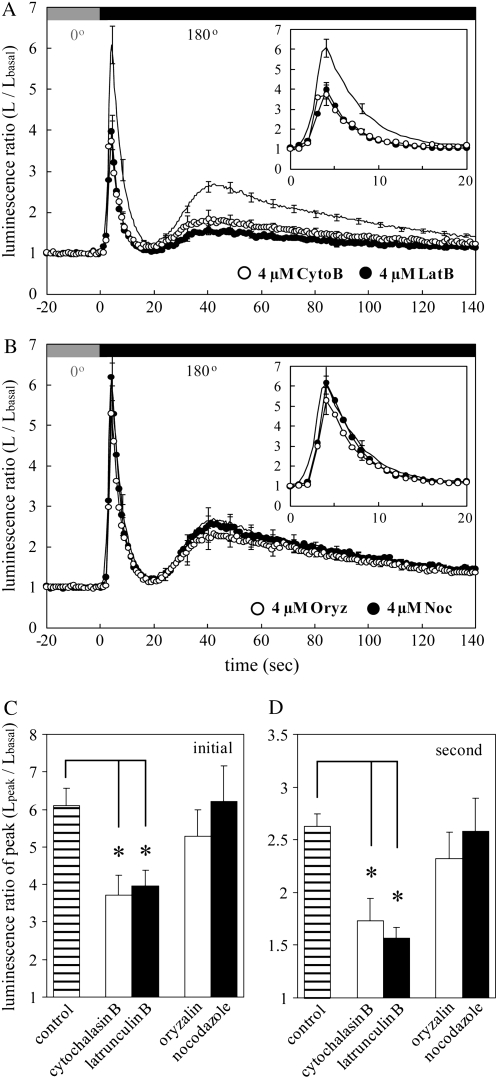
Previous studies indicate that the cytoskeleton plays a certain role in the mechanosensitivity of MSCCs (Hamill and Martinac, 2001). Recent evidence showed that activation of MSCC by stretch force imposed in the plasma membrane is modulated by actin filaments in human leukemia cells (Staruschenko et al., 2005) and guard cells of Vicia faba (Zhang et al., 2007), suggesting that MSCCs are coupled with the cortical actin filaments functionally and/or structurally. Internal or external forces resulting from mechanical stimuli (e.g. gravistimulation) are hypothesized to be effectively transmitted to MSCCs thorough the cytoskeleton (Fasano et al., 2002; Perbal and Driss-Ecole, 2003). The involvement of cytoskeleton, especially actin filaments and microtubules, in the biphasic [Ca2+]c transient induced by gravistimulation was examined in this study. The graviinduced [Ca2+]c transient was attenuated by actin-disrupting drugs, latrunculin B, and cytochalasin B but not by the microtubule-disrupting drugs oryzalin and nocodazole (Fig. 6, A, B, and D), suggesting that actin filaments are partially involved in the hypothesized activation of MSCCs, leading to the graviinduced [Ca2+]c transient. The initial [Ca2+]c transient was also slightly attenuated by latrunculin B and cytochalasin B but not by oryzalin and nocodazole (Fig. 6, A–C), suggesting an actin filament-dependent machinery for the initial [Ca2+]c transient. Forces generated by the rotational motion may also be effectively transduced into MSCCs in the plasma membrane via actin filaments.
Figure 6.
The effect of cytoskeleton-disrupting drugs on the initial and second [Ca2+]c transients. A and B, Actin-disrupting drugs (cytochalasin B and latrunculin B) and microtubule-disrupting drugs (oryzalin and nocodazole) were extracellularly applied to Arabidopsis seedlings at the final concentration of 4 μm. Each averaged trace shows changes in luminescence ratio induced by gravistimulation at the speed of 6 rpm in control (solid line) and drug-treated seedlings (white and black circles). The inset in each figure shows an enlargement of the initial [Ca2+]c transients. C and D, The peak amplitudes of the initial (C) and second (graviinduced) [Ca2+]c transients (D) in control (hatched bar) and drug-treated seedlings (white and black bars) are shown. Symbols and concentrations of the chemicals are presented in each figure (A and B) or below the bar graphs (C and D). Data represent means ± ses, n = 19, 11, 18, 19, and 11 from the left bar in A and B, respectively. *, P < 0.01, the two-tailed Student's t test.
DISCUSSION
Changes in [Ca2+]c induced by various endo- and exogenous signals have been extensively investigated in plants (Gilroy et al., 1993; Sanders et al., 2002; Scrase-Field and Knight, 2003). Recent work demonstrated that changes in the gravity vector induced a [Ca2+]c transient in hundreds of Arabidopsis seedlings expressing apoaequorin (Plieth and Trewavas, 2002). On the other hand, no [Ca2+]c change to gravistimulation was detected in Arabidopsis roots, in which indo-1 was used to probe [Ca2+]c levels (Legue et al., 1997). It has been controversial whether gravistimulation induces changes in [Ca2+]c in Arabidopsis seedlings, because the organs where the intensity of aequorin luminescence increased have remained to be determined. In this study, we confirmed the previous observation (Plieth and Trewavas, 2002) and gain new insights into the debated issue by utilizing advanced optical techniques.
We clarified that gravistimulation induced a [Ca2+]c increase in hypocotyls and petioles (Fig. 2), which is not contradictory to the previous results (Legue et al., 1997). In our setup, we did not detect aequorin luminescence from roots due to the incomplete reconstitution of aequorin in roots growing inside the agar. We tried to detect [Ca2+]c changes in response to gravistimulation in roots by reconstituting aequorin in another way (see “Materials and Methods”). However, we still did not detect it in the roots during gravistimulation using PCC (data not shown). In general, gravistimulation may cause an increase in [Ca2+]c at a detectable level in shoots, as reported previously (Gehring et al., 1990), but not in roots. Recently, transient increases in cytoplasmic pH induced by gravistimulation were observed in columella cells of Arabidopsis roots (Fasano et al., 2001; Hou et al., 2004). Cytoplasmic pH rather than [Ca2+]c may play an important role in gravity response of roots.
Leaf petioles as well as hypocotyls generally show gravitropic responses (Hangarter, 1997; Fukaki et al., 1998). The conventional gravitropic mutants in hypocotyls, phosphoglucomutase and shoot gravitropism2, also exhibit reduced gravitropism in petioles, suggesting that petioles and hypocotyls share the same mechanism of gravity response (Mano et al., 2006). The mechanism of the [Ca2+]c increase may also be shared in hypocotyls and petioles, because we observed [Ca2+]c increases with comparable amplitude in both the organs (Fig. 2C). There are a large number of endodermal cells that are believed to be a gravity perceptive cell in hypocotyls and petioles (Fukaki et al., 1998; Mano et al., 2006). Genetic studies using gravitropic mutants in Arabidopsis suggest that sedimentation of highly dense, starch-filled plastids (amyloplasts) in shoot endodermal cells is involved in gravity sensing (Sack, 1997; Tasaka et al., 1999; Kiss, 2000). According to this hypothesis, [Ca2+]c would increase in the shoot endodermal cells in response to sedimentation of amyloplasts, as discussed previously (Perbal and Driss-Ecole, 2003; Morita and Tasaka, 2004). However, we could not resolve the responsive cells because of the limitation of optical resolution with our setup. To uncover the relationship between the [Ca2+]c increase and amyloplasts, we examined [Ca2+]c changes in response to gravistimulation in the endodermal-amyloplast-less1 (eal1) mutant that has no intact amyloplast in shoot endodermal cells (Fujihira et al., 2000; Morita et al., 2007). Our preliminary experiments showed that both the amplitude and decay time constant of the [Ca2+]c increase in eal1 were almost the same as those in wild-type seedlings, implying that intact amyloplasts in endodermal cells of hypocotyls were not necessary for the [Ca2+]c increase observed in this study. During gravistimulation, both the side and end cell walls are supposed to be exposed to mechanical stress. The [Ca2+]c increase may be initiated by interaction between the cell wall and the plasma membrane via adhesion molecules (Pickard, 2007) rather than by sedimentable organelles.
Gravistimulation caused a biphasic [Ca2+]c increase consisting of the initial and second [Ca2+]c transients with different characters (Fig. 3A). The initial [Ca2+]c transient looks similar in its kinetics to the wind- or touch-induced [Ca2+]c spikes in seedlings of Nicotiana plumbaginifolia (Knight et al., 1991, 1992). The wind- and touch-induced [Ca2+]c spikes are supposed to arise from intracellular Ca2+ release, because they were inhibited by RR but not by Gd3+ or La3+ in Nicotiana seedlings (Knight et al., 1992) and Arabidopsis roots (Legue et al., 1997). However, the initial [Ca2+]c transient in this study was inhibited by Gd3+ and La3+ but not by RR (Fig. 5, A–E), which is diametrically opposite to the above-mentioned results. Collectively, it is suggested that the initial [Ca2+]c transient is pharmacologically distinct from the wind- and touch-induced [Ca2+]c spikes. Certainly, the effect of wind during rotation must be extremely small in this study, because the seedlings are grown in a capped petri dish.
The peak amplitude of the initial [Ca2+]c transient was dependent on the rotational speed (Fig. 4B) but not on the angle (Fig. 3B), suggesting that the initial [Ca2+]c transient is induced by forces related to the rotational speed. Centripetal acceleration during rotation depends on the rotational speed and exerts a centrifugal force on the seedlings. However, the centripetal acceleration during rotation at the speed of 6 rpm was 5.6 × 10−3g in our setup (Fig. 1A), which was much smaller than the gravitational acceleration (1g). To exclude the effect of prospected centrifugal force, the seedlings were turned at the speed of 6 rpm on the rotation axis, which did not affect the initial [Ca2+]c transient at all (data not shown). This suggests that the initial [Ca2+]c transient is not induced by the centrifugal force during rotation. All gravistimuli here were performed at the rotational acceleration of 3.6 × 10−2g (Fig. 1C). To examine the effect of the rotational acceleration on the initial [Ca2+]c transient, the seedlings were turned at the rotational acceleration of 7.2 × 10−1g, which did not affect the initial [Ca2+]c transient (data not shown). This suggests that the initial [Ca2+]c transient is not dependent on the rotational acceleration. The acceleration time to reach the speed of 6 rpm at 7.2 × 10−1g was 0.01 s, which might not be long enough to induce a response in the seedlings. Rotation causes a shift in weight bearing of the seedlings, leading to a slight deformation in hypocotyls and petioles, because they are supported on one end. The mechanical stress resulting from the deformation might induce the initial [Ca2+]c transient.
The second [Ca2+]c transient showed a rotational angle dependency (Fig. 3C), whereas the rotational speed had no significant effect on its peak amplitude (Fig. 4C). A single rotation around 360° did not induce the second [Ca2+]c transient, whereas the initial one was observed (Plieth and Trewavas, 2002). These results suggest that the second [Ca2+]c transient is specific for changes in the gravity vector but not for the rotation. As the rotational speed was decreased, the peak amplitude of the second [Ca2+]c transient was slightly increased and the time to the peak was delayed (Fig. 4, A and C). When the seedlings were turned 135° and returned back to the vertical position (0°) within 5 s, a small but distinguishable second [Ca2+]c transient was observed (Plieth and Trewavas, 2002), suggesting that the presentation time, the time of gravistimulation to elicit the second [Ca2+]c transient, is less than 5 s. It requires approximately 50 s to reach 180° at the speed of 0.6 rpm (Fig. 4A). Because the presentation time is much shorter than the above rotational time, the seedlings might be gravistimulated at each angle during the slow rotation, resulting in an apparent increase in the second [Ca2+]c transient (Fig. 4A). The delayed time to the peak is probably due to the delay to reach 180°, because the second [Ca2+]c transient is induced by changes in the gravity vector, as discussed above. Recently, Toyota et al. (2007) demonstrated that increases in the gravitational acceleration caused a monophasic [Ca2+]c transient in Arabidopsis seedlings, which resembles the second [Ca2+]c transient in terms of time course. The monophasic [Ca2+]c was inhibited by La3+ or Gd3+ and was also attenuated by repetitive hypergravity stimuli. [Ca2+]c spikes like the initial [Ca2+]c transient were not evoked by hypergravity stimuli, implying that gravity-related stimuli (e.g. changes in the gravity vector or increases in the gravitational acceleration) cause a long lasting [Ca2+]c increase. Based on this perspective, our observations further support the idea that the second [Ca2+]c transient was induced by changes in the gravity vector.
The second (graviinduced) [Ca2+]c transient was inhibited by La3+, Gd3+, RR, and BAPTA (Fig. 5), suggesting that it arises from Ca2+ influx via putative MSCCs in the plasma membrane and Ca2+ release from intracellular Ca2+ stores. Plant MS channels have been characterized by the patch clamp technique in V. faba guard cells (Cosgrove and Hedrich, 1991), epidermal cells of Allium cepa (Ding and Pickard, 1993), and Arabidopsis mesophyll cells (Qi et al., 2004). Recently, several genes of putative MS channels were identified in Arabidopsis. Nakagawa et al. (2007) isolated MID1-COMPLEMENTING ACTIVITY1 encoding a plasma membrane protein that is responsible for Ca2+ influx in response to mechanical stress such as hypoosmotic shock. Haswell and Meyerowitz (2006) characterized two MscS-like (MSL) proteins, MSL2 and MSL3, which are homologous to the well-known bacterial MS channel MscS and are localized to the plastids such as chloroplasts. A homolog of MscS expressing in chloroplasts (MSC1) was also cloned in Chlamydomonas and its functional reconstitution was succeeded in Escherichia coli (Nakayama et al., 2007). These putative MS channels may be involved not only in mechanosensing in the plasma and endomembrane but also in the [Ca2+]c transient in Arabidopsis seedlings, which is induced by changes in the gravity vector, as proposed here.
Intracellular levels of inositol 1,4,5-trisphosphate (InsP3) increased within 15 s of gravistimulation and peaked at around 60 s in the lower pulvinus of the horizontally placed oat shoot (Perera et al., 2001). Arabidopsis inflorescence stems also showed increases in the InsP3 level within 5 min of gravistimulation (Perera et al., 2006). The graviinduced [Ca2+]c transient could be observed at approximately 20 s after the onset of rotation and lasted for more than 5 min, which resembles the InsP3 increase in terms of time course. Thus, the graviinduced [Ca2+]c transient may be related to the increased level of InsP3 that leads to an InsP3-induced Ca2+ release (Bush, 1995). Plieth and Trewavas (2002) demonstrated that both the initial and second [Ca2+]c transients were attenuated by auxin transport inhibitors (naphthylphthalamic acid, NPA; and 2,3,5-triiodobenzoic acid, TIBA) and raised the possibility that [Ca2+]c increases while auxin is redistributed asymmetrically. We do not exclude this possibility, because we also confirmed that NPA and TIBA affected the [Ca2+]c transient with our setup (data not shown). The biphasic [Ca2+]c transient may be related to auxin via its multiple signaling pathways (Woodward and Bartel, 2005).
Recently, an intriguing model for gravity sensing was proposed based on a careful analysis of gravitropism in maize roots (LaMotte and Pickard, 2004). In this model, maize roots require at least two gravity sensing steps, termed gravifacilitation and vectorial graviinduction steps. Pickard (2007) speculates that the biphasic [Ca2+]c transient is involved in the gravifacilitation step that does not induce gravitropism directly but operates the second vectorial graviinduction step. The pharmacological properties of the biphasic [Ca2+]c transient in this study are almost consistent with the speculation. The roles of the biphasic [Ca2+]c transient in gravitropism remain to be solved.
MATERIALS AND METHODS
Plant Materials and Growth Condition
We used the transgenic Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 (Col-0) expressing cytoplasmic apoaequorin under the control of the 35S promoter of Cauliflower mosaic virus (Knight et al., 1996) for all experiments. Approximately 40 surface-sterilized seeds of homozygous luminous plants were sown on a 0.3% [w/v] gelrite (Wako Pure Chemicals) plate containing plant growth medium (Murashige and Skoog salts, 1% [w/v] Suc, 0.01% [w/v] myoinositol, and 0.05% [w/v] MES, pH 5.8, adjusted with 1 m KOH) in petri dishes (diameter, 6 cm). The plates were incubated at 4°C in the dark for 2 d and subsequently cultivated at 22°C in a growth chamber under continuous light at approximately 80 μmol m−2 s−1 with daylight fluorescent lamps (model FL32S NA-G, Matsushita Electric Industrial). All plates of seedlings light grown on the agar were used for experiments 5 to 7 d after cultivation.
Reconstitution of Aequorin
Chemically synthesized o-fluoro-dehydrocoelenterazine (F-DCT; Isobe et al., 2002), a kind gift from Dr. M. Kuse and Prof. M. Isobe, Nagoya University, was prepared in ethanol to give a 1-mm stock solution and then added into the plant growth medium to make a 2.5-μm solution. To reconstitute aequorin, Arabidopsis seedlings grown on the agar were filled with 3 mL of the plant growth medium containing 2.5 μm F-DCT for approximately 8 h at 22°C in the dark. After incubation, the plant growth medium was removed from the dish 2 h before experiments.
Recently, a coelenterazine was considered as a chemiluminescent indicator for reactive oxygen species (ROS) in addition to [Ca2+]c (Plieth, 2005). We examined whether the transient increase in intensity of aequorin luminescence during gravistimulation is induced by an increase in ROS. Untransformed Col-0 seedlings treated with F-DCT showed no changes in luminescence intensity during gravistimulation (data not shown), indicating that the increase in luminescence intensity is induced by [Ca2+]c increases but not by ROS.
Treatments with Chemical Agents
La3+ or Gd3+, potential MSCC inhibitors; RR, a potential endomembrane Ca2+-permeable channel inhibitor; and BAPTA, a Ca2+ chelator were prepared in distilled water to give a 10-mm stock solution. Cytochalasin B or latrunculin B, actin-disrupting drugs, and oryzalin or nocodazole, microtubule-disrupting drugs, were prepared in dimethyl sulfoxide to give a 10-mm stock solution. Each drug was added into the plant growth medium containing F-DCT in a petri dish 1 h prior to removal of the medium. The plant growth medium containing F-DCT and drugs was removed from the dish 2 h before the experiments. The final dimethyl sulfoxide concentration did not exceed 0.1%, which had no effect on the [Ca2+]c increases induced by gravistimulation (data not shown).
Gravistimulation and [Ca2+]c Monitoring
Approximately 40 Arabidopsis seedlings cultivated on the agar in a petri dish were mounted under an ultrasensitive PCC (a CCD camera equipped with an image intensifier, model C2741-35A, or a Peltier cooled image intensifier, model C8600-04, Hamamatsu Photonics) and a PMT (model RP1942, Hamamatsu Photonics) in a light-tight dark box (Fig. 1A). The box was set on the folder and turned by a computer-controlled stepping motor system (model RK569BA, Oriental Motor) to change the gravity vector against the seedlings, termed gravistimulation (Fig. 1B). The radius of rotation, the length from the rotation axis to seedlings, was approximately 0.14 m. All gravistimuli were performed at the rotational acceleration of 0.35 m s−2 (Fig. 1C, black circle) and at the rotational speed of 6 rpm except for one experiment (Fig. 4). In this system, the relative position between Arabidopsis seedlings and the detector was not shifted during gravistimulation, which made it possible to monitor aequorin luminescence with a spatial resolution.
To visualize the organ that increases [Ca2+]c, photons of aequorin luminescence from individual plants attached on the agar surface were integrated for 7 min using PCC with a 25-mm lens (F = 0.95; model CM 120, Schneider Kreuznach) before and during gravistimulation as described previously (Furuichi et al., 2001). The integrated images were processed with the image analysis software, MetaMorph (Universal Imaging) or HPD-LIS (Hamamatsu Photonics) and presented through low-pass filtering (Fig. 2A). Aequorin luminescence was integrated from 20 s after the start of rotation to exclude the luminescence of the initial [Ca2+]c transient. Bright-field images of Arabidopsis seedlings were taken with a digital camera (model D70s, Nikon) under approximately 80 μmol m−2 s−1 white light at the end of each experiment.
The intensity of aequorin luminescence from a group of Arabidopsis seedlings in a petri dish were monitored with PMT with a 50-mm lens (F = 0.95; model YMV5095, Yakumo). The signals from the seedlings were processed by a photon counter (model PHC3000-1, Scientex) at 0.5-s intervals and stored in a computer (Fig. 1B).
Data Analysis
For the statistical analysis of the data obtained from different gravistimuli, the luminescence ratio (Lpeak/Lbasal) was calculated by dividing the initial and second peak intensities of aequorin luminescence (Lpeak) with the steady luminescence intensity before gravistimulation (Lbasal). The relative luminescence (Lpeak/Lmax) was obtained by dividing Lpeak by the Lmax induced by the addition of 20% (v/v) ethanol plus 2 m CaCl2 at the end of each experiment. Almost the same statistical significances were obtained using either Lpeak/Lbasal or Lpeak/Lmax (data not shown) in this study. All experiments were repeated more than three times independently with similar results. All data represent means ± se.
The levels of aequorin reconstitution in hypocotyls, petioles, cotyledons, and roots were estimated by the measurements of the relative steady luminescence (Lbasal/Lmax). The Lbasal/Lmax was monitored with the PCC and almost the same level was observed in hypocotyls, petioles, and cotyledons but was quite small in roots (data not shown). When the reconstitution of aequorin was performed by floating seedlings in the growth medium containing F-DCT, as described previously (Knight et al., 1991), we could detect almost the same levels of the Lbasal/Lmax in all organs (data not shown). These results suggest that the reconstitution of aequorin is not completed in roots in the agar, probably due to poor penetration of F-DCT into the agar. Thus, aequorin luminescence from seedlings was mainly originated from shoots in our system.
Acknowledgments
We thank Dr. M. Kuse and Professor M. Isobe (Nagoya University, Japan) for synthesizing coelenterazine, Professor A. Trewavas (University of Edinburgh, UK) for providing seeds of transgenic Arabidopsis Col-0 expressing apoaequorin, and Professor K. Yamamoto (Hokkaido University, Japan), Dr. Y. Narusaka, and Dr. M. Narusaka (Research Institute for Biological Sciences Okayama, Japan) for technical support. We are also grateful to Professor B. Pickard (Washington University), Professor M. Terao-Morita, Professor M. Tasaka (Nara Institute of Science and Technology, Japan), and Professor H. Iida (Tokyo Gakugei University, Japan) for helpful discussions.
This work was supported by the Japan Society for the Promotion of Science for Young Scientists (research fellowships to M.T.), by International Cooperative Research Project/Solution Oriented Research for Science and Technology (Japan Science and Technology Agency; grant to M.S.), by the Ministry of Education, Science, Sports and Culture (grant-in-aid for General Scientific Research nos. 13480216 to M.S. and 14580769 to H.T., grant-in-aid for Scientific Research on Priority Areas no. 15086270 to M.S., and grant-in-aid for Creative Research no. 16GS0308 to M.S.), and by the Japan Space Forum (to H.T. and M.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Masahiro Sokabe (msokabe@med.nagoya-u.ac.jp).
References
- Bauer CS, Plieth C, Bethmann B, Popescu O, Hansen UP, Simonis W, Schonknecht G (1998) Strontium-induced repetitive calcium spikes in a unicellular green alga. Plant Physiol 117 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewell MA, Maathuis FJ, Allen GJ, Sanders D (1999) Calcium-induced calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet. FEBS Lett 458 41–44 [DOI] [PubMed] [Google Scholar]
- Björkman T, Cleland RE (1991) The role of extracellular free-calcium gradients in gravitropic signalling in maize roots. Planta 185 379–384 [PubMed] [Google Scholar]
- Blancaflor EB, Masson PH (2003) Plant gravitropism: unraveling the ups and downs of a complex process. Plant Physiol 133 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DS (1995) Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol 46 95–122 [Google Scholar]
- Chen R, Rosen E, Masson PH (1999) Gravitropism in higher plants. Plant Physiol 120 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ, Hedrich R (1991) Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta 186 143–153 [DOI] [PubMed] [Google Scholar]
- Daye S, Biro RL, Roux SJ (1984) Inhibition of gravitropism in oat coleoptiles by the calcium chelator, ethyleneglycol-bis-(beta-aminoethyl ether)-N,N′-tetraacetic acid. Physiol Plant 61 449–454 [DOI] [PubMed] [Google Scholar]
- Ding JP, Pickard BG (1993) Mechanosensory calcium-selective cation channels in epidermal cells. Plant J 3 83–110 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Massa GD, Gilroy S (2002) Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul 21 71–88 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihira F, Kurata T, Watahiki K, Karahara I, Yamamoto K (2000) An agravitropic mutant of Arabidopsis, endodermal-amyloplast less 1, that lacks amyloplasts in hypocotyl endodermal cell layer. Plant Cell Physiol 41 1193–1199 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M (1998) Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 14 425–430 [DOI] [PubMed] [Google Scholar]
- Furuichi T, Mori I, Takahashi K, Muto S (2001) Sugar-induced increase in cytosolic Ca2+ in Arabidopsis thaliana whole plants. Plant Cell Physiol 42 1149–1155 [DOI] [PubMed] [Google Scholar]
- Gehring CA, Williams DA, Cody SH, Parish RW (1990) Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature 345 528–530 [DOI] [PubMed] [Google Scholar]
- Gilroy S, Bethke PC, Jones RL (1993) Calcium homeostasis in plants. J Cell Sci 106 453–461 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Martinac B (2001) Molecular basis of mechanotransduction in living cells. Physiol Rev 81 685–740 [DOI] [PubMed] [Google Scholar]
- Hangarter RP (1997) Gravity, light and plant form. Plant Cell Environ 20 796–800 [DOI] [PubMed] [Google Scholar]
- Haswell ES, Meyerowitz EM (2006) MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol 16 1–11 [DOI] [PubMed] [Google Scholar]
- Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17 2142–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Kramer VL, Wang YS, Chen R, Perbal G, Gilroy S, Blancaflor EB (2004) The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant J 39 113–125 [DOI] [PubMed] [Google Scholar]
- Isobe M, Fujii T, Kuse M, Miyamoto K, Koga K (2002) 19F-Dehydrocoelenterazine as probe to investigate the active site of symplectin. Tetrahedron 58 2117–2126 [Google Scholar]
- Kiss JZ (2000) Mechanisms of the early phases of plant gravitropism. CRC Crit Rev Plant Sci 19 551–573 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352 524–526 [DOI] [PubMed] [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ (1992) Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA 89 4967–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte CE, Pickard BG (2004) Control of gravitropic orientation. II. Dual receptor model for gravitropism. Funct Plant Biol 31 109–120 [DOI] [PubMed] [Google Scholar]
- Lee JS, Mulkley TJ, Evans ML (1983. a) Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science 220 1375–1376 [DOI] [PubMed] [Google Scholar]
- Lee JS, Mulkley TJ, Evans ML (1983. b) Gravity induced polar transport of calcium across root tips of maize. Plant Physiol 73 874–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S (1997) Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol 114 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano E, Horiguchi G, Tsukaya H (2006) Gravitropism in leaves of Arabidopsis thaliana (L.) Heynh. Plant Cell Physiol 47 217–223 [DOI] [PubMed] [Google Scholar]
- Millet B, Pickard BG (1988) Early wrong-way response occurs in orthogravitropism of maize roots treated with lithium. Physiol Plant 72 555–559 [DOI] [PubMed] [Google Scholar]
- Morita MT, Saito C, Nakano A, Tasaka M (2007) endodermal-amyloplast less 1 is a novel allele of SHORT-ROOT. Adv Space Res 39 1127–1133 [Google Scholar]
- Morita MT, Tasaka M (2004) Gravity sensing and signaling. Curr Opin Plant Biol 7 712–718 [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami K, Sokabe M, Kojima I, Sato S, et al (2007) Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA 104 3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Fujiu K, Sokabe M, Yoshimura K (2007) Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci USA 104 5883–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal G, Driss-Ecole D (2003) Mechanotransduction in gravisensing cells. Trends Plant Sci 8 498–504 [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Chang SC, Boss WF, Kaufman PB (2001) A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat shoot pulvini. Plant Physiol 125 1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IY, Hung CY, Brady S, Muday GK, Boss WF (2006) A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol 140 746–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BG (2007) Delivering force and amplifying signals in plant mechanosensing. Curr Top Membr 58 361–392 [Google Scholar]
- Plieth C (2005) Calcium: just another regulator in the machinery of life? Ann Bot (Lond) 96 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C, Trewavas AJ (2002) Reorientation of seedlings in the earth's gravitational field induces cytosolic calcium transients. Plant Physiol 129 786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin II, Dobrovinskaya OR, Muniz J (1999) Cooperative block of the plant endomembrane ion channel by ruthenium red. Biophys J 77 1973–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Kishigami A, Nakagawa Y, Iida H, Sokabe M (2004) A mechanosensitive anion channel in Arabidopsis thaliana mesophyll cells. Plant Cell Physiol 45 1704–1708 [DOI] [PubMed] [Google Scholar]
- Sack FD (1997) Plastids and gravitropic sensing. Planta 203 S63–S68 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14 S401–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrase-Field SA, Knight MR (2003) Calcium: just a chemical switch? Curr Opin Plant Biol 6 500–506 [DOI] [PubMed] [Google Scholar]
- Sinclair W, Trewavas AJ (1997) Calcium in gravitropism: a re-examination. Planta 203 S85–90 [DOI] [PubMed] [Google Scholar]
- Slocum RD, Roux SJ (1983) Cellular and subcellular localization of calcium in gravistimulated oat coleoptiles and its possible significance in the establishment of tropic curvature. Planta 157 481–492 [DOI] [PubMed] [Google Scholar]
- Staruschenko A, Negulyaev YA, Morachevskaya EA (2005) Actin cytoskeleton disassembly affects conductive properties of stretch-activated cation channels in leukaemia cells. Biochim Biophys Acta 1669 53–60 [DOI] [PubMed] [Google Scholar]
- Tasaka M, Kato T, Fukaki H (1999) The endodermis and shoot gravitropism. Trends Plant Sci 4 103–107 [DOI] [PubMed] [Google Scholar]
- Toyota M, Furuichi T, Tatsumi H, Sokabe M (2007) Hypergravity stimulation induces changes in intracellular calcium concentration in Arabidopsis seedlings. Adv Space Res 39 1190–1197 [Google Scholar]
- Trewavas AJ, Knight MR (1994) Mechanical signalling, calcium and plant form. Plant Mol Biol 26 1329–1341 [DOI] [PubMed] [Google Scholar]
- Trewavas AJ, Malho R (1998) Ca2+ signalling in plant cells: the big network! Curr Opin Plant Biol 1 428–433 [DOI] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6 669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR (2003) Calcium in plants. Ann Bot (Lond) 92 487–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Fan LM, Wu WH (2007) Osmo-sensitive and stretch-activated calcium-permeable channels in Vicia faba guard cells are regulated by actin dynamics. Plant Physiol 143 1140–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]