Abstract
Increased levels of proinflammatory cytokines, TNF-α and IL-6, predict mortality and morbidity. In cardiovascular disease patients, they are observed in atherosclerotic lesions and serum. Factors behind the increased levels of these cytokines are multifaceted and may include latent herpesviruses, such as Epstein-Barr virus (EBV) that can be reactivated by stress. Previously, we showed that the EBV-encoded deoxyuridine triphosphate nucleotidohydrolase (dUTPase), a protein synthesized in the early phase of virus replication, can induce human monocytes/macrophages to produce TNF-α and IL-6. In this study, we modeled the interactions that take place between macrophages and endothelial cells in vivo using human umbilical vein endothelial cells (HUVEC). HUVEC were stimulated by soluble factors induced by EBV dUTPase-treated monocyte-derived macrophages (MDM) that resulted in the upregulation of VCAM-1 and ICAM-1. These changes were related to MDM production of TNF-α following the activation of NF-κB. In a previous study, chronically stressed dementia caregivers had elevations in plasma IL-6 levels, a risk for cardiovascular disease. We found a relationship between plasma IL-6 levels and neutralizing antibody titers to EBV dUTPase suggesting that one source of the plasma IL-6 observed in our previous study could be related to the effect of EBV-encoded dUTPase on macrophages. The results suggest that EBV-encoded dUTPase can enhance production of proinflammatory cytokines by monocytes/macrophages in contact with endothelial cells of blood vessels, and may play a role in cardiovascular pathology and chronic inflammation.
Keywords: EBV, dUTPase, proinflammatory cytokines, endothelial cells, monocytes/macrophages
Introduction
Inflammation plays a central role in driving the evolution of atherosclerosis and coronary artery disease (Binder et al., 2002; Danesh, 1997; Rattazzi et al., 2005; Ridker et al., 2005a). Clinical investigations indicate that inflammation is an independent risk factor for the development of atherosclerosis and also interacts with established risk factors such as hypercholesterolemia (Binder et al., 2002; Ridker et al., 2005b; Steinberg, 2002). Evidence from model systems and patient studies have demonstrated increases in the concentration and activity of factors that drive inflammation and innate immunity, including the well described increase in C-reactive protein (CRP), as well as upstream factors, such as interleukin-1 beta (IL-1β), IL-6, and TNF-α (Binder et al., 2002; Danesh, 1997; Rattazzi et al., 2005; Ridker et al., 2005a). These cytokines are increased in circulating plasma in patients with cardiovascular disease and are present within atherosclerotic lesions themselves (Rattazzi et al., 2005). In particular, TNF-α promotes the expression of pattern recognition receptors such as scavenger receptors and Toll-like receptors that facilitate the interaction between monocytes/macrophages and circulating lipid, thus leading to the formation of early atherosclerotic lesions (Rattazzi et al., 2005).
The factors that promote the activation of these inflammatory mechanisms are incompletely defined. Viruses, such as the latent herpes viruses Epstein Barr virus (EBV) or cytomegalovirus (CMV), are a likely stimulus to inflammation, considering their persistence in the host and prevalence throughout the population; however, investigations have not provided consistent evidence that they play a significant role in atherogenesis (Danesh, 1998; Horvath et al., 2000; Ibrahim et al., 2005). The failure to consistently link viruses with inflammation does not exclude the possibility that their proatherogenic role may be obscured by the fact that a significant percentage of adults are latently infected with EBV and CMV. Such apparent dormant states may exert proatherogenic effects through mechanisms that are yet to be defined (Glaser, 2006).
Depression is among the comorbid conditions that contribute to the evolution and progression of atherosclerosis and is, itself, influenced by proinflammatory pathways (Empana et al., 2005; Ferketich, 2005a, b; Ferketich et al., 2000; Jones et al., 2003; Schulz et al., 2000). Depression can increase serum levels of cytokines including IL-1β, IL-6, and TNF-α, and plasma proteins, such as CRP. Furthermore, depression enhances mortality in patients who have suffered a myocardial infarction or who have congestive heart failure (Rumsfeld et al., 2005). Depression may promote mortality by further activation of proinflammatory pathways in part through stress hormones, such as the catecholamines which have been shown to activate NF-κB (Bierhaus et al., 2003), thus accelerating the progression of cardiovascular disease. Accordingly, the common proinflammatory pathways in atherosclerosis and depression imply a possible shared causal mechanism for the activation of these pathways.
Depression and psychological stressors can interrupt the steady-state expression of latent EBV and reactivate the virus (Glaser, 2005b, 1991; Kasl et al., 1979; Payne, 1999; Sarid, 2002). Moreover, reactivation of latent EBV may be abortive in that only some early viral proteins, e.g. viral-encoded enzymes, are synthesized (Glaser, 2005b, 1991). The EBV encodes for six enzymes which are involved in virus replication (Glaser, 2005a; Glaser, 2006). These viral enzymes are part of the early antigen (EA) complex and can be synthesized by the endogenous virus genome prior to, and independent of, virus DNA synthesis.
One of these viral proteins, the EBV-encoded dUTPase (Williams, 1985), can produce immune dysregulation in vitro and in vivo. For example, the EBV-encoded dUTPase induced unstimulated monocytes to upregulate the production of TNF-α, IL-1β, IL-6, IL-8, and IL-10 (Glaser, 2006). Accordingly, we are proposing that latent EBV plays a role in cardiovascular disease risk by the production of viral proteins during lytic and/or abortive replication following reactivation of the latent virus. The interaction of one or more EBV-encoded proteins (such as dUTPase) with monocytes/macrophages would contribute to the production of proinflammatory cytokines that would add to the process of chronic inflammation.
The interactions among aging, endothelial cells, macrophages and EBV could have implications for chronically distressed older people. In a previous study, we found an age-related association between higher antibody titers to EBV EA and virus capsid antigen (VCA) in a group of healthy older subjects, average age of 72, compared to a younger group of 23 year old medical students (Glaser, 1985). In a follow-up study of husbands and wives providing care for a spouse with dementia (average age 67), the caregivers had even higher levels of IgG antibody titers to EBV VCA than well matched controls of the same age (Kiecolt-Glaser et al., 1991). The differences in antibody titers to latent EBV are thought to reflect differences in virus-specific T-cell immunity(Glaser, 2005b). Higher antibody titers reflect a decrease in the control over the replication of the latent virus, which results in the synthesis of viral proteins and the immune response to the proteins. The end result is an increase in antibody titers to viral proteins (Glaser, 2005b). Caregivers’ higher antibody titers to latent EBV (compared to non-caregiving controls) may be associated with the interaction between aging and chronic distress and their impact on the cellular immune response (Glaser, 1985; Kiecolt-Glaser et al., 1991). Moreover, distressed caregivers also showed four times the rate of increase in serum levels of IL-6 over a six-year longitudinal study compared to well matched noncaregivers (Kiecolt-Glaser, 2003). Plasma IL-6 levels are a risk factor for cardiovascular disease (Papanicolaou et al., 1998), and dementia caregivers have a higher incidence of cardiovascular disease than noncaregivers (Lee et al., 2003). Thus, the current study addressed the connection between EBV-encoded dUTPase stimulation of proinflammatory cytokines, and the consequent TNF-α-mediated induction of adhesion molecules, ICAM-1 and VCAM-1; a setting that models the in vivo interactions between macrophages and endothelial cells.
Materials and Methods
Endothelial Cells and Monocyte-Derived Macrophages (MDM)
Human umbilical vein endothelial cells (HUVEC) were isolated and propagated as previously described (Sedmak et al., 1990). Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque (Histopaque, Sigma) density gradient centrifugation from buffy coats (n=7) purchased from the American Red Cross as previously described (Waldman et al., 1992). Because complete donor anonymity is a strict condition of this arrangement, no IRB human subjects protocol is required, as specified by the NIH and OSU IRB guidelines. To promote monocyte differentiation into the macrophage phenotype, PBMC were suspended in RPMI 1640 medium (GIBCO) supplemented with 10% pooled human serum and incubated for 5–7 days in Teflon plates at 37°C in a humidified atmosphere of 5% CO2/95% air, transferred to plastic tissue culture plates, and incubated 24 hours prior to removal of non-adherent cells. Cells prepared in this manner routinely marked 90–95% positive for CD14 with undetectable levels of CD3+ T cell contamination as determined by immunofluorescence flow cytometry.
Purification of EBV-encoded dUTPase
Detailed methods for the purification of the EBV-encoded dUTPase have been previously reported (Glaser, 2006; Williams, 1985). All EBV-encoded dUTPase preparations used were tested as described previously (Glaser, 2006) and were free of detectable levels of lipopolysaccharide (Rosenstein et al.), peptidoglycan (SLP-HS), DNA or RNA. Protein concentration was determined with a Coomassie Brilliant Blue dye-binding assay (Bio-Rad Laboratories) using bovine serum albumin as the standard. The purified EBV-encoded dUTPase used in these studies was stored at 4°C at stock concentrations of 0.5 and 1 mg per ml.
Assay of Macrophage-Mediated Endothelial Activation
To determine the impact of soluble factors induced by the EBV-encoded dUTPase-treated MDM upon proximal endothelia, MDM in 24-well culture plates were incubated for 24 hours at 37°C in a humidified atmosphere of 5% CO2/95% air, with various concentrations of EBV-encoded dUTPase (0.125–15 μg/mL) or culture medium alone (0.5 mL/well)and analyzed as described below. This concentration range was found to be optimum to induce proinflammatory cytokines in our previous study (Glaser, 2006).
Confluent HUVEC monolayers in 24-well culture plates were treated with MDM supernatants, culture medium alone, medium supplemented with 15 μg/mL EBV-encoded dUTPase, or medium supplemented with 300 IU/mL human recombinant TNF-α (R & D Systems, Minneapolis, MN) as a positive control for adhesion molecule induction. Cells were incubated for 8 (VCAM-1) or 24 (ICAM-1) hours, harvested by brief trypsin digestion, and assayed for ICAM-1 and VCAM-1 expression by immunofluorescence flow cytometry as previously described (Kristovich et al., 2004).
TNF-α and IL-6 Quantification in MDM Supernatants
All donors were presumed to be in generally good health since they qualified for donation by Red Cross standards; only HIV and hepatitis-C serum negative blood samples were provided. Since donor anonymity is part of the arrangement, donor age and gender were not known. Supernatants from EBV-encoded dUTPase-treated MDMs were analyzed for TNF-α and IL-6 production using the BD™ Human Inflammation Cytometric Bead Array kit (BD Biosciences, CA) according to the manufacturer’s instructions. TNF-α was measured because it has been shown that this cytokine can modulate the expression of ICAM-1 and VCAM-1; IL-6 does not have the capacity to regulate the expression of these adhesion molecules, but it is an important cytokine in the pathophysiology of cardiovascular disease as previously discussed (Kiecolt-Glaser, 2003; Pober and Cotran, 1990). The lower limit of detection by this method for TNF-α is 3.7 pg/ml and 20 pg/ml for IL-6.
We have previously demonstrated EBV-encoded dUTPase-mediated induction of pro-inflammatory cytokines in freshly isolated PBMCs. Studies of CD14-depleted populations and of the adherent fraction of PBMCs implicated the monocytic fraction as a responsive component (Glaser, 2006). To determine whether differentiated macrophages are similarly responsive, MDM purified and differentiated from buffy coats obtained from seven individual blood donors were incubated for 24 hours with various concentrations of EBV-encoded dUTPase (0.125–15 μg/mL). These concentrations were chosen based upon the results of our previous study (Glaser, 2006). Culture supernatants were recovered for measurement of TNF-α and IL-6 content by flow cytometry.
NF-κB Activation
Human PBMCs were isolated and subsequently treated with EBV-encoded dUTPase as described (Glaser, 2006). At indicated times, nuclear extracts were prepared (Guttridge et al., 1999) and electrophorectic mobility shift assay (EMSA) was performed probing for the DNA binding activity of NF-κB. Briefly, 2μg of nuclear extract were incubated with 1 mM phenylmethylsulfonyl fluoride and 1 μg of poly(dI-dC)-poly(dI-dC) (Amersham Biosciences) for 10 min at room temperature. To this mixture 2×104 cpm of a 32P-labeled oligonucleotide probe corresponding to the MHC promoter was added in a buffer consisting of 10 mM Tris-HCI, pH 7.7, 50 mM NaCl, 0.5 mM EDTA, 1 mM dithiothreitol, and 10% glycerol. Complexes were resolved on a non-denaturing 5% polyacrylamide gel, and then subsequently exposed on phosphoimaging screen (Kodak). For supershifts, antibodies raised against specific subunits of NF-κB, p65 (Rockland), p50 (NLS, Santa Cruz Biotechnology), and c-Rel (Santa Cruz Biotechnology) were preincubated with nuclear extracts for 10 min at room temperature before the addition of phenylmethlsulfonyl fluoride and poly(dI-dC)-poly(dI-dC).
To confirm that NF-κB contributed to cytokine production, dUTPase-treated PBMCs were incubated in the presence of NF-κB inhibitors, NBD and PS1145, which target the IkappaB kinase (IKK) responsible for NF-κB-controlled activity. PBMCs at 0.5 million cells/ml were pre-incubated with IKK inhibitors (NBD peptide, 100 μM; or PS1145, 10 μM) for 60 minutes followed by dUTPase treatment at 10 μg/ml for an additional 1 h and 3 h. At this time, culture medium and cell pellets were collected for cytokine analyses and EMSA analysis, respectively, as described above.
Subjects
The subjects studied were part of a previous six-year longitudinal study on caregiving, stress, and health in older adults (Kiecolt-Glaser, 2003) in which serum levels of IL-6 were assessed (by ELISA) and depressive symptoms were assessed using the Beck Depression Index (Bonaccorso et al.) (Beck et al., 1988). The study was approved by our institutional review board and the subjects gave informed consent. The average age of the 34 men and 60 women chosen for this subsample was 72.18 (SD=8.92) and 10 were non-white. The majority of older adults take some medication; in this sample the most common medications were aspirin and other over-the-counter analgesics (n=27), diuretics (n=19), estrogen (n=13), thyroid (n=14), calcium channel blockers (n=11), and beta blockers (n=10). Health problems included arthritis (n=61), hypertension (n=40), prostate problems (n=15), digestive disorders (n=13), and oral health problems (n=11).
Levels of depression were positively correlated with plasma IL-6 levels (Kiecolt-Glaser, 2003). Serum neutralizing antibody titers to the EBV-encoded dUTPase were measured as previously described (Williams, 1985). Neutralizing antibody titers should be related to amount of EBV-encoded dUTPase being synthesized (Levitsky, 2002).
Statistical Analysis
To account for the correlations with the immune markers, mixed effects models were used to study the association between log(TNF-α) or log(IL-6) and log(dUTPase) concentrations. First, we tested the significance of a quadratic trend in (log-transformed) dose for each of the endpoints. Next, we performed all pairwise comparisons (using Tukey’s adjustment) among the dose levels, using repeated measures analysis of variance (ANOVA).
For the study to confirm that NF-κB activation by EBV-encoded dUTPase was related to the induction of TNF-α and IL-6, two sample tests (with unequal variances) were used to compare TNF-α levels between groups with and without inhibitors. For the study involving antibody titers to the EBV-encoded dUTPase, linear regression analyses were performed to determine if there was an association between plasma IL-6 levels and antibody titers to the EBV-encoded dUTPase. Of the 94 subjects studied, 62 (66%) had no measurable level of antibodies against the EBV-encoded dUTPase. Values were determined for the remaining 32 subjects. An indicator variable that took on the value 0 for all patients with non-measurable and 1 for those patients with measurable dUTPase levels was created. We used this variable to stratify the population and compare IL-6 levels within the two groups. IL-6 levels were log transformed so as to better conform to the normality and equal variance assumption.
Results
EBV-encoded dUTPase induces TNF-α and IL-6 production by differentiated macrophages
Data generated by these experiments are presented in Figure 1 as the mean ± 1 standard deviation (solid symbols and error bars) for log TNF-α among the 7 donors at each concentration of dUTPase. Open symbols represent log TNF-α values for each individual donor. As evident in the figure, MDM produced TNF-α in response to EBV-encoded dUTPase treatment in a dose-dependent manner. Repeated measures analysis of variance supports the fact that mean log(TNF-α) levels differ significantly with different concentrations of dUTPase (p<0.0001) and multiple comparison methods suggest that the baseline levels using the two highest concentrations of dUTPase do not differ significantly from one another. For IL-6, the baseline level (0.05) differs significantly from all higher concentrations of dUTPase. Log(IL-6) levels at the five highest concentrations of dUTPase do not differ significantly from one another suggesting that we had reached saturation.
Figure 1.
Figure 1A-EBV-encoded dUTPase stimulates TNF-α production by human differentiated macrophages. Human macrophages isolated and differentiated from 7 individual donors were treated for 24 hours with various concentrations of EBV-encoded dUTPase. TNF-α content in culture supernatants was measured by flow cytometry using the BD™ Human Inflammation Cytometric Bead Array. Open symbols represent log TNF-α values for each individual donor. Closed symbols represent mean log TNF-α values among donors at each dUTPase concentration ± 1 standard deviation.
Figure 1B-EBV-encoded dUTPase stimulates IL-6 production by human differentiated macrophages.The conditions were the same as described in Figure 1A.
EBV-encoded dUTPase induces macrophage-mediated endothelial inflammatory activation
To simulate the impact of soluble factors produced by EBV-encoded dUTPase-exposed-differentiated macrophages upon proximal vascular endothelium, HUVEC were incubated with supernatants of MDM treated for 24 hours with various concentrations of dUTPase (0.125 – 15 μg/mL). Controls included untreated HUVEC, HUVEC treated with 15 μg/mL dUTPase, and HUVEC treated with 300 IU/mL human recombinant TNF-α as a positive control for adhesion molecule induction. We measured TNF-α and IL-6 levels in the supernatants. Following 8 (VCAM-1) or 24 (ICAM-1) hours of incubation, HUVEC were harvested and assayed forVCAM-1 or ICAM-1 expression by immunofluorescence flow cytometry. These incubation times were based upon numerous studies which demonstrate that endothelial cells treated with TNF-α express peak levels of VCAM-1 at 8 hours and of ICAM-1 at 24 hours (Pober and Cotran, 1990). Data generated by these experiments are presented in Figure 2 as the mean ± 1 standard deviation (solid symbols and error bars) for log endothelial ICAM-1 (2A) or VCAM-1 (2B) mean fluorescence intensity among the 7 MDM donors at each concentration of dUTPase. Open symbols represent log mean fluorescence intensity values for each individual donor. As was true for TNF-α and IL-6 values shown in Figures 1A and 1B, the magnitude of endothelial ICAM-1 and VCAM-1 induction by supernatants of EBV-encoded dUTPase-treated MDM varied among individual MDM donors, but exhibited a dose-response which plateaus at the higher levels of dUTPase. The association between each of these markers and log(dUTPase) is quadratic (in all cases p < 0.0011), and in all cases the p-value for the repeated measures analysis of variance is significant at the p < 0.0001 level. For ICAM-1, the baseline level (0.05) differs significantly from all but the 0.125 and 0.25 levels of dUTPase. Log(ICAM-1) levels at the highest concentrations of dUTPase does not differ significantly from either of the next two highest concentrations suggesting that we had reached saturation. For VCAM-1, the baseline level (0.05) differs significantly from all higher levels of dUTPase. Log(VCAM-1) levels at the four highest concentrations of dUTPase do not differ significantly from one another again suggesting that we had reached saturation. The failure of direct dUTPase treatment of HUVEC to induce ICAM-1 or VCAM-1 verifies that endothelial adhesion molecules were induced by soluble factors produced by EBV-encoded dUTPase-treated MDM and not directly by residual dUTPase in MDM culture supernatants (data not shown).
Figure 2. Figure 2A & 2B- Soluble factors elaborated by EBV-encoded dUTPase-treated macrophages induce inflammation-associated adhesion molecule expression on human endothelial cells.
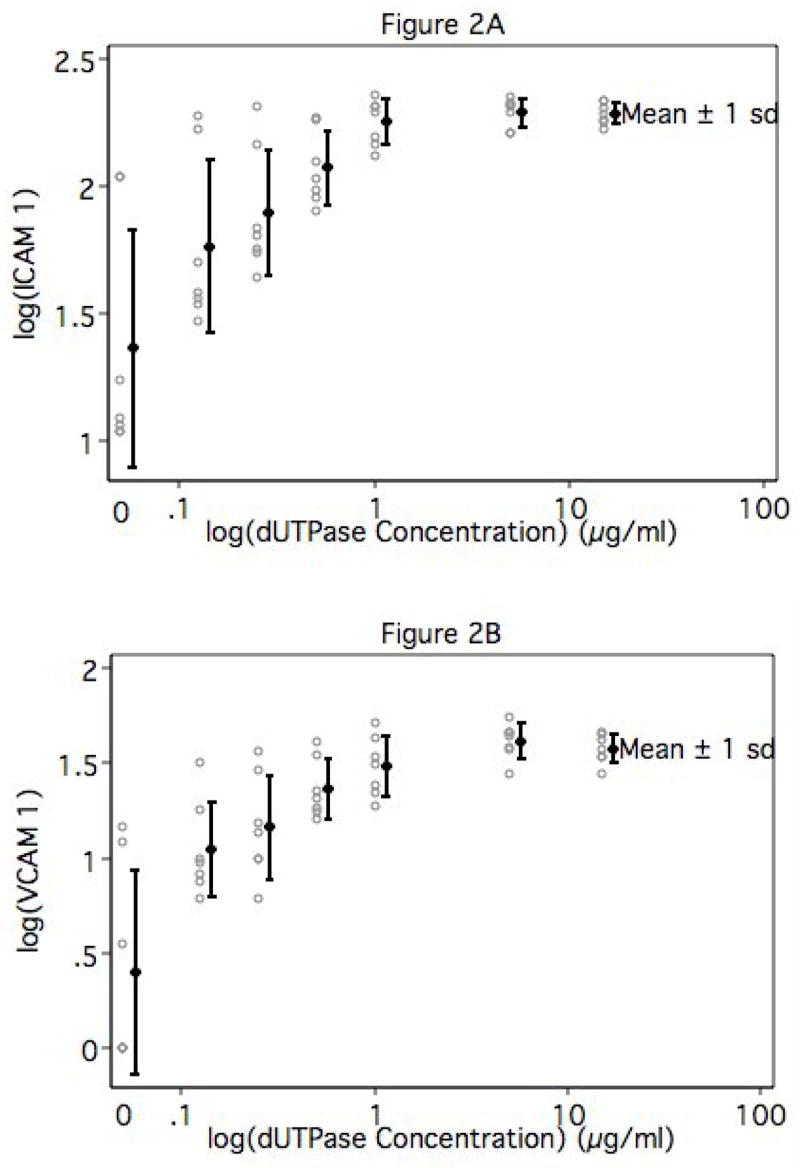
Human umbilical vein endothelial cells were incubated for 8 (VCAM-1) or 24 (ICAM-1) hours with supernatants recovered from EBV dUTPase-treated macrophages, harvested and stained with FITC-conjugated monoclonal antibodies specific for ICAM-1 (A) or VCAM-1 (B), and analyzed by fluorescence flow cytometry. Open symbols represent log mean fluorescence intensity values for each individual donor. Closed symbols represent mean log mean fluorescence intensity values among donors at each dUTPase concentration ± 1 standard deviation.
EBV-encoded dUTPase activates NF-κB in macrophages
As a first step in elucidating mechanisms by which EBV-encoded dUTPase induces macrophage pro-inflammatory cytokine production, we measured NF-κB activation in dUTPase-treated PBMCs, since this signaling pathway is known to be a major positive regulator of a number of inflammatory responses, in particular the activation of TNF-α and IL-6 transcription. Furthermore, catecholamine stress hormones are elevated in depressed individuals and they can upregulate the production of proinflammatory cytokines through the activation of NF-κB. Results showed that NF-κB activity was induced at 1 hour by EBV-encoded dUTPase treatment, which returned close to basal levels after 4 hours of stimulation (Figure 3). Furthermore, EMSA demonstrated that activation of NF-κB comprised primarily of p50/c-Rel heterodimers with a slight contribution from p50/p65 complexes (Figure 4).
Figure 3. NF-κB is activated by dUTPase treatment of macrophages.

Cells were treated with EBV-encoded dUTPase and, at indicated times, nuclear extracts were prepared and EMSA analysis was performed.
Figure 4. dUTPase activates the p50/c-Rel complex of NF-κB.
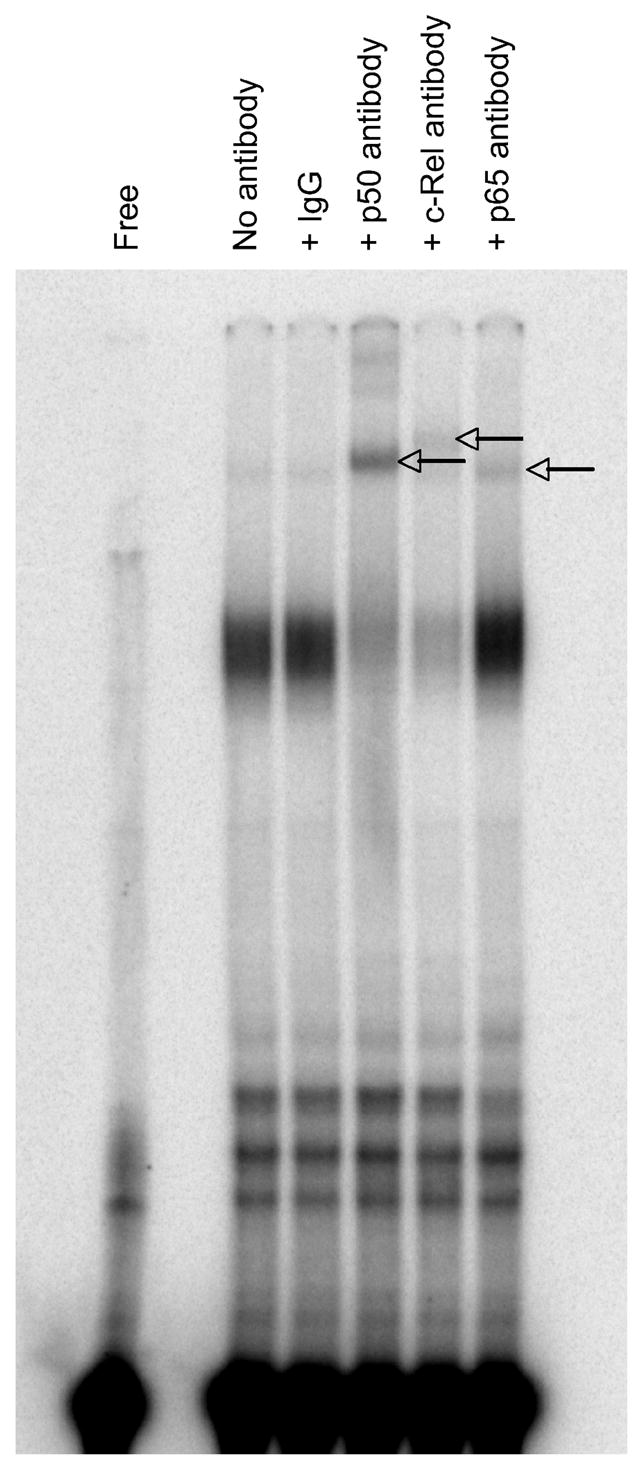
Nuclear extracts were pre-incubated with antibodies specific to NF-κB subunits and EMSA analysis was subsequently performed. Arrows indicate supershifted complexes.
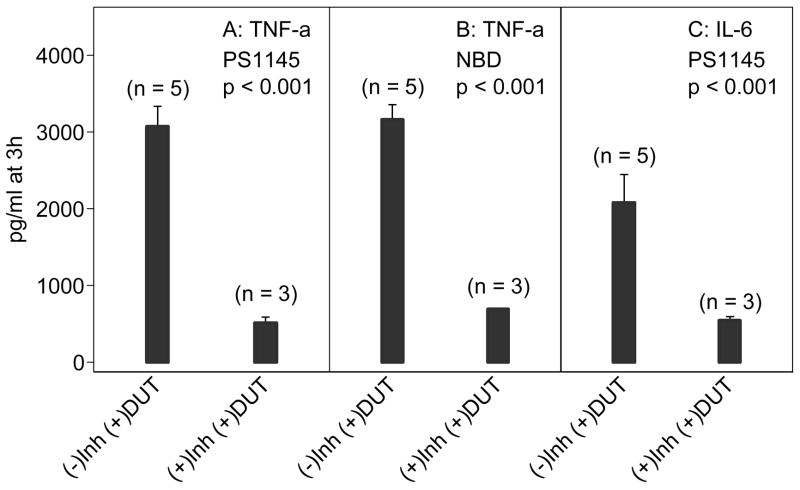
To confirm that NF-κB contributed to cytokine production, dUTPase-treated PBMCs were incubated in the presence of NF-κB inhibitors, PS1145 and NBD, that target the IKK complex responsible for NF-κB controlled activity. In the presence of the inhibitors, TNF-α production was markedly reduced (p < 0.001), which corresponded to a concomitant reduction of NF-κB DNA binding activity (p <0.001), and similar reductions were also observed for IL-6 (Figure 5 and data not shown). Together, these data suggest that expression of inflammatory cytokines is mediated through dUTPase-induced activation of NF-κB transcriptional activity.
Figure 5. dUTPase-induced secretion of TNF-α and IL-6 from PBMCs is NF-κB dependent.
TNF-α (A, B) and IL-6 (C) released from dUTPase-treated PBMCs pre-incubated with and without NF-κB inhibitor compounds (Lynch, 1972), EBV dUTPase treatment at 10 μg/ml, PS1145 (10 μM) and NBD (100 μM). Inh=inhibitor, DUT=dUTPase
Antibody titers to the EBV-encoded dUTPase are related to plasma IL-6 levels
All subjects were part of our earlier longitudinal study on caregivers in which plasma IL-6 levels were measured (Kiecolt-Glaser, 2003). Because circulating IL-6 levels can be a co-factor for depression and we have shown that the EBV-encoded dUTPase can upregulate the production of IL-6 in monocyte/macrophages, we assessed the association between plasma IL-6 levels and EBV dUTPase antibody titers. The subjects’ plasma IL-6 values were below 15 pg/ml, with the exception of two participants whose values were 240.58 pg/ml and 831.49 pg/ml, or at least 90 SDs above the group mean; they were excluded from the sample. Both of these subjects were in the group with measurable EBV-encoded dUTPase neutralizing antibody titers; they were removed from the database so as to better conform to the assumptions of equal variance and normality of the IL-6 data within subgroups for the t-test, and because their extreme values could have reflected unreported health problems, e.g., acute illness that was either unknown to the subject or not reported. Subjects with measurable anti-EBV-encoded dUTPase neutralizing antibody levels had generally higher mean IL-6 levels (mean = .41, SEM = 0.06) than did the group whose anti-EBV-encoded dUTPase antibody levels were unmeasurable (mean = 0.34, SEM = 0.03), but the difference between the groups did not achieve statistical significance (p = 0.26). While not statistically significant, the direction of the difference is suggestive. Figure 6A presents a scatterplot of log(plasma IL-6 levels) on log(EBV dUTPase antibody titers) for only those patients with measurable levels of anti-EBV-encoded dUTPase antibody with the two outliers removed. The scatterplot demonstrates that, for those subjects with measurable EBV-encoded dUTPase antibody titers, IL-6 levels tend to increase as the antibody titers increase. This association is significant (p = 0.04), with log (dUTPase) antibody titers explaining r2 = 14% of the variability in log(IL-6). After controlling for age, the association is still significant (p = 0.047, R2=14.5%).
Figure 6.
Figure 6A: Scatterplot and linear regression line of ln(IL-6) on log(dUTPase) Figure 6A presents a scatter plot of log(IL-6) vs. log(dUTPase) along with the linear regression model (log(IL-6) = 0.464 + 0.243 × log(dUTPase)) based on n = 30 subjects with no missing data and eliminating 2 subjects with log(IL-6) > 3. This association was statistically significant (p = 0.039).
Figure 6B: Scatterplot and linear regression line of Beck Depression Scale on log(dUTPase). Figure 6B presents a scatter plot of the Beck Depression Scale vs. log(dUTPase) along with the linear regression model (Beck = 6.278 + 3.004 × log(dUTPase)) based on the same n = 30 subjects as were used in Figure 6A. This association was statistically significant (p = 0.023).
Figure 6B presents a scatterplot of BDI scores obtained in our previous study (Kiecolt-Glaser, 2003) on log EBV-encoded dUTPase antibody titers (only for those subjects with measurable EBV-encoded dUTPase neutralizing antibody titers and eliminating the two subjects with extreme IL-6 levels). The relationship shown in the graph is significant (p = 0.0233), with log (EBV dUTPase antibody titers) explaining r2 = 17% of the variability in depression scores.
This observed relationship between BDI scores and log EBV-encoded dUTPase antibody titers remains significant in a linear model (p = 0.032) even after controlling for ln IL-6 levels. IL-6 levels were neither a confounder nor an effect modifier of the strong relationship between the depression scores and log EBV dUTPase.
Discussion
In this study the EBV-encoded dUTPase stimulated monocytes/macrophages to upregulate the expression of TNF-α through a NF-κB mechanism and resulting in the upregulation of the surface expression of inflammation-associated endothelial adhesion molecules, VCAM-1 and ICAM-1; an upregulation of IL-6 was also observed. These data support the evidence that EBV induces an inflammatory cascade.
A recent study showing that EBV DNA can be detected (by PCR) in atherosclerosis plaques is consistent with the interpretation that EBV can lytically replicate in the monocytes/macrophages (Savard et al., 2000), which have become associated with the plaques (de Boer, 2006). EBV can induce macrophages to synthesize the macrophage inflammatory protein-1 alpha (MIP-1α), which can attract B- and T-lymphocytes to the site of inflammation (McColl, 1997); a recent study by Tugizov et al found that EBV-infected B-cells can provide a source of EBV that can act as a source of the virus to infect monocytes (Tugizov et al., 2007). Furthermore, the deBoer et al study showed that EBV-specific T-cells are generated at the site of the plaque as well, confirming that a local EBV-specific T-cell response can contribute to the inflammatory process presumably related to the EBV-infected macrophages(de Boer, 2006). This investigation provides a plausible basis for the initiation of pro-atherogenic inflammatory responses by latent viral infections, such as EBV.
Supporting the in vitro data, which provide a possible mechanism, distressed caregivers had significant elevations in plasma IL-6 levels (compared to noncaregivers), a known risk factor for cardiovascular disease. In accord with these data, other laboratories have documented caregivers’ greater risk for cardiovascular disease (Lee et al., 2003; Schulz et al., 2001). The significant relationship between plasma IL-6 levels and neutralizing antibody titers to the EBV-encoded dUTPase in our subjects suggest that at least one source of plasma IL-6 could be related to the effect of the EBV-encoded dUTPase on monocyte/macrophage-induced inflammatory cytokines.
Although some subjects had antibody to the EBV-encoded dUTPase and some did not, all were latently infected with EBV as demonstrated by the presence of antibody to EBV VCA, consistent with previous reports (Glaser, 1985; Jones, 1988). Despite the fact that unique antibody patterns to several EBV-encoded early proteins in different groups of EBV-infected subjects have been demonstrated by our laboratory and others, the reason for these patterns is not known (Cheng et al., 1980; Glaser, 1998; Glaser, 2006; Glaser, 2005b; Jones, 1988; Laichalk and Thorley-Lawson, 2005).
The data support the hypothesis that EBV-encoded proteins play a role in the pathogenesis of atherosclerosis and that distress-associated upregulation of the steady state expression of latent EBV could contribute to the increase in plasma IL-6 levels observed in our caregiver studies (Kiecolt-Glaser, 2003; Kiecolt-Glaser et al., 1991). The significant association of BDI depression scores with antibody titers to the EBV-encoded dUTPase supports the relationships among depressive symptoms, virus reactivation, and the upregulation of proinflammatory cytokine synthesis. The data also suggest a possible mechanism by which viral protein(s) could participate in this process. These data provide the foundation for considering new strategies for the prevention and treatment of peripheral arteriosclerosis and coronary artery disease.
Supplementary Material
Acknowledgments
We thank Bryon Laskowski and Monica Litsky for their technical help in generating the plasma IL-6 data. We thank Christopher Jones for his help with the statistical analysis and the NF-κB experiments. We also thank Gary Phillips for his assistance in preparing the graphics for this manuscript. This study was supported in part by the Ohio State University Comprehensive Cancer Center Core Grant CA16058, the Gilbert and Kathryn Mitchell endowment and grant P01 AG16321 from the National Institute on Aging/NIH. Philip Binkley’s work is supported by National Institute of Biomedical Imaging and Bioengineering/NIH grant K24-H104208 and the James H. and Ruth J. Wilson Professorship.
Funding: This study was supported in part by the Ohio State University Comprehensive Cancer Center Core Grant CA16058, the Gilbert and Kathryn Mitchell endowment and grant P01 AG16321 from the National Institute on Aging/NIH. Philip Binkley’s work is supported by National Institute of Biomedical Imaging and Bioengineering/NIH grant K24-H104208 and the James H. and Ruth J. Wilson Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder CJ, Chang MK, Shawn PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Lin A, Verkerk R, Van Hunsel F, Libbrecht I, Scharpe S, DeClerck L, Biobdi M, Janca A, Maes M. Immune markers in fibromyalgia: Comparison with major depressed patients and normal volunteers. Journal of Affective Disorders. 1998;48:75–82. doi: 10.1016/s0165-0327(97)00144-4. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Chen YY, Glaser R, Henle W. Frequency and levels of antibodies to Epstein-Barr virus specific DNase are elevated in patients with nasopharyngeal carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:6162–6165. doi: 10.1073/pnas.77.10.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Appleby P. Persistent infection and vascular disease: a systematic review. Expert Opin Investig Drugs. 1998;7:691–713. doi: 10.1517/13543784.7.5.691. [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: Is there a link. Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- de Boer OJ, Teeling P, Idu MM, Becker AE, van der Wal AC. Epstein Barr virus specific T-cells generated from unstable human atherosclerotic lesions: Implications for plaque inflammation. Atherosclerosis. 2006;184:322–329. doi: 10.1016/j.atherosclerosis.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- Ferketich AK, Binkley PF. Psychological distress and cardiovascular disease: Results from the 2002 National Health Interview Survey. Eur Heart J. 2005a;26:1923–1929. doi: 10.1093/eurheartj/ehi329. [DOI] [PubMed] [Google Scholar]
- Ferketich AK, Ferguson JP, Binkley PF. Depressive symptoms and inflammation among heart failure patients. Am Heart J. 2005b;150:132–136. doi: 10.1016/j.ahj.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch Intern Med. 2000;160:1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-Induced Immune Dysfunction: Implications for Health. Nature Reviews Immunology. 2005a;5:10–18. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-associated immune modulation: Relevance to viral infections and chronic fatique syndrome. American Journal of Medicine. 1998;105:35S–42S. doi: 10.1016/s0002-9343(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Glaser R, Litsky ML, Padgett DA, Baiocchi RA, Yang EV, Chen M, Yeh PE, Green-Church KB, Caligiuri MA, Williams MV. EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology. 2006;346:205–218. doi: 10.1016/j.virol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Glaser R, Litsky ML, Padgett DA, Baiocchi RA, Yang EV, Chen M, Yeh PE, Klimas NG, Marshall GD, Whiteside T, Herberman R, Williams MV. Stress-associated changes in the steady state expression of latent Epstein-Barr Virus: Implications for Chronic Fatigue Syndrome and Cancer. Brain Behavior & Immunity. 2005b;19:91–103. doi: 10.1016/j.bbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearson GR, Jones JF, Hillhouse J, Kennedy S, Mao HY, Kiecolt-Glaser JK. Stress-related activation of Epstein-Barr virus. Brain Behavior & Immunity. 1991;5:219–232. doi: 10.1016/0889-1591(91)90018-6. [DOI] [PubMed] [Google Scholar]
- Glaser R, Strain EC, Tarr K, Holliday JE, Donnerberg RL, Kiecolt-Glaser JK. Changes in Epstein-Barr virus antibody titers associated with aging. Proceedings of the Society of Experimental Biology and Medicine. 1985;179:352–355. doi: 10.3181/00379727-179-42108. [DOI] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin ASJ. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R, Cerny J, Benedik J, Jr, Hokl J, Jelinkova I, Benedik J. The possible role of human cytomegalovirus (HCMV) in the origin of atherosclerosis. J Clin Virol. 2000;16:17–24. doi: 10.1016/s1386-6532(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Ibrahim AI, Obeid MT, Jouma MJ, Moasis GA, Al-Richane WL, Kindermann I, Boehm M, Roemer K, Mueller-Lantzsch N, Gartner BC. Detection of herpes simplex virus, cytomegalovirus and Epstein-Barr virus DNA in atherosclerotic plaques and in unaffected bypass grafts. J Clin Virol. 2005;32:29–32. doi: 10.1016/j.jcv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Bromberger JT, Sutton-Tyrell K, Matthews KA. Lifetime history of depression and carotid atherosclerosis in middle-aged women. Arch Gen Psychiatry. 2003;60:153–160. doi: 10.1001/archpsyc.60.2.153. [DOI] [PubMed] [Google Scholar]
- Jones JF, Williams M, Schooley RT, Robinson C, Glaser R. Antibodies to Epstein-Barr virus specific DNase and DNA polymerase in the chronic fatigue syndrome. Archives of Internal Medicine. 1988;148:1957–1960. [PubMed] [Google Scholar]
- Kasl SV, Evans AS, Niederman JC. Psychosocial risk factors in the development of infectious mononucleosis. Psychosomatic Medicine. 1979;41:445–466. doi: 10.1097/00006842-197910000-00002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Preacher K, MacCallum R, Atkinson C, Malarkey W, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kristovich R, Knight DA, Long JF, Williams MV, Dutta PK, Waldman WJ. Macrophage-mediated endothelial inflammatory responses to airborne particulates: impact of particulate physicochemical properties. Chemical Research in Toxicology. 2004;17:1303–1312. doi: 10.1021/tx049893p. [DOI] [PubMed] [Google Scholar]
- Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. Journal of Virology. 2005;79:1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in US women - A prospective study. American Journal of Preventive Medicine. 2003;24:113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- Levitsky V, Masucci MG. Manipulation of immune responses by Epstein-Barr virus. Virus Research. 2002;88:71–86. doi: 10.1016/s0168-1702(02)00121-1. [DOI] [PubMed] [Google Scholar]
- Lynch RG, Graff RJ, Sirisinha S, Simms ES, Eisen HN. Myeloma proteins as tumor-specific transplantation antigens. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl SR, Roberge CJ, Larochelle B, Gosselin J. EBV induces the production and release of IL-8 and macrophage inflammatory protein-1 alpha in human neutrophils. Journal of Immunology. 1997;159:6164–6168. [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Annals of Internal Medicine. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Payne DA, Mehta SK, Tyring SK, Stowe RP, Pierson DL. Incidence of Epstein-Barr virus in astronaut saliva during spaceflight. Aviation space and environmental medicine. 1999;70:1211–1213. [PubMed] [Google Scholar]
- Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiological Reviews. 1990;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Rattazzi M, Faggin E, Pertipaglia B, Pauletto P. Innate immunity and atherogenesis. Lupus. 2005;14:747–751. doi: 10.1191/0961203305lu2213oa. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005a;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005b;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- Rosenstein BS, Phelps RG, Weinstock MA, Bernstein JL, Gordon ML, Rudikoff D, Kantor I, Shelton R, Lebwohl MG. p53 Mutations in basal cell carcinomas arising in routine users of sunscreens. Photochemistry and Photobiology. 1999;70:798–806. [PubMed] [Google Scholar]
- Rumsfeld JS, Jones PG, Whooley MA, Sullivan MD, Pitt B, Weintraub WS, Spertus JA. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J. 2005;150:961–967. doi: 10.1016/j.ahj.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Sarid O, Anson O, Yaari A, Margalith M. Epstein-Barr virus specific salivary antibodies as related to stress caused by examinations. J Med Virol. 2002;64:149–156. doi: 10.1002/jmv.1030. [DOI] [PubMed] [Google Scholar]
- Savard M, Belanger C, Tardif M, Gourde P, Flamand L, Gosselin J. Infection of primary human monocytes by Epstein-Barr virus. Journal of Virology. 2000;74:2612–2619. doi: 10.1128/jvi.74.6.2612-2619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Archives of Internal Medicine. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Lind B, Martire LM, Zdaniuk B, Hirsch C, Jackson S, Burton L. Involvement in caregiving and adjustment to death of a spouse: findings from the caregiver health effects study. JAMA : the Journal of the American Medical Association. 2001:285, 3123–3129. doi: 10.1001/jama.285.24.3123. [DOI] [PubMed] [Google Scholar]
- Sedmak DD, Roberts WH, Stephens RE, Buesching WJ, Morgan LA, Davis DH, Waldman WJ. Inability of cytomegalovirus infection of cultured endothelial cells to induce HLA class II antigen expression. Transplantation. 1990;49:458–462. doi: 10.1097/00007890-199002000-00043. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- Tugizov S, Herrera R, Veluppillai P, Greenspan J, Greenspan D, Palefsky JM. Epstein-Barr Virus (EBV)-Infected Monocytes Facilitate Dissemination of EBV within the Oral Mucosal Epithelium. Journal of Virology. 2007;81:5484–5496. doi: 10.1128/JVI.00171-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman WJ, Adams PW, Orosz CG, Sedmak DD. T lymphocyte activation by cytomegalovirus-infected, allogeneic cultured human endothelial cells. Transplantation. 1992;54:887–896. doi: 10.1097/00007890-199211000-00024. [DOI] [PubMed] [Google Scholar]
- Williams MV, Holliday JE, Glaser R. Induction of a deoxyuridine triphosphate nucleotidohdrolase activity in Epstein-Barr virus-infected cells. Virology. 1985;142:326–333. doi: 10.1016/0042-6822(85)90341-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.