Abstract
Water-soluble carbohydrates (WSCs; composed of mainly fructans, sucrose [Suc], glucose [Glc], and fructose) deposited in wheat (Triticum aestivum) stems are important carbon sources for grain filling. Variation in stem WSC concentrations among wheat genotypes is one of the genetic factors influencing grain weight and yield under water-limited environments. Here, we describe the molecular dissection of carbohydrate metabolism in stems, at the WSC accumulation phase, of recombinant inbred Seri/Babax lines of wheat differing in stem WSC concentrations. Affymetrix GeneChip analysis of carbohydrate metabolic enzymes revealed that the mRNA levels of two fructan synthetic enzyme families (Suc:Suc 1-fructosyltransferase and Suc:fructan 6-fructosyltransferase) in the stem were positively correlated with stem WSC and fructan concentrations, whereas the mRNA levels of enzyme families involved in Suc hydrolysis (Suc synthase and soluble acid invertase) were inversely correlated with WSC concentrations. Differential regulation of the mRNA levels of these Suc hydrolytic enzymes in Seri/Babax lines resulted in genotypic differences in these enzyme activities. Down-regulation of Suc synthase and soluble acid invertase in high WSC lines was accompanied by significant decreases in the mRNA levels of enzyme families related to sugar catabolic pathways (fructokinase and mitochondrion pyruvate dehydrogenase complex) and enzyme families involved in diverting UDP-Glc to cell wall synthesis (UDP-Glc 6-dehydrogenase, UDP-glucuronate decarboxylase, and cellulose synthase), resulting in a reduction in cell wall polysaccharide contents (mainly hemicellulose) in the stem of high WSC lines. These data suggest that differential carbon partitioning in the wheat stem is one mechanism that contributes to genotypic variation in WSC accumulation.
Water-soluble carbohydrates (WSCs) can accumulate in the stem and leaf sheath of cool-season cereals (e.g. wheat [Triticum aestivum], barley [Hordeum vulgare], and oats [Avena sativa]) during the period from stem elongation to the early phase of grain filling and serve as temporary carbohydrate reserves, commonly called the stem carbohydrate reserves (Schnyder, 1993; Wardlaw and Willenbrink, 1994; Blum, 1998; Gebbing, 2003). WSCs in wheat stems are mainly composed of fructans, Suc, Glc, and Fru, with fructans being the major component at the late stage of WSC accumulation phase (Ruuska et al., 2006). Fructans are soluble linear or branched β-2,1- or β-2,6-linked fructosyl-oligosaccharides that are derived from Suc and synthesized in the vacuole, and are present in 15% of angiosperm species (Vijn and Smeekens, 1999; Van Laere and Van den Ende, 2002; Chalmers et al., 2005). Fructans in cereals are mainly the graminan type, that is predominantly β-2,6-linked fructosyl-units with shorter β-2,1-linked branches (Bancal and Triboï, 1993; Ritsema and Smeekens, 2003; Chalmers et al., 2005).
Because cereal stem tissue at the early reproductive stage is the predominant organ by weight, stem WSC reserve is an important carbon source for grain yield in wheat and barley (Bonnett and Incoll, 1992; Schnyder, 1993). WSCs can accumulate in wheat stems to more than 40% of total stem dry weight (McCaig and Clarke, 1982; Blacklow et al., 1984; Housley, 2000). WSCs mobilize from the stem during the later phase of grain filling (Willenbrink et al., 1998) and can potentially contribute to about 20% of grain yield under normal conditions (Schnyder, 1993; Blum, 1998; Wardlaw and Willenbrink, 2000). A similar level of stem WSC contribution to grain yield has been shown in barley (Bingham et al., 2007). The stem WSCs become more important for grain yield in cereal crops under abiotic stress (Blum et al., 1991; Kiniry, 1993; Schnyder, 1993; Blum, 1998; Van Herwaarden et al., 1998a, 1998b). In wheat crops with drought stress during the grain-filling period, the stem WSCs could potentially contribute to >50% of grain yield (Brooks et al., 1982; Aggarwal and Sinha, 1984). This is because the carbon supply from photosynthesis is reduced during drought stress due to both stomatal closure in the leaves (Chaves et al., 2002) and coordinated down-regulation of genes involved in the Calvin cycle (our unpublished data).
Stem WSC accumulation is influenced by environmental factors (Blum, 1998; van Herwaarden et al., 1998a; Ehdaie et al., 2006; Ruuska et al., 2006, 2008). However, considerable genotypic variation in stem WSC concentration has been observed in barley and wheat (Schnyder, 1993; Foulkes et al., 2002; Ehdaie et al., 2006; Ruuska et al., 2006). Genotypic ranking among wheat genotypes in stem WSC concentration is generally consistent across environments (Foulkes et al., 2002; Ruuska et al., 2006), with large broad-sense heritability (H = 0.9) in wheat (Ruuska et al., 2006). Analysis of the physiological basis of recent genetic gains in wheat breeding for grain yield in Australia and the United Kingdom has shown an increase in stem WSC concentration (Van Herwaarden and Richards, 2002; Shearman et al., 2005). Positive relationships between stem WSC concentration at anthesis and grain weight or yield in wheat have been observed in many studies, particularly under water-limited environments (Foulkes et al., 2002; Asseng and Van Herwaarden, 2003; Ruuska et al., 2006). Therefore, high WSC concentration is considered to be a potentially useful trait for improving grain weight and yield in water-limited wheat production environments (Blum, 1998; Asseng and Van Herwaarden, 2003; Shearman et al., 2005; Ruuska et al., 2006; Foulkes et al., 2007). However, the molecular mechanisms that underlie genotypic variation in the WSC trait are essentially unknown.
Differences in the accumulation of WSCs in stems among genotypes could potentially result from various factors such as photosynthesis capacity, carbon use efficiency, and carbon partitioning between stem reserve deposition and other physiological processes (e.g. maintenance respiration, growth, and cell wall synthesis). These processes involve many carbohydrate metabolic genes in a number of major carbohydrate metabolic pathways: the Calvin cycle, gluconeogenic, glycolytic, Suc, and fructan synthetic pathways, etc. Fructans and Suc are the major components of wheat stem WSCs (Ruuska et al., 2006) and the rate of fructan synthesis appears to be dependent on Suc levels in the tissue (Kühbauch and Thome, 1989; Pollock and Cairns, 1991; Smouter and Simpson, 1991). Therefore, genotypic differences in WSC accumulation could center primarily in differences in the influx and/or efflux rate of carbon into the stem Suc pool. To dissect the molecular basis of genotypic variation in stem WSC concentration, we performed Affymetrix GeneChip expression analysis in stems of recombinant inbred Seri/Babax (SB) lines of wheat varying in stem WSC concentrations. This study focused on genes involved in metabolic pathways of glycolysis, gluconeogenesis, Suc, and fructan synthesis and hydrolysis, as well as genes involved in diverting carbohydrate metabolites from the Suc synthetic pathway to cell wall polysaccharide synthesis. Because the stem WSC concentration at anthesis or shortly after anthesis (i.e. at the stem WSC accumulation phase) is a good indicator of positive association between WSC level and grain weight or yield in wheat (Ruuska et al., 2006; Foulkes et al., 2007), we analyzed the association of gene expression levels with stem WSC concentrations at anthesis. We identified that expression levels of a number of key carbohydrate metabolic enzymes relevant to WSC metabolism were correlated with stem WSC concentrations. The associations of key differentially expressed carbohydrate metabolic genes with genotypic variation in WSC accumulation in the stem were validated by analyses of genotypic differences in their enzyme activities or end product contents.
RESULTS
Variation in Stem WSC Concentration and Positive Correlation between WSC Concentration and Grain Weight or Grain Yield in SB Progeny
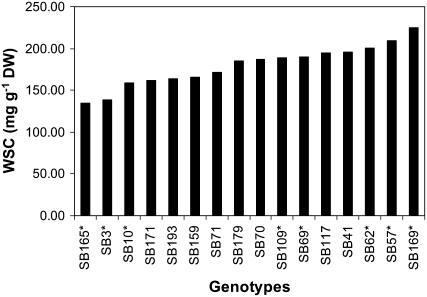
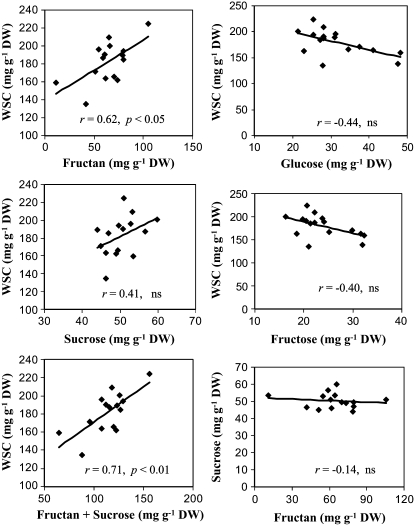
Variation in stem WSC concentration was examined at anthesis among 16 recombinant inbred SB progeny lines of wheat, grown in 2005 under rain-fed conditions with a small amount of supplementary irrigation in southern Queensland, Australia, where wheat production is prone to terminal drought stress. These SB lines were selected based on variation in stem WSC concentration, but similar in anthesis date. This trial experienced both pre- and postanthesis drought stress. However, to obtain stem samples with uniform plant water status for comparative analysis of gene expression in the progeny lines, samples were harvested about 2 d after rain such that no drought stress symptoms were observed in these plants at the time of sampling. Figure 1 shows variation in stem WSC concentration among these progeny lines. The WSC level in the highest WSC line was about 2 times higher than that in the lowest line. A similar level of difference in stem WSC concentration among these lines was also observed in field trials of previous years and in 2006 and 2007 (data not shown), indicating that the variation was largely genotypic. Fructan was the major component that contributed to genotypic variation in WSC concentration. In addition, Suc made some positive contribution to the WSC trait, but was not statistically significant (Fig. 2). The combined levels of fructan and Suc were highly correlated with the WSC concentrations (r = 0.71, P < 0.01). However, Suc levels were not correlated with fructan levels in the stem (Fig. 2). Stem Glc or Fru levels in these SB lines were inversely associated with WSC concentrations, but were not statistically significant (Fig. 2).
Figure 1.
The WSC concentrations in the top two internodes (peduncle and penultimate with leaf sheath) of 16 SB lines at anthesis. Values are means of two field replicates. The asterisks (*) refer to SB lines used for Affymetrix GeneChip expression analysis. DW, Dry weight.
Figure 2.
Relationships between WSC, fructan, Suc, Glc, and Fru concentrations in stems of SB lines at anthesis differing in WSC concentrations. Each point is the genotypic mean of two field replicates. DW, Dry weight; ns, not significant.
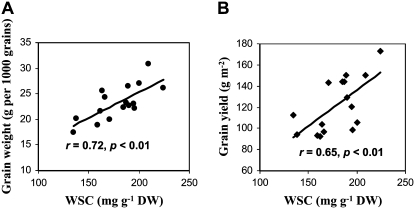
To examine the relationships between WSC accumulation and grain weight or yield, the stem WSC levels of individual lines were plotted with grain weight or yield. A relatively strong correlation (r = 0.72, P < 0.01) between the WSC levels and grain weight was observed among these 16 progeny lines (Fig. 3A). The WSC levels were also significantly correlated with grain yields (r = 0.65, P < 0.01) among these SB lines (Fig. 3B).
Figure 3.
Relationships between stem WSC concentration and grain weight (A) or grain yield (B) in 16 SB lines. Each value is the genotypic mean of two field replicates. DW, Dry weight.
Annotation of Wheat Carbohydrate Metabolic Genes Related to WSC Metabolism
To dissect the molecular basis underlying genotypic variation in WSC accumulation, we used the wheat genome array (Affymetrix GeneChip) containing >61,000 probe sets representing 55,052 transcripts to examine genotypic differences in the transcript levels of carbohydrate metabolic genes involved in major pathways related to WSC metabolism. This requires clear annotation of carbohydrate metabolic genes related to WSC metabolism, including bioinformatic prediction of the subcellular location of enzymes, because carbohydrate metabolic enzymes present in the cytoplasm are involved in different metabolic pathways from their chloroplast counterparts. We used the T. aestivum gene index (TaGI) database (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=wheat) as the first step in annotating the gene identity of each probe set present in the Affymetrix wheat GeneChip. In the TaGI database, wheat ESTs from PlantGDB database (www.plantgdb.org/) have been assembled into tentative consensus (TC) sequences of individual genes. Identified putative carbohydrate metabolic genes potentially involved in the major pathways related to WSC metabolism were then subjected to extensive bioinformatic analyses for their potential biochemical function based on sequence homology with known-function proteins and prediction of subcellular location.
A list of carbohydrate metabolic genes related to WSC metabolism identified through the above bioinformatic analyses is given in Supplemental Table S1. These carbohydrate metabolic genes are grouped into gene families based on their sharing the same metabolic function. In this sense chloroplast Fru-bisP genes are excluded from the cytoplasmic Fru-bisP family, as the former is involved in the Calvin cycle and the latter is involved in gluconeogenesis. Extensive reannotation is given for genes from glycosyltransferase family 32, which contains cell wall and vacuolar invertases, fructosyltransferases, and fructan exohydrolases (FEHs), based on recent publications (Chalmers et al., 2003, 2005; Van den Ende et al., 2003, 2005, 2006; De Coninck et al., 2005; Kawakami and Yoshida, 2005; Kawakami et al., 2005; Ritsema et al., 2005, 2006; Francki et al., 2006; Lasseur et al., 2006; Van Riet et al., 2006; Ji et al., 2007; Le Roy et al., 2007; Lothier et al., 2007; Verhaest et al., 2007).
Affymetrix Wheat GeneChip Analysis for Dissecting Genotypic Variation in Expression Levels of the Carbohydrate Metabolic Genes Related to WSC Accumulation
To examine the relationship between the transcript levels of individual carbohydrate metabolic genes and stem WSC levels, 16 Affymetrix wheat GeneChips were used to determine the transcript levels of carbohydrate metabolic genes in the stem of eight SB lines at anthesis with two field replicates of each line. Correlation analysis between the transcript levels of individual genes and stem WSC concentrations was performed using both genotypic means (n = 8) and individual samples (8 genotypes × 2 field replicates, n = 16; Supplemental Table S1). This analysis showed that the majority of the enzyme families related to WSC metabolism had at least one significantly correlated isoenzyme gene (Supplemental Table S1). In addition, many enzyme families had members that showed differential mRNA-WSC correlation patterns (Supplemental Table S1; Supplemental Fig. S1). The physiological significance of individual isoenzyme genes in contribution to a given enzymatic reaction is partly related to its relative mRNA abundance among the members of a given multigene enzyme family, as the mRNA abundance of many carbohydrate metabolic genes differs markedly among family members (Supplemental Table S1). Owing to conflicting expression profiles of individual isoenzymes in many enzyme families in relation to WSC accumulation, the total transcript level of an enzyme family that shares the same metabolic function is likely to provide a useful indicator of the potential contribution of a given enzyme to WSC accumulation despite potential error due to the absence of knowledge of relative translation efficiency and specific activity among these isoenzymes.
To evaluate whether relative hybridization signals of individual isoenzyme genes from the Affymetrix GeneChip data are proportional to their relative mRNA levels among members of a given gene family, we quantified the relative mRNA levels of individual isoenzyme genes from Suc-P synthase and Suc synthase families (six genes selected from each family) using quantitative reverse transcription (RT)-PCR. The relative mRNA abundance among members within an enzyme gene family was estimated as apparent expression levels (AELs) relative to an internal control gene, TaRPII36 encoding T. aestivum RNA polymerase II 36-kD subunit (Stephenson et al., 2007). This analysis showed a strong correlation (r = 0.99 for the Suc synthase family; 0.95 for the Suc-P synthase family) between mRNA level data derived from quantitative RT-PCR and Affymetrix GeneChip (data not shown). Therefore, we used the total hybridization signal (i.e. total transcript level) of individual enzyme families as the first step to assess their relationships with the WSC levels. With this approach, we found that expression levels of 11 enzyme families showed a significant correlation (P < 0.05) with stem WSC concentrations at both genotypic (n = 8) and genotype/field-replicate (n = 16) levels, as summarized in Table I (enzymes consisting of multicomponents, such as pyruvate dehydrogenase complex and cellulose synthase, are considered here as one enzyme family). The roles of these enzymes in carbohydrate metabolic pathways and differences in their total transcript levels between the high and low WSC lines are illustrated in Figure 4. In each of these 11 enzyme families, the mRNA level of at least one predominantly expressed isoenzyme was significantly correlated with the WSC concentrations at both genotypic and genotype/field-replicate levels. However, differences in the expression levels of two enzyme families (Fru-bisphosphatase and Suc-P synthase) among the SB lines were very small. For both families the total mRNA level in the SB line containing the highest mRNA level was <30% higher than that with the lowest level. As such a difference would be technically difficult to verify by quantitative RT-PCR, we did not consider them as differentially expressed gene families. In addition to the genotypic component, the mRNA levels of many carbohydrate metabolic genes, as well as stem WSC concentrations, were affected by environmental factors, as seen by differences in field replicates (data not shown). Significant correlation between the mRNA levels of a given enzyme family and stem WSC concentration at the genotype/field-replicate level (n = 16) provides further support for association of the genotypically correlated enzymes with the WSC trait, as this ensures that the mRNA level of a given enzyme family in each sample is linked to the WSC concentration.
Table I.
Correlation analysis between the transcript levels of enzyme families and WSC concentrations in the stems of eight SB lines
The total transcript levels of each enzyme family derived from the Affymetrix GeneChip data were used for this analysis. The mean value of hybridization signals from two field replicates of each line was used for correlation analysis between the total mRNA levels of each enzyme family and WSC concentrations at the genotypic level (n = 8). Variation in mRNA levels and WSC concentrations in two field replicates is implicated in correlation analysis at the genotype/replicate level (n = 16). Enzyme families that are statistically significant at both levels are in bold letters. Statistical significance of correlation coefficients was indicated by * (P < 0.05) or ** (P < 0.01). Mito, Mitochondrion; Cyto, cytoplasm; Vac, vacuole; PM, plasma membrane; Apo, apoplast; PDH, pyruvate dehydrogenase complex; GAPDH, glyceraldehyde-3-P dehydrogenase; PFP, diphosphate-Fru-6-P 1-phosphotransferase; P, phosphate.
| EC No. | Enzyme Name | No. of Genes Included | Predicted Subcellular Location | Correlation Coefficient (r) between Total mRNA Level and WSCs
|
Ratio of the Maximum to Minimum mRNA Level | |
|---|---|---|---|---|---|---|
| Genotype (n = 8) | Genotype and Replicate (n = 16) | |||||
| 1.2.4.1 | PDH E1α | 2 | Mito | −0.75* | −0.48 | 1.18 |
| 1.2.4.1 | PDH E1β | 2 | Mito | −0.79* | −0.64** | 1.41 |
| 2.3.1.12 | PDH E2 | 4 | Mito | −0.35 | −0.32 | 2.46 |
| 1.8.1.4 | PDH E3 | 1 | Mito | −0.80* | −0.61* | 1.54 |
| 2.7.1.40 | Pyruvate kinase | 7 | Cyto | −0.49 | −0.42 | 1.28 |
| 4.2.1.11 | Phosphopyruvate hydratase | 5 | Cyto | 0.08 | 0.03 | 1.30 |
| 5.4.2.1 | Phosphoglycerate mutase | 11 | Cyto | 0.50 | 0.22 | 1.13 |
| 2.7.2.3 | Phosphoglycerate kinase | 3 | Cyto | −0.60 | −0.16 | 1.12 |
| 1.2.1.12 | NAD-dependent GAPDH | 6 | Cyto | −0.30 | −0.03 | 1.13 |
| 1.2.1.9 | NADP-dependent GAPDH | 1 | Cyto | 0.32 | 0.43 | 1.23 |
| 4.1.2.13 | Fru-P2 aldolase | 12 | Cyto | −0.72* | −0.46 | 1.24 |
| 3.1.3.11 | Fru-bisphosphatase | 3 | Cyto | 0.72* | 0.66** | 1.29 |
| 2.7.1.90 | PFP, α-subunit | 3 | Cyto | −0.68 | −0.49 | 1.66 |
| 2.7.1.90 | PFP, β-subunit | 1 | Cyto | −0.58 | −0.36 | 1.29 |
| 2.7.1.11/2.7.1.90 | ATP- or diphosphate-dependent 6-phosphofructokinase | 6 | Cyto | 0.55 | 0.36 | 1.30 |
| 5.3.1.9 | Glc-6-P isomerase | 2 | Cyto | −0.64 | −0.60* | 1.21 |
| 1.1.1.49 | Glc-6-P 1-dehydrogenase | 4 | Cyto | −0.51 | −0.45 | 1.33 |
| 5.4.2.2 | Phosphoglucomutase | 2 | Cyto | −0.63 | −0.27 | 1.15 |
| 2.7.7.9 | UDP-Glc pyrophosphorylase | 6 | Cyto | −0.75* | −0.49 | 1.30 |
| 1.1.1.22 | UDP-Glc 6-dehydrogenase | 6 | Cyto | −0.88** | −0.68** | 2.09 |
| 4.1.1.35 | UDP-glucuronate decarboxylase | 9 | Cyto | −0.86** | −0.67** | 1.83 |
| 2.4.1.12 | CesA1 | 4 | PM | −0.84** | −0.57* | 1.5 |
| 2.4.1.12 | CesA3 | 1 | PM | −0.72* | −0.42 | 1.42 |
| 2.4.1.12 | CesA4 | 2 | PM | −0.55 | −0.47 | 1.69 |
| 2.4.1.12 | CesA4-like | 1 | PM | −0.82** | −0.62* | 1.72 |
| 2.4.1.12 | CesA7 | 3 | PM | −0.49 | −0.46 | 1.51 |
| 2.4.1.12 | CesA8 | 2 | PM | −0.58 | −0.50 | 1.70 |
| 2.4.1.12 | CesA10 | 1 | PM | −0.86** | −0.68** | 1.94 |
| 2.4.1.14 | Suc-P synthase | 7 | Cyto | 0.82** | 0.69** | 1.22 |
| 3.1.3.24 | Suc phosphatase | 5 | Cyto | 0.52 | 0.11 | 1.13 |
| 2.4.1.13 | Suc synthase | 10 | Cyto | −0.77* | −0.70** | 1.86 |
| 3.2.1.26 | Neutral invertase | 7 | Cyto | 0.65 | 0.58* | 1.21 |
| 3.2.1.26 | Apoplastic invertase | 3 | Apo | 0.09 | −0.11 | 3.12 |
| 2.7.1.4 | Fructokinase | 7 | Cytoa | −0.75* | −0.56* | 1.84 |
| 2.7.1.1 | Hexokinase | 5 | Cytoa | −0.11 | 0.09 | 1.08 |
| 3.2.1.26 | Soluble acid invertase | 4 | Vac | −0.87** | −0.74** | 3.50 |
| 2.4.1.99 | 1-SST | 1 | Vac | 0.86** | 0.85** | 5.29 |
| 2.4.1.10 | 6-SFT | 2 | Vac | 0.92** | 0.86** | 3.13 |
| 2.4.1.100 | 1,2-β-Fructan 1F-fructosyltransferase | 2 | Vac | 0.61 | 0.63** | 6.26 |
| 3.2.1.- and 3.2.1.80 | Fructan 6-exohydrolase and 1-exohydrolase | 9 | Apo/vac | −0.36 | −0.37 | 1.35 |
Isoenzymes predicted to have a signal sequence potentially targeting to apoplast or nucleus (potentially dual subcellular locations) are also included.
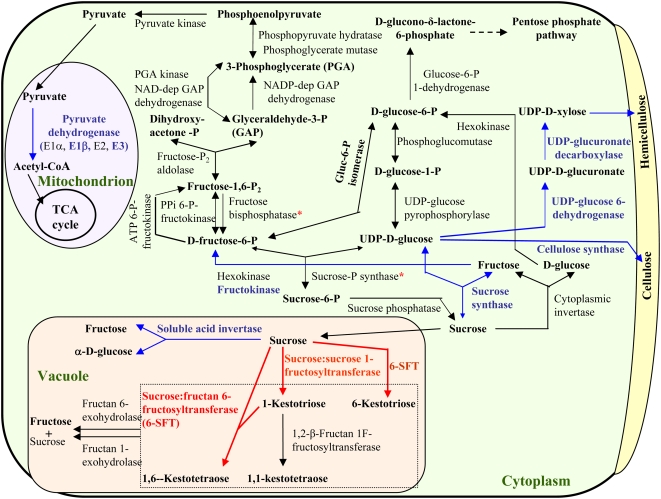
Figure 4.
Illustration of major WSC metabolic pathways and WSC-correlated enzyme families. The total mRNA levels of individual enzyme families were determined by Affymetrix GeneChip analysis as shown in Table I. Red color indicates enzyme families with the total mRNA levels positively correlated with stem WSC concentrations; blue indicates enzyme families with the total mRNA levels inversely correlated with WSCs; black indicates enzyme families that showed no significant correlations. The asterisks (*) show potentially positively WSC-correlated enzyme families (see description in “Results”).
Of the nine gene families that showed significant correlations with WSCs at both levels, two showed a positive correlation between mRNA and WSC levels and seven exhibited an inverse correlation (Table I). The positively correlated gene families are Suc:Suc 1-fructosyltransferase (1-SST) and Suc:fructan 6-fructosyltransferase (6-SFT), both of which are involved in fructan synthesis (Chalmers et al., 2005). The seven inversely correlated gene families are Suc-hydrolyzing enzymes (Suc synthase and soluble acid invertase), enzymes involved in sugar catabolic pathways (fructokinase and pyruvate dehydrogenase complex), and enzymes capable of diverting carbon to cell wall synthesis pathways (UDP-Glc 6-dehydrogenase, UDP-glucuronate decarboxylase, and cellulose synthase). In plants, cellulose synthase contains multiple subunits (Djerbi et al., 2004; Aspeborg et al., 2005; Persson et al., 2005). Two functional cellulose synthase complexes are known and each functional cellulose synthase complex contains at least three different subunits (Persson et al., 2005). In Arabidopsis (Arabidopsis thaliana), CesA1, CesA3, and CesA6 form an enzyme complex responsible for primary cell wall synthesis, whereas a CesA4-CesA7-CesA8 complex is responsible for secondary cell wall synthesis (Persson et al., 2005). TaCesA1, TaCesA3, TaCesA4, TaCesA7, TaCesA8, and TaCesA10 (see Table I) are designated based on their sequences that share the highest amino acid homology with their respective Arabidopsis cellulose synthase subunits. TaCesA4-like (a partial cDNA) has the highest homology with AtCesA4, but shares only about 80% amino acid identity with other members of TaCesA4. The total mRNA levels of TaCesA1, TaCesA4-like, and TaCesA10 showed a significant inverse correlation with WSC concentrations.
Validation of Key WSC-Correlated Genes Derived from Affymetrix GeneChip Data in an Extended Group of SB Lines
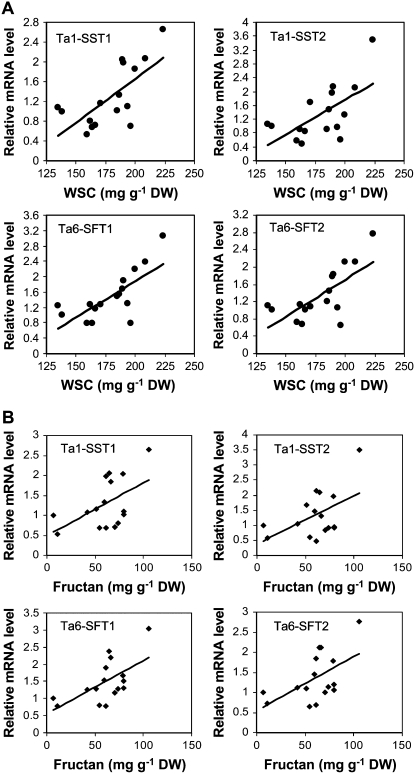
To validate the WSC-correlated carbohydrate metabolic genes derived from the Affymetrix GeneChip data based on eight SB lines, we extended the correlation analysis of gene expression levels in association with the stem WSC concentrations to the 16 SB lines as shown in Figure 1, which included the eight SB lines that were used in the Affymetrix GeneChip analysis. One or two predominantly expressed isoenzyme members were selected from each WSC-correlated gene family based on the Affymetrix GeneChip data for quantitative RT-PCR analysis. As shown in Figure 5, the expression levels of four fructosyltransferase genes were significantly correlated with stem WSC levels in the 16 SB lines (r = 0.63–0.73 and P < 0.01) as well as with stem fructan levels (r = 0.51–0.52 and P < 0.05). Correlations of the mRNA levels of fructosyltransferase genes with fructan levels appear to be lower than with WSC levels. Particularly, when the highest WSC and fructan line (SB169) was excluded from analysis, correlations between fructosyltransferase mRNA levels and fructan levels fell below the significant level (P > 0.05). In contrast, correlations were still statistically significant between fructosyltransferase mRNA levels and WSC levels, even when SB169 was excluded.
Figure 5.
Fructosyltransferase genes positively correlated with stem WSC (A) and fructan (B) concentrations in 16 SB lines. Relative expression levels of individual genes were determined by quantitative RT-PCR. Each value is the mean of two field replicate samples. Correlations between the expression levels of these genes and WSC or fructan concentrations are all statistically significant (P < 0.05). Ta1-SST1 gene is not present in the Affymetrix GeneChip. TC number of each gene is provided in Supplemental Table S3. DW, Dry weight.
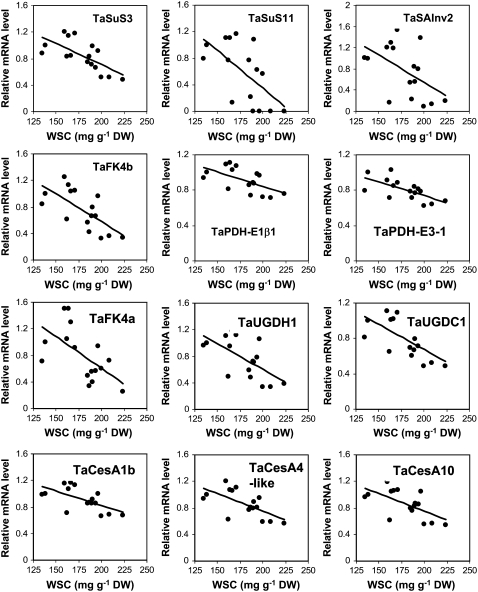
The expression levels of two major Suc synthase isoenzyme genes (TaSuS3 and TaSuS11) and one major soluble acid invertase gene (TaSAInv2) were inversely correlated with WSC levels (r = 0.52–0.68, P < 0.05; Fig. 6). The other predominantly expressed isoenzyme genes that showed a significant inverse correlation (r = 0.63–0.68, P < 0.01) with stem WSC concentrations in these 16 SB lines were two fructokinases (TaFK4a and TaFK4b), pyruvate dehydrogenase complex subunits (TaPDH-E1β1 and TaPDH-E3-1), UDP-Glc dehydrogenase (TaUGDH1), UDP-glucuronate decarboxylase (TaUGDC1), and three cellulose synthase subunit genes (TaCesA1b, TaCesA4-like, and TaCesA10; Fig. 6). These data provide strong evidence at the transcript level for the role of these carbohydrate metabolic gene families in genotypic variation in WSC accumulation.
Figure 6.
Carbohydrate metabolic and cellulose synthase genes that were inversely correlated with WSC concentrations in the stems of 16 SB lines. Relative expression levels of individual genes were determined by quantitative RT-PCR. Each value is the mean of two field replicates. Correlations between the expression levels of these genes and WSC concentrations are all statistically significant (P < 0.05). TC number of each gene is provided in Supplemental Table S3. SuS, Suc synthase; SAInv, soluble acid invertase; FK, fructokinase; PDH, pyruvate dehydrogenase; UGDH, UDP-Glc dehydrogenase; UGDC, UDP-glucuronate decarboxylase; CesA, cellulose synthase; DW, dry weight.
Genotypic Variation in the mRNA Levels of Suc Synthase, Soluble Acid Invertase, and 1-SST Families Is Supported by Genotypic Difference in Their Enzyme Activities
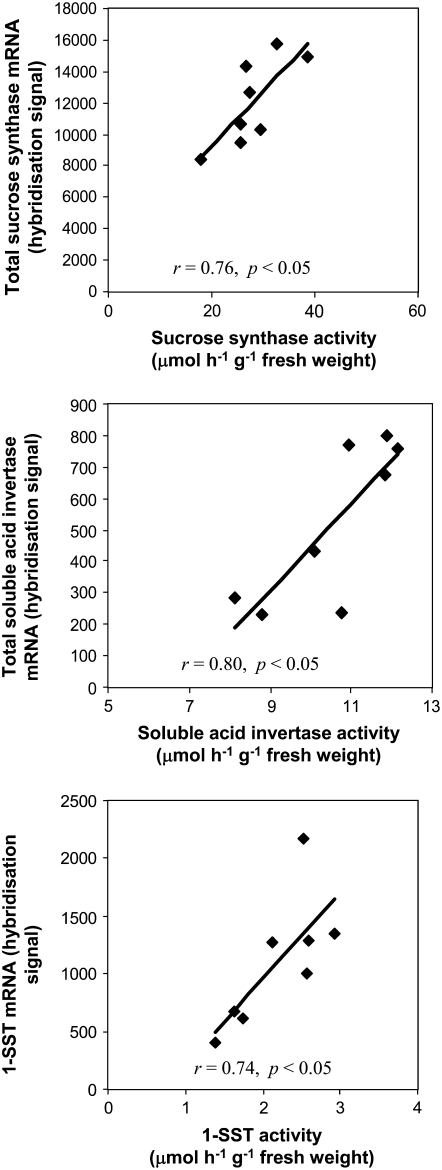
Because Suc synthase, soluble acid invertase, and 1-SST are three important differentially expressed enzyme families with relatively high differences in expression level between high and low WSC lines, we measured the activities of these three enzymes in the stem of the eight SB lines that were used in Affymetrix GeneChip analysis. The enzyme activities of both Suc synthase and soluble acid invertase in these eight SB lines were significantly correlated with the total mRNA levels of their respective enzyme families derived from the Affymetrix GeneChip data (Fig. 7). The correlation coefficients for Suc synthase and soluble acid invertase were 0.76 and 0.80 (P < 0.05), respectively. The activities of these two enzymes were inversely correlated with stem WSC concentrations in these SB lines (r = −0.74 [P < 0.05] for Suc synthase and r = −0.71 [P < 0.05] for soluble acid invertase; data not shown). The relative enzyme activity of 1-SST in the stem of eight SB lines was also significantly correlated with its mRNA level, WSC concentration, and fructan concentration (r = 0.74 [P < 0.05] between its enzyme activity and mRNA level [Fig. 7], r = 0.84 [P < 0.01] between the enzyme activity and WSC concentration, r = 0.82 [P < 0.05] between the enzyme activity and fructan concentration [data not shown]).
Figure 7.
Correlations between the total mRNA levels and enzyme activities of Suc synthase, soluble acid invertase, and 1-SST in the stems of eight SB lines. The total mRNA level of each enzyme family was determined using the Affymetrix GeneChip. Each point represents the mean of two field replicate samples. The enzyme activity is expressed as micromoles of Fru or Glc released from Suc by respective enzymes as described in “Materials and Methods.”
Stem Cell Wall Polysaccharide Contents Are Inversely Associated with WSCs
Expression analysis showed that the transcript levels of UDP-Glc 6-dehydrogenase, UDP-glucuronate decarboxylase, and some subunits of cellulose synthase were inversely correlated with the stem WSC levels, suggesting potentially differential partitioning of carbon to the cell wall components between high and low WSC lines. Therefore, we performed a comparative analysis of stem hemicellulose and cellulose contents between the three highest and three lowest WSC SB lines (Fig. 1). The high and low WSC groups had a similar anthesis date (see the caption of Table II); particularly, the highest WSC line (SB169) and the lowest line (SB165) had the same anthesis date. Cellulose and hemicellulose are the major components of cell walls in cereal stems. As shown in Table II, both cellulose and hemicellulose contents in the stem were significantly lower in the high WSC lines than in the low WSC lines, which agrees with the transcript data of enzyme genes related to the synthesis of these cell wall polysaccharides. Relative difference in carbon partitioning between WSCs and cell wall polysaccharides can be expressed as the ratio of WSCs to hemicellulose plus cellulose, which markedly differs between high and low WSC groups (Table II).
Table II.
Comparative analysis of stem hemicellulose, cellulose, and WSC concentrations between high and low WSC SB lines
Values are means ± sd of three SB lines with values for each line derived from the mean of two field replicates. High WSC lines are SB57, SB62, and SB169 with a mean anthesis date of 77.6 d and low WSC lines are SB3, SB10, and SB165 with a mean anthesis date of 78.1 d. DW, Dry weight.
| Genotype | WSC Concentration | Hemicellulose Content | Cellulose Content | Ratio of WSCs to Hemicellulose and Cellulose |
|---|---|---|---|---|
| mg g−1 DW | mg g−1 DW | mg g−1 DW | ||
| High WSC lines | 211.4 ± 12.2 | 185.0 ± 6.7 | 184.4 ± 4.8 | 0.572 |
| Low WSC lines | 143.9 ± 13.1 | 223.0 ± 13.2 | 199.2 ± 4.6 | 0.341 |
| P value | 0.003 | 0.01 | 0.02 |
DISCUSSION
Stem WSCs serve as a carbohydrate reserve for grain filling in wheat and other cool-season cereals (Schnyder, 1993; Wardlaw and Willenbrink, 1994; Blum, 1998; Gebbing, 2003). This study represents a comprehensive investigation in molecular dissection of genotypic differences in WSC metabolism in stems. We demonstrated here that genotypic differences in WSC accumulation in wheat stems were determined at least in part by differential regulation of some carbohydrate metabolic genes at the transcript level and identified a number of key enzyme families that appear to be associated with the variation in WSC accumulation.
Fructan is the major component of stem WSCs that accounts for variation in the WSC concentrations among these SB progeny lines. It is synthesized from Suc in the vacuole and is one of the major temporary reserve carbohydrates in the vegetative organs of cool-season cereals and other grass species (Pollock and Cairns, 1991; Ritsema and Smeekens, 2003; Chalmers et al., 2005). The β-2,6-linked fructan is synthesized by the consecutive action of 1-SST and 6-SFT (Ritsema and Smeekens, 2003; Chalmers et al., 2005). The enzymes responsible for the synthesis of β-2,1-linked fructan are 1-SST and 1,2-β-fructan 1F-fructosyltransferase. Ta1-SST and Ta6-SFT genes were differentially expressed among SB genotypes differing in WSC concentrations. Strong positive correlations between the Ta1-SST/Ta6-SFT transcript levels and the stem WSC or fructan concentrations clearly indicate that these two enzyme families are associated with genotypic variation in fructan accumulation. This is in line with the fact that fructans in cereal stems are predominantly of the β-2,6-linked type (Bancal and Triboï, 1993). We also demonstrated that the enzyme activity of Ta1-SST was positively correlated with its mRNA level and fructan concentration in the stem.
It has been shown in some studies that exogenous Suc can enhance fructan accumulation and fructosyltransferase mRNA level or enzyme level (Wagner et al., 1986; Müller et al., 2000; Ruuska et al., 2008). Suc levels were also positively associated (though not statistically significant) with WSC concentrations in the stem among these SB lines. However, there was no correlation between the Suc and fructan levels in the stem. Thus, the stem Suc levels did not appear to contribute to genotypic variation in fructan accumulation unless there is genotypic variation in Suc compartmentation in the stem of these lines. No difference in the total mRNA level of vacuolar Suc transporter genes, based on high-sequence homology with a previously characterized barley vacuolar transporter (Endler et al., 2006), between high and low WSC lines was observed (data not shown). In addition, the mRNA levels of fructosyltransferase genes were not correlated with stem Suc levels among the 16 SB lines (r < 0.3). Whether there is genotypic difference in intercellular Suc compartmentation or the presence of other factors controlling fructosyltransferase expression awaits further investigation.
Another factor that potentially contributes to fructan accumulation is the fructan hydrolysis rate. Fructan is hydrolyzed to Fru and Suc by fructan 1-exohydrolase (1-FEH) and/or fructan 6-exohydrolase (6-FEH; Van den Ende et al., 2005; Van Riet et al., 2006). The transcript levels of TaFEH genes did not appear to be associated with genotypic variation in the stem WSCs at anthesis.
Suc is central to WSC metabolism. Up- or down-regulation of genes involved in Suc synthesis or hydrolysis is likely to play an important role in determining the pool size of WSCs or fructan in the stem. We found that the transcript levels of the enzymes (Suc synthase and soluble acid invertase) involved in Suc hydrolysis were inversely correlated with the stem WSC levels among the SB progeny lines. Analysis of enzyme activities of these two enzymes supports these expression data. This suggests that a high level of WSC accumulation is associated with a reduced rate of Suc hydrolysis.
A lower rate of Suc hydrolysis in high WSC lines, as indicated by reduced levels of Suc synthase and soluble acid invertase activities, appears to be accompanied by a reduced expression level of fructokinase. Fructokinase controls the rate of Fru entering the glycolytic pathway. Although no significant correlations between the total mRNA levels of enzymes involved in glycolysis and stem WSC concentrations were observed, the transcript levels of two enzyme components (E1β and E3) from the mitochondrion pyruvate dehydrogenase showed a significant inverse correlation with WSCs. The pyruvate dehydrogenase in the mitochondrion is a key enzyme group that controls the rate of carbohydrates entering the tricarboxylic acid cycle (Tovar-Méndez et al., 2003). However, as genotypic differences in the mRNA levels of these differentially expressed components of the pyruvate dehydrogenase were relatively small, a demonstration of differences in the enzyme activity would be technically difficult.
It is interesting to note that unlike fructokinase, no decrease in the total mRNA level of the hexokinase family was observed along with the significant reduction in soluble acid invertase mRNA and activity in high WSC lines. This may be explained by the possibility that the reduced rate of Glc production by acid invertase in the high WSC lines is compensated by an increased amount of Glc released from fructan synthetic reactions, in which the fructosyl-moiety of Suc is incorporated into fructan (Pollock and Cairns, 1991). Furthermore, the difference in differential transcript regulation between fructokinase and hexokinase may be partly related to another fact that Fru is a common product of Suc hydrolysis by both acid invertase and Suc synthase.
The other product of Suc hydrolysis by Suc synthase is UDP-Glc. The total mRNA levels of the other two UDP-Glc utilizing enzymes, cellulose synthase (subunits TaCesA1, TaCesA4-like, and TaCesA10) and UDP-Glc dehydrogenase, were highly correlated with that of the Suc synthase family (r > 0.9; data not shown). Genetic studies on cellulose synthase subunit mutants in Arabidopsis have shown that CesA1 is one of the essential subunits of the cellulose synthase complex involved in primary cell wall synthesis (Arioli et al., 1998) and CesA4 in secondary cell wall synthesis (Taylor et al., 2003). In addition, the mRNA level of the enzyme family (UDP-glucuronate decarboxylase), involved in removal of the product of the UDP-Glc dehydrogenase-catalyzed reaction and diverting carbohydrates to hemicellulose synthesis (Harper and Bar-Peled, 2002), was also highly correlated with that of the Suc synthase family (r = 0.91). The mRNA levels of the major isoenzymes of these enzymes were positively correlated with the major isoenzyme (TaSuS3) of Suc synthase (r = 0.8–0.9; data not shown) and inversely correlated with the stem WSC levels in the 16 SB lines. These enzymes divert carbon from the Suc pool to cell wall components (cellulose and hemicellulose). Comparative analysis of hemicellulose and cellulose contents in wheat stems between high (three highest lines) and low (three lowest lines) WSC groups showed that the high WSC lines contained significantly lower amounts of these cell wall polysaccharides (mainly hemicellulose) than the low WSC lines, thus supporting the data on the inverse relationships between WSCs and transcript levels of the genes involved in cell wall polysaccharide synthesis. This difference in the cell wall polysaccharide contents based on total dry matter may be partly due to WSC concentrations. When values are expressed on the basis of total dry matter minus the WSC component, no difference in cellulose content between high and low WSC groups was observed, but the hemicellulose content in the high WSC group is still significantly lower than that in the low WSC group (data not shown). Nevertheless, relative partitioning of carbon between WSCs and cell wall polysaccharides in the stem, as expressed as the ratio of WSCs to hemicellulose and cellulose, considerably differed between high and low WSC lines. The reduced carbon partitioning to cell wall polysaccharides in the high WSC lines did not lead to apparent reduction in plant growth or weakness in stem strength. Genotypic variation in cellulose and hemicellulose contents in plants is known from previous studies. For example, in a legume forage Cyamopsis tetragonoloba, cellulose and hemicellulose contents varied from 16.6% to 28.1% and 3.7% to 9.3% of dry matter among 14 genotypes, respectively (Das et al., 1975). The arabinoxylan content in wheat grains ranged between 4.07% and 6.02% of dry matter among five genotypes (Lempereur et al., 1997). The range of the neutral detergent fiber content (composed of mainly cellulose, hemicellulose, and lignin) in wheat straw is between 68.18% and 81.97% of dry matter and the range of the calculated hemicellulose content is between 24.91% and 30.70% among 15 genotypes (Habib et al., 1995). Interestingly, an inverse relationship between WSCs and neutral detergent fiber contents has been observed in the shoot of ryegrass (Lolium perenne) varieties (Smith et al., 2002).
Overall, the data derived from a combined analysis of global transcript profiling and key enzyme activities or end product contents in stems suggest that the high stem WSC trait in wheat is associated with the following factors: (1) enhanced fructan deposition, (2) reduced Suc hydrolysis, and (3) reduced carbon partitioning into cell wall polysaccharides. In addition, a decrease in the transcript levels of fructokinase and some components of the pyruvate dehydrogenase in the high WSC lines implicates a potentially decreased flux of carbohydrates to the tricarboxylic acid cycle, which could positively contribute to WSC accumulation. These represent biochemical mechanisms for the enhanced WSC accumulation in the stem of some wheat genotypes. Strong correlation between the WSC concentrations and the transcript levels of the major isoenzyme genes from some of these differentially expressed enzyme families suggest that some of these genes are potential targets for metabolic engineering to improve stem WSC concentrations in wheat. However, a complete picture of the molecular basis of the WSC trait and its contribution to grain yield awaits further molecular analyses of genotypic differences in WSC metabolism in the source leaf organ and its mobilization from stem to grain during the grain-filling period.
MATERIALS AND METHODS
Plant Materials and Field Growth Conditions
A set of 194 SB recombinant inbred lines from the Seri M82/Babax cross (Olivares-Villegas et al., 2007) was evaluated in a series of trials from 2002 to 2004 at various locations in Queensland, Australia. For each line, WSC concentration in aboveground plant material at anthesis (DC65) was determined in several of these trials. From these data, 16 SB lines with a similar anthesis date, differing in WSC concentration, were chosen in this study. These 16 lines were grown under rain-fed conditions, with some supplementary irrigation because of low plant-available water in the soil, with two field replicates of each line in 2005 at the CSIRO Cooper Laboratory at Gatton (latitude 27°34′S; longitude 152°17′E). The site was on a deep fertile prairie loam soil developed on alluvium with a plant-available water holding capacity of about 250 mm to a depth of 1.5 m. The trial was sown on July 12, at the end of the normal sowing window for the region, and harvested for grain yield assessment on November 21. Plot size was 6 × 1.76 m (eight rows) with an interrow spacing of 22 cm. Plots were sprayed with herbicides to control weeds and fungicide to prevent foliar diseases. At sowing, the plant-available water holding to 1.5-m depth was 112 mm (i.e. 47% of plant-available water holding capacity). From sowing to before anthesis, 67 mm of water was added through rain (17 mm) and some irrigation (50 mm); 2 d before anthesis there was 30 mm of rain; from anthesis to midgrain fill a further 5 mm of rain; and from midgrain fill to harvest a further 332 mm of rain. All plots exhibited considerable drought stress before anthesis (the mean value of anthesis biomass in this rain-fed trial was 70% of that in an adjacent trial of the same genotypes that received 100 mm of irrigation in the preanthesis period), and during the early to midgrain fill period.
The stem tops (peduncle and penultimate internode with leaf sheath attached) were sampled at 50% anthesis between 12 and 1 pm about 2 d after 30 mm of rain (otherwise, prior irrigation would be required to relieve drought stress). At the time of sampling the surface of the soil in these plots was still wet and plants were in a full hydration status. Each sample contained seven to eight stems from main tillers that were randomly sampled from each plot, immediately dropped into liquid nitrogen, stored at −80°C, and used for RNA isolation, WSC, enzyme, and cell wall polysaccharide analyses.
WSC Extractions and Measurements
WSCs were extracted from 100 mg of lyophilized and powdered stems (peduncle and penultimate internode with leaf sheath attached) with 10 mL of 80% (v/v) ethanol at 80°C followed by two extractions of the same volume of water at 60°C. WSC level in the combined extracts was measured using the anthrone method (Yemm and Willis, 1954). The WSC composition (Suc, Glc, Fru, and fructan) in the combined extracts was determined by high-performance anion-exchange chromatography as described by Ruuska et al. (2006).
Total RNA Extraction
Frozen fresh stems (peduncle and penultimate internode with leaf sheath attached) were ground to fine powder in liquid nitrogen. Total RNA was isolated from wheat (Triticum aestivum) stems using Plant RNA Reagent (Invitrogen), according to the manufacturer's instruction. RNA was further purified through an RNeasy column (Qiagen) after pretreatment with RNase-free DNase I (Xue and Loveridge, 2004).
Expression Analysis Using Affymetrix GeneChip Wheat Genome Array
In the Affymetrix GeneChip expression experiment, a subset of eight of the 16 SB lines covering the range of WSC levels was selected (see Fig. 1). The wheat genome array (Affymetrix GeneChip) contains 61,127 probe sets representing 55,052 transcripts for all 42 chromosomes in the wheat genome. RNA quality check, cRNA preparation, labeling, hybridization, and data acquisition of Affymetrix wheat GeneChips were performed by the microarray service at the Australian Genome Research Facility, Melbourne, Australia. A total of 16 Affymetrix genechips for 16 RNA samples from the eight SB lines with two field replicates of each line were used in this study. The raw GeneChip data were normalized using robust multiarray average, a log scale measurement of expression developed by Irizarry et al. (2003), using the default settings for the Affymetrix package within Bioconductor, running within the R-statistical programming environment (http://www.r-project.org/). The full data set has been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and is accessible through GEO Series accession number GSE9767. The normalized expression data for genes (probe sets) from enzyme families related to WSC metabolism (see Supplemental Table S1) were retrieved and carbohydrate metabolic genes with normalized hybridization signals of <20 were discarded. The sequences of the retrieved probe sets were searched for corresponding TC sequences in the TaGI database (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=wheat). When multiple probe sets fell into the same TC sequence, the mean expression value was used for analysis. The sequences of these retrieved genes went through further bioinformatic analyses for checking correctness of their annotations against NCBI BLAST databases (http://www.ncbi.nlm.nih.gov/BLAST/) and prediction of potential subcellular location as described at the latter section. The reannotated carbohydrate metabolic genes were then grouped into enzyme families. The expression values for these WSC metabolism-related genes are presented in Supplemental Table S1. Pearson correlation analysis was used for the identification of carbohydrate metabolic genes for their potential association with the WSC trait. Correlations between mRNA levels and stem WSC concentrations in eight SB lines were analyzed at both genotypic (using the mean value of two field replicates of each line; n = 8) and genotype/field-replicate levels (8 genotypes × 2 field replicates, n = 16). Differences in mRNA and WSC levels between two field replicates of each line represent both biological and environmental variation. Genes with correlation coefficients that were statistically significant (P < 0.05) at both levels were considered to be potentially associated with the WSC trait. The reliability of the Affymetric GeneChip data was confirmed by quantitative RT-PCR analysis of 20 genes with a mean correlation coefficient of 0.95 between the two methods (Supplemental Table S2). The Affymetrix GeneChip data on the relative transcript abundance of individual isoenzymes from a given enzyme gene family were also assessed by quantitative RT-PCR analysis of two enzyme families (Suc-P synthase and Suc synthase).
Bioinformatic Prediction of Subcellular Locations of Wheat Proteins
A combination of BaCello (http://gpcr.biocomp.unibo.it/bacello/pred.htm), Plant-PLoc 2.0 (http://chou.med.harvard.edu/bioinf/plant/), LOCtree (http://cubic.bioc.columbia.edu/cgi-bin/var/nair/loctree/query), WoLF PSORT (http://wolfpsort.org/), TargetP 1.1 (www.cbs.dtu.dk/services/TargetP/), SignalP 3.0 (www.cbs.dtu.dk/services/SignalP/), MITOPROT (http://ihg.gsf.de/ihg/mitoprot.html), ChloroP 1.1 (www.cbs.dtu.dk/services/ChloroP/), TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0/), and PredictNLS (http://cubic.bioc.columbia.edu/cgi/var/nair/resonline.pl) was used to predict subcellular location. The subcellular location of each protein was predicted first using BaCello, Plant-PLoc 2.0, LOCtree, and WoLF PSORT and was then further analyzed by other prediction tools as detailed below. Chloroplast location was predicted by ChloroP 1.1 and TargetP 1.1, based on the presence of a chloroplast transit peptide. Mitochondrial location was predicted by TargetP 1.1 and MITOPROT, based on the presence of a mitochondrial targeting peptide. Extracellular location was predicted by SignalP 3.0 (based on the presence of a secretory pathway signal peptide, but without a signal anchor sequence). Nuclear location was predicted using PredictNLS, based on the presence of nuclear localization signal. Vacuolar location was predicted based on the presence of signal anchor sequences near the N terminus with or without a signal peptide (SignalP 3.0) and high homology with known vacuolar proteins in the SWISS-PROT database using WoLF PSORT. Many plant vacuolar invertases are known to have no N-terminal signal peptide, but contain a single hydrophobic subterminal transmembrane segment near the N terminus (Ji et al., 2005). FEHs encoded by the TaFEH genes listed in Supplemental Table S1 all contain a hydrophobic N-terminal signal peptide with no signal anchor sequences, as predicted by SignalP. Although the presence of FEH enzymes and fructans in the apoplast has been demonstrated (Livingston and Henson, 1998; Van den Ende et al., 2005), the vacuole is probably a primary subcellular location for most of FEH enzymes (Wagner and Wiemkem, 1986; Van den Ende et al., 2003; Lothier et al., 2007). Many proteins have experimentally been shown to have more than one subcellular location, such as cytoplasmic and nuclear locations of a yeast hexokinase (Randez-gil et al., 1998). To date, no known vacuolar targeting signals have been identified in 6- and 1-FEHs. Plasma membrane location and presence of transmembrane helices are predicted using WoLF PSORT and TMHMM. When a wheat TC sequence or EST was not full length, the sequence of a highly homologous gene (>90% amino acid similarities in the available sequence region) from other plant species was used for prediction.
Quantitative RT-PCR Analysis
cDNA was synthesized using an oligo(dT)20 primer from total RNA samples that had been pretreated with RNase-free DNase I (Xue and Loveridge, 2004) and purified through an RNeasy column (Qiagen). The transcript levels of wheat genes were quantified with real-time PCR with an ABI Prism 7900 sequence detection system (Applied Biosystems) using SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions. An external reference mRNA (626 nt) in vitro transcribed from a bovine cDNA (CF767388; Xue et al., 2006a) was added to each RNA sample (0.04 pg of the external reference mRNA per microgram of total RNA purified through a Qiagen column) before cDNA synthesis to check the similar efficiencies in cDNA synthesis among RNA samples. The sequences of primer pairs used for real-time PCR are listed in Supplemental Table S3. The gene-specific primers were designed based on the multiple sequence alignment of ESTs from each TC with primer sequence selection mainly targeting the EST sequence used in Affymetrix wheat GeneChip. The gene specificity of primers for each gene during primer designing was checked by blasting primer sequences in the TaGI database (using an expect value setting at 10,000), matching only with the sequence of the targeted gene, except those (indicated in Supplemental Table S3) where the presence of extremely highly homologous TC sequences makes designing of the gene-specific primer pair impossible.
Two wheat genes (TaRPII36, RNA polymerase II 36-kD subunit [TC235230; Xue et al., 2006b] and TaRP15, RNA polymerases I, II, and III, 15-kD subunit [TC265122]) were used as internal reference genes for calculation of relative transcript levels of the genes under study. The mRNA levels of these internal reference genes were almost the same in the stem among samples of the 16 SB lines, as judged using the external reference gene (CF767388). The PCR efficiency of each primer pair was determined by a dilution series of samples. The specificity of real-time PCR amplification was confirmed by the following criteria: (1) a single peak in Tm curve analysis of real-time PCR-amplified products and (2) a single band on gel electrophoretic analysis. For the accuracy of quantitative RT-PCR data, single-channel pipettes were used for setting up real-time PCR assays.
The AEL of each gene relative to an internal reference gene, TaRPII36, was calculated using the following formula (Stephenson et al., 2007): ErCt/EtCt × F, where Ct is cycle threshold (PCR cycle number required for reaching the signal point used for detection across samples), Er is reference gene amplification efficiency (TaRPII36), Et is target gene amplification efficiency, and F is amplicon size factor (reference gene amplicon size divided by target gene amplicon size). We tentatively used AEL values here to provide an approximate estimation of relative expression levels among various genes under the situation where the absolute quantification of mRNA levels for a large number of genes using a cRNA (or cDNA) calibration curve is not possible.
Measurements of Suc Synthase, Soluble Acid Invertase, and 1-SST Activities
Stem (peduncle and penultimate internode with leaf sheath attached) samples were homogenized to fine powder in liquid nitrogen. The powdered sample (0.5 g) was homogenized in 2 mL of cold homogenization buffer (25 mm HEPES-KOH, pH 7.4, 50 mm KCl, 5 mm MgC12, 1 mm EDTA, 1 mm EGTA, 1 mg mL−1 bovine serum albumin, 0.1% Triton X-100, and 5 mm 1,1,1,-trichloro-2,2-bis(p-chlorophenyl)ethane). The homogenate was centrifuged at 13,000g and 4°C for 10 min. Suc synthase and soluble acid invertase in the supernatant were precipitated by 15% polyethylene glycol (8,000 kD) as described by Albertson and Grof (2007). The precipitate was washed once with 15% polyethylene glycol and then dissolved in 1 mL of enzyme buffer (25 mm HEPES-KOH, pH 7.4, 50 mm KCl, 5 mm MgC12, 1 mm EDTA, 1 mm EGTA, and 1 mm 1,1,1,-trichloro-2,2-bis(p-chlorophenyl)ethane) for 30 min. The dissolved enzyme preparation (1 mL) was centrifuged at 13,000g and 4°C for 10 min to remove insoluble material and then concentrated by ultrafiltration using Centricon-30 (Millipore). The filtrate was washed once with 1 mL of the enzyme buffer and used for enzyme assays.
Suc synthase activity was measured in Suc degradation direction in the following reaction mixture: 100 mm Suc and 8 mm UDP, 25 mm HEPES-KOH, pH 7.0, 50 mm KCl, and 5 mm MgC12. The reaction without UDP was used as control. The reaction was incubated at 30°C for 60 min and terminated by heating at 85°C for 10 min. The Fru and Glc released from Suc were determined by the HPLC method and the amount of Fru over an equal molar amount of Glc was used for measurement of Suc synthase activity (Albertson and Grof, 2007). This is because in this assay system Suc is also hydrolyzed to Fru and Glc by neutral invertase.
Soluble acid invertase activity was measured in the following reaction mixture: 100 mm Suc, 50 mm sodium citrate, pH 5.0, and 50 mm KCl. The reaction with heat-inactivated enzyme preparation was used as control. The reaction was incubated at 30°C for 60 min and terminated by heating at 85°C for 10 min. The Glc and Fru released from Suc were determined by the HPLC method (Albertson and Grof, 2007). Acid invertase activity was calculated based on the amount of Fru production. This is because, in wheat stem, 1-SST is able to release the Glc moiety of Suc while it incorporates the Fru moiety into fructan.
1-SST activity was measured as for the soluble acid invertase assay. The excess molar amount of Glc over Fru produced from Suc in the 1-SST and soluble acid invertase reaction was used to estimate 1-SST activity.
For all three enzymes, the amount of enzyme solution and reaction time were previously determined to be in the linear range of the reaction.
Measurements of Hemicellulose and Cellulose Contents
Frozen fresh stems (peduncle and penultimate internode with leaf sheath attached) were ground to fine powder in liquid nitrogen and dried at 60°C. Dried stem powder (0.5 g) was used for fractionation of hemicellulose and cellulose. Water- and ethanol-soluble materials were removed by sequential extraction twice with water at 70°C (14 and 3 h), twice with 80% ethanol at 80°C (2 h each) and then once with water at 70°C (16 h). Cell wall material was extracted sequentially with 100% ethanol at 85°C, then with acetone, methanol:chloroform mixture (1:1, v/v), and ethanol at room temperature (1 h each extraction), and dried at 60°C. The dried cell wall material was suspended in 10 mL of 50 mm sodium citrate buffer (pH 6.5) containing 6 units mL−1 porcine pancreatic α-amylase (Sigma) and incubated at 37°C for 4 h to remove starch.
After digestion with α-amylase, samples were washed twice with water and dried at 60°C. Hemicellulose was dissolved from the α-amylase-treated samples by three-time extractions with 17.5% (w/v) NaOH containing 0.02% (w/v) NaBH4 at 22°C (12–14 h each extraction). The hemicellulose extracts were neutralized with 100% acetic acid and hemicellulose was precipitated by adding 2.5 volumes of 100% ethanol at 4°C. The hemicellulose precipitate, after washing four times with 75% ethanol containing 1 mm acetic acid, was dissolved (partially) in 25 mm NaOH and hydrolyzed in a 2.5% (v/v) H2SO4 solution at 92°C for 8 h. The reducing sugars released from hemicellulose were determined by the colorimetric method of Lever (1972) using Xyl and oat-spelled xylan hydrolysate as standards. The alkaline insoluble fraction of the cell wall material was incubated with nitric/acetic acid mixture (nitric acid, 80% acetic acid; 1:10 by volume) at 90°C for 1 h, washed twice with water, and then dissolved in 67% (v/v) H2SO4. The cellulose content in the nitric/acetic acid-treated samples was determined by the anthrone method as described by Updegraff (1969).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Illustration of differential regulation of isoenzyme members within an enzyme gene family between high and low WSC SB lines.
Supplemental Table S1. Correlation between stem WSC concentrations and the transcript levels of genes related to WSC metabolism in eight SB lines.
Supplemental Table S2. Comparison of relative expression levels of genes estimated between Affymetrix GeneChip and quantitative RT-PCR.
Supplemental Table S3. Primers for real-time PCR analysis of wheat genes.
Supplementary Material
Acknowledgments
We thank Andrew Spriggs and Gavin Kennedy for helping in the normalization and analysis of Affymetrix GeneChip data. We are grateful to Drs. Scott Chapman and Fernanda Dreccer for helpful discussion of the field experiment, and Dr. Chris Grof for his advice in measurement of Suc synthase and acid invertase activities. We also thank Dr. Graham Bonnett for his helpful suggestions on the manuscript. The excellent technical assistance of Janneke Drenth is very much appreciated.
This work was supported by the Australian Grains Research & Development Corporation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Gang-Ping Xue (gang-ping.xue@csiro.au).
The online version of this article contains Web-only data.
References
- Aggarwal PK, Sinha SK (1984) Effect of water stress on grain growth and assimilate partitioning in two cultivars of wheat contrasting in their yield stability in a drought-environment. Ann Bot (Lond) 53 329–340 [Google Scholar]
- Albertson P, Grof CPL (2007) Application of high performance anion exchange-pulsed amperometric detection to measure the activity of key sucrose metabolising enzymes in sugarcane. J Chromatogr B Analyt Technol Biomed Life Sci 845 151–156 [DOI] [PubMed] [Google Scholar]
- Arioli T, Peng L, Betzner A, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, Birch R, et al (1998) Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279 717–720 [DOI] [PubMed] [Google Scholar]
- Aspeborg H, Schrader J, Coutinho PM, Stam M, Kallas Å, Djerbi S, Nilsson P, Denman S, Amini B, Sterky F, et al (2005) Carbohydrate-active enzymes involved in the secondary cell wall biogenesis in hybrid aspen. Plant Physiol 137 983–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseng S, Van Herwaarden AF (2003) Analysis of the benefits to wheat yield from assimilates stored prior to grain filling in a range of environments. Plant Soil 256 217–229 [Google Scholar]
- Bancal P, Triboï E (1993) Temperature effect on fructan oligomer contents and fructan-related enzyme activities in stems of wheat (Triticum aestivum L.) during grain filling. New Phytol 123 247–253 [Google Scholar]
- Bingham IJ, Blake J, Foulkes MJ, Spink J (2007) Is barley yield in the UK sink limited? I. Post-anthesis radiation interception, radiation-use efficiency and source-sink balance. Field Crops Res 101 198–211 [Google Scholar]
- Blacklow WM, Darbyshire B, Pheloung P (1984) Fructans polymerized and depolymerized in internodes of winter wheat as grain-filling progressed. Plant Sci Lett 36 213–218 [Google Scholar]
- Blum A (1998) Improving wheat grain filling under stress by stem reserve mobilization. Euphytica 100 77–83 [Google Scholar]
- Blum A, Shpiler L, Golan G, Mayer J, Sinmena B (1991) Mass selection of wheat for grain filling without transient photosynthesis. Euphytica 54 111–116 [Google Scholar]
- Bonnett GD, Incoll LD (1992) The potential pre-anthesis and post-anthesis contributions of stem internodes to grain yield in crops of winter barley. Ann Bot (Lond) 69 219–225 [Google Scholar]
- Brooks A, Jenner CF, Aspinall D (1982) Effect of water deficit on endosperm starch granules and grain physiology of wheat and barley. Aust J Plant Physiol 9 423–436 [Google Scholar]
- Chalmers J, Johnson X, Lidgett A, Spangenberg G (2003) Isolation and characterisation of a sucrose:sucrose 1-fructosyltransferase gene from perennial ryegrass (Lolium perenne). J Plant Physiol 160 1385–1391 [DOI] [PubMed] [Google Scholar]
- Chalmers J, Lidgett A, Cummings N, Cao Y, Forster J, Spangenberg G (2005) Molecular genetics of fructan metabolism in perennial ryegrass. Plant Biotechnol J 3 459–474 [DOI] [PubMed] [Google Scholar]
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot (Lond) 89 907–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Arora SK, Luthra YP (1975) Variability in structural carbohydrates and in vitro digestibility of forages. J Dairy Sci 58 1347–1351 [DOI] [PubMed] [Google Scholar]
- De Coninck B, Le Roy K, Francis I, Clerens S, Vergauwen R, Halliday AM, Smith SM, Van Laere A, Van Den Ende W (2005) Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant Cell Environ 28 432–443 [Google Scholar]
- Djerbi S, Aspeborg H, Nilsson P, Sundberg B, Mellerowicz E, Blomqvist K, Teeri TT (2004) Identification and expression analysis of genes encoding putative cellulose synthases (CesA) in the hybrid aspen, Populus tremula (L.) × P. tremuloides (Michx.). Cellulose 11 301–312 [Google Scholar]
- Ehdaie B, Alloush GA, Madore MA, Waines JG (2006) Genotypic variation for stem reserves and mobilization in wheat: II. Postanthesis changes in internode water-soluble carbohydrates. Crop Sci 46 2093–2103 [Google Scholar]
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG (2006) Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes MJ, Scott RK, Sylvester-Bradley R (2002) The ability of wheat cultivars to withstand drought in UK conditions: formation of grain yield. J Agric Sci 138 153–169 [Google Scholar]
- Foulkes MJ, Snape JW, Shearman VJ, Reynolds MP, Gaju O, Sylvester-Bradley R (2007) Genetic progress in yield potential in wheat: recent advances and future prospects. J Agric Sci 145 17–29 [Google Scholar]
- Francki MG, Walker E, Forster JW, Spangenberg G, Appels R (2006) Fructosyltransferase and invertase genes evolved by gene duplication and rearrangements: rice, perennial ryegrass, and wheat gene families. Genome 49 1081–1091 [DOI] [PubMed] [Google Scholar]
- Gebbing T (2003) The enclosed and exposed part of the peduncle of wheat (Triticum aestivum)—spatial separation of fructan storage. New Phytol 159 245–252 [DOI] [PubMed] [Google Scholar]
- Habib G, Shah SBA, Inayat K (1995) Genetic variation in morphological characteristics, chemical composition and in vitro digestibility of straw from different wheat cultivars. Anim Feed Sci Technol 55 263–274 [Google Scholar]
- Harper AD, Bar-Peled M (2002) Biosynthesis of UDP-xylose. Cloning and characterization of a novel Arabidopsis gene family, uxs, encoding soluble and putative membrane-bound UDP-glucuronic acid decarboxylase isoforms. Plant Physiol 130 2188–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley TL (2000) Role of fructans redistributed from vegetative tissues in grain filling of wheat and barley. In AK Gupta, N Kaur, eds, Carbohydrate Reserves in Plants: Synthesis and Regulation. Developments in Crop Science, Vol 26. Elsevier, Amsterdam, pp 207–221
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 249–264 [DOI] [PubMed] [Google Scholar]
- Ji X, Van den Ende W, Schroeven L, Clerens S, Geuten K, Cheng S, Bennett J (2007) The rice genome encodes two vacuolar invertases with fructan exohydrolase activity but lacks the related fructan biosynthesis genes of the Pooideae. New Phytol 173 50–62 [DOI] [PubMed] [Google Scholar]
- Ji X, Van den Ende W, Van Laere A, Cheng S, Bennett J (2005) Structure, evolution, and expression of the two invertase gene families of rice. J Mol Evol 60 615–634 [DOI] [PubMed] [Google Scholar]
- Kawakami A, Yoshida M (2005) Fructan:fructan 1-fructosyltransferase, a key enzyme for biosynthesis of graminan oligomers in hardened wheat. Planta 223 90–104 [DOI] [PubMed] [Google Scholar]
- Kawakami A, Yoshida M, Van den Ende W (2005) Molecular cloning and functional analysis of a novel 6 &1-FEH from wheat (Triticum aestivum L.) preferentially degrading small graminans like bifurcose. Gene 358 93–101 [DOI] [PubMed] [Google Scholar]
- Kühbauch W, Thome U (1989) Nonstructural carbohydrates of wheat stems as influenced by sink source manipulations. J Plant Physiol 134 243–250 [Google Scholar]
- Kiniry JR (1993) Nonstructural carbohydrate utilization by wheat shaded during grain growth. Agron J 85 844–849 [Google Scholar]
- Lasseur B, Lothier J, Djoumad A, De Coninck B, Smeekens S, Van Laere A, Morvan-Bertrand A, Van den Ende W, Prud'homme MP (2006) Molecular and functional characterization of a cDNA encoding fructan:fructan 6G-fructosyltransferase (6G-FFT)/fructan:fructan 1-fructosyltransferase (1-FFT) from perennial ryegrass (Lolium perenne L.). J Exp Bot 57 2719–2734 [DOI] [PubMed] [Google Scholar]
- Lempereur I, Rouau X, Abecassis J (1997) Genetic and agronomic variation in arabinoxylan and ferulic acid contents of durum wheat (Triticum durum L.) grain and its milling fractions. J Cereal Sci 25 103–110 [Google Scholar]
- Le Roy K, Lammens W, Verhaest M, De Coninck B, Rabijns A, Van Laere A, Van den Ende W (2007) Unraveling the difference between invertases and fructan exohydrolases: a single amino acid (Asp-239) substitution transforms Arabidopsis cell wall invertase1 into a fructan 1-exohydrolase. Plant Physiol 145 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M (1972) A new reaction for colorimetric determination of carbohydrates. Anal Biochem 47 273–279 [DOI] [PubMed] [Google Scholar]
- Livingston DP, Henson CA (1998) Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol 116 403–408 [Google Scholar]
- Lothier J, Lasseur B, Le Roy K, Van Laere A, Prud'homme MP, Barre P, Van den Ende W, Morvan-Bertrand A (2007) Cloning, gene mapping, and functional analysis of a fructan 1-exohydrolase (1-FEH) from Lolium perenne implicated in fructan synthesis rather than in fructan mobilization. J Exp Bot 58 1969–1983 [DOI] [PubMed] [Google Scholar]
- McCaig TN, Clarke JM (1982) Seasonal changes in nonstructural carbohydrate levels of wheat and oats grown in a semiarid environment. Crop Sci 22 963–970 [Google Scholar]
- Müller J, Aeschbacher RA, Sprenger N, Boller T, Wiemken A (2000) Disaccharide-mediated regulation of sucrose:fructan-6-fructosyltransferase, a key enzyme of fructan synthesis in barley leaves. Plant Physiol 123 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villegas JJ, Reynolds MP, McDonald GK (2007) Drought-adaptive attributes in the Seri/Babax hexaploid wheat population. Funct Plant Biol 34 189–203 [DOI] [PubMed] [Google Scholar]
- Persson S, Wei H, Milne J, Page GP, Somerville CR (2005) Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proc Natl Acad Sci USA 102 8633–8638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CJ, Cairns AJ (1991) Fructan metabolism in grasses and cereals. Annu Rev Plant Physiol Plant Mol Biol 42 77–101 [Google Scholar]
- Randez-gil F, Herrero P, Sanz P, Prieto JA, Moreno F (1998) Hexokinase II has a double cytosolic-nuclear localisation in Saccharomyces cerevisiae. FEBS Lett 425 475–478 [DOI] [PubMed] [Google Scholar]
- Ritsema T, Hernández L, Verhaar A, Altenbach D, Boller T, Wiemken A, Smeekens S (2006) Developing fructan-synthesizing capability in a plant invertase via mutations in the sucrose-binding box. Plant J 48 228–237 [DOI] [PubMed] [Google Scholar]
- Ritsema T, Smeekens S (2003) Fructans: beneficial for plants and humans. Curr Opin Plant Biol 6 223–230 [DOI] [PubMed] [Google Scholar]
- Ritsema T, Verhaar A, Vijn I, Smeekens S (2005) Using natural variation to investigate the function of individual amino acids in the sucrose-binding box of fructan:fructan 6G-fructosyltransferase (6G-FFT) in product formation. Plant Mol Biol 58 597–607 [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Lewis DC, Kennedy G, Furbank RT, Jenkins CLD, Tabe LM (2008) Large scale transcriptome analysis of the effects of nitrogen nutrition on accumulation of stem carbohydrate reserves in reproductive stage wheat. Plant Mol Biol 66 15–32 [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Rebetzke GJ, van Herwaarden AF, Richards RA, Fettell NA, Tabe L, Jenkins CLD (2006) Genotypic variation in water-soluble carbohydrate accumulation in wheat. Funct Plant Biol 33 799–809 [DOI] [PubMed] [Google Scholar]
- Schnyder H (1993) The role of carbohydrate storage and redistribution in the source-sink relations of wheat and barley during grain filling. New Phytol 123 233–245 [Google Scholar]
- Shearman VJ, Sylvester-Bradley R, Scott RK, Foulkes MJ (2005) Physiological processes associated with wheat yield progress in UK. Crop Sci 45 175–185 [Google Scholar]
- Smith KF, Culvenor RA, Humphreys MO, Simpson RJ (2002) Growth and carbon partitioning in perennial ryegrass (Lolium perenne) cultivars selected for high water-soluble carbohydrate concentrations. J Agric Sci 138 375–385 [Google Scholar]
- Smouter H, Simpson RJ (1991) Fructan metabolism in leaves of Lolium rigidum Gaudin. II: Fructosyltransferase, invertase and fructan hydrolase activity. New Phytol 119 517–526 [Google Scholar]
- Stephenson TJ, McIntyre CL, Collet C, Xue GP (2007) Genome-wide identification and expression analysis of the NF-Y family of transcription factors in Triticum aestivum. Plant Mol Biol 65 77–92 [DOI] [PubMed] [Google Scholar]
- Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR (2003) Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc Natl Acad Sci USA 100 1450–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Méndez A, Miernyk JA, Randall DD (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem 270 1043–1049 [DOI] [PubMed] [Google Scholar]
- Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32 420–424 [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Clerens S, Vergauwen R, Boogaerts D, Le Roy K, Arckens L, Van Laere A (2006) Cloning and functional analysis of a high DP fructan:fructan 1-fructosyl transferase from Echinops ritro (Asteraceae): comparison of the native and recombinant enzymes. J Exp Bot 57 775–789 [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Clerens S, Vergauwen R, Van Riet L, Van Laere A, Yoshida M, Kawakami A (2003) Fructan 1-exohydrolases. β-(2,1)-trimmers during graminan biosynthesis in stems of wheat? Purification, characterization, mass mapping, and cloning of two fructan 1-exohydrolase isoforms. Plant Physiol 131 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W, Yoshida M, Clerens S, Vergauwen R, Kawakami A (2005) Cloning, characterization and functional analysis of novel 6-kestose exohydrolases (6-KEHs) from wheat (Triticum aestivum). New Phytol 166 917–932 [DOI] [PubMed] [Google Scholar]
- Van Herwaarden AF, Angus JF, Richards RA, Farquhar GD (1998. a) ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertilizer. II. Carbohydrate and protein dynamics. Aust J Agric Res 49 1083–1093 [Google Scholar]
- Van Herwaarden AF, Richards RA (2002) Water soluble carbohydrate accumulation in stems is related to breeding progress in Australia wheats. In Proceedings 12th Australasian Plant Breeding Conference. Australian Plant Breeding Association, Perth, Australia, pp 878–882
- Van Herwaarden AF, Richards RA, Farquhar GD, Angus JF (1998. b) ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertilizer. III. The influence of water deficit and heat shock. Aust J Agric Res 49 1095–110 [Google Scholar]
- Van Laere A, Van den Ende W (2002) Inulin metabolism in dicots: chicory as a model system. Plant Cell Environ 25 803–813 [Google Scholar]
- Van Riet L, Nagaraj V, Van den Ende W, Clerens S, Wiemken A, Van Laere A (2006) Purification, cloning and functional characterization of a fructan 6-exohydrolase from wheat (Triticum aestivum L.). J Exp Bot 57 213–223 [DOI] [PubMed] [Google Scholar]
- Verhaest M, Lammens W, Le Roy K, De Ranter CJ, Van Laere A, Rabijns A, Van den Ende W (2007) Insights into the fine architecture of the active site of chicory fructan 1-exohydrolase: 1-kestose as substrate vs sucrose as inhibitor. New Phytol 174 90–100 [DOI] [PubMed] [Google Scholar]
- Vijn I, Smeekens S (1999) Fructan: more than a reserve carbohydrate? Plant Physiol 120 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Wiemkem A (1986) Properties and subcellular localization of fructan hydrolase in the leaves of barley (Hordeum vulgare L. cv Gerbel). J Plant Physiol 123 429–439 [Google Scholar]
- Wagner W, Wiemken A, Matile P (1986) Regulation of fructan metabolism in leaves of barley (Hordeum vulgare L. cv Gerbel). Plant Physiol 81 444–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw IF, Willenbrink J (1994) Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Aust J Plant Physiol 21 255–271 [Google Scholar]
- Wardlaw IF, Willenbrink J (2000) Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytol 148 413–422 [DOI] [PubMed] [Google Scholar]
- Willenbrink J, Bonnett GD, Willenbrink S, Wardlaw IF (1998) Changes of enzyme activities associated with the mobilization of carbohydrate reserves (fructans) from the stem of wheat during kernel filling. New Phytol 139 471–478 [Google Scholar]
- Xue GP, Bower NI, McIntyre CL, Riding GA, Kazan K, Shorter R (2006. a) TaNAC69 from the NAC superfamily of transcription factors is up-regulated by abiotic stresses in wheat and recognises two consensus DNA-binding sequences. Funct Plant Biol 33 43–57 [DOI] [PubMed] [Google Scholar]
- Xue GP, Loveridge CW (2004) HvDRF1 is involved in abscisic acid-mediated gene regulation in barley and produces two forms of AP2 transcriptional activators, interacting preferably with a CT-rich element. Plant J 37 326–339 [DOI] [PubMed] [Google Scholar]
- Xue GP, McIntyre CL, Chapman S, Bower NI, Way H, Reverter A, Clarke B, Shorter R (2006. b) Differential gene expression of wheat progeny with contrasting levels of transpiration efficiency. Plant Mol Biol 61 863–881 [DOI] [PubMed] [Google Scholar]
- Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57 508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.