Abstract
Vasoactive intestinal polypeptide (VIP) is a potent vasodilator, and has been successfully used to alleviate hypertension. Consistently, disruption of VIP gene in mice leads to hypertension. However, its downstream targets in the vascular regulation are still not well demonstrated. To test the hypothesis that the vascular smooth muscle isoform of KATP channels is a downstream target of the VIP signaling, we performed the studies on the Kir6.1/SUR2B channel expressed in HEK293 cells. We found that the channel was strongly activated by VIP. Through endogenous VIP receptors, the channel activation was reversible and dependent on VIP concentrations with the midpoint-activation concentration ∼10 nM. The channel activation was voltage-independent and could be blocked by KATP channel blocker glibenclamide. In cell-attached patches, VIP augmented the channel open-state probability with modest suppression of the single channel conductance. The VIP-induced Kir6.1/SUR2B channel activation was blocked by PKA inhibitor RP-cAMP. Forskolin, an adenylyl cyclase activator, activated the channel similarly as VIP. The effect of VIP was further evident in the native tissues. In acutely dissociated mesenteric vascular smooth myocytes, VIP activated the KATP currents in a similar manner as in HEK293 cells. In endothelium-free mesenteric artery rings, VIP produced concentration-dependent vasorelaxation that was attenuated by glibenclamide. These results therefore indicate that the vascular isoform (Kir6.1/SUR2B) of KATP channels is a target of VIP. The channel activation relies on the PKA pathway and produces mesenteric arterial relaxation.
Keywords: VIP, K+ channel, antagonist, second messenger, vascular tones
Introduction
Vasoactive intestinal polypeptide (VIP) is a 28-amnio-acid peptide hormone and neurotransmitter present in multiple organs and systems. VIP has broad effects on cellular functions including vasodilation, water reabsorption, neurotransmission, insulin secretion and immunomodulation [1-3]. These biological effects are mediated by specific VIP receptors (VPAC1 and VPAC2), both of which are coupled to G-proteins, primarily the GS proteins [4].
As a potent vasodilator, VIP containing nerve terminals innervate a variety of blood vessels in systemic and pulmonary circulations [5]. VIP released from the nerve terminals produces vascular smooth muscle relaxation. Such a vasodilation effect is 50−100 times more potent than acetylcholine [5]. Administration of VIP to patients with severe cardiovascular diseases such as primary pulmonary hypertension results in substantial improvement in their conditions without adverse side-effects [6]. Moderate pulmonary arterial hypertension has been observed in mice lacking the VIP gene [7,8]. By taking the advantage of the vasodilation effects, several species of animals have developed VIP-like peptides that are lethal vasodilatory toxins serving for defense and predatory purposes [9-11].
The downstream molecules of VIP in the vasodilation effect are still not fully understood [5]. In the vascular smooth muscle cells, the influx of Ca++ through voltage-dependent Ca++ channels contributes to vessel constriction, while the preclusion of this event leads to the vasodilation [12,13]. The opening and closure of these Ca++ channels is largely controlled by membrane potentials. Activation of the vascular KATP channels hyperpolarizes the vascular smooth muscle cells, prevents the Ca++ influx, and relaxes blood vessels. Therefore, the vascular KATP channels play an important role in vascular tone regulation [14].
Functional KATP channels are made of four pore-forming subunits Kir6.x (Kir6.1 or Kir6.2) and four regulatory subunits sulfonylurea receptor SURx (SUR1, SUR2A or SUR2B) [15]. The former belongs to the inwardly rectifying K+ channels, and the latter is a member of the ATP-binding cassette (ABC) protein family [16-18]. A combination of deferent Kir6.x and SURx results in distinct KATP channels, such as the Kir6.2/SUR1 channel in pancreatic β-cells and the Kir6.2/SUR2A channel in cardiac muscles [19]. The Kir6.1/SUR2B channel is the major isoform of KATP channels in vascular smooth muscles, although there is evidence that the Kir6.2 also form functional channels with SUR2B in blood vessels [20-22]. The biophysical and pharmacological properties of the Kir6.1/SUR2B channel are comparable to those of KNDP channels found in native coronary and mesenteric arteries [23,24]. Consistently, disruptions of the Kcnj8 (Kir6.1) or ABCC9 (SUR2) genes in mice cause abnormalities in coronary circulation, sudden cardiac death and fatal susceptibility to endotoxemia [25-27].
Previous studies have suggested that the vascular KATP channels are subjected to phosphorylation regulation by protein kinases A, C and G (PKA, PKC, PKG), allowing them to respond to several vasoactive hormones and neurotransmitters [28-30, 31,32]. Since the PKA signaling system can be activated by VIP, it is possible that the vascular smooth muscle KATP channels play a role in vasodilation effect of VIP. To test the hypothesis, we performed these studies on the Kir6.1/SUR2B channel expressed in HEK293 cells, cell-endogenous KATP channels from dissociated smooth myocytes and isolated mesenteric arterial rings.
Methods and materials
Expression Kir6.1/Sur2B channel in HEK293 cells
Human embryonic kidney cells (HEK293) were used for expression of Kir6.1/SUR2B channel. The HEK293 cells were cultured in DMEM/F12 medium with 10% fetal bovine serum and Penicillin/streptomycin at 37°C with 5% CO2. A eukaryotic expression vector pcDNA3.1 containing Rat Kir6.1 (GenBank No. D42145) or Sur2B cDNAs (GenBank No. D86038, mRNA isoform NM_011511) was co-transfected to the cells. A 35 mm petri dish of cells was transfected with 1 μg Kir6.1 and 3 μg SUR2B using Lipofectamine2000 (Invitrogen Inc., Carlsbad, CA). To facilitate the identification of positively transfected cells, 0.5 μg green fluorescent protein (GFP) cDNA (pEGFP-N2, Clontech, Palo Alto, CA) was included in the cDNA mixture. 24h after transfection, cells were disassociated with 0.25% trypsin, split and transferred to cover slips. Experiments were performed on the cells in the following 6−48h.
Electrophysiology
Patch clamp experiments were carried out at room temperature as described previously [31-33]. In brief, fire-polished patch pipettes with 2−5 MΩ resistance were made from 1.2 mm borosilicate glass capillaries. Whole-cell currents were recorded in single-cell voltage clamp using the Axopatch 200B amplifier (Axon Instruments Inc., Foster City, CA), low-pass filtered (2 kHz, Bessel 4-pole filter, −3 dB), and digitized (10 kHz, 16-bit resolution) with Clampex 9 (Axon Instruments Inc.). Data was analyzed using Clampfit 9 (Axon Instruments Inc.). The bath solution contained (in mM): KCL 10, potassium gluconate 135, EGTA 5, glucose 5, and HEPES 10 (pH=7.4). The pipette was filled with a solution containing: KCl 10, potassium gluconate 133, EGTA 5, glucose 5, K2ATP 1, NaADP 0.5, MgCl21, and HEPES 10 (pH=7.4). To avoid nucleotide degradation, all intracellular solutions were freshly made and used within 4 hrs.
All reagents and chemicals were purchased from Sigma unless otherwise stated. Pinacidil and glibenclamide were prepared as stock solution of 10 mM in DMSO. VIP was prepared in 1% acetic acid (v/v). All solvents were tested and showed no detectable effect on the KATP channels.
Mesenteric artery preparation and tension measurement
All animal experiments were performed in compliance with an approved protocol by the Institutional Animal Care and Use Committees (IACUC) at Georgia State University. Male Sprague-Dawley rats (200−250g body weight) were deeply anesthetized followed by decapitation. Mesenteric arteries were dissected free and placed in PSS containing (in mM): NaCl 140, KCl 4.6, CaCl2 1.5 MgCl2 1, glucose 10, HEPES 5, pH 7.3. The arteries were cut into small rings (2 mm in length) and transferred to ice-cold Krebs solution containing: NaCl 118.0, NaHCO3 25.0, KCl 3.6, MgSO4 1.2, KH2PO4 1.2, glucose 11.0, CaCl2 2.5. The endothelium-free rings were prepared by rubbing with a sanded polyethylene tubing, and confirmed with vasodilation response to acetylcholine (ACh) as described previously [31].The arterial ring was mounted on a force-electricity transducer (Model FT-302, iWorx/CBSciences, Inc. Dover, NH) for measurements of isometric force contraction in a 5-ml tissue bath filled with the air bubbled Krebs solution. All rings were pre-tested with phenylephrine (PE) to ensure the tissue vitality. When endothelium needed to be removed, the rings were tested by PE for contraction followed by an exposure to ACh (1 μM). The rings were considered to be endothelium-free if more than 90% relaxation was eliminated. PE and ACh then were washed out, and the rings were allowed to equilibrate in the Krebs solution for another 30−60 min before experiments.
Acute dissociation of mesenteric vascular smooth cells
Single vascular smooth cells were prepared with two-step enzyme digestions. Main branch of mesenteric arteries were obtained as mentioned above. After clearance of connective tissue, 1−2 mm small segments were cut and placed in 5ml solution containing (in mM): NaCl 140, KCl 5.4, MgCl2 1, CaCl2 0.1, HEPES 10 and D-glucose 10 for 10 min in room temperature. The tissues were then placed in 1ml of the same solution with 20U of papain (Worthington, New Jersey), 1.25mg dithiothreitol (DTT) and 1% fetal bovine serum. After 25min digestion at 35°C, the tissues were washed and incubated with 440U collagenase (CLS II, Worthington), 1.25mg trypsin inhibitor (Sigma) and 1% fetal bovine serum for 10 min. After thorough washes, the tissues were triturated with a fire-polished Pasteur pipette to yield single cells. The dissociated smooth muscle cells were placed in a petri dish and allowed to attach to the dish surface before recordings. Patch clamp experiments were carried out in the cells that show clear smooth muscle morphology, and had no sign of swelling and shrinkage
Data analysis
Data were presented as means ± s.e. (standard error). Differences were evaluated using Student t-tests or ANOVA, and statistical significance was accepted if P<0.05
Result
1. Activation of the Kir6.1/SUR2B channel by VIP in HEK293 cells
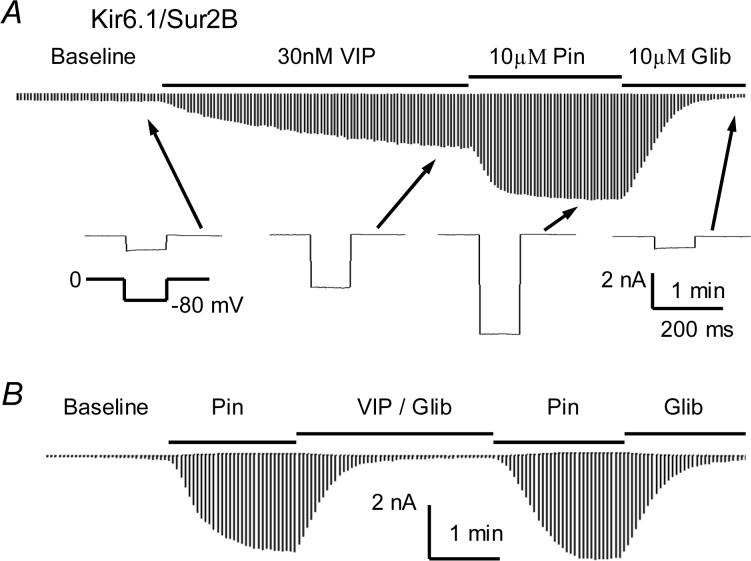
The Kir6.1/SUR2B channel was transiently expressed in the HEK293 cells, and whole cell voltage-clamp was performed on the GFP-positive cells. The cell was exposed to an extracellular perfusion solution after whole-cell configuration formation. Most cells showed small stable baseline currents, and the currents in some cells increased slightly reaching a steady state within a few minutes (Fig.1A). When VIP (30 nM) was applied to the cell in the perfusion solution, the whole-cell currents increased rapidly and reached a plateau in about 2−3 min. KATP channel opener pinacidil (Pin, 10 μM) strongly augmented the currents that was subsequently inhibited by glibenclamide (Glib, 10 μM) (Fig. 1A). Thus we used 10 μM Pin and 10 μM Glib throughout the study unless otherwise stated. Another KATP channel opener, diazoxide (100 μM), opened the channel to the similar degree as Pin, and its effect was also totally blocked by Glib (Online Figure 1). The effect of VIP was completely blocked in the presence of 10 μM Glib suggesting that Kir6.1/SUR2B channel is targeted by VIP (Fig. 1B). For quantitative analysis, the effect of VIP was normalized between the baseline current and the current activated by 10 μM Pin. Under such a condition, the currents activated by 30 nM VIP averaged 58.0±3.4% (n=5).
Fig 1.
Activation of the Kir6.1/SUR2B channel by VIP in HEK293 cells. A. Whole-cell currents were recorded from a cell transfected with Kir6.1/SUR2B. The bath solutions contained 145 mM K+ so that the reversal potential of K+ currents is close to 0 mV. The recording pipette was filled with the same solution with the addition of 1 mM ATP, 0.5 mM ADP, and 1 mM free Mg++. Application of VIP (30 nM) increased the whole-cell currents rapidly and reached a plateau in about 3 min. KATP channel opener pinacidil (Pin, 10 μM) strongly augmented the currents that was potently inhibited by glibenclamide subsequently (Glib, 10 μM). Note that arrows point to where each bottom trace was taken from. B. The effect of VIP was totally blocked in the presence of 10 μM Glib.
Several control experiments were done. The outward currents studied together with the inward currents using depolarizing (−80 mV) and hyperpolarizing (80 mV) command pulses were affected by VIP and other K+ channel blockers in a similar proportion as the inward currents (Online Figures 1 and 2). Both the Pin-activated inward and outward currents were insensitive to 30 μM 4-aminopyridine (4-AP) and 100 nM charybdotoxin (ChTX), specific blockers of Kv and BK channels, respectively, but inhibited by 1 mM tetrabutylammonium (TBA) (Online Figure 2). Finally, the Pin- and Glib-sensitive currents were not observed in cells transfected with the expression vector alone (Fig.3B), indicating that the VIP-elicited current is conducted through KATP channels.
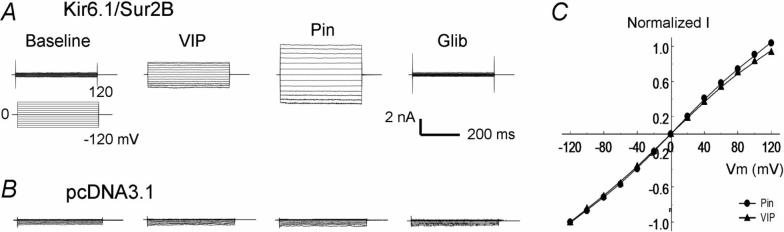
Fig 3.
Voltage independence. A. Voltage dependence was studied with step command pulses from −120mV to 120mV from a holding potential of 0 mV. The current activation by VIP was observed across the whole voltage range in the HEK cell. B. Similar current activation was not seen in another cell transfected with the vector alone. C. The I-V relationship of VIP and Pin was plotted by normalized to the current at −120 mV. The I-V plots superimposed with each other almost completely indicating the effect of VIP is not voltage-dependent.
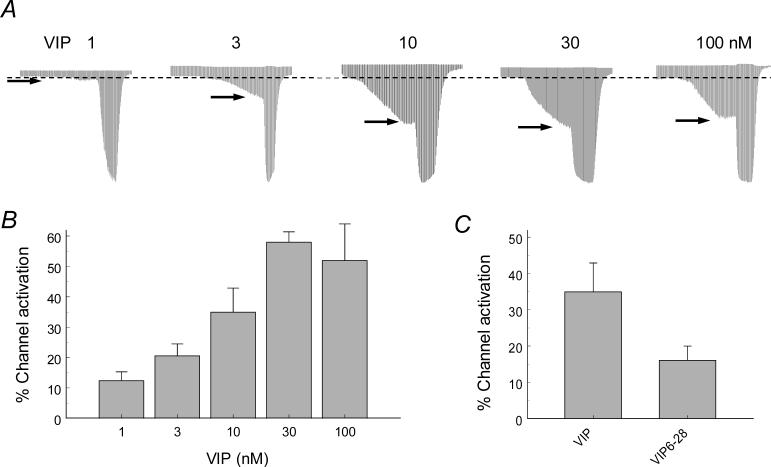
The activation of the Kir6.1/SUR2B channel by VIP showed clear concentration-dependence. Evident increase in the current amplitude was observed with the VIP concentration as low as 1 nM, and stronger activation occurred with higher concentrations (Fig. 2A). The maximum effect (58.0±3.4%) was reached with 30 nM VIP (Fig. 2B).
Fig 2.
A-B. The activation of the Kir6.1/SUR2B channel by VIP was concentration-dependent with VIP concentration for a half current activation ∼10nM. C. The effect of VIP (10 nM) was significantly diminished in the presence of VIP receptor blocker VIP6−28 (100 nM).
Previous studies have shown that the VIP receptor VPAC1 is constitutively expressed in HEK293 cells [34,35]. We found that the Kir6.1/SUR2B channel was activated by VIP when expressed in HEK293 cells without exogenous VIP receptors. Such a channel activation was significantly diminished by the VIP receptor antagonist VIP6−28 (16.1±3.9% 10 nM VIP with the presence of VIP 6−28 vs 35.0±7.9% 10 nM VIP without VIP 6−28; P<0.05, n=15) (Fig. 2C). When the HEK293 cells were transfected with the VPAC2 receptor (the VPAC1 receptor cDNA is not commercially available), VIP (100 nM) did not show any significant additional effect on the Kir6.1/SUR2B channel (49.2±4.5% with the VPAC2 receptor vs. 52.8±3.4% without; P>0.05, n=7). These results suggest that the endogenous VIP receptors in the HEK293 cells mediate the VIP effect, consistent with previous observations on the presence of endogenous VIP receptors in HEK293 cells [34].
2. Biophysical basis for the Kir6.1/SUR2B channel activation
When step voltage protocols were applied, the current activation by VIP was observed across the whole voltage range from −120 mV to 120 mV (Fig.3A). The I-V relationship of the VIP-activated and Pin-activated currents was normalized to the current amplitude at −120 mV and plotted in the same X-Y axis system. Under the condition, the two I-V plots superimposed with each other nicely, indicating that the current activation by VIP is voltage-independent (Fig. 3B,C). Such currents were not seen in cells transfected with the expression vector alone (Fig. 3D).
To understand the biophysical basis for the Kir6.1/SUR2B channel activation, single-channel recordings were performed in cell-attached patches. Exposure to VIP (100 nM) augmented the channel open-state probability (NPo 0.005±0.002 at baseline, 0.048±0.006 with VIP, and 0.116±0.017 with Pin, n=5) (Fig. 4A). The single-channel conductance was slightly inhibited by VIP (34.9±0.9pS before vs. 31.7±1.7pS after VIP treatment, P<0.05, n=10; Fig. 4B,C). At baseline, the closed time was 164.7±74.1 ms (n=6), the level-1 open time was 1.77±0.53 ms (n=6), and the level 2 open time was 0.73±0.19 ms (n=6). During the VIP exposure, the closed time was reduced to 59.1±7.6 ms (n=7, P<0.05), while the level 1 open time (2.28±0.45 ms, n=7) and level 2 open time (1.06±0.15 ms, n=7) did not change significantly, indicating that VIP increases the NPo via suppression of the closed time.
Fig 4.
Effect of VIP on single-channel properties. A. Single-channel currents were recorded in a cell-attached patch. The lower trace in each panel is an expansion from the record of upper trace between arrows. Two active channels were seen at baseline with rather low channel activity (NPo 0.001). When the cell was exposed to 100nM VIP, the single-channel activity was augmented (NPo 0.028). The channel activity was further stimulated with 10μM (NPo 0.152). B. Single channel conductance was measured in a cell-attached patch from another cell with a ramp voltage from −100 to 100 mV. An active channel was observed with 100 nM VIP. The straight line represents a slope conductance of 36pS. C. The slope conductance increased modestly, and an additional active channel was recruited in the presence of pinacidil. The slope conductance is 40pS for both.
3. PKA dependence
Previous studies have shown that KATP channels are regulated by both PKA and PKC [28,29,36,37]. VIP receptors (VPAC1 and VPAC2) are primarily coupled to GS, stimulation of which can lead to activation of PKA pathway [4,38]. To elucidate whether the activation of the Kir6.1/SUR2B channel by VIP depends on the PKA pathway, 8-(4-chlorophenylthio) adenosine-3′,5′-cyclic monophosphorothioate Rp-isomer (RP-cAMP), a PKA inhibitor, was applied in both the pipette solution (200μM) and in perfusion solution (100μM). In the presence of RP-cAMP, the current activation by 100 nM VIP was significantly reduced to 11.9±6.9% (P<0.01, n=10 in comparison to control) (Fig. 5A,E).
Fig 5.
PKA dependence of the Kir6.1/SUR2B channel activation by VIP. A. RP-cAMP, a potent PKA inhibitor, was applied in both the pipette solution (200 μM) and the perfusion solution (100 μM). The VIP-activated Kir6.1/SUR2B currents were greatly attenuated in the presence of RP-cAMP. B. The Kir6.1/SUR2B channel was activated by 10 μM forskolin, an adenylyl cyclase activator. C. The forskolin-activated current had similar characteristics as the VIP-activated current showing no evident voltage-dependence. D. After the currents were activated by VIP, forskolin had no additional effect. E. Summary of VIP effects in the presence or absence of RP-cAMP and forskolin. The VIP-induced channel activation was significantly reduced in the presence of RP-cAMP (**, P<0.01, n=10). Forskolin and VIP did not produce any significant additional activation in comparison to the effect of VIP alone.
As the adenylyl cyclase is activated by GS, we studied the Kir6.1/SUR2B currents with forskolin, a potent adenylyl cyclase activator. Exposure of the cell to 10 μM forskolin strongly activated the Kir6.1/SUR2B channel. Such an effect was not significantly different from the channel activation by 100 nM VIP (P>0.05, Fig. 5B,E). The forskolin-activated currents also showed identical characteristics to the current activated by VIP in their I-V relationship (Fig. 5D in comparison with Fig. 3A). After the currents were activated by VIP, forskolin had no additional effect (Fig. 5C,E), further suggesting that the VIP and forskolin share the same intracellular signaling pathway in regulating the channel activity.
4. Effects of VIP on cell-endogenous KATP channels in vascular smooth myocytes
The effect of VIP on cell-endogenous KATP channels of the vascular smooth myocytes (VSM) dissociated acutely from mesenteric arterials. Under the same recording condition as for the HEK293 cells, the inward K+ currents were recorded from the VSMs. The VSM-endogenous current had single-channel conductance 34.8±1.1 (n=4) similar to the Kir6.1/SUR2B current expressed in HEK cells (34.9±0.9, n=11). The currents were activated with the treatment of 100 nM VIP, further augmented by Pin (10 μM), and subsequently inhibited by Glib (10 μM) (Fig. 6), consistent with the Kir6.1/SUR2B channel activation observed in HEK293 cells.
Fig 6.
Activation of endogenous KATP channels in vascular smooth myocytes. A. K+ currents were recorded in the same condition as in Fig. 1. The currents were augmented with an exposure to 100 nM VIP. The currents were further activated by Pin and inhibited by Glib. B. A acutely dissociated vascular smooth myocyte with the recording pipette on the right side. C. The current amplitude increased significantly with VIP exposure. Data are presented as mean ± s.e. (n=4). **, P=0.01.
To further understand whether the regulation of KATP channel by VIP affects vascular tone, we studied isometric contractions of endothelium-free mesenteric artery rings. Administration of phenylephrine (PE) led to a rapid contraction of the arterial rings which lasted over 30 min without clear desensitization. The failure of 1 μM carbamylcholine to dilate the ring after contraction with PE was the evidence of functional endothelial ablation (Fig.7A). When VIP was applied, the rings relaxed in a concentration-dependent manner (Fig. 7B,D). Such a VIP-induced vasorelaxation was markedly attenuated by a pretreatment with 1 μM Glib (Fig. 7C,D), indicating that the KATP channels activation play an important role in the VIP-induced relaxation.
Fig 7.

Involvement of KATP channel-dependent in the VIP-induced vasorelaxation. A. The effect of VIP was studied in an endothelium-free mesenteric arterial ring in-vitro. Administration of 1 μM phenylephrine (PE) led to a strong and persistent contraction of the arterial ring. A subsequent exposure to 1 μM carbamylcholine relaxed the ring only slightly. B. The application of VIP in the presence of PE dilated the artery rings in a concentration-dependent manner. C. Such vasorelaxation effect was markedly attenuated by a 30min pretreatment with 1 μM glibenclamide (Glib). D. Summary of the vasodilation effect of VIP on PE-induced vasoconstriction in the presence or the absence of Glib. Data are presented as mean ± s.e. (n=7). *, P<0.05; ***, P<0.001.
Discussion
Our studies have shown that the Kir6.1/SUR2B channel is a downstream target of VIP. The channel is strongly activated by VIP with the midpoint concentration for the channel activation ∼10 nM. The channel activation is likely mediated through the VIP receptor, adenylyl cylcase and PKA signaling system. Such KATP channel activation tends to hyperpolarize the cell and relaxes the vascular smooth muscle, which is consistent with our data showing that VIP produces KATP channel-dependent relaxation of the mesenteric arteries.
Several potential downstream effector molecules have been suggested for the vasodilation effect of VIP. In the isolated perfused rat heart, selective activation of VIP receptors produces vasodilation of the coronary circulation. Such a vasodilation effect can be blocked by glibenclamide but not 4-aminopyridine, suggesting that KATP channels are involved [39, 40 ]. Another studies suggest that the vasodilation effect of VIP is mediated through glibenclamide-insensitive channels [41,42]. Clearly, VIP may affect vascular tones via multiple mechanisms that remain to be demonstrated, as these previous studies relied on sulfonylureas that are known to affect other ion channels as well (43, 44). Using the cloned channel in the HEK expression system, we have revealed that Kir6.1/SUR2B channel is one of the downstream molecules. In the mesenteric VSMs, activation of Maxi-K channels also contributes to the vasorelaxation response to VIP, in which the cAMP-dependent signaling pathways seem to be involved [45]. In the gastrointestinal system, VIP can produce relaxation of the sphincter of Oddi which can be attenuated by glibenclamide [46]. Consistent with these previous studies, our results show that VIP relaxes isolated mesenteric rings, an effect that can be blocked by glibenclamide. Our studies in a heterologous expression system have indicated that the cloned Kir6.1/SUR2B channel indeed is activated by VIP. As the Kir6.1/SUR2B channel is expressed in vascular smooth muscles [33,47,48], and as the channel protein may be phosphorylated by PKA [28,29], it is likely that that the activation of the Kir6.1/SUR2B channel contributes to the vasodilation effect of VIP.
The KATP channels play an important role in membrane potentials and cell activity [15,17]. These K+ channels are inhibited by physiological concentrations of ATP, and are open when ATP level drops during metabolic stress. Such a property allows them to regulate several cellular functions during metabolic stress, including vascular tone regulation, myocardium excitability control, neuronal responses to hypoxic ischemia, insulin-secretion in pancreatic β-cells, and glucose uptake in striated muscles [15,17,19]. The Kir6.1/SUR2B channel is likely to be the major isoform of KATP channels in vascular smooth muscles, whose pharmacological properties resemble those of the native KNDP channels in vascular smooth myocytes [49-51]. The involvement of Kir6.1/SUR2B in vascular tone regulation has been demonstrated in both physiological and pathological conditions. Activation of these KATP channels in response to local regulators hyperpolarizes vascular smooth muscles and dilates resistance arteries leading to redistribution of the blood flow [15], while the disruption of the Kcnj8 (Kir6.1) or ABCC9 (SUR2) genes in mice causes abnormalities in coronary circulation, sudden cardiac death and fatal susceptibility to endotoxemia [25-27]. The vascular KATP channel is regulated by a variety of vasoactive substances especially circulating hormones and neurotransmitters. Several studies have suggested that the regulation of the vascular KATP channel is achieved via phosphorylation of the channel proteins by protein kinases that are activated through specific intracellular singling pathways downstream to the VIP receptors [52,53]. Therefore, the understanding of the KATP channel function in vascular tone control depends on the demonstration of the precise signal pathway underlying the channel modulation.
Several other intracellular signaling pathways may be involved in the vasodilation effect of VIP. The VIP effect has been shown to rely on the VPAC2 receptor in the coronary circulation [39]. Because of the lack of specific receptor blockers, we could not differentiate VPAC1 from VPAC2 in our studies. According to previous studies, the VPAC1 is expressed in the HEK293 cells [34,35], suggesting that the Kir6.1/SUR2B channel activation may be produced by VPAC1. However, we cannot rule out the involvement of VPAC2 as both VPAC1 and VPAC2 receptors are coupled to GS leading to activation of adenylyl cyclase and PKA.
Although the VIP receptor - adenylyl cylcase – PKA system seems critical, VIP also can activate PKG pathway [54,56]. In addition, the VIP-induced relaxation of aorta and uterine arteries has been shown to be produced by nitric oxide (NO) released from the endothelial cells [56,57]. Another study however suggests that NO synthesis is not affected by VIP, while stimulation of endogenous NO production provokes VIP release from nerve terminals [53]. Since endothelium-free arterial rings were used, our results from the present study suggest that the effect of VIP on vasorelaxation is NO-independent. Because of the presence of these diverse signaling molecules, distinct intracellular signaling pathways appear to play a role in the VIP signaling targeting at perhaps different effector molecules. Thus, the demonstration of the adenylyl cyclase – PKA – Kir6.1/SUR2B pathway in the present study is of significance in the understanding the vascular effect of VIP.
In conclusion, our results indicate that the Kir6.1/SUR2B channel is a downstream target of VIP signaling. The activation of the major vascular isoform of KATP channels can produce hyperpolarization and relaxation of vascular smooth muscles, which is consistent with the effect of VIP on mesenteric arteries. The information that we have found for the VIP signaling pathway may be useful for the manipulation of specific membrane and intracellular signal molecules in the control of vascular tones.
Acknowledgments
The authors are grateful to Dr. S. Seino and Y. Kurachi for their gifts of the Kir6.1 and SUR2B cDNAs, respectively. This work was supported by the NIH (HL067890) and Georgia State University Research Program Enhancement Fund. SG, SZ and DZ were supported by National Natural Science Foundation of China (Grants 30370578, 30470752).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Online Figure 1. Activation of the Kir6.1/SUR2B channel by diazoxide (DZX) and pinacidil in HEK293 cells. A. DZX (100 μM) activated the Kir6.1/SUR2B channel similarly as 10 μM Pin, which was also inhibited by 10 μM Glib. B. Summary of the currents activated by DZX and Pin. Data are presented as mean ± s.e. (n=3).
Online Figure 2. The effect of different K+ channel blockers on K+ currents recorded in HEK293 cells. A. The pretreatment of 4-aminopyridine did not block the effect of VIP- and diazoxide (DZX)-induced currents. B. Pin-induced K+ currents were insensitive to charybdotoxin. C. TBA (non-selective K+ channel blocker) partially blocked the Pin-induced K+ currents. D. Summary of effects of the K+ channel blockers. E. The VIP-induced channel activation was not blocked by 4-AP treatment. Data are presented as mean ± s.e. (n=3∼5).
Reference List
- 1.Delgado M, Abad C, Martinez C, Laceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nature Medicine. 2001;7:563. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 2.Straub SG, Sharp GWG. A wortmannin-sensitive signal transduction pathway is involved in the stimulation of insulin release by vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide. J. Biol. Chem. 1996;271:1660. doi: 10.1074/jbc.271.3.1660. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsumi M, Claus TH, Liang Y, Li YX, Yang L, Zhu J, Dela Cruz F, Peng XB, Chen HX, Yung SL, Hamren S, Livingston JN, Pan CQ. A potent and highly selective VPAC2 agonist enhances glucose-induced insulin release and glucose disposal - A potential therapy for type 2 diabetes. Diabetes. 2002;51:1453. doi: 10.2337/diabetes.51.5.1453. [DOI] [PubMed] [Google Scholar]
- 4.Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regulatory Peptides. 2002;108:165. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 5.Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovascular Research. 2001;49:27. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 6.Petkov V, Mosgoeller W, Ziesche R, Raderer M, Stiebellehner L, Vonbank K, Funk GC, Hamilton G, Novotny C, Burian B, Block LH. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. Journal of Clinical Investigation. 2003;111:1339. doi: 10.1172/JCI17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamidi SA, Szema AM, Lyubsky S, Dickman KG, Degene A, Mathew SM, Waschek JA, Said SI. Clues to VIP function from knockout mice, Vip, Pacap, and Related Peptides: From Gene to Therapy. 2006;1070:5. doi: 10.1196/annals.1317.035. [DOI] [PubMed] [Google Scholar]
- 8.Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal Peptide gene. Circulation. 2007;115:1260. doi: 10.1161/CIRCULATIONAHA.106.681718. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino M, Yanaihara C, Hong YM, Kishida S, Katsumaru Y, Vandermeers A, Vandermeers-Piret MC, Robberecht P, Christophe J, Yanaihara N. Primary structure of helodermin, a VIP-secretin-like peptide isolated from Gila monster venom. FEBS Lett. 1984;178:233. doi: 10.1016/0014-5793(84)80607-9. [DOI] [PubMed] [Google Scholar]
- 10.Blank MA, Brown JR, Hunter JC, Bloom SR, Tyers MB. Effects of VIP and related peptides and Gila monster venom on genitourinary smooth muscle. Eur. J. Pharmacol. 1986;132:155. doi: 10.1016/0014-2999(86)90600-x. [DOI] [PubMed] [Google Scholar]
- 11.Pohl M, Wank SA. Molecular cloning of the helodermin and exendin-4 cDNAs in the lizard. Relationship to vasoactive intestinal polypeptide/pituitary adenylate cyclase activating polypeptide and glucagon-like peptide 1 and evidence against the existence of mammalian homologues. J. Biol. Chem. 1998;273:9778. doi: 10.1074/jbc.273.16.9778. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson H. Interactions between membrane potential and intracellular calcium concentration in vascular smooth muscle. Acta Physiologica Scandinavica. 1998;164:559. doi: 10.1046/j.1365-201X.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 13.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Signal transduction in smooth muscle - Invited review: Arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. Journal of Applied Physiology. 2001;91:973. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- 14.Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clinical and Experimental Pharmacology and Physiology. 2002;29:312. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- 15.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiological Reviews. 1997;77:1165. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 16.Babenko AP, Aguilar-Bryan L, Bryan J. A view of SUR/Kir6.X, KATP channels. Annual Review of Physiology. 1998;60:667. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 17.Seino S. ATP-sensitive potassium channels: A model of heteromultimeric potassium channel/receptor assemblies. Annual Review of Physiology. 1999;61:337. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- 18.Deeley RG, Westlake C, Cole SPC. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiological Reviews. 2006;86:849. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 19.Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. American Journal of Physiology-Cell Physiology. 1998;43:C25–C37. doi: 10.1152/ajpcell.1998.274.1.C25. [DOI] [PubMed] [Google Scholar]
- 20.Cao K, Tang GH, Hu DH, Wang R. Molecular basis of ATP-sensitive K+ channels in rat vascular smooth muscles. Biochemical and Biophysical Research Communications. 2002;296:463. doi: 10.1016/s0006-291x(02)00892-6. [DOI] [PubMed] [Google Scholar]
- 21.Jansen-Olesen I, Mortensen CH, El Bariaki N, Ploug KB. Characterization of KATP-channels in rat basilar and middle cerebral arteries: Studies of vasomotor responses and mRNA expression. European Journal of Pharmacology. 2005;523:109. doi: 10.1016/j.ejphar.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Tricarico D, Mele A, Lundquist AL, Desai RR, George AL, Jr., Conte CD. Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc. Natl. Acad. Sci. U. S A. 2006;103:1118. doi: 10.1073/pnas.0505974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Bolton TB. Activation by Intracellular Gdp, Metabolic Inhibition and Pinacidil of A Glibenclamide-Sensitive K-Channel in Smooth-Muscle Cells of Rat Mesenteric-Artery. British Journal of Pharmacology. 1995;114:662. doi: 10.1111/j.1476-5381.1995.tb17190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang HL, Bolton TB. Two types of ATP-sensitive potassium channels in rat portal vein smooth muscle cells. British Journal of Pharmacology. 1996;118:105. doi: 10.1111/j.1476-5381.1996.tb15372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chutkow WA, Pu JL, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. Journal of Clinical Investigation. 2002;110:203. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nature Medicine. 2002;8:466. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 27.Kane GC, Lam CF, O'Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. Gene knockout of the KCNJ8-encoded Kir6.1 KATP channel imparts fatal susceptibility to endotoxemia. Faseb Journal. 2006;20:2271. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- 28.Quinn K, Giblin JP, Tinker A. Protein kinase C regulates Kir6.1/SUR2B current but not Kir6.2/SUR2B current in stably transfected HEK293 cells. Journal of Physiology-London. 2001;536:20P. [Google Scholar]
- 29.Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circulation Research. 2004;94:1359. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 30.Thorneloe KS, Maruyama Y, Malcolm AT, Light PE, Walsh MP, Cole WC. Protein kinase C modulation of recombinant ATP-sensitive K+ channels composed of Kir6.1 and/or Kir6.2 expressed with SUR2B. Journal of Physiology-London. 2002;541:65. doi: 10.1113/jphysiol.2002.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C. Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol. 2007;293:R191–R199. doi: 10.1152/ajpregu.00047.2007. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007:00337. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XR, Wu JP, Li L, Chen FX, Wang RP, Jiang C. Hypercapnic acidosis activates KATP channels in vascular smooth muscles. Circulation Research. 2003;92:1225. doi: 10.1161/01.RES.0000075601.95738.6D. [DOI] [PubMed] [Google Scholar]
- 34.Simmons NL. A cultured human renal epithelioid cell line responsive to vasoactive intestinal peptide. Exp. Physiol. 1990;75:309. doi: 10.1113/expphysiol.1990.sp003406. [DOI] [PubMed] [Google Scholar]
- 35.Reubi JC. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues - Clinical implications, Vip, Pacap, Glucagon, and Related Peptides. 2000;921:1. doi: 10.1111/j.1749-6632.2000.tb06946.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. Embo Journal. 2000;19:942. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human KATP channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J. 1999;18:4722. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shreeve SM. Identification of G-proteins coupling to the vasoactive intestinal peptide receptor VPAC1 using immunoaffinity chromatography: Evidence for precoupling. Biochemical and Biophysical Research Communications. 2002;290:1300. doi: 10.1006/bbrc.2002.6342. [DOI] [PubMed] [Google Scholar]
- 39.Sawmiller DR, Ashtari M, Urueta H, Leschinsky M, Henning RJ. Mechanisms of vasoactive intestinal peptide-elicited coronary vasodilation in the isolated perfused rat heart. Neuropeptides. 2006;40:349. doi: 10.1016/j.npep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Horikawa N, Ishiro H, Kataha K, Nakazawa T, Watanabe N, Ishii K, Nakayama K, Yanaihara N, Shigenobu K. Glibenclamide-sensitive mechanism is involved in helodermin-produced vasodilatation in rat mesenteric artery. Research Communications in Molecular Pathology and Pharmacology. 1997;98:141. [PubMed] [Google Scholar]
- 41.Hattori Y, Nagashima M, Endo Y, Kanno M. Glibenclamide Does Not Block Arterial Relaxation Caused by Vasoactive Intestinal Polypeptide. European Journal of Pharmacology. 1992;213:147. doi: 10.1016/0014-2999(92)90246-z. [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki J, Kobayashi S, Miyagi Y, Nishimura J, Fujishima M, Kanaide H. The mechanisms of the relaxation induced by vasoactive intestinal peptide in the porcine coronary artery. British Journal of Pharmacology. 1997;121:977. doi: 10.1038/sj.bjp.0701206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishida-Takahashi A, Otani H, Takahashi C, Washizuka T, Tsuji K, Noda M, Horie M, Sasayama S. Cystic fibrosis transmembrane conductance regulator mediates sulphonylurea block of the inwardly rectifying K+ channel Kir6.1. Journal of Physiology-London. 1998;508:23. doi: 10.1111/j.1469-7793.1998.023br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konstas AA, Dabrowski M, Korbmacher C, Tucker SJ. Intrinsic sensitivity of Kir1.1 (ROMK) to glibenclamide in the absence of SUR2B - Implications for the identity of the renal ATP-regulated secretory K+ channel. Journal of Biological Chemistry. 2002;277:21346. doi: 10.1074/jbc.M202005200. [DOI] [PubMed] [Google Scholar]
- 45.Liu YC, Patel HJ, Khawaja AM, Belvisi MG, Rogers DR. Neuroregulation by vasoactive intestinal peptide (VIP) of mucus secretion in ferret trachea: activation of BKCa channels and inhibition of neurotransmitter release. British Journal of Pharmacology. 1999;126:147. doi: 10.1038/sj.bjp.0702288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sari R, Peitl B, Kovacs P, Lonovics J, Palvolgyi A, Hegyi P, Nagy I, Nemeth J, Szilvassy Z, Porszasz R. Cyclic GMP-mediated activation of a glibenclamide-sensitive mechanism in the rabbit sphincter of Oddi. Digestive Diseases and Sciences. 2004;49:514. doi: 10.1023/b:ddas.0000020513.34670.c0. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Wu J, Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. Journal of Membrane Biology. 2003;196:61. doi: 10.1007/s00232-003-0625-z. [DOI] [PubMed] [Google Scholar]
- 48.Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar CP, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC. Physiol. 2005;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. Journal of Physiology-London. 1997;499:715. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada M, Kurachi Y. The nucleotide-binding domains of sulfonylurea receptor 2A and 2B play different functional roles in nicorandil-induced activation of ATP-sensitive K+ channels. Molecular Pharmacology. 2004;65:1198. doi: 10.1124/mol.65.5.1198. [DOI] [PubMed] [Google Scholar]
- 51.Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. Journal of Physiology-London. 2006;572:617. doi: 10.1113/jphysiol.2006.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin-Gene-Related Peptide Activated ATP-Sensitive K+ Currents in Rabbit Arterial Smooth-Muscle Via Protein-Kinase-A. Journal of Physiology-London. 1994;475:9. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. Journal of Physiology-London. 1998;507:117. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurjak M, Fritsch R, Saur D, Schusdziarra V, Allescher HD. Functional coupling between nitric oxide synthesis and VIP release within enteric nerve terminals of the rat: involvement of protein kinase G and phosphodiesterase 5. Journal of Physiology-London. 2001;534:827. doi: 10.1111/j.1469-7793.2001.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim BJ, Lee JH, Jun JY, Chang IY, So I, Kim KW. Vasoactive intestinal polypeptide inhibits pacemaker activity via the nitric oxide-cGMP-protein kinase G pathway in the interstitial cells of Cajal of the murine small intestine. Molecules and Cells. 2006;21:337. [PubMed] [Google Scholar]
- 56.Pomerleau F, Fournier A, Cadieux A. Mouse aorta: A preparation highly sensitive to the vasodilatory action of cGRP. Journal of Cardiovascular Pharmacology. 1997;30:343. doi: 10.1097/00005344-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Pesic S, Grbovic L, Radenkovic M, Stojic D, Nikolic V, Cvetkovic Z. The relaxant effect of vasoactive intestinal polypeptide in the isolated canine uterine artery: The role of endothelium. Journal of Veterinary Medicine Series A-Physiology Pathology Clinical Medicine. 2004;51:394. doi: 10.1111/j.1439-0442.2004.00668.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure 1. Activation of the Kir6.1/SUR2B channel by diazoxide (DZX) and pinacidil in HEK293 cells. A. DZX (100 μM) activated the Kir6.1/SUR2B channel similarly as 10 μM Pin, which was also inhibited by 10 μM Glib. B. Summary of the currents activated by DZX and Pin. Data are presented as mean ± s.e. (n=3).
Online Figure 2. The effect of different K+ channel blockers on K+ currents recorded in HEK293 cells. A. The pretreatment of 4-aminopyridine did not block the effect of VIP- and diazoxide (DZX)-induced currents. B. Pin-induced K+ currents were insensitive to charybdotoxin. C. TBA (non-selective K+ channel blocker) partially blocked the Pin-induced K+ currents. D. Summary of effects of the K+ channel blockers. E. The VIP-induced channel activation was not blocked by 4-AP treatment. Data are presented as mean ± s.e. (n=3∼5).