Abstract
The Venus flytrap (Dionaea muscipula) possesses an active trapping mechanism to capture insects with one of the most rapid movements in the plant kingdom, as described by Darwin. This article presents a detailed experimental investigation of trap closure by mechanical and electrical stimuli and the mechanism of this process. Trap closure consists of three distinctive phases: a silent phase with no observable movement; an accelerated movement of the lobes; and the relaxation of the lobes in their closed state, resulting in a new equilibrium. Uncouplers and blockers of membrane channels were used to investigate the mechanisms of different phases of closing. Uncouplers increased trap closure delay and significantly decreased the speed of trap closure. Ion channel blockers and aquaporin inhibitors increased time of closing. Transmission of a single electrical charge between a lobe and the midrib causes closure of the trap and induces an electrical signal propagating between both lobes and midrib. The Venus flytrap can accumulate small subthreshold charges, and when the threshold value is reached, the trap closes. Repeated application of smaller charges demonstrates the summation of stimuli. The cumulative character of electrical stimuli points to the existence of electrical memory in the Venus flytrap. The observed fast movement can be explained by the hydroelastic curvature model without invoking buckling instability. The new hydroelastic curvature mechanism provides an accurate description of the authors' experimental data.
Plants can react to mechanical stimuli (Ksenzhek and Volkov, 1998; Braam, 2005) with the use of mechanosensitive channels. These channels are found in different types of cells—animal, plant, fungal, and bacterial. The omnipresence of these channels indicates their important physiological function in the regulation of osmolarity, cell volume, and growth (Markin and Sachs, 2004). They are ideal transducers of physiologically relevant mechanical forces (Benolken and Jacobson, 1970). Mechanosensory ion channels in plants are activated by mechanical stress and transduce the sensed information into electrical signals (Volkov and Haack, 1995). In higher plants, these channels are involved in the response to environmental stress (Volkov et al., 1998; Roberts, 2006; Volkov, 2006a, 2006b; Volkov and Brown, 2006a, 2006b).
The response of Venus flytrap (Dionaea muscipula) to mechanical stimulation has long been known (Burdon-Sanderson, 1873; Darwin, 1875; Burdon-Sanderson and Page, 1876; Brown, 1916; Sibaoka, 1969). Perhaps all plants react in response to mechanical stimuli, but only certain plants with rapid and highly noticeable touch-stimulus response, such as the Venus flytrap, have received much attention (Hodick and Sievers, 1986, 1988, 1989; Juniper et al., 1989; Fagerberg and Allain, 1991; Fagerberg and Howe, 1996). This small plant consists of five to seven leaves with each leaf divided into two parts: the upper leaf and the lower leaf. The upper leaf has a pair of trapezoidal lobes held together by a blade (midrib). The center of each lobe contains three sensitive trigger hairs and a red anthocynanin pigment that attracts insects. The edge of each lobe is lined with hair-like projections (cilia). The lower leaf, also known as the footstalk, has an expanded leaf-like structure. Each trap reaches a maximum size of 3 to 7 cm (Lloyd, 1942). We discovered the trap closure using electrical stimulation between the lobes and midrib of the Venus flytrap (Volkov et al., 2007). Time and speed of closing do not depend on the type of stimuli; both mechanically and electrically stimulated Venus flytraps close in 0.3 s.
Touching trigger hairs protruding from the upper leaf epidermis of the Venus flytrap activates mechanosensitive ion channels and generates receptor potentials, which induce an action potential (AP; Burdon-Sanderson, 1874, 1882; Burdon-Sanderson and Page, 1876; Stuhlman and Darden, 1950; Jacobson, 1965; Sibaoka, 1966; Volkov et al., 2007). A receptor potential always precedes an AP and couples the mechanical stimulation step to the AP step of the preying sequence (Jacobson, 1974). A possible pathway of AP propagation includes vascular bundles and plasmodesmata in the upper leaf (Volkov et al., 2007). Within the last 130 years, plant electrophysiologists have measured Venus flytrap action potentials with extremely slow registration systems and without anti-aliasing low-pass filters (Table I). Due to the electronic effects of aliasing and the differing time constants of analog voltmeters (τ = RC), previous researchers published AP propagation velocities ranging from 6 to 20 cm/s, AP amplitudes from 8.4 to 200 mV, and AP durations from 300 ms to 10 s (Burdon-Sanderson, 1874, 1882; Burdon-Sanderson and Page, 1876; Stuhlman and Darden, 1950; DiPalma et al., 1966; Sibaoka, 1966, 1969; Hodick and Sievers, 1986, 1988; Krol et al., 2006). Plant physiologists correctly criticized these results (Sachs, 1887). Using an ultrafast data acquisition system with measurements in real time, Volkov et al. (2007) found that the generated AP has the duration time of 1.5 ms and the velocity of 10 m/s.
Table I.
APs in Venus flytrap
| Amplitude | Duration | Speed | Length | Low-Pass Anti-Aliasing Filter | Citation | |
|---|---|---|---|---|---|---|
| mV | s | cm/s | cm | |||
| 1 | Unknown | 0.5–0.8 | 20 | 10–16 | No | Burdon-Sanderson (1873, 1874, 1882) |
| 2 | 130 | 1 | 20 | 20 | No | Stuhlman and Darden (1950) |
| 3 | 8.4–14.6 | 1 | Unknown | Unknown | No | DiPalma et al. (1961) |
| 4 | 15–20 | 0.3–0.8 | Unknown | Unknown | No | DiPalma et al. (1966) |
| 5 | 100 | 1 | 6–17 | 6–17 | No | Sibaoka (1966) |
| 6 | 140–200 | 2–10 | 17 | 34–170 | No | Hodick and Sievers (1988) |
| 7 | 150–200 | 2–5 | 10 | 20–50 | No | Trebacz and Sievers (1998) |
| 8 | 67–68 | 10 | 10 | 100 | No | Krol et al. (2006) |
| 9 | 150 | 0.0010–0.0014 | 1,000 | 1–1.4 | Yes | Volkov et al. (2007) |
Upon closure, the cilia protruding from the edge of each lobe form an interlocking wall that is impenetrable to all except the smallest prey. The trap shuts when the prey touches its trigger hairs, which are arranged in a triangular pattern, three in a lobe. Partial closure allows the cilia to overlap, but the lobes are still held slightly ajar. This partial closure occurs in a fraction of a second, and several minutes may be required for the lobes to come fully together. When an insect is caught, the lobes seal tightly and remain so for 5 to 7 d, allowing digestion to take place (Scala et al., 1969). The stalk and basal cells containing lipid globules and the common wall between these two cells are traversed by numerous plasmodesmata. Electron micrographs of the trigger hair reveal three regions where the cells differ in size, shape, and cytoplasmic content (Williams and Mozingo, 1971). The basal walls of the indentation cells contain many plasmodesmata. Plasmodesmata found in anticlinal and podium cells pass through constricted zones in the cell wall, and there are numerous plasmodesmata in the peripheral podium cells.
Uncouplers and ion channel blockers inhibit APs in the Venus flytrap. Hodick and Sievers (1988) reported an excitability inhibition of the Dionaea leaf mesophyll cells using uncoupler 2,4-dinitrophenol. Resting potential and excitability are completely restored after 30 min of washing with a standard medium. Anthracene-9-carboxylic acid (9-AC), neomycin, ruthenium red, lanthanum ions, EGTA, and NaN3 inhibit the AP in the Venus flytrap lobes (Hodick and Sievers, 1988; Krol et al., 2006; Volkov et al., 2007).
The lobes of the Venus flytrap move because of changes in the shape, curvature, and volume of cells. In the case of the osmotic motor, water flux is linked to ion fluxes. If water follows H+ flux by osmosis, then the rate of flux will determine the rate of volume change in the lobes. Rapid movement of each lobe requires water cotransporters or contractile proteins (Lea, 1976; Morillon et al., 2001). During the last few years it has become clear that water flux across biological membranes not only reflects a passive diffusion across the lipid bilayer, but also facilitates by aquaporins, which may play a pivotal role in osmoregulation in both animals and plants (Maurel, 1997; Maurel and Chrispeels, 2001; Tyerman et al., 2002; Detmers et al., 2006; Törnroth-Horsefield et al., 2006). Aquaporins play a crucial role in water transport through membranes of plant cells. The rate of cellular movement is determined by the water flux. This flux is induced by a rapid change in osmotic pressure and is monitored by a fast and transient opening of aquaporins. K+ channel blockers tetraethylammonium and nonyltriethylammonium as well as anion channel blocker Zn2+ inhibit water channel activity. Gating behavior of aquaporins is poorly understood but evidence is mounting that phosphorylation, pH, and osmotic gradients can affect water channel activity.
We propose a new hydroelastic curvature mechanism based on the assumption that the lobes possess curvature elasticity and comprise upper and lower hydraulic layers with different hydrostatic pressures. The open state of the trap contains high elastic energy due to the hydrostatic pressure difference between the hydraulic layers of the lobe. Stimuli open pores connecting both hydraulic layers, water rushes from one hydraulic layer to another, and the trap relaxes to the equilibrium configuration corresponding to the closed state. Our report analyzes the kinetics and mechanism of trap closure induced by mechanical or electrical stimuli.
RESULTS
Speed of Closing
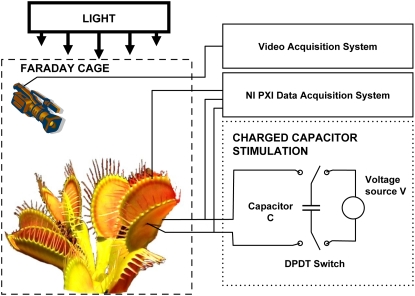
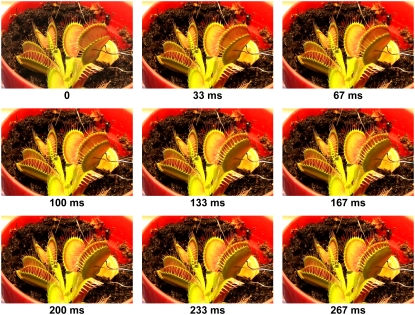
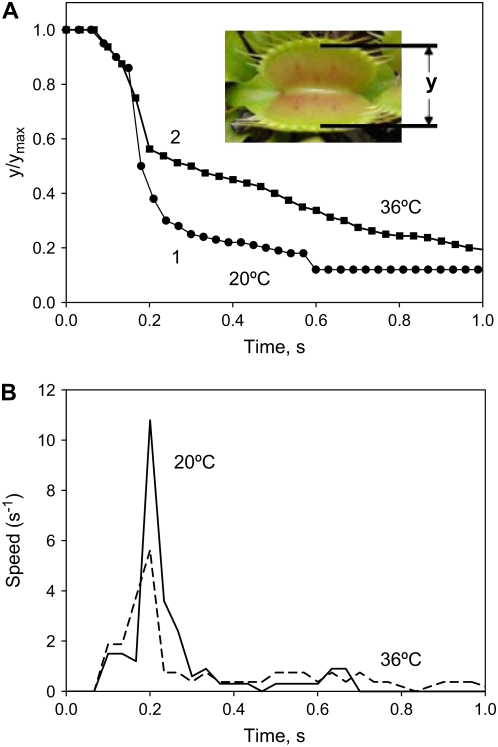
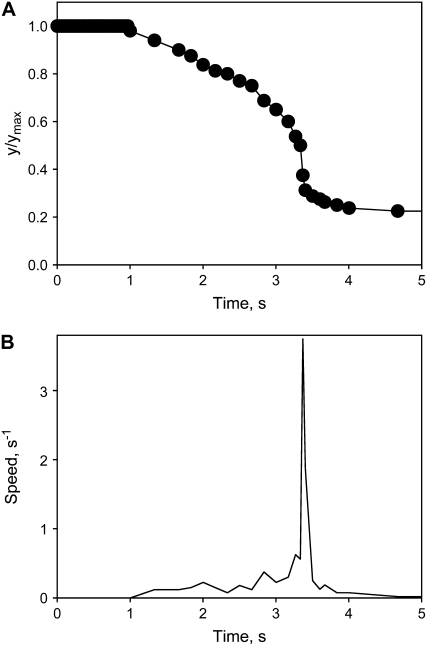
The trap closure of the Venus flytrap was studied with mechanical and electrical stimulation at different temperatures. We used the charge injection method (Fig. 1) and found that the trap was closed by an electrical charge of 14 μC delivered between the midrib and a lobe of the upper leaf (Fig. 2). Figure 3 demonstrates the closing of the Venus flytrap at two different temperatures. We measured the distance y(t) between the edges of the trap leaf in the closing process. In the open state, the distance between the edges of the trap leaf is ymax. Individual plants have various distances between the edges of each trap. We therefore used the normalized (dimensionless) distance x = y/ymax. The speed of trap closure was calculated as 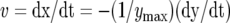 ; it has the dimension of s−1, as represented in Figure 3.
; it has the dimension of s−1, as represented in Figure 3.
Figure 1.
Experimental setup. [See online article for color version of this figure.]
Figure 2.
Closing of the trap with a 14-μC electrical stimulus.
Figure 3.
Kinetics of trap closure at 20°C (1) and 36°C (2) applying a 14-μC charge injection to the midrib; y is the distance between the edges of the lobes. A, Dependencies of distances between the edges of the lobes at 20°C (1) and 36°C (2) on time after electrical stimulation. B, Dependencies of the speed of trap closure 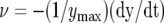 on time after electrical stimulation at 20°C (solid line) and 36°C (dashed line). [See online article for color version of this figure.]
on time after electrical stimulation at 20°C (solid line) and 36°C (dashed line). [See online article for color version of this figure.]
As one can see, trap closure consists of three distinctive phases: a silent phase with no observable movement; an accelerated movement of the lobe; and the relaxation of the lobe to its closed state. To describe this process, we developed the hydroelastic curvature model.
The Model
It was assumed that the leaf includes upper and lower layers of cells where different hydrostatic pressures are maintained. The driving force of the closing process is the elastic curvature energy stored and locked in the leaves due to a pressure difference between the upper and lower layers. The trigger signal opens the water pores between these layers and the fluid transfers from the upper to the lower layer. As a result, the leaf changes its configuration and relaxes to its equilibrium state, corresponding to the closed state.
Immediately after the stimulus application at the moment t = 0, there is no visible reaction until time tth when the trigger reaches the threshold value. After tth, closing begins. In this period the distance between the edges of the lobes can be described by the following equation:
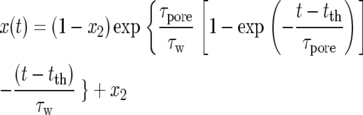 |
(1) |
where τpore is the characteristic time of pore opening; τw is the characteristic time of fluid transfer between two layers; t is time; and x2 is the final relative distance between the edges of two lobes in the closed state. Both distance and mean curvature of the leaf are described by the same function of time. The mean curvature changes from a positive to a negative value during the process of trap closure while the Gaussian curvature stays positive: it decreases in the beginning, reaches zero, and then begins to increase.
The speed v of trap closure can be obtained from Equation 1 as follows:
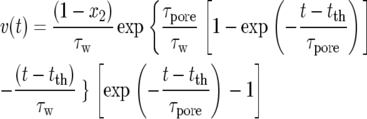 |
(2) |
Effect of Temperature
The speed of trap closure depends on temperature (Fig. 3). At 20°C, the speed of trap closure is twice as fast as it is at 36°C. At temperatures between 15°C and 25°C, two mechanical stimuli are required for trap closure (Darwin, 1875), whereas at higher temperatures, between 35°C and 40°C, only one stimulus is required (Brown and Sharp, 1910). We found significant differences in the kinetics (Fig. 3A) and speed of the trap during closing (Fig. 3B).
The pulse with inverted polarity of negative voltage applied to the midrib was not able to close the plant at either temperature.
Blockers of Ion and Water Channels
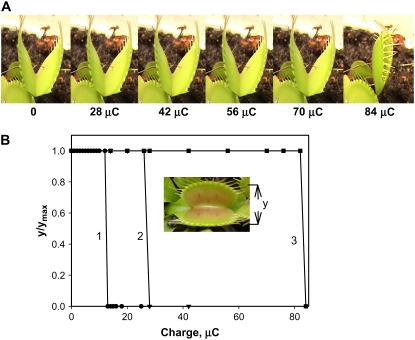
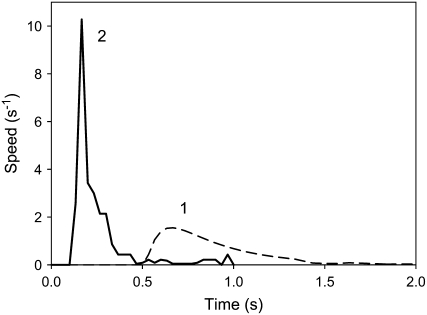
Ion channel blockers tetraethylammonium chloride and Ba2+ as well as uncouplers carbonylcyanide-3-chlorophenylhydrazone (CCCP), carbonylcyanide-4-trifluoromethoxyphenyl hydrazone, pentachlorophenol, and 2,4-dinitrophenol increase the time of trap closure and require a significantly larger electrical charge to close the trap (Volkov et al., 2007). Figure 4A shows that if the soil around the Venus flytrap is treated by a blocker of plant anion channels, 9-AC, the trap will require a charge significantly larger than 14 μC to close. Figure 4 shows the kinetics of trap closure in the presence of 9-AC. In the presence of 9-AC, trap closure is 17 times slower (Fig. 5) and the maximal speed of closing is three times less in comparison to a nontreated Venus flytrap (Fig. 3).
Figure 4.
The effect of anion channel blockers on charge-induced stimulation using two Ag/AgCl electrodes located in the midrib (+) and in one of the two lobes (−). The soil was treated by 25 mL of 10 mm 9-AC 24 h before experiments. A, Photos of the trap closing by different charge stimulations. B, Dependencies of the distance between the edges of the lobes (y) on injected charges. An additional electrical charge was injected to the plant every 5 s. The soil was treated by 25 mL of 10 mm 9-AC 4 h (2) or 24 h (3) before experiments. The capacitor was charged by a 1.5-V battery. [See online article for color version of this figure.]
Figure 5.
Kinetics of trap closing at 20°C by a 42-μC charge injection to the midrib; y is the distance between rims of lobes. A, Variation of distance between the edges of the lobes at 20°C after electrical stimulation. B, Variation of trap closure speed 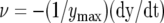 with time after electrical stimulation at 20°C. The soil was treated by 25 mL of 10 mm 9-AC 4 h before experiments. The capacitor was charged by a 1.5-V battery.
with time after electrical stimulation at 20°C. The soil was treated by 25 mL of 10 mm 9-AC 4 h before experiments. The capacitor was charged by a 1.5-V battery.
Figure 6 demonstrates the inhibitory effect of uncoupler CCCP on the speed of trap closure. Electrically induced trap closure in the presence of CCCP can be inhibited by depolarization of a membrane or dissipation of a proton gradient during ATP hydrolysis. In the presence of CCCP, trap closure is significantly slower: the speed of closing decreases and there is a delay before the start of closing (Fig. 6, curve 1). This effect is reversible. After a thorough washing of the soil treated by CCCP with distilled water, the closing speed of the trap returns to 10 s−1, but a higher electrical charge is needed for trap closure (Fig. 6, curve 2).
Figure 6.
Kinetics of a trap closing 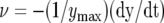 after 70 μC electrical stimulation (1). Fifty milliliters of 10 μm CCCP was added to the soil 4.5 h before experiments. The soil around the Venus flytrap was washed with distilled water to decrease CCCP concentration (2).
after 70 μC electrical stimulation (1). Fifty milliliters of 10 μm CCCP was added to the soil 4.5 h before experiments. The soil around the Venus flytrap was washed with distilled water to decrease CCCP concentration (2).
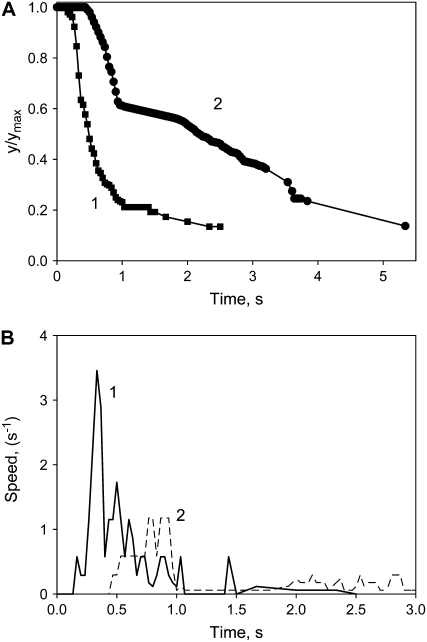
Zn2+ is known as a blocker of aquaporins, Ca-permeable anion channels, and possibly H+ channels in plants. Figure 7 shows that Zn2+ inhibits the closing process of a trap stimulated both mechanically and electrically. In the case of mechanostimulation, Zn2+ can block the propagation of electrical signals and trap closure. In the case of electrostimulation, Zn2+ directly inhibits closing of the trap. Electrostimulation causes the trap to close more quickly than mechanostimulation by a small piece of gelatin (Fig. 7).
Figure 7.
The kinetics of trap closure after stimulation of the trigger hairs by a small piece of gelatin (2) or by 28 μC electrical stimulation (1). Fifty milliliters of 10 mm ZnCl2 was added to the soil 4.5 h before experiments. A, Dependencies of distances between the edges of the lobes on time at 20°C after electrical stimulation. B, Dependencies of trap closure speed 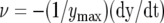 on time after electrical stimulation at 20°C.
on time after electrical stimulation at 20°C.
Plant Electrical Memory
Transmission of a single electrical charge (mean 13.63 μC, median 14.00 μC, sd 1.51 μC, n = 41) causes the trap to close and induces an electrical signal that propagates between the lobe and the midrib (Volkov et al., 2007). Figure 4B illustrates that the Venus flytrap can accumulate small charges, and when the threshold value is reached, the trap closes. A summation of stimuli is demonstrated through the repetitive application of smaller charges. If we apply two or more consecutive injections of electrical charge within a period of less than 50 s, the trap will close when a total of 14 μC charge is reached. In the presence of 9-AC, a significantly larger charge is required for trap to close (Fig. 4B, curves 2 and 3).
In our experiment, we applied the charge of 14 μC between the upper and the lower leaves, but the trap did not close. The same was true when we increased the injected charge to 1 mC. When we applied 14 μC between the midrib and lobe, the trap closed.
DISCUSSION
The fast movement of the Venus flytrap has intrigued scientists since it was first described by Darwin (1875), and since then it has caused periodic bursts of research activity. The experimental technique pioneered by Darwin (1875) was employed in the most recent analysis of this movement (Forterre et al., 2005). The mechanism of the trap movement is still debated, with ideas stretching from the expansion of cell walls through acid increase to the snap-buckling instability. Conflicting models have been proposed about the mechanism of Venus flytrap closure. Darwin (1875) was the first to observe that the lobes of each trap are convex when held open and concave when held shut. Brown (1916) noted that the underside of the lobes expands during closure and the inner sides of the lobes increase upon reopening. This model helps to explain the flipping action of “the most wonderful plant” as described by Darwin (1875). By painting the surface with dots, Darwin (1875) was able to prove that during the process of closing, the superficial layer of leaf cells contracts over the whole upper surface.
The rapid trap closure of the Venus flytrap has been explained by a loss of turgor in the upper epidermis or by a sudden acid-induced wall loosening of motor cells. Another plausible explanation is an expansion of the cell wall through acid growth (Williams and Bennet, 1982). Several recent articles have linked trap closure with a rapid decrease in pH: traps have been shown to close when immersed in solutions to a pH of 4.5 and below (Williams and Bennet, 1982). The low pH can activate enzymes that expand the lobes' cell walls. Leaves infiltrated with neutral buffers keeping the pH above 4.5 do not close in response to stimulation of their trigger hairs although APs are generated.
The closing process essentially involves a change of the leaf's geometry. The upper leaf is convex in the open position and concave in its closed position. Forterre et al. (2005) presented comprehensive analysis of upper leaf geometry during closing. Bobji (2005) considered the Venus flytrap as a bistable vibrator, which can be in an open or closed state. This last proposition is tempting, but Bobji and Forterre's model does not take into account a number of experimental facts: (1) the trap is stable and does not close spontaneously without stimuli; (2) two mechanical stimuli with an interval of up to 35 s are required for trap closure; (3) the trap does not close during rain or by blasts of air; and (4) reopening of the trap is a slow process, during which the lobes change their shapes from flat to concave and finally to convex (Darwin, 1875). This means that significant changes in the structure and mechanics of the trap occur during closing.
Trap closure is believed to represent nonmuscular movement based on hydraulics and mechanics (Brown, 1916; Skotheim and Mahadevan, 2005). The nastic movement in various plants involves a large internal pressure (turgor). It is quite likely that these movements are driven by differential turgor that is actively regulated by the plants. Trap closure occurs via quick changes in the curvature of each lobe rather than movement of the leaf as a whole. The cell walls of the upper and lower epidermis and adjacent mesophyll feature a preferential microfibril orientation in the direction of the applied stress (Hodick and Sievers, 1989). These anatomical features were selected as the basis of the hydroelastic curvature model presented above.
The driving force of the closing process is most likely the elastic curvature energy stored and locked in the leaves due to a pressure difference between the upper and lower layers of the leaf. The trigger signal opens the water pores between these layers and the fluid transfers from the upper to the lower layer. The leaf relaxes to its equilibrium state, corresponding to the closed configuration. This process develops very quickly; we found that it takes a small fraction of a second.
To close the trap, an electrical charge of 14 μC can either be submitted as a single pulse or be applied cumulatively as a sequence of small charges applied during a short period of time. Trap closure by electrical stimulus obeys the all-or-none law: there is no reaction for under-threshold stimulus, and the speed of closing does not depend on stimulus strength above threshold.
Electrostimulation and mechanical stimulation using a string or a piece of gelatin cause the trap to close in 0.3 s with the same speed. In the presence of an inhibitor of anion channels 9-AC, accumulation of an 84-μC electrical charge is required to close the trap (Fig. 4A). Uncouplers increase delay in trap closure and significantly decrease the speed of closing. A 70-μC electrical charge is required for trap closure in the presence of CCCP. CCCP concentration decreases when the soil is washed out by distilled water, and the speed of the trap closure increases. Ion channel blockers and aquaporin inhibitors tetraethylammonium and Zn2+ also decrease the speed and increase the time of trap closure.
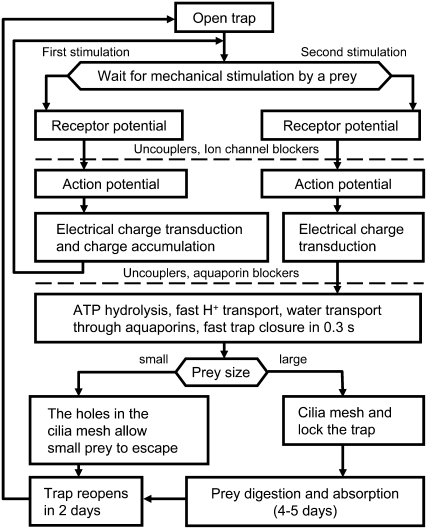
The possible mechanism of trap closure is shown on Figure 8. When trigger hairs in the open trap receive mechanical stimuli, a receptor potential is generated. Two mechanical stimuli are required for closing the trap in vivo. However, at high temperatures (36°C–40°C) only one stimulus is required for trap closure. Receptor potentials generate APs, which can propagate in the plasmodesmata of the plant to the midrib. Uncouplers and blockers of fast anion and potassium channels can inhibit AP propagation in the Venus flytrap. The trap accumulates the electrical charge delivered by an AP. Once a threshold value of the charge is accumulated, ATP hydrolysis (Jaffe, 1973) and fast proton transport start (Williams and Bennet, 1982; Rea, 1983), and aquaporin opening is initiated. Fast proton transport induces transport of water and a change in turgor.
Figure 8.
The mechanism of trap closure based on experimental and theoretical analysis of this work and experimental data from Volkov et al. (2007).
The trap possesses curvature elasticity and consists of outer and inner hydraulic layers where different hydrostatic pressures can build up. The open state of the trap contains high elastic energy accumulated due to the hydrostatic pressure difference between the outer and inner layers of the lobe. Applied stimuli open pores connecting the two layers, water rushes from one hydraulic layer to another, and the trap relaxes to the equilibrium configuration, its closed state.
Uncouplers can inhibit H+ transport, and blockers of aquaporins can inhibit water flow. In the absence of inhibitors, the trap closes in 0.3 s. After the trap closes, the cilia slowly mesh and lock the trap to capture its prey. Digestion takes place within 4 to 5 d. During trap reopening, the convex shape of each lobe is restored.
Our results demonstrate the role and kinetics of electrical, biochemical, and mechanical events leading to the fast trap closure induced by mechanical or electrical stimuli. The reception of electrical stimulus has a cumulative character, indicating the existence of electrical memory in this plant. There are many quick mechanical movements in plants, and this new hydroelastic curvature theory can be used for understanding and estimating their exact mechanisms. The new noninvasive charge capacitor method permits the study of different steps in signal transmission and responses in the plant kingdom.
MATERIALS AND METHODS
Data Acquisition
All measurements were conducted at a constant room temperature inside a Faraday cage mounted on a vibration-stabilized table. To estimate possible high-frequency content of the evoked responses, a high-performance National Instruments data acquisition system was used. High-speed data acquisition of low-pass filtered signals was performed using a simultaneous multifunction I/O plug-in data acquisition board NI-PXI-6115 or NI-PCI-6115 (National Instruments) interfaced through an NI-SCB-68 shielded connector block to 0.1-mm-thick nonpolarizable reversible Ag/AgCl electrodes (Fig. 1). The results were reproduced on a workstation with data acquisition board NI-6052E-DAQ with input impedance of 100 GΩ interfaced through an NI-SC-2040 Simultaneous Sample and Hold. The system integrates standard low-pass anti-aliasing filters to eliminate all signal frequencies over one-half of the sampling frequency. The multifunction data acquisition board NI-PXI-6115 provides high resolution and a wide gain range. Any single channel can be sampled at any gain at up to 10 million samples/s. The system integrates standard low-pass anti-aliasing filters at half of the sampling frequency.
Electrodes
Ag/AgCl electrodes were prepared from Teflon-coated silver wires (Volkov, 2000, 2006a; Volkov and Mwesigwa, 2001). After insertion of the electrodes into each lobe and midrib, the traps closed due to the mechanical stimulation. We allowed the plants to rest until the traps were completely open.
Plant Electrostimulation
The Charge Injection Method (Fig. 1) has been used to estimate precisely the amount of electrical energy necessary to cause trap closure. Two critical parameters have been analyzed: the amount of charge and the applied voltage. Both parameters are tested to determine the minimum amount of charge and the minimum voltage sufficient to close the plants' trap. A double-pole, double-throw switch was used to connect the known capacitor to the voltage source during charging, and then to the plant during electrical stimulation. Because the charge of the capacitor Q is related to the voltage source V in the equation Q = CV, we can precisely regulate the amount of charge using different capacitors and applying various voltages. By changing the switch position, we can instantaneously connect the charged capacitor to the plant and induce an evoked response.
Mechanostimulation
String Stimulus
Mechanical stimulation was performed by using a cotton thread to gently touch one or two of the six trigger hairs inside the upper leaf of the Venus flytrap (Dionaea muscipula). The thread was then immediately removed before the leaves closed.
Gelatin Stimulus
Plants were fed a 6- × 6- × 2-mm cube of 4% (w/v) gelatin and induced to close by stimulating two of the six trigger hairs of the Venus flytrap, as suggested by Jaffe (1973).
Images
Digital video recorders Sony DCR-HC36 and Canon ZR300 were used to monitor the Venus flytraps and to collect digital images, which were analyzed frame by frame. The National Television Standards Committee format consists of 30 interlaced frames of video per second, which represents the maximum sampling frequency of parameters extracted from the video stream.
Chemicals
CCCP, carbonylcyanide-4-trifluoromethoxyphenyl hydrazone, 2,4-dinitrophenol, 2,3,4,5,6-pentachlorophenol, gelatin, ZnCl2, and tetraethylammonium chloride were obtained from Fluka; 9-AC was purchased from American Tokyo Kasei.
Plants
Three hundred bulbs of Venus flytrap were purchased for this experimental work from Fly-Trap Farm and grown in a well-drained peat moss in plastic pots at 22°C with a 12:12-h light:dark photoperiod. The soil was treated with distilled water. All experiments were performed on healthy adult specimens.
This work was supported by the National Science Foundation (grant no. DMR–0521611) and the National Aeronautics and Space Administration (grant no NAG8–1888).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alexander George Volkov (agvolkov@yahoo.com).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Benolken RM, Jacobson SL (1970) Response properties of a sensory hair excised from Venus's flytrap. J Gen Physiol 56 64–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobji MS (2005) Springing the trap. J Biosci 30 143–146 [DOI] [PubMed] [Google Scholar]
- Braam J (2005) In touch: plant responses to mechanical stimuli. New Phytol 165 373–389 [DOI] [PubMed] [Google Scholar]
- Brown WH (1916) The mechanism of movement and the duration of the effect of stimulation in the leaves of Dionaea. Am J Bot 3 68–90 [Google Scholar]
- Brown WH, Sharp LW (1910) The closing response in Dionaea. Bot Gaz 49 290–302 [Google Scholar]
- Burdon-Sanderson J (1873) Note on the electrical phenomena which accompany stimulation of the leaf of Dionaea muscipula Ellis. Philos Trans R Soc Lond B Biol Sci 21 495–496 [Google Scholar]
- Burdon-Sanderson J (1874) Venus fly-trap (Dionaea muscipula). Nature 10 105–107, 127–128 [Google Scholar]
- Burdon-Sanderson J (1882) On the electromotive properties of the leaf of Dionaea in the excited and unexcited states. Philos Trans R Soc Lond B Biol Sci 173 1–55 [Google Scholar]
- Burdon-Sanderson J, Page FJM (1876) On the mechanical effects and on the electrical disturbance consequent on excitation of the leaf of Dionaea muscipula. Philos Trans R Soc Lond B Biol Sci 25 411–434 [Google Scholar]
- Darwin C (1875) Insectivorous Plants. Murray, London
- Detmers FJM, De Groot BL, Mueller EM, Hinton A, Konings IBM, Sze M, Flitsch SL, Grubmueller H, Deen PMT (2006) Quaternary ammonium compounds as water channel blockers: specificity, potency, and site of action. J Biol Chem 281 14207–14214 [DOI] [PubMed] [Google Scholar]
- DiPalma JR, McMichael R, DiPalma M (1966) Touch receptor of Venus flytrap, Dionaea muscipula. Science 152 539–540 [DOI] [PubMed] [Google Scholar]
- DiPalma JR, Mohl R, Best W (1961) Action potential and contraction of Dionaea muscipula (Venus flytrap). Science 133 878–879 [DOI] [PubMed] [Google Scholar]
- Fagerberg WR, Allain D (1991) A quantitative study of tissue dynamics during closure in the traps of Venus's flytrap Dionaea muscipula Ellis. Am J Bot 78 647–657 [Google Scholar]
- Fagerberg WR, Howe DG (1996) A quantitative study of tissue dynamics during closure in the traps of Venus's flytrap Dionaea muscipula (Droseraceae). 2. Trap reopening. Am J Bot 83 836–842 [Google Scholar]
- Forterre Y, Skothelm JM, Dumals J, Mahadevan L (2005) How the Venus flytrap snaps. Nature 433 421–425 [DOI] [PubMed] [Google Scholar]
- Hodick D, Sievers A (1986) The influence of Ca2+ on the action potential in mesophyll cells of Dionaea muscipula Ellis. Protoplasma 133 83–84 [Google Scholar]
- Hodick D, Sievers A (1988) The action potential of Dionaea muscipula Ellis. Planta 174 8–18 [DOI] [PubMed] [Google Scholar]
- Hodick D, Sievers A (1989) On the mechanism of trap closure of Venus flytrap (Dionaea muscipula Ellis). Planta 179 32–42 [DOI] [PubMed] [Google Scholar]
- Jacobson SL (1965) Receptor response in Venus's flytrap. J Gen Physiol 49 117–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SL (1974) The effect of ionic environment on the response of the sensory hair of Venus's flytrap. Can J Bot 52 1293–1302 [Google Scholar]
- Jaffe MJ (1973) The role of ATP in mechanically stimulated rapid closure of the Venus's flytrap. Plant Physiol 51 17–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper BE, Robins RJ, Joel DM (1989) The Carnivorous Plants. Academic Press, London
- Krol E, Dziubinska H, Stolarz M, Trebacz K (2006) Effects of ion channel inhibitors on cold- and electrically-induced action potentials in Dionaea muscipula. Biol Plant 50 411–416 [Google Scholar]
- Ksenzhek OS, Volkov AG (1998) Plant Energetics. Academic Press, San Diego
- Lea HW (1976) A muscle contracting substance from a plant's closing fly-trap. Planta 129 39–41 [DOI] [PubMed] [Google Scholar]
- Lloyd FE (1942) The Carnivorous Plants. Ronald, New York
- Markin VS, Sachs F (2004) Thermodynamics of mechanosensitivity. Phys Biol 1 110–124 [DOI] [PubMed] [Google Scholar]
- Maurel C (1997) Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol 48 399–429 [DOI] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ (2001) Aquaporins. A molecular entry into plant water relations. Plant Physiol 125 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon R, Lienard D, Chrispeels MJ, Lassalles JP (2001) Rapid movements of plants organ require solute-water cotransporters or contractile proteins. Plant Physiol 127 720–723 [PMC free article] [PubMed] [Google Scholar]
- Rea PA (1983) The dynamics of H+ efflux from the trap lobes of Dionaea muscipula Ellis (Venus's flytrap). Plant Cell Environ 6 125–134 [Google Scholar]
- Roberts SK (2006) Plasma membrane anion channels in higher plants and their putative functions in roots. New Phytol 169 647–666 [DOI] [PubMed] [Google Scholar]
- Sachs J (1887) Lectures on the Physiology of Plants. Clarendon Press, Oxford
- Scala J, Iott K, Schwab DW, Semersky FE (1969) Digestive secretion of Dionaea muscipula (Venus's flytrap). Plant Physiol 44 367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibaoka T (1966) Action potentials in plant organs. Symp Soc Exp Biol 20 49–74 [PubMed] [Google Scholar]
- Sibaoka T (1969) Physiology of rapid movements in higher plants. Annu Rev Plant Physiol 20 165–184 [Google Scholar]
- Skotheim JM, Mahadevan L (2005) Physical limits and design principles for plant and fungal movements. Science 308 1308–1310 [DOI] [PubMed] [Google Scholar]
- Stuhlman O, Darden E (1950) The action potential obtained from Venus's-flytrap. Science 111 491–492 [DOI] [PubMed] [Google Scholar]
- Törnroth-Horsefield S, Wang Y, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P (2006) Structural mechanism of plant aquaporin gating. Nature 439 688–694 [DOI] [PubMed] [Google Scholar]
- Trebacz K, Sievers A (1998) Action potentials evoked by light in traps of Dionaea muscipula Ellis. Plant Cell Physiol 39 369–372 [Google Scholar]
- Tyerman SD, Niemietz CM, Bramley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25 173–194 [DOI] [PubMed] [Google Scholar]
- Volkov AG (2000) Green plants: electrochemical interfaces. J Electroanal Chem 483 150–156 [Google Scholar]
- Volkov AG (2006. a) Electrophysiology and phototropism. In F Balushka, S Manusco, D Volkman, eds, Communication in Plants. Neuronal Aspects of Plant Life. Springer-Verlag, Berlin, pp 351–367
- Volkov AG, editor (2006. b) Plant Electrophysiology. Springer, Berlin
- Volkov AG, Adesina T, Jovanov E (2007) Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signal Behav 2 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Brown CL (2006. a) Electrochemistry of plant life. In AG Volkov, ed, Plant Electrophysiology—Theory & Methods. Springer, Berlin, pp 437–459
- Volkov AG, Brown CL (2006. b) Nanodevices in nature. In CSSR Kumar, ed, Nanodevices for Life Sciences. Wiley-VCH, Weinheim, Germany, pp 440–463
- Volkov AG, Deamer DW, Tanelian DL, Markin VS (1998) Liquid Interfaces in Chemistry and Biology. Wiley, New York
- Volkov AG, Haack RA (1995) Insect induces bioelectrochemical signals in potato plants. Bioelectrochem Bioenerg 35 55–60 [Google Scholar]
- Volkov AG, Mwesigwa J (2001) Electrochemistry of soybean: effects of uncouplers, pollutants, and pesticides. J Electroanal Chem 496 153–157 [Google Scholar]
- Williams ME, Mozingo HN (1971) The fine structure of the trigger hair in Venus's flytrap. Am J Bot 58 532–539 [Google Scholar]
- Williams SE, Bennet AB (1982) Leaf closure in the Venus flytrap: an acid growth response. Science 218 1120–1121 [DOI] [PubMed] [Google Scholar]