Abstract
Ebola virus budding is mediated by two proline-rich motifs, PPxY and PTAP, within the viral matrix protein VP40. We have previously shown that a Nedd4-like protein BUL1, but not Nedd4, positively regulates budding of type D retrovirus Mason-Pfizer monkey virus (J. Yasuda, E. Hunter, M. Nakao, and H. Shida, EMBO Rep. 3:636-640, 2002). Here, we report that the cellular E3 ubiquitin ligase Nedd4 regulates budding of VP40-induced virus-like particles (VLPs) through interaction with the PPxY motif. Mutation of the active site cysteine (C894A), resulting in abrogation of ubiquitin ligase activity, impaired the function of Nedd4 on budding. In addition, the WW domains of Nedd4 are essential for binding to the viral PPxY motif, and a small fragment of Nedd4 containing only WW domains significantly inhibited Ebola VLP budding in a dominant-negative manner. Our findings suggest that the viruses containing PPxY as an L-domain motif specifically use E3 in the process of virus budding. We also examined the effects of overexpression of Tsg101 and its mutant. As expected, Tsg101 enhanced VP40-induced VLP release, and TsgΔC, which lacks its C-terminal half, inhibited VLP release. These results indicate that Nedd4, together with Tsg101, plays an important role in Ebola virus budding.
Ebola virus is a nonsegmented negative-strand RNA virus that causes a hemorrhagic disease resulting in high mortality (5). Its RNA genome encodes seven viral proteins: NP, VP35, VP40, GP, VP30, VP24, and L. VP40 is the matrix protein and is essential for virus assembly and release from host cells. Expression of VP40 in mammalian cells is sufficient to generate extracellular virus-like particles (VLPs), which resemble authentic virions (12, 15, 25).
Recent studies have revealed that retroviral Gag and rhabdoviral M proteins play critical roles during a late stage of virus budding and, when expressed alone in cells, they are released in the form of VLPs. These viral proteins possess a so-called late (or L) domain containing PT/SAP, PPxY, or YxxL, which are critical motifs for efficient budding (9, 11, 14, 16, 21, 23, 27, 28, 29, 32). VP40 contains overlapping PTAP and PPxY motifs near its amino terminus.
PTAP motif was first identified in human immunodeficiency virus (HIV) p6Gag and has recently been reported to bind Tsg101, a ubiquitin-conjugating E2 enzyme variant (UEV) and a component of the vesicular protein-sorting machinery (8, 9, 14, 26). The interaction between p6Gag and Tsg101 is required for HIV budding and Tsg101 appears to facilitate this budding by linking the p6 late domain to the vacuolar protein sorting (Vps) pathway (8, 26). Martin-Serrano et al. recently showed that the PTAP sequence within Ebola virus VP40 (Ebola VP40) is also necessary and sufficient to recruit Tsg101 to sites of particle assembly and is required for the efficient formation and release of VLPs (20).
Another motif within the VP40 L-domain, PPxY, has also been determined as the principal sequence that binds to the WW domain consisting of 38 to 40 amino acids containing two widely spaced tryptophans (24). In fact, it has been shown that the viral PPxY sequence can interact with WW domain-containing cellular proteins (7, 11, 13, 17). In addition, we recently demonstrated that a Nedd4-like protein, BUL1, which contains WW domains and possesses the ability to ubiquitinate target proteins containing a PPxY motif, interacts with the PPxY sequence of the Mason-Pfizer monkey virus (M-PMV) Gag and facilitates viral budding (31). Although Harty et al. reported that Ebola VP40 could also bind to Rsp5, a yeast homologue of Nedd4 (12), the functional role of Nedd4 or a Nedd4-like protein in Ebola virus budding remains unknown. Therefore, to better understand the role of a cellular protein affecting Ebola viral budding, we examined the functional involvement of Nedd4 in Ebola VP40-mediated particle formation and determined the domains and activity of Nedd4 that are required for the regulation of virus budding.
MATERIALS AND METHODS
Plasmids.
Plasmids that express Nedd4, C894A, BUL1, KIAA1301, Ebola VP40, or VP40AAXY have been described previously (1, 15, 31). A cDNA encoding Tsg101 was amplified by PCR from the human spleen Marathon-Ready cDNA library (Clontech). BamHI sites were introduced by standard PCR into Tsg101, TsgΔC, Nedd4ΔC2, and WW constructs (described below) at both 5′ and 3′ ends and subcloned into pBj-Myc-hNedd4, which was constructed for Nedd4 expression (1) by replacing Nedd4 with each DNA fragment by using BamHI. The Nedd4 mutants possessing a tryptophan-to-glycine substitution at the first tryptophan residue of each WW domain were generated by site-directed mutagenesis by using the QuickChange site-directed mutagenesis kit (Stratagene).
Radiolabeling and immunoprecipitation.
COS-7 cells (60-mm-diameter plates) were maintained at 37°C in a 5% CO2 incubator in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. The expression plasmid for Nedd4-like proteins, Tsg101, or their mutants (3.6 μg), along with 1.8 μg of pCEboZVP40 (15), was transfected into COS-7 cells (6 × 105) by using 15.6 μl of Trans-IT LT-1 liposomal reagent (Panvera). At 48 h after transfection, cells were labeled for 7 h with [35S]methionine-[35S]cysteine mixture (Perkin-Elmer). Radiolabeled VLP released from cells into the culture medium were prepared by centrifugation for 30 min at 75,000 rpm in a Beckman TLA110 rotor at 4°C after clarification by centrifugation for 15 min at 5,000 × g at 4°C. As described previously (29, 30), radiolabeled cells and VLPs were lysed and then subjected to immunoprecipitation with rabbit anti-VP40 antiserum (15). Immunoprecipitated proteins were electrophoresed on a sodium dodecyl sulfate (SDS)-polyacrylamide gel (10 to 20%). The intensity of the band for VP40 was quantified by using a FLA-3000 imaging system (Fujifilm).
Detection of VP40 interaction with Nedd4 or Tsg101 in VLPs.
VLPs produced from COS-7 cells by coexpressing Nedd4 or Tsg101 with VP40 as described above were immunoprecipitated with rabbit anti-VP40 antiserum. Immunoprecipitated proteins were separated on a 7.5% resolving gel by SDS-polyacrylamide gel electrophoresis, followed by Western blotting with mouse anti-Myc monoclonal antibody (9E10; Santa Cruz Biotechnology) to detect Myc-tagged Nedd4 and Tsg101.
Coimmunoprecipitation assay.
COS-7 cells were cotransfected with pCEboZVP40 or pCEboZVP40AAXY and expression plasmid for Nedd4 or WG1234. At 48 h after transfection, cells were lysed with TNE buffer consisting of 10 mM Tris-HCl (pH 7.8), 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 10 μg of aprotinin/ml. After clarification by centrifugation, lysates were used for immunoprecipitation with rabbit anti-VP40 antiserum. Coimmunoprecipitated proteins with wild-type or mutant VP40 were separated on a 10% resolving gel by SDS-PAGE, followed by Western blotting with a mouse anti-Myc monoclonal antibody (9E10) to determine the presence of Myc-tagged Nedd4 or WG1234.
RESULTS
Effect of overexpression of Nedd4-like proteins on Ebola VLP production.
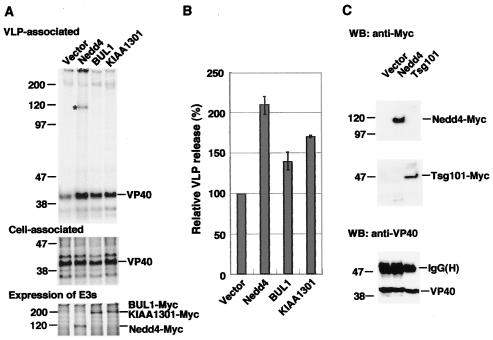
To determine the effect of Nedd4-like proteins on the egress of the Ebola virus, we overexpressed Nedd4 or the previously identified Nedd4-like proteins BUL1 or KIAA1301 (31) and monitored for impact on Ebola VP40-induced VLP budding. COS-7 cells were cotransfected with pCEboZVP40 and the expression plasmid for each Nedd4-like molecule. VLP release from these transfected cells into the culture media were compared by radioimmunoprecipitation analysis. As shown in Fig. 1A and B, overexpression of Nedd4, BUL1, or KIAA1301 apparently promoted VLP release from cells. Among them, Nedd4 had the strongest effect on the enhancement of VLP release, although Nedd4, BUL1, and KIAA1301 were expressed at similar level in cells. Overexpression of Nedd4 or Nedd4-like proteins had no effect on viral protein VP40 synthesis and stability (Fig. 1A, middle panel). Interestingly, in a VLP preparation from cells expressing Nedd4, a protein with a molecular weight similar to Nedd4, was detected (Fig. 1A, asterisk in Nedd4 lane). To determine the identity of this protein, we carried out immunoblot analysis with anti-Myc antibody to detect Myc-tagged Nedd4. As shown in Fig. 1C, the anti-Myc monoclonal antibody recognized this band, indicating that Nedd4 was efficiently incorporated into VLPs through interaction with VP40. These results show that Nedd4 and other Nedd4-like proteins promote Ebola virus budding.
FIG. 1.
Effect of the overexpression of Nedd4 or Nedd4-like proteins on Ebola VP40-induced VLP budding. (A) COS-7 cells were cotransfected with pCEboZVP40 and the empty vector as a control (Vector) or with expression plasmids for Nedd4, BUL1, or KIAA1301. Extracellular VLPs were pelleted from the culture fluids of the labeled cells. VLP-associated (upper panel) or cell-associated (middle panel) VP40 was immunoprecipitated with rabbit anti-VP40 antiserum. To examine the expression of Myc-tagged Nedd4, BUL1, or KIAA1301, cell lysates were also immunoprecipitated with mouse anti-Myc monoclonal antibody (lower panel). The position of VP40 is indicated on the right. The asterisk on the left indicates the protein detected only in the VLPs from Nedd4-expressing cells. (B) Intensity of the band for VLP-associated VP40 in panel A was quantified as described in Materials and Methods. The extent of VP40 in the VLPs released from cells cotransfected with pCEboZVP40, and the control vector was set to 100%. The data represent averages and standard deviations (SD) of three independent experiments. (C) To confirm that Nedd4 and Tsg101 were efficiently incorporated into VLPs through interaction with VP40, VLPs from cells coexpressing Nedd4 or Tsg101 with VP40 were immunoprecipitated with rabbit anti-VP40 antiserum and subjected to Western blot analysis with mouse anti-Myc monoclonal antibody (upper panel). To examine whether all immunoprecipitated samples contain equal amounts of VP40, one-fifth of each immunoprecipitated sample was also analyzed by Western blot analysis with rabbit anti-VP40 antiserum (lower panel).
Analyses of Nedd4 mutants.
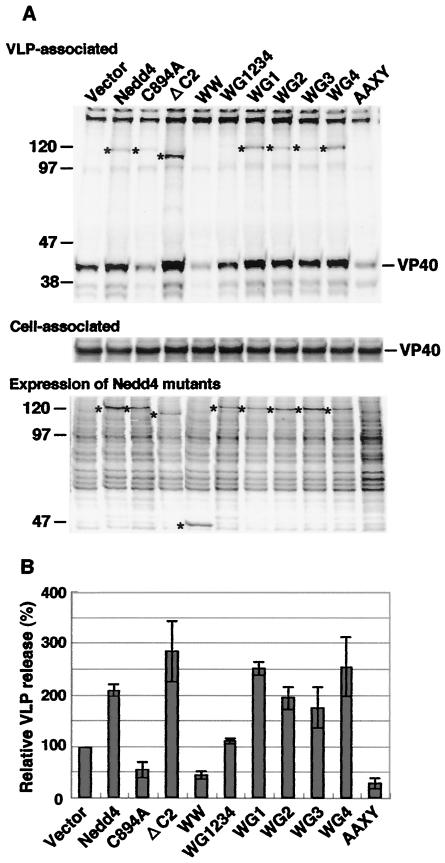
To determine the domains of Nedd4 involved in VLP production, we constructed various Nedd4 mutants (Fig. 2) and examined their effects on Ebola VLP budding. Nedd4 contains a N-terminal C2 domain, four WW domains, and a C-terminal HECT domain. The C2 domain is believed to be responsible for Ca2+-dependent binding of Nedd4 to membrane phospholipids. As shown in Fig. 3, ΔC2, which completely lacked the C2 domain, enhanced VLP budding much stronger than did wild-type Nedd4. The deletion of the C2 domain may change the conformation of this protein to a form more suitable for target binding.
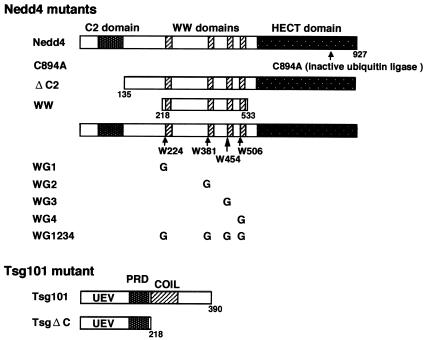
FIG. 2.
Schematic representation of the Nedd4 and Tsg101 mutants. Nedd4 contains an amino-terminal C2 domain, four WW domains, and a carboxyl-terminal HECT domain. Tsg101 contains the ubiquitin-conjugating E2 enzyme variant domain (UEV), the proline-rich domain (PRD), and the putative coiled-coil domain (COIL) (6, 22). All constructs were expressed as proteins containing a Myc tag at their N termini.
FIG. 3.
Effect of Nedd4 mutants on Ebola VLP production. (A) VLPs produced from cells coexpressing VP40 and each Nedd4 mutant were analyzed as in Fig. 1A (upper panel). The expression of VP40 (middle panel) and Nedd4 mutants (lower panel) were also examined. AAXY indicates the VLP sample from cells cotransfected with pCEboZVPAAXY and a control vector. The position of VP40 is indicated on the right. Asterisks on the left indicate the migration position of the putative Nedd4 or Nedd4 mutant proteins incorporated into VLPs in the upper panel and indicate the actual position of Nedd4 or Nedd4 mutants in the lower panel. (B) The intensity of the band for VLP-associated VP40 in panel A was quantified as described in Materials and Methods. The extent of VP40 in the VLPs released from cells cotransfected with pCEboZVP40 and a control vector was set to 100%. The data represent averages and SD of three independent experiments.
The WW domains appear to be important for interaction with the PPxY sequence within the viral L domain (7, 12). We constructed various mutants, introducing a W-to-G mutation in the WW domains (Fig. 2) and then determined which WW domain was critical for interaction with VP40. The introduction of a W-to-G substitution at any single WW site (WG1, WG2, WG3, and WG4) failed to alter the Nedd4 activity needed to enhance VLP budding (Fig. 3); the amounts of extracellular VLP from mutant-expressing cells were similar to those from wild-type Nedd4-expressing cells. In contrast, WG1234, which has mutations at all WW domains, abolished the ability of Nedd4 to enhance VLP budding. In addition, WG1234 was not incorporated into VLPs. These results indicated that Nedd4 bound the PPxY motif within VP40 via its WW domains and that several or all of the WW domains could function as interaction sites with the VP40 L domain.
The HECT domain is critical for ubiquitin ligase activity and contains a conserved cysteine residue that can form a thioester with ubiquitin. Interestingly, the C894A mutant, which has a cysteine-to-alanine substitution at the active site cysteine in the HECT domain, reduced VLP release to 55% of the amount observed in the vector control, suggesting that ubiquitin ligase activity is required for Nedd4 to function in viral budding and that the C894A mutant reduces VLP production in a dominant-negative manner.
We previously showed that a truncated BUL1 containing only the WW domains inhibits M-PMV budding in a dominant-negative manner (31). Therefore, it seemed likely that the WW fragment containing only the four WW domains of Nedd4 would also inhibit Ebola VLP budding. In cells cotransfected with a WW mutant expressing only the region containing the four WW domains (Fig. 2), the culture supernatant contained significantly less virion-associated protein (<45%) than that seen with the wild-type Nedd4 plasmid (Fig. 3). The amount of released VLP was similar to that from cells expressing the VP40AAXY mutant, which disrupted L-domain function, indicating that this construct efficiently inhibited Ebola VLP budding in a dominant-negative manner.
Functional involvement of Tsg101 in VLP release.
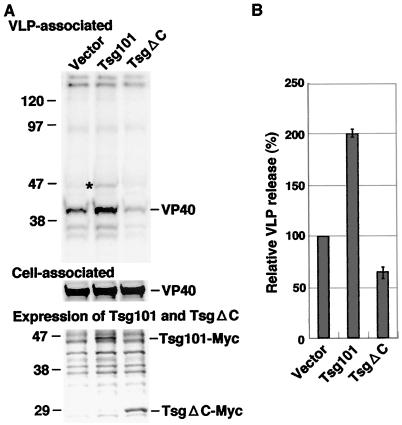
Martin-Serrano et al. have shown that the PTAP sequence within Ebola VP40 is required for interaction with Tsg101 and for efficient virion egress (20). We therefore examined the effect of overexpression of Tsg101 on Ebola VLP budding as was carried out for Nedd4. As expected, overexpression of Tsg101 enhanced Ebola VLP egress more than twofold (Fig. 4A and B). As seen with VLPs from Nedd4-expressing cells, efficient incorporation of Tsg101 was also observed in VLP from cells overexpressing Tsg101 (Fig. 1C and 4A).
FIG. 4.
Effect of overexpression of Tsg101 or its mutant on Ebola VP40-induced VLP budding. (A) Extracellular VLPs from cells cotransfected with pCEboZVP40 and either the expression plasmid for Tsg101 or the expression plasmid for TsgΔC were analyzed as in Fig. 1A (upper panel). The expression levels of VP40 (middle panel) and Tsg101 or TsgΔC (lower panel) were also determined. The position of VP40 is indicated on the right. The asterisk on the left indicates the protein detected only in VLPs from cells expressing Tsg101. (B) The intensity of the band for VLP-associated VP40 in panel A was quantified as described in Materials and Methods. The extent of VP40 in the VLPs released from cells cotransfected with pCEboZVP40 and a control vector was set to 100%. The data represent averages and SD of three independent experiments.
Tsg101 contains the UEV, proline-rich, and putative coiled-coil domains (6, 22). The N-terminal fragment of Tsg101 containing the UEV domain may act as a dominant-negative inhibitor of Ebola virus budding, since the N-terminal UEV domain interacts with the PTAP sequence (8, 20, 26). Thus, we examined whether this putative dominant-negative mutant of Tsg101 reduced Ebola VLP release. As shown in Fig. 4, TsgΔC, which expresses only the N-terminal half of the Tsg101 containing UEV domain (Fig. 2), inhibited VLP release by >40% in a dominant-negative manner, suggesting that the N-terminal region of Tsg101 is useful as a dominant-negative inhibitor, similar to the WW fragment of Nedd4.
Interaction between VP40 and Nedd4 in the cells.
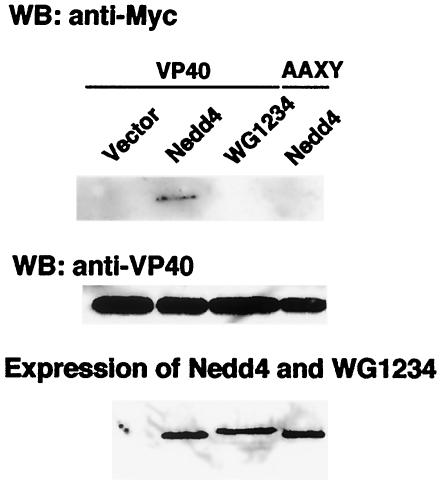
We observed the interaction between VP40 and Nedd4 in VLP (Fig. 1). To further confirm the interaction between VP40 and Nedd4 in the cells, we carried out coimmunoprecipitation analyses. COS-7 cells expressing wild-type or mutant VP40, together with Nedd4 or its mutant, were lysed with TNE buffer and then immunoprecipitated with a rabbit anti-VP40 antiserum. The immunoprecipitates were analyzed by Western blotting with either anti-Myc or anti-VP40 antibodies. As shown in Fig. 5A, wild-type Nedd4, but not WG1234, was coimmunoprecipitated with VP40 and not detected in the coimmunoprecipitate with VP40-AAXY mutant. In this experiment, the expression of Nedd4 and WG1234 mutant in the cells was similar level (lower panel), and all of the immunoprecipitated samples contained similar amounts of wild-type or AAXY VP40 (middle panel). Taken together, these data indicate that Nedd4 interacts with VP40 in cells and that the WW domains of Nedd4 and the PPxY sequence of VP40 are critically required for this interaction.
FIG. 5.
Detection of the interaction between VP40 and Nedd4 in vivo. (A) COS-7 cells were cotransfected with pCEboZVP40 or pCEboZVP40AAXY and the expression plasmid for Nedd4 or WG1234. At 48 h after transfection, cells were lysed with TNE buffer and then immunoprecipitated with rabbit anti-VP40 antiserum. (Upper panel) To examine the interaction of Myc-tagged Nedd4 or WG1234 with VP40, proteins coimmunoprecipitated with wild-type or mutant VP40 were analyzed by Western blotting with mouse anti-Myc monoclonal antibody (9E10). (Middle panel) To demonstrate that VP40 and AAXY-VP40 are present in equivalent amounts in all samples, a lower portion of the membrane in the upper panel was used for the detection of VP40 by rabbit anti-VP40 antiserum. (Lower panel) To examine the expression of Nedd4 and WG1234 in transfected cells, the cell lysates were also analyzed by Western blotting with anti-Myc antibody.
DISCUSSION
In this study, we demonstrated that overexpression of the E3 ubiquitin ligase Nedd4 apparently enhances VLP budding induced by Ebola VP40 expression. We have previously shown that a Nedd4-like protein, BUL1, positively regulates M-PMV budding as a host factor and that Nedd4 suppresses M-PMV budding (31). Therefore, these results indicate that viruses containing PPxY as an L-domain motif have a different specificity for the use of E3(s) in the process of virus budding.
The ability of Nedd4 to enhance Ebola VLP budding was completely abolished by the W-to-G substitution at all four WW domains (WG1234), suggesting, as predicted, that Nedd4 associates with the L domain of VP40 via the WW domain. Although the C894A mutant appears to be able to bind to VP40, as suggested by its incorporation into VLPs (Fig. 3A), VLP budding was suppressed by overexpression of C894A. The C894 mutant cannot accept ubiquitin from the E2 ubiquitin-conjugating enzyme (1). Thus, abolishment of Nedd4-enhanced viral budding was concomitant with the loss of ubiquitin ligase activity. It has not been examined whether VP40 is actually ubiquitinated in cells infected with Ebola viruses. However, Harty et al. previously demonstrated that Rsp5, a yeast homolog of Nedd4, could ubiquitinate Ebola VP40 in vitro (12). Ubiquitination of VP40 or cellular proteins by Nedd4 may be critical for Ebola virus budding.
The C2 domain was first identified in protein kinase C and regulates the function of proteins by mediating translocation to phospholipid membranes in response to increased cytosolic Ca2+ (10, 18). Although the function of the C2 domain in Nedd4 is unknown, the C2 domain was dispensable for Nedd4 enhancement of Ebola VLP budding.
Nedd4 and Tsg101 were efficiently incorporated into VLPs from cells overexpressing Nedd4 or Tsg101, supporting the concept that VP40 interacts with Nedd4 and Tsg101 in cells. In fact, we could confirm the interaction between VP40 and Nedd4 in cells by coimmunoprecipitation assay. Bavari et al. recently reported that assembly and budding of Ebola virus occurs at lipid rafts in the cytoplasmic membrane (2). It has also been shown that the ubiquitin ligases Cbl and Nedd4 colocalize with FcɛRI, a high-affinity receptor for immunoglobulin E (IgE), and become associated with lipid rafts after activation of IgE signaling (19). In addition, Tsg101 has an important role in the cellular Vps pathway, which coordinates the ubiquitin-mediated sorting of membrane-associated proteins through a series of endosomal compartments for eventual delivery to the lysosome (8, 22). Therefore, Tsg101 and Nedd4 may play important roles in intracellular transport by the Vps pathway and/or raft trafficking and partitioning of viral protein and cellular factors involved in virus budding.
Previous reports, along with the present study, showed that Nedd4 and Tsg101 could independently bind to VP40 (12, 20). It is also possible that Nedd4 directly interacts with Tsg101 (3) and that this interaction is required for viral budding, since Tsg101 is a UEV containing an E2-like domain. Further examination of the functional link between Nedd4 and Tsg101 and their role in virus budding is crucial for elucidating the molecular mechanisms of virus budding.
Here we also showed that small fragments of Nedd4 containing only the WW domains or Tsg101 lacking the C-terminal half functions as a dominant-negative mutant. Recently, Demirov et al. also reported that overexpression of the N-terminal domain of Tsg101 inhibits HIV-1 budding (4). Virus budding inhibitors that mimic the structures of these regions of Nedd4 and Tsg101 may have important implications for the development of antiviral strategies targeting virus budding.
Acknowledgments
We thank Kantou Nakajima and Junko Hioki for excellent technical assistance and Krisna Wells for editing the manuscript.
This work was supported by the grants from the Japan Society for the Promotion of Science, the Japan Health Sciences Foundation, the Naito Foundation, the Akiyama Foundation, the National Institute of Allergy and Infectious Diseases Public Health Service, and CREST (Japan Science and Technology Corp.).
REFERENCES
- 1.Anan, T., Y. Nagata, H. Koga, Y. Honda, N. Yabuki, C. Miyamoto, A. Kuwano, I. Matsuda, F. Endo, H. Saya, and M. Nakao. 1998. Human ubiquitin-protein ligase Nedd4: expression, subcellular localization, and selective interaction with ubiquitin-conjugating enzymes. Genes Cells 3:751-763. [DOI] [PubMed] [Google Scholar]
- 2.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter, C. A. 2002. Tsg101: HIV-1's ticket to ride. Trends Microbiol. 10:203-205. [DOI] [PubMed] [Google Scholar]
- 4.Demirov, D. G., A. Ono, J. M. Orenstein, and E. O. Freed. 2002. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. USA 99:955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman, H., and H. D. Klenk. 1996. Marburg and Ebola viruses. Adv. Virus Res. 47:1-52. [DOI] [PubMed] [Google Scholar]
- 6.Feng, G. H., C. J. Lih, and S. N. Cohen. 2000. TSG101 protein steady-state level is regulated posttranslationally by an evolutionarily conserved COOH-terminal sequence. Cancer Res. 60:1736-1741. [PubMed] [Google Scholar]
- 7.Garnier, L., J. W. Wills, M. F. Verderame, and M. Sudol. 1996. WW domains and retrovirus budding. Nature 381:744-745. [DOI] [PubMed] [Google Scholar]
- 8.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 9.Gottlimger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine. 1991. Effect of mutations affecting the p6 Gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 88:3195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey, K. F., and S. Kumar. 1999. Nedd4-like proteins: an emerging family of ubiquitin-protein ligases implicated in diverse cellular functions. Trends Cell Biol. 9:166-169. [DOI] [PubMed] [Google Scholar]
- 11.Harty, R. N., J. Paragas, M. Sudol, and P. Palese. 1999. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J. Virol. 73:2921-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harty, R. N., M. E., Brown, G. Wang, J. Huibregtse, and F. P. Hayes. 2000. A PPxY motif within the VP40 protein of ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. USA 97:13871-13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harty, R. N., M. E. Brown, J. P. Mcgettigan, G. Wang, H. R. Jayakar, J. Huibregtse, M. A. Whitt, and M. J. Schnell. 2001. Rhabdoviruses and the cellular ubiquitin-proteasome system: a budding interaction. J. Virol. 75:10623-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, M., J. M. Orenstein, M. A. Martin, and E. O. Freed. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasenosky, L. D., G. Neumann, I. Lukashevich, and Y. Kawaoka. 2001. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 75:5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayakar, H. R., K. G. Murti, and M. A. Whitt. 2000. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release. J. Virol. 74:9818-9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikonyogo, A., F. Bouamr, M. L. Vana, Y. Xiang, A. Aiyar, C. Carter, and J. Leis. 2001. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA 98:11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knopf, J. L., M. H. Lee, L. A. Sultzman, R. W. Kriz, C. R. Loomis, R. M. Hewick, and R. M. Bell. 1986. Cloning and expression of multiple protein kinase C cDNAs. Cell 46:491-502. [DOI] [PubMed] [Google Scholar]
- 19.Lafont, F., and K. Simons. 2001. Raft-partitioning of the ubiquitin ligases Cbl and Nedd4 upon IgE-triggered cell signaling. Proc. Natl. Acad. Sci. USA 98:3180-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 21.Parent, L. J., R. P. Bennett, R. C. Craven, T. D. Nelle, N. K. Krishna, J. B. Bowzard, C. B. Wilson, B. A. Puffer, R. C. Montelaro, and J. W. Wills. 1995. Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol. 69:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pornillos, O., S. L. Alam, R. L. Rich, D. G. Myszka, D. R. Davis, and W. I. Sundquist. 2002. Structure and functional interactions of the Tsg101 UEV domain. EMBO J. 21:2397-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puffer, B. A., L. J. Parent, J. W. Wills, and R. C. Montelaro. 1997. Equine infectious anemia virus utilizes a YXXL motif within the late assembly domain of the Gag p9 protein. J. Virol. 71:6541-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudol, M. 1996. Structure and function of the WW domain. Prog. Biophys. Mol. Biol. 65:113-132. [DOI] [PubMed] [Google Scholar]
- 25.Timmins, J. T., S. Scianimanico, G. Schoehn, and W. Weissenhorn. 2001. Vesicular release of Ebola virus matrix protein VP40. Virology 283:1-6. [DOI] [PubMed] [Google Scholar]
- 26.VerPlank, L., F., Bouamr, T. J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C. A. Carter. 2001. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55Gag. Proc. Natl. Acad. Sci. USA 98:7724-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills, J. W., C. E. Cameron, C. B. Wilson, Y. Xiang, R. P. Bennett, and J. Leis. 1994. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J. Virol. 68:6605-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang, Y., C. E. Cameron, J. W. Wills, and J. Leis. 1996. Fine mapping and characterization of the Rous sarcoma virus Pr76gag late assembly domain. J. Virol. 70:5695-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda, J., and E. Hunter. 1998. A proline-rich motif (PPPY) in the Gag polyprotein of Mason-Pfizer monkey virus plays a maturation-independent role in virion release. J. Virol. 72:4095-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda, J., and E. Hunter. 2000. Role of matrix protein in the type D retrovirus replication cycle: importance of the arginine residue at position 55. Virology 268:533-538. [DOI] [PubMed] [Google Scholar]
- 31.Yasuda, J., E. Hunter, M. Nakao, and H. Shida. 2002. Functional involvement of a novel Nedd4-like ubiquitin ligase on retrovirus budding. EMBO Rep. 3:636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan, B., X. Li, and S. P. Goff. 1999. Mutations altering the Moloney murine leukemia virus p12 Gag protein affect virion production and early events of the virus life cycle. EMBO J. 18:4700-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]