Abstract
Peripheral nerve injury models are used to investigate processes that can potentially be exploited in CNS injury. A consistent change that occurs in injured peripheral neurons is an induction in expression of PACAP, a neuropeptide with putative neuroprotective and neuritogenic actions. PACAP-deficient mice were used here to investigate actions of endogenous PACAP after facial nerve injury. Although motor neuron survival after axotomy was not significantly different in PACAP deficient vs. wild type mice, recovery of axon regeneration after crush injury was significantly delayed. The impaired regeneration was associated with 8- to 12-fold increases in gene expression of proinflammatory cytokines TNF-α, IFN-γ, IL-6, and a 10-fold decrease in the anti-inflammatory cytokine IL-4 at the injury site. Similar cytokine changes and an increased microglial response were observed in the brainstem facial motor nucleus. Because immunocompromised animals such as SCID mice are known to exhibit peripheral nerve regeneration defects, the observations raise the novel hypothesis that PACAP is critically involved in a carefully controlled immune response that is necessary for proper nerve regeneration after injury.
Introduction
It is well recognized that neurons survive poorly and axons do not appreciably regenerate after CNS injury. Thus, investigators have utilized peripheral nerve injury models to identify mechanisms that may be exploitable in CNS repair. For example, transplantations of macrophages that had been activated with segments of sciatic, but not optic nerve were found to greatly improve optic nerve regeneration after crush (Lazarov-Spiegler et al., 1998). Facial nerve injury is a simple peripheral nerve injury model that is highly amenable to mechanistic studies on neuron survival and repair (Moran and Graeber, 2004, Makwana and Raivich, 2005). Although facial motor neurons start to degenerate after axotomy, showing the classical features of retrograde reaction (chromatolysis; nuclear eccentricity; increased basophilia; perikaryal, nuclear and nucleolar swelling), a great majority recover, resulting in 80% or higher survival in most murine strains. Moreover, axon regeneration is robust. Because of these features, facial nerve injury is one of the most well characterized peripheral nerve injury models. For example, the post axotomy time courses of expression of more than 100 genes in the facial motor nucleus (FMN) (where the cell bodies reside) have been reported, including those of neuropeptides, growth factors, cytokines, and cell adhesion molecules (Moran and Graeber, 2004). Much less studied are the changes that occur at the injury site.
PACAP is a 38 amino acid neuropeptide originally discovered as a hypothalamic peptide that stimulates cAMP production in the pituitary. Although this peptide functions as a neurotransmitter and/or neuromodulator in diverse processes such as regulation of circadian rhythms (Colwell et al., 2004), considerable data indicate that PACAP has growth factor-like actions in neural development and injury (reviews (Vaudry et al., 2000, Waschek, 2002)). For example, PACAP has been shown to regulate cortical progenitor mitosis and differentiation in vivo (Suh et al., 2001), and to promote neuron survival during cerebellar development (Vaudry et al., 1999) and in the injured brain (Takei et al., 2000). Moreover, PACAP has been shown to regulate axonogenesis in neurite outgrowth and growth cone turning assays (Guirland et al., 2003), and to promote axonal regeneration after facial nerve injury in vivo, as determined by analyses of retrograde tracers (Suarez et al., 2006) and numbers of myelinated axons distal to the site of injury (Kimura et al., 2003). Finally, two different groups have shown that mice with targeted deletions of the PACAP gene exhibit greater infarct volumes and neurological deficits in cerebral artery occlusion models of stroke (Chen et al., 2006, Ohtaki et al., 2006).
PACAP mRNA levels strongly induced (more than 20-fold) in facial motor neurons after axotomy, with a significant increase occurring as early as six hours after injury (Zhou et al., 1999). Thus, it seems possible that PACAP might function in this injury model to promote neuron survival or regeneration of axons. PACAP might act locally within the FMN to affect these processes or on cells at the peripheral nerve injury site. With respect to the latter, PACAP was found to be axonally transported and released at a crush site in another peripheral nerve injury model (ex vivo nodose ganglia/vagus nerve explant) (Reimer et al., 1999).
Another possible function of PACAP in nerve injury is to regulate the immune response. Receptors which bind both PACAP and the closely related peptide vasoactive intestinal peptide (VIP) are expressed on microglia and on many different types of immune cells, and mediate a rage of actions, including the production of inflammatory mediators, T cell activation and differentiation, and chemotaxis (reviewed in (Delgado et al., 2004). The effects on T cell activation/differentiation are of special interest here because motor neuron survival and regeneration after facial nerve injury was shown to be impaired in SCID mice (Serpe et al., 1999, Serpe et al., 2002). Moreover, the effect on survival in this model appears to be dependent primarily on T helper (Th)2 cell differentiation (Deboy et al., 2006), a process shown to be promoted by PACAP (Delgado et al., 1999). Although the immunomodulatory actions of PACAP have not been well studied in the context of in nerve injury, VIP was reported reduce neuron cell death induced by intraventricular administration of the bacterial endotoxin lipopolysaccharide in association with reductons in microglia activation and proinflammatory cytokine production (Delgado and Ganea, 2003).
We examined here the effects of PACAP deficiency on facial motor neuron survival after axotomy and on axon regeneration after nerve crush. We also compared microglial activation and specific cytokine responses in the brain stem, where the motor neurons cell bodies reside, and compared the cytokine responses with in the injured nerves.
Experimental procedures
Animals
PACAP deficient (KO) mice, were previously generated by targeted mutagenesis (Colwell et al., 2004). The gene targeting strategy removed sequences encoding PACAP and also PACAP-related peptide, a peptide of unknown biological significance. These mice were found by sensitive radioimmunoassay to not express PACAP in the brain or peripheral sites (Colwell et al., 2004). All mice used in these experiments were 2–4 month old females, and were generated after backcrossing the mutation to C57BL/6 mice for at least six generations. Wild type (WT) female littermates or other age-matched female WT C57BL/6 mice in the colony were used as controls. Experiments were conducted in accordance with the recommendations for animal use by the UCLA Division of Laboratory Animals, and the guidelines from the National Institutes of Health.
Facial nerve axotomy and crush
Animals were anesthetized with isofluorane and the faces were shaved, washed with surgical scrub and 70% ethanol, and then under aseptic conditions an incision was made on the right face, exposing the facial nerve. For assessment of motor neuron survival, the nerve was severed and a 1 mm section near the stylomastoid foramen was removed. The axotomy was performed unilaterally so that the other side served as a control. All other studies utilized the crush procedure. In these cases, the exposed nerve was crushed with a pair of jeweler’s forceps for a period of 30 seconds. In most cases, the crush was performed at a location 1 mm from the stylomastoid foramen. In cases which required subsequent dissection of the crush site and/or distal nerve to study axonal regrowth or remyelination, the crush was performed on the marginal mandibular branch of the facial nerve, 1 mm away from the bifurcation point. In these cases, the inner surface of the forceps was first coated with a layer of sterile India Ink in order to mark the crush site. After procedures, wounds were closed with sutures.
Motor neuron survival
Axotomized WT and PACAP KO mice (n=6 per group) were allowed to recover for a period of 28 days before being sacrificed. Mice were perfused with 4% PFA/1xPBS. Brains were removed, postfixed overnight in the perfusate solution, cryoprotected in a solution of 30% sucrose/1x PBS for 12–18 hours, then transferred to a −70 °C freezer, where they are kept until sectioning. Sections were cut at 12 μm on a cryostat, then stained with cresyl violet. All sections passing through the FMN were assessed by two separate blinded raters. Neuron profiles were scored if they were darkly stained and had a clear nucleus. Neuron size was quantified using NIH Image. The number of motor neuron profiles on the ipsilateral and contralateral of each section was determined. The total number of motor neurons per section was calculated from the number and sizes of motor neuron profiles using the Abercrombie method (Abercrombie, 1946). The means and standard errors of the means (SEM) per section were calculated. Percent survival was determined as the overall mean number of surviving motor neurons on the ipsilateral side divided by the mean number of surviving neurons on the contralateral side.
Assessment of reinnervation as determined by retrograde transport with Fluorogold (FG)
Injections of FG (2 μl of a 2% water solution) were made bilaterally into both the whisker pad and the upper lip (4 μl total for each side of the face) at 6, 7, 9, and 12 days after crush injury (n=3–5 per group at each time point). The mice were then anesthetized, perfused with 4% paraformaldehyde (PFA), and brains were prepared for cryostat sectioning as previously described (Armstrong et al., 2004b). Images of the fluorescent neurons were taken with an Olympus IX-80 camera on a Zeiss microscope using fluorescent illumination and a DAPI filter. Fluorescent neurons were counted by two separate blind raters.
GAP-43 and galanin immunofluorescence
Mice were sacrificed 1 and 3 days after nerve injury, and the nerve and surrounding musculature was surgically removed for immunofluorescence assay. Facial nerves were isolated by cutting a relatively small portion of the musculature with a scalpel in a triangular shape with the most distal region coming almost to a point. In this way the orientation of the facial nerve, as well as the location of the crush, marked as stated above with India Ink, could be visualized after the immunofluorescent assay was performed. The tissue was then washed in phosphate buffered saline (PBS), fixed in 4% PFA for one hr, cryoprotected in 20% sucrose overnight, and embedded in OCT. Sections were cut on a cryostat (14μm for longitudinal, and 10 μm for cross sections) and thaw mounted onto Superfrost slides, and then stored at −70°C. Immunofluorescence procedures for visualizing re-growing axons were carried out as follows. Slides were warmed for 30 minutes and then washed 3×5 minutes in PBS, blocked with 3% normal goat serum for 1 hour. GAP-43 antibody was applied (Chemicon International, catalog # AB5220, lot 25050080, dilution of 1:1000 and 1:2000 for longitudinal and cross sections, respectively) and slides were then incubated overnight at 4°C. A secondary antibody conjugated with FITC was applied (1:200; Jackson Immuno Research Labs) for 1 hour. Slides were then washed in PBS and coverslipped using Vectashield (Vector Labs) mounting medium. Pictures were taken with a Zeiss camera on an Olympus microscope using fluorescent illumination with a FITC filter. Immunohistochemistry for NF160 (Sigma, cat# N5264, clone NN18, 1:100) was as described above for GAP-43. Immunohistochemistry for galanin (Peninsula Labs, BACHEM, catalog # T-4334 (IHC7141), lot # 030790-3) was performed as described above and previously (Werner et al., 2000) using DAB as the chromagen.
On longitudinal sections, images were digitized and labeled axons counted with the use of the NIH Image (Scion) program. The number of labeled axons in the nerve was obtained for the first 300 μm, and from 300 to 600 μm, past the site of the crush. The optical density was also measured using these same distances. To access the background, the optical density of a region with no specific signal was determined and subtracted that from the optical density of the region of the nerve measured. This was done on each section to account for the picture to picture variation in brightness. On cross sections, images were taken on a Zeiss Camera on an Olympus microscope using fluorescent illumination with a FITC filter. Photos were taken at a magnification of 10X using the AxioVision software. Labeled axons were counted using the NIH ImageJ software after a threshold size of 30 pixels was set. The number of labeled axons was obtained starting at the crush site, as evident from the presence of the India Ink, at increments of 300 μm, until labeled axons were no longer present.
Cytokine gene expression
Mice (n=8–9 WT and KO) were sacrificed 1 and 7 days after nerve crush and the nerve containing the crush site was surgically removed for RNA extraction. Ipsilateral facial motor nuclei were obtained as follows: Brains were fast frozen on dry ice and cut on a cryostat from the anterior side. When the anterior end of the FMN started to appear, the brain was removed from the cryostat and a 1 mm section was manually cut with an autoclaved razor blade. Using an atlas and available landmarks as a guide, further cuts were made just dorsal and medial to the FMN, and finally at the midline. While still frozen, nerve segments and ipsilateral FMN blocks were transferred to microfuge tubes, and total RNA isolated using the RNeasy Micro kit from Qiagen (Valencia, CA), reverse transcribed, and analyzed by real-time PCR. To obtain primers, cDNA encoding TNF-α, IL-6, IL-4 and IFN-γ were first analysed for secondary structures using M-fold software (BioRad). Portions of sequence lacking secondary structure were imported into Oligo6 software (Molecular Biology Insights) to design highly stringent primer sets. The following sequences of primers were used: for murine TNF-α, sense 5′-CATCTTCTCAAAATTCGAGTGACA-3′ and antisense 5′-TGGGAGTAGACAAGGTACAACCC-3′, for murine IL-6, sense 5′-TTCCATCCAGTTGCCTTCTTG-3′ and antisense 5′-TTGGGAGTGGTATCCTCTGTGA-3′, for murine IL-4, sense 5′-CGAGGTCACAGGAGAAGGGA-3′ and antisense 5′-AAGCCCTACAGACGAGCTCACT-3′, and for murine IFN-γ, sense 5′-TGCTGATGGGAGGAGAGATGTCT-3′ and antisense 5′-TTTCTTTCAGGGACAGCCTGTT-3′. The GenBank accession numbers and numbers for the 5′ and 3′ ends of the nucleotides for the PCR products are as follows: TNF-α, NM_013693, 424-575; IL-6, NM_031168, 76-156; IL-4, NM_021283, 201-301; IFN-γ, NM_008337. To standardize the experiments, we designed, using the same approach, a primer set (5′-CCGGCTTGTATGCTATC-3′ and 5′-AGTTCATGTTCGGCTTC-3′, as sense and antisense, respectively), for the mouse β-2-microglobulin gene. These primers amplified an 87-bp region encoding the nucleotides 99–185 of the published sequence (MM2BMR) of the mouse mRNA. Amplified TNF-α, IL-6, IL-4, IFN-γ and β2-microglobulin bands were cloned into PCRII and sequenced to confirm identity. Real-time PCR was set up using Syber Green-containing supermix from Biorad, for 50 cycles of a three-step procedure including a 30-s denaturation at 96 °C, a 30-s annealing at 60 °C, followed by a 30-s extension at 72°C. Amplification specificity was assessed by melting curve. The formula used was 2−ΔCt, where ΔCt is the difference in the Ct values for the target gene and the reference gene, β2-microglobulin (in each sample assayed).
Assessment of microglia response and gliosis in the FMN
The microglia and astrocyte response in the FMN were assessed seven days after nerve crush using antibodies to CD68 and GFAP, respectively (n=3 WT and KO). After perfusion in 4% PFA, brains were post-fixed, cryoprotected in 20% sucrose overnight, and embedded in OCT. Sections were cut on a cryostat at 12 μm and thaw mounted onto Superfrost slides, and then stored at −70°C. Slides were warmed for 30 minutes to room temperature and washed 3×5 minutes in 1X PBS and blocked with 3% normal goat serum for 1 hour, and then either CD68 antibody (Serotec, catalog # MCA1957, clone FA-11) at a dilution of 1:100 or GFAP antibody (Lab Vision Corporation, catalog # RB-087) at a dilution of 1:200 was applied to the sections and then incubated overnight at 4°C. A secondary antibody conjugated to either FITC or Texas Red was applied (1:200; Jackson Immuno Research Labs) for 1 hour. Slides were then washed in PBS and coverslipped with Vectashield (Vector Labs) mounting medium. Cells were counted on an Olympus microscope using fluorescent illumination.
Statistics
Except where otherwise indicated, differences between PACAP KO and WT mice were compared using two-tailed Student’s t-tests. Differences were considered significant at p < .05.
Results
Motor neuron survival after axotomy in PACAP-deficient mice
PACAP has been shown to act as a neuronal survival factor on cultured cerebellar granule, sensory, autonomic and other types of neurons, and has been shown to promote neuron survival in various in vivo experimental CNS injury paradigms (reviewed in (Waschek, 2002, Atlasz et al., 2007, Botia et al., 2007)). Thus, it was of considerable interest to determine if lack of endogenous PACAP results in impaired neuron survival after facial nerve axotomy. However, we found no difference between the PACAP KO and WT mice with regards to motor neuron survival 28 days after facial nerve axotomy. The mean numbers (+/− SEM) of surviving facial motor neurons per section in WT mice on ipsilateral and contralateral sides (corrected by the Abercrombie method for motor neuron profile size) were 5.5 +/− 0.3 and 7.9 +/− 1.0, respectively in WT mice (survival = 70%), and 5.0+/−0.4 and 6.3+/0.7, respectively in PACAP KO mice (survival = 79%); p > 0.05). Thus, in this model of motor nerve injury, deletion of the PACAP gene resulted in no detectable differences in neuron survival.
Recovery of reinnervation is delayed in PACAP KO mice
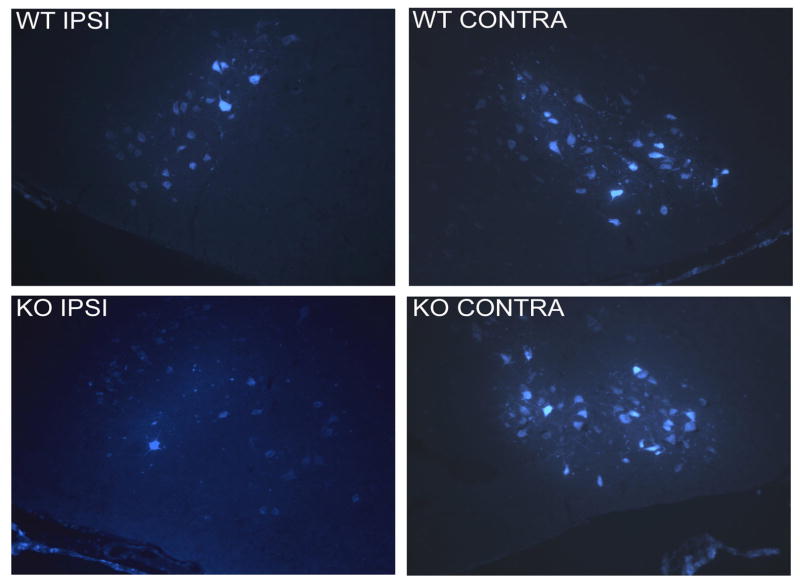
As a measure of nerve regeneration, we compared after crush injury the number of motor neurons whose axons re-entered the whisker pads over time in WT and PACAP KO mice. This was assessed by administering the retrograde tracer fluorogold into the whisker pad and lip at 6, 7, 9, and 12 days after nerve crush. In WT mice, recovery of retrograde transport was found to begin at 7 days and be complete by 12 days. PACAP KO mice showed a significantly reduced number of retrogradely labeled motor neurons at 7 days (Fig. 1 and 2), although by 12 days, there clearly was no difference between these two groups (Fig. 2). These data indicate that PACAP KO mice exhibit a delay in recovery of retrograde axonal transport to the whisker pads after facial nerve crush.
Fig. 1. FG retrograde labeling of facial motor neurons 7 days after nerve crush in WT and PACAP KO mice.
FG (2 μl of a 2% water solution) was injected bilaterally into both the whisker pad and the upper lip seven days after nerve crush. Two days later, animals were perfused and brain sections visualized.
Fig. 2. Time course of recovery of FG retrograde transport in WT and PACAP KO mice subjected to facial nerve crush.
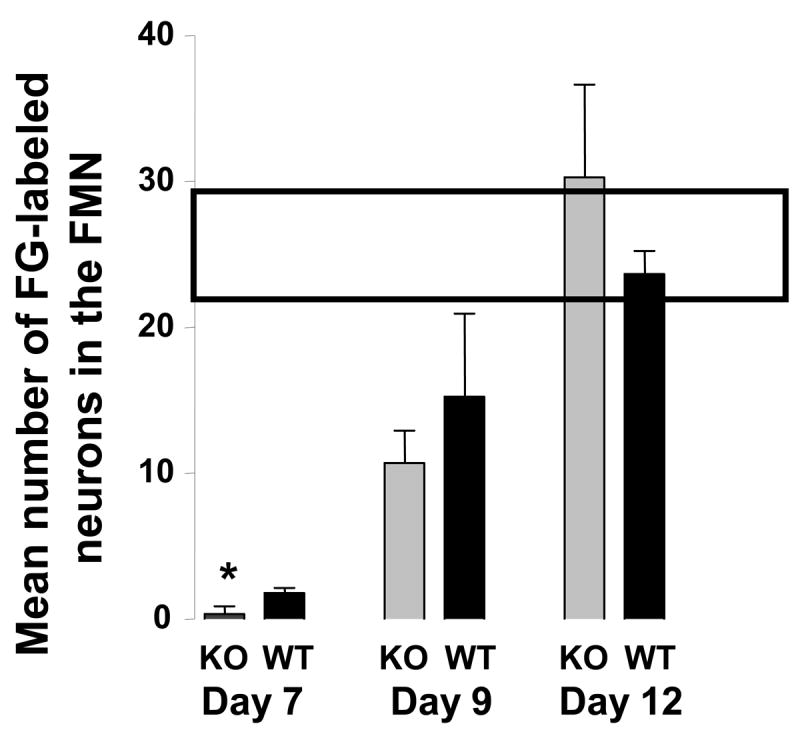
FG was injected as described in the Fig. 1 legend at 6, 7, 9, and 12 days after nerve crush. No labeling was observed in mice on day 6. The box across the top indicates the mean number +/− SEM of labeled neurons on the contralateral side over the course of the experiment.
Axonal growth is impaired in PACAP KO mice
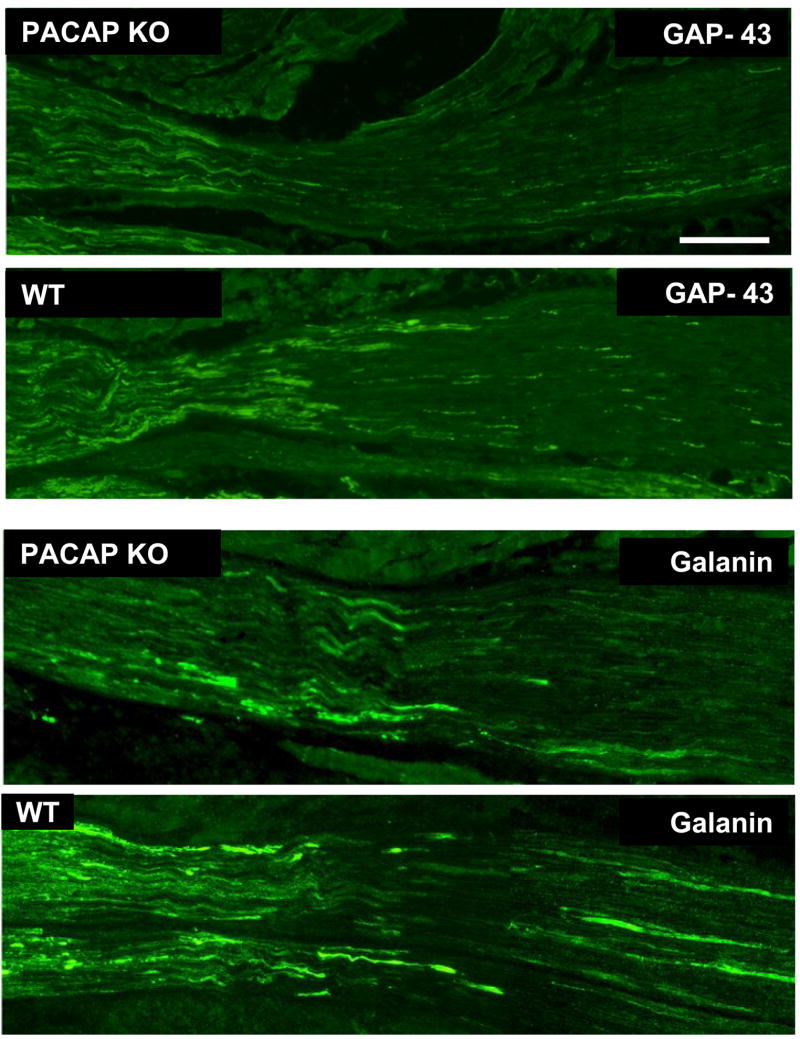
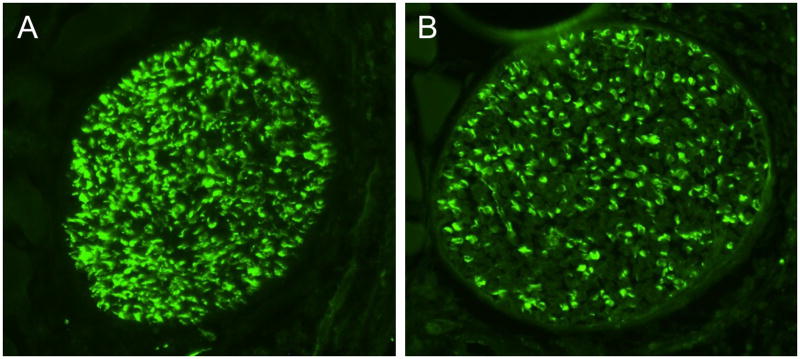
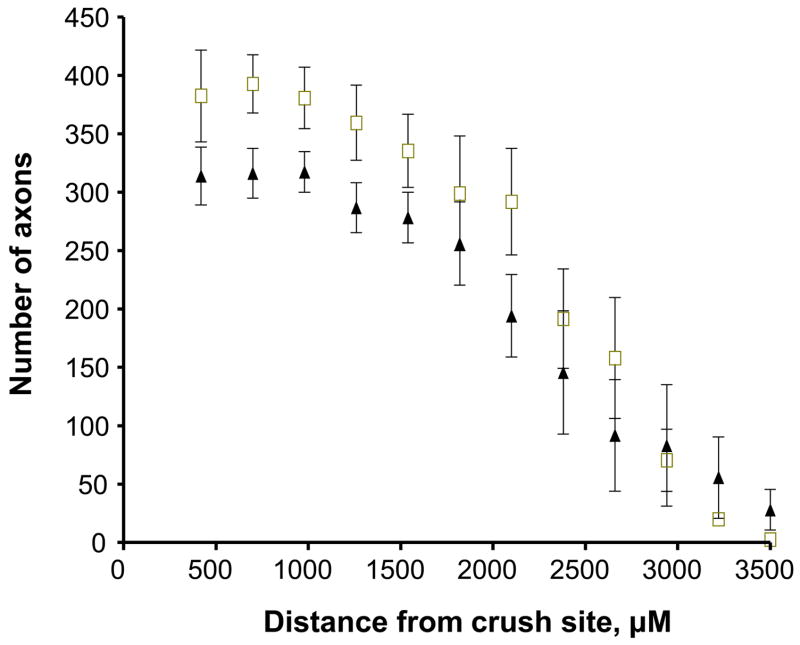
The above results suggested that the growth of axons across and beyond the site of injury might be impaired early after facial nerve crush. We thus performed immunohistochemistry using GAP-43 on longitudinal sections to label regenerating axons just distal to the lesion site 24 hours after crush. The immunofluorescence assay revealed bright GAP-43 labeled regenerating axons in the facial nerve distal to the crush site (Fig. 3, top two panels). Scoring of GAP-43 axons indicated that PACAP KO mice had significantly fewer labeled axons compared to WT mice in the nerve segment 300 to 600 μm from the crush site, but not in the more proximal segment (0 to 300 μm from the crush site) (Fig. 4A). In agreement with this result, quantification of overall fluorescence optical density in the nerve segments was decreased in PACAP KO mice, specifically in the more distal portion of the nerve (Fig. 4B). To confirm this result using another marker, we used galanin immunoreactivity to label regenerating axons (Fig. 3 bottom two panels). Again, this showed that PACAP KO mice had significantly reduced numbers of regenerating axons than WT mice specifically in the nerve segment 300 to 600 μm from the crush site (Fig. 5). Finally, to corroborate these data and to obtain quantitative information on the number of regenerating axons in WT vs. PACAP KO mice, we performed GAP-43 immunohistochemistry on cross sections of regenerating nerve at multiple distances distal to the nerve crush site three days after injury. These studies revealed that the mean number of regenerating axons was decreased by about 20% in PACAP KO mice at all distances examined to about 2.7 mm distal to the injury site (Fig. 6 and 7). On the other hand, the number of axons in non-axotomized facial nerves, measured by NF160 immunohistochemistry, did not differ between WT and PACAP KO mice (data not shown). Thus, direct visualization of regenerating axons in the injured nerve provided evidence that axon regenerating is delayed in PACAP KO mice, supporting the data obtained with the retrograde tracer fluorogold.
Fig. 3. Visualization of regenerating nerves after nerve crush in WT and PACAP KO mice by GAP-43 and galanin immunofluorescence.
24 hr after WT and PACAP KO mice were subjected to nerve crush, the nerves and surrounding musculature were surgically removed and fixed. Sections were stained with anti-GAP-43 (upper two panels) and anti-galanin (lower panels). Pictures were taken with a Zeiss camera on an Olympus microscope using fluorescent illumination with a FITC filter. The size bar in the top panel is 100 μM and corresponds to all photos. The crush site is near, or just to the left of, the center of each photo. The distal regenerating part of the nerve is to the right.
Fig. 4. Quantification of regenerating axons in longitudinal nerve segments of WT and PACAP KO mice by GAP-43 immunofluorescence.
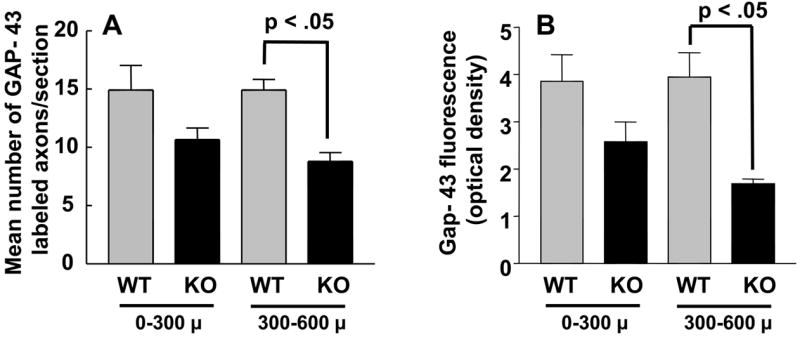
Nerve segments from WT and PACAP KO mice (n= 4 each) were dissected 24 hour after crush and subjected to Gap-43 immunohistochemistry as in Fig. 3 Serial images were digitized and quantified using the NIH Image (Scion) program. Panel A shows the mean number of GAP-43 immunofluorescent fibers in segments 0–300 and 300–600 microns distal to the nerve crush site in WT and KO mice. Panel B shows the overall fluorescence (optical density) in the same segments.
Fig. 5. Quantification of regenerating axons in longitudinal nerve segments of WT and PACAP KO mice by galanin immunofluorescence.
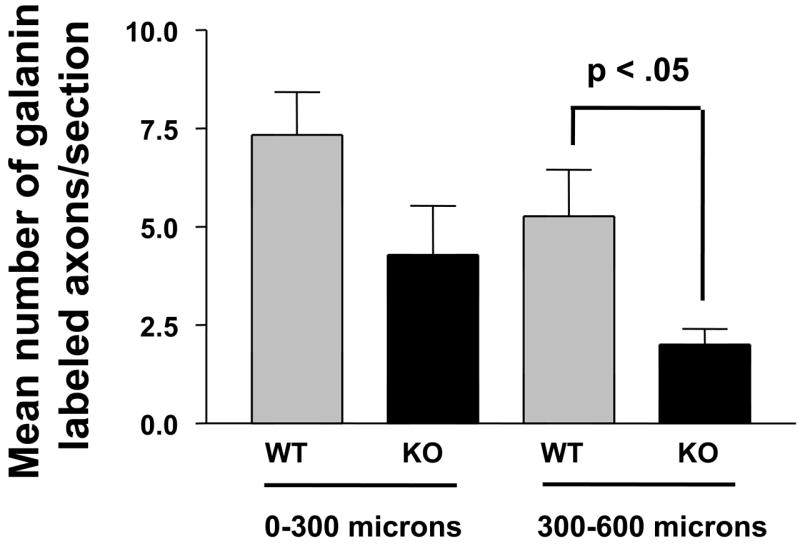
Nerve segments from WT (n=6) and PACAP KO (n=4) mice were dissected 24 hour after crush and subjected to galanin immunohistochemistry as in Fig. 3 Serial images were digitized and quantified using the NIH Image (Scion) program. Panel A shows the mean number of galanin immunofluorescent fibers in segments 0–300 and 300–600 microns distal to the nerve crush site in WT and KO mice.
Fig. 6. GAP-43 immunofluorescence on cross sections of regenerating facial nerves.
Photomicrographs show representative sections of regenerating nerves from a WT (A) and PACAP KO mouse (B) at a distance 1.5 mm distal to the crush site.
Fig. 7. Quantification of regenerating axons in nerve cross sections by GAP-43 immunofluorescence at various distances distal to the crush site of WT and PACAP KO mice.
Nerve segments from WT (n=4) and PACAP KO mice (n=5) were dissected 24 hour after crush, cut as cross sections, and subjected to GAP-43 immunohistochemistry. Serial images were digitized and quantified using the NIH ImageJ program. Differences between WT (squares) and PACAP KO mice (triangles) were significant at all distances less than 2.7 mm from the injury site at the level of p<0.01 (mixed model for repeated measures).
Altered microglial and cytokine response and normal astrogliosis in the FMN in PACAP KO mice
The axon regeneration defect observed in PACAP KO mice could be due to an altered reaction to injury in either the FMN where the motor neuron cell bodies reside, in the nerve crush site, or both. Two well-characterized responses in the FMN after facial nerve injury are astrogliosis and microglial proliferation/activation, both of which are confined to the ipsilateral FMN (Moran and Graeber, 2004). To assess these responses, we performed immunohistochemistry seven days after nerve crush using antibodies to glial fibrillary acidic protein (GFAP) and the activated microglia marker CD68, respectively. These studies showed that astrogliosis did not differ (Fig. 8A), but that the CD68+ microglia staining was increased in PACAP KO mice (Fig. 8B). Quantification of these responses determined by scoring the mean number GFAP and CD68 positive cells showed that while the number of GFAP+ cells did not differ between WT and PACAP KO mice (84 +/− 9 vs. 91 +/− 10, respectively, p > 0.05), WT animals had an average of 71.7+/−4.6 CD68 labeled cells per 12 μm section while KO animals had an average of 88.5 +/− 7.1 positive cells (p < 0.05). On the contralateral side, essentially no cells were labeled with the GFAP antibody, and only 5-10 cells/section were CD68+.
Fig. 8. Nerve crush-induced glial response in the FMN of WT vs. PACAP KO mice.
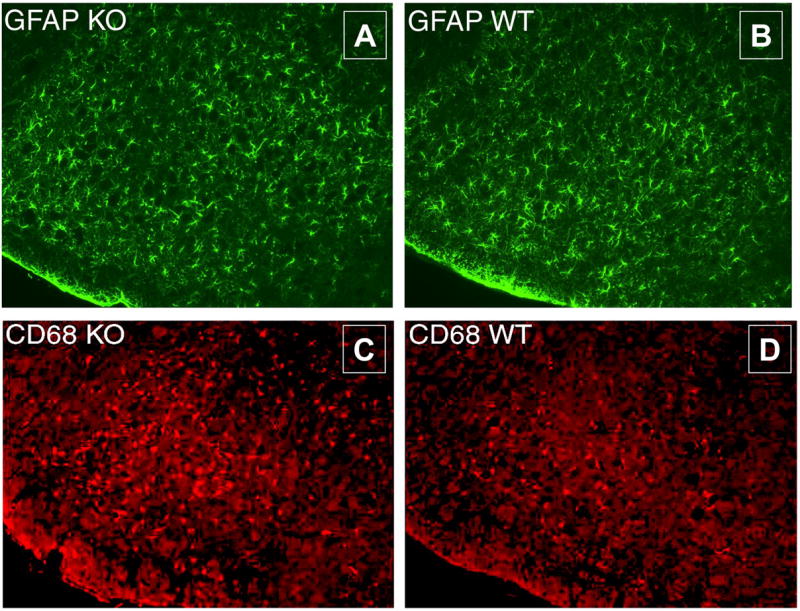
Seven days after nerve crush, WT and PACAP KO mice were perfused. Brains were removed, post-fixed, and embedded in OCT. Sections containing the FMN were stained with GFAP (A, B) and CD68 antibodies (C, D). Secondary antibodies conjugated to FITC or Texas Red were used, respectively. The ipsilateral nucleus is shown. The mean numbers of CD68+ and GFAP+ cells on contralateral side were less than 10 and 2, respectively, and did not differ between WT and PACAP KO mice.
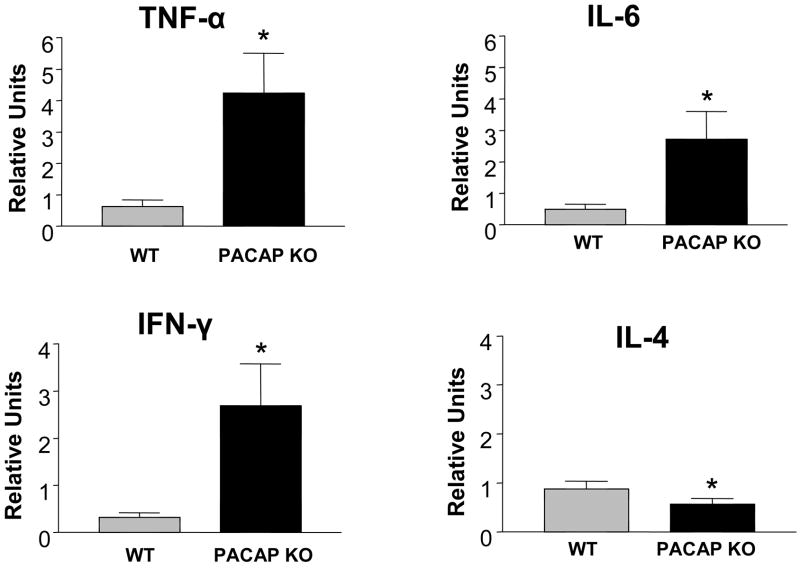
The fact that the response of microglia (resident macrophages in the CNS) was altered in PACAP KO mice suggested that the immune response to nerve injury might be different in WT vs. PACAP deficient mice. We thus measured in the ipsilateral FMN changes in gene expression of four representative cytokines, three which are considered proinflammatory (TNF-α, IL-6, and IFN-γ), and one which is considered anti-inflammatory (IL-4). Remarkably, the levels of proinflammatory cytokine mRNAs were eight to ten-fold higher in PACAP KO compared to WT mice (Fig. 9). Conversely, mRNA levels of the anti-inflammatory cytokine IL-4 were significantly reduced, albeit moderately. Thus PACAP KO mice exhibited an amplified inflammatory response in the FMN after facial nerve crush.
Fig. 9. Cytokine gene expression profiles in the facial motor nucleus of WT and PACAP KO mice.
WT and PACAP KO mice were subjected to nerve crush. Seven days later, brains were dissected, fast frozen on dry ice, and cut on a cryostat from the anterior side to the level of the facial motor nucleus with the aid of an atlas and landmarks. Cuts were made with an autoclaved razor blade just dorsal and medial to the facial motor nucleus, and finally about 1 mm deep. Samples were transferred to microfuge tubes, and total RNA was isolated, reverse transcribed, and analyzed by real-time PCR using primers for TNF-α, IFN-γ, IL-6, and IL-4 mRNAs. The units given are for the cytokine genes relative to β2-microglobin on the ipsilateral side. The graph represents data obtained from one experiment with n=8–9 mice. Statistical analysis was performed by Student’s t-test with *p<0.05, **p<0.01.
PACAP KO mice show an exaggerated immune response in the regenerating nerve
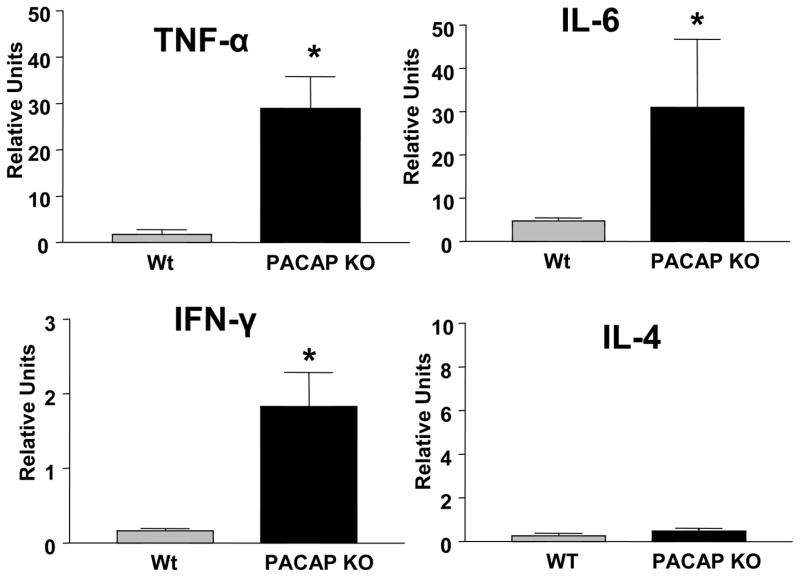
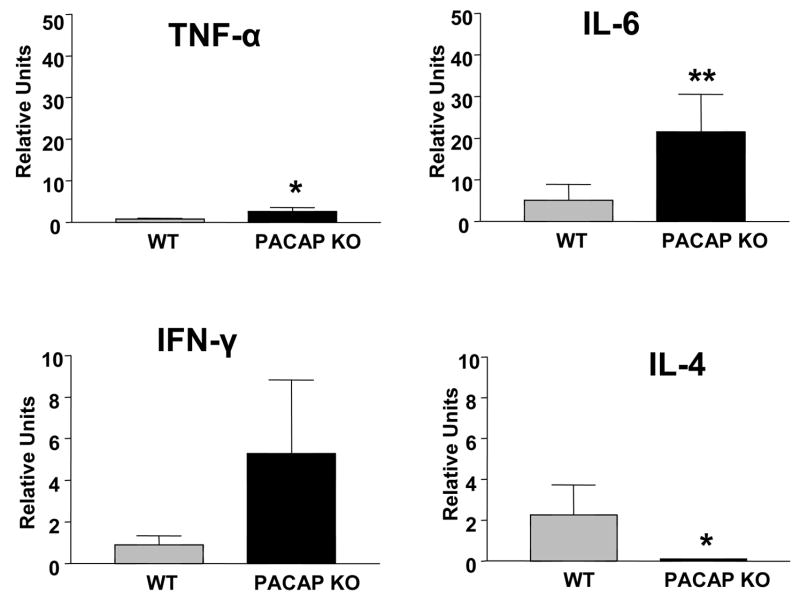
The fact the cytokine response in the FMN was altered in PACAP KO mice suggested that the immune response in the injured nerve might be also be affected. We thus examined the cytokine response at the crush site one and seven days after crush. The cytokine responses observed were similar to that observe in the FMN. One day after nerve crush, mRNA levels for the proinflammatory cytokines TNF-α, IL-6, and IFN-γ were 8- to 12-fold higher in PACAP KO vs. WT mice (Fig. 10). Levels of IL-4 gene expression were very low at this time, and not different between genotypes. Seven days after injury, the differences in proinflammatory cytokine mRNA levels were less pronounced between WT and PACAP KO mice. In contrast, levels of the anti-inflammatory cytokine IL-4 mRNA were greatly reduced in PACAP KO mice at this time (Fig. 11). Thus, like in the FMN, PACAP KO mice exhibited a greatly amplified inflammatory response at the peripheral nerve crush site
Fig. 10. Cytokine mRNA profiles in WT and PACAP KO mice 24 hours after nerve crush.
Regenerating nerve segments were collected from WT and PACAP KO mice and transferred to microfuge tubes. Total RNA was isolated, reverse transcribed, and analyzed by real-time PCR using primers for TNF-α, IFN-γ, IL-6, and IL-4 mRNAs. The units given are for the cytokine genes relative to β2-microglobin on the ipsilateral side. The graph represents data obtained from one experiment with n=8–9 mice. Statistical analysis was performed by Student’s t-test with *p<0.05, **p<0.01.
Fig. 11. Cytokine mRNA profiles in WT and PACAP KO mice seven days after nerve crush.
Regenerating nerve segments were collected from WT and PACAP KO mice and transferred to microfuge tubes. Total RNA was isolated, reverse transcribed, and analyzed by real-time PCR using primers for TNF-α, IFN-γ, IL-6, and IL-4 mRNAs. The units given are for the cytokine genes relative to β2-microglobin on the ipsilateral side. The graph represents data obtained from one experiment with n=8–9 mice. Statistical analysis was performed by Student’s t-test with *p<0.05, **p<0.01.
Discussion
An abundance of in vivo and in vitro data indicates that exogenous PACAP can act on developing or injured neurons to promote their survival (Vaudry et al., 2000, Waschek, 2002). Tissue cultures studies also indicate that PACAP can stimulate neurite outgrowth (Waschek, 2002), guide growth cones from immature neurons (Guirland et al., 2003) and regulate myelinogenesis (Lee et al., 2001), suggesting that PACAP might also act in the process of axon regeneration after injury. The generation of a PACAP deficient mouse model provides an opportunity to determine if endogenous PACAP plays a role these processes. We chose to utilize the facial nerve injury paradigm to study the role of PACAP in injury because 1) PACAP gene expression is strongly induced in motor neurons in this model (Armstrong et al., 2003), 2) the lesions (crush or axotomy) are simple, reproducible, and occur outside the brain, and 3) the response to both crush and axotomy are well characterized in the literature. Our results show that although loss of PACAP did not lead to a reduction in motor neuron survival, axon regeneration was significantly delayed. The delayed regeneration was associated with an enhanced microglial reaction in the FMN, and significantly increased levels of proinflammatory cytokine gene expression in both the FMN and nerve crush site. In contrast, mRNA levels were reduced for IL-4, a cytokine known to inhibit inflammation by blocking macrophage activity. However, the relationship between the observed inflammation changes and the nerve regeneration defect in PACAP KO mice is unknown. Additional study will be required to determine if the inflammatory differences are mechanistically linked to the regeneration defect.
The altered cytokine response observed in PACAP KO mice is consistent with data indicating that PACAP exerts immunomodulatory actions via high affinity receptors expressed on macrophages and T lymphocytes (reviewed in (Delgado et al., 2004). T lymphocyte accumulation into injured peripheral nerves and into the FMN of facial nerve axotomized rodents has been demonstrated (Raivich et al., 1998, Moalem et al., 1999). Two well-studied immunomodulatory actions of PACAP and VIP (acting primarily via receptors which interact with both peptides) are their ability to inhibit the production and release of proinflammatory cytokines like TNF-α and IL-6 from macrophages, and to promote the balance of T helper (Th) cytokines from Th1 to Th2 (characterized by the prototype cytokines IFN-γ and IL-4, respectively). It is notable that PACAP KO mice exhibited an enhanced Th1/Th2 cytokine ratio in injured tissue, as exhibited by higher IFN-γ and lower IL-4 mRNA levels compared to WT mice (Figs. 9–11). Thus, the cytokine response in nerve-injured PACAP KO mice was altered, with excess production of proinflammatory cytokines. Although it remains to be determined if the altered cytokine response is responsible for the facial nerve regeneration defect in PACAP KO mice, certain evidence points to this possibility. First, it is known that SCID mice, which lack functional lymphocytes, show reduced facial motor neuron survival after axotomy (Serpe et al., 1999), and a strong delay in nerve regeneration in the facial crush model, a defect that is rescued by preinfusion of splenocytes from normal mice (Serpe et al., 2002). Thus, the immune system clearly plays a role in axonal regeneration in this model. Second, in the experimental autoimmune encephalomyelitis (EAE), Th1 cytokines predominate and mediate inflammatory damage, whereas Th2 cytokines have been associated with remissions and recovery from disease (Khoury et al., 1992). In fact, PACAP administration was recently shown to inhibit the clinical symptoms and pathological manifestations in MOG35-55-induced EAE, and inhibit the production of the Th1 cytokine IFN-γ in MOG-specific T lymphocytes (Kato et al., 2004). Our data, on the other hand, shows that loss of PACAP results in an unbalanced cytokine response after nerve crush, with a bias towards a pro-inflammatory Th1 phenotype. Thus, one function of PACAP after nerve and perhaps other types of injury might be to delimit and/or provide temporal control of the inflammatory immune response.
As already discussed, it remains to be determined if the altered immune response in PACAP KO mice is causally related to the nerve regeneration defect. The impairment might also be related to the ability of PACAP to act directly on neurons to induce axonal regrowth. PACAP has been shown to induce neuritogenesis in at least three different primary cell culture models, sympathetic neuroblasts, cortical precursors and cerebellar granule cells (reviewed in (Waschek, 2002)) and to induce turning of growth cones of embryonic Xenopus spinal cord neurons (Guirland et al., 2003). Moreover, VIP has been shown to increase the in vitro Schwann cell production of laminin, which is a potential substrate for regenerating axons (Zhang et al., 1996). On the other hand, exogenous PACAP was unable to stimulate neurite outgrowth after nerve crush in nodose ganglia/vagal explants, a model which does not allow influx of immune cells into the injured nerve (Reimer et al., 1999).
We did not observe an effect of loss of PACAP on either motor neuron survival after axotomy. This was surprising given the data indicating that exogenous PACAP is neuroprotective in in vivo stroke and other CNS injury models (Reglodi et al., 2000, Takei et al., 2000, Reglodi et al., 2002), and that PACAP has been shown to act as a survival factor on numerous types of immature neurons, including motor neurons (Arimura et al., 1994). Among the many reasons that could explain this negative finding, the loss of PACAP could be compensated for by another peptide, such as VIP. This peptide is 70% homologous to PACAP, shares two if its receptors (VPAC1 and VPAC2), and is also strongly induced in this model (Armstrong et al., 2003). We observed that PACAP-preferring receptor (PAC1) gene expression decreased by about 80% in facial motor neurons after facial nerve axotomy in rats, whereas VPAC2 mRNA levels did not significantly change (Zhou et al., 1999).
While the data presented here indicate that PACAP can modulate the inflammatory response after nerve injury, other data indicate that inflammation itself can induce PACAP expression in neurons. For example, administration of complete Freund’s adjuvant to the rat paw was shown to induce PACAP expression in sensory neurons (Zhang et al., 1998). On the basis of those findings, we investigated in other studies the possibility that the immune response after facial nerve injury contributes to the upregulation of PACAP in the affected facial motor neurons. First we found that that induction of PACAP mRNA after facial nerve injury was mimicked by local application of an inflammatory stimuli to the nerve (Armstrong et al., 2004b), and that the PACAP inductions after either axotomy or nerve inflammation were blocked in SCID mice (Armstrong et al., 2003, Armstrong et al., 2004b). The loss of PACAP gene expression in SCID mice after axotomy was fully reversed by an infusion of normal splenocytes (Armstrong et al., 2003) or by an infusion of CD4+ T lymphocytes (Armstrong et al., 2004a). The latter suggested that the induction of PACAP mRNA requires inflammatory mediators from T helper lymphocytes. In the most recent experiments, the induction of PACAP was found to be attenuated in STAT-6, but not STAT-4 KO mice (Armstrong et al., 2006), suggesting that the Th2 response is necessary for the axotomy-induced upregulation of PACAP gene expression. The deficient Th2 response observed here in nerve-lesioned PACAP KO mice suggest that one action of endogenous PACAP is to promote or facilitate a Th2 response. Thus a Th2-mediated induction of PACAP might serve as a positive regulatory loop to amplify the Th2 response and thereby reinforce anti-inflammatory actions of PACAP on macrophages and/or microglia.
Acknowledgments
This work was supported by NIH Grants HD06576 and HD04612, and grants from the Norman Cousins Center for Psychoneuroimmunology, and the Roman Reed Spinal Cord Injury Research Fund of California. We thank Dr. He-Jing Wang for help with statistical analyses of the data.
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- FG
fluorogold
- FMN
facial motor nucleus
- GFAP
glial fibriallary acidic protein
- IFN
interferon
- IL
interleukin
- KO
knockout
- NF
neurofilament
- PACAP
pituitary adenylyl cyclase activating peptide
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- Th
T helper
- TNF
tumor necrosis factor
- VIP
vasoactive intestinal peptide
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anatomy Record. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Weill C, Fiore RC, Tatsuno I, Bay V, Brenneman DE. PACAP functions as a neurotrophic factor. Ann N Y Acad Sci. 1994;739:228–243. doi: 10.1111/j.1749-6632.1994.tb19825.x. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Coute AC, Ngo DH, Waschek JA. Impairment of axotomy-induced pituitary adenylyl cyclase-activating peptide gene expression in T helper 2 lymphocyte-deficient mice. Neuroreport. 2006;17:309–312. doi: 10.1097/01.wnr.0000199465.54907.74. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Rodriguez W, Cheung-Lau G, Trinh V, Waschek JA. Restoration of axotomy-induced PACAP gene induction in SCID mice with CD4+ T-lymphocytes. Neuroreport. 2004a;15:2647–2650. doi: 10.1097/00001756-200412030-00018. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Lee M, Chhith S, Gomariz RP, Waschek JA. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience. 2004b;129:93–99. doi: 10.1016/j.neuroscience.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- Atlasz T, Babai N, Kiss P, Reglodi D, Tamas A, Szabadfi K, Toth G, Hegyi O, Lubics A, Gabriel R. Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. Gen Comp Endocrinol. 2007;153:108–114. doi: 10.1016/j.ygcen.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Botia B, Basille M, Allais A, Raoult E, Falluel-Morel A, Galas L, Jolivel V, Wurtz O, Komuro H, Fournier A, Vaudry H, Burel D, Gonzalez BJ, Vaudry D. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides. 2007 doi: 10.1016/j.peptides.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Chen Y, Samal B, Hamelink CR, Xiang CC, Chen Y, Chen M, Vaudry D, Brownstein MJ, Hallenbeck JM, Eiden LE. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul Pept. 2006;137:4–19. doi: 10.1016/j.regpep.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Waschek JA. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1194–1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- Deboy CA, Xin J, Byram SC, Serpe CJ, Sanders VM, Jones KJ. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exp Neurol. 2006;201:212–224. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. Faseb J. 2003;17:1922–1924. doi: 10.1096/fj.02-1029fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide stimulate the induction of Th2 responses by up-regulating B7.2 expression. J Immunol. 1999;163:3629–3635. [PubMed] [Google Scholar]
- Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Guirland C, Buck KB, Gibney JA, DiCicco-Bloom E, Zheng JQ. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J Neurosci. 2003;23:2274–2283. doi: 10.1523/JNEUROSCI.23-06-02274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, Ueda R, Suzumura A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Kawatani M, Ito E, Ishikawa K. Effects of pituitary adenylate cyclase-activating polypeptide on facial nerve recovery in the Guinea pig. Laryngoscope. 2003;113:1000–1006. doi: 10.1097/00005537-200306000-00016. [DOI] [PubMed] [Google Scholar]
- Lazarov-Spiegler O, Solomon AS, Schwartz M. Peripheral nerve-stimulated macrophages simulate a peripheral nerve-like regenerative response in rat transected optic nerve. Glia. 1998;24:329–337. [PubMed] [Google Scholar]
- Lee M, Lelievre V, Zhao P, Torres M, Rodriguez W, Byun JY, Doshi S, Ioffe Y, Gupta G, de los Monteros AE, de Vellis J, Waschek J. Pituitary adenylyl cyclase-activating polypeptide stimulates DNA synthesis but delays maturation of oligodendrocyte progenitors. J Neurosci. 2001;21:3849–3859. doi: 10.1523/JNEUROSCI.21-11-03849.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. Febs J. 2005;272:2628–2638. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- Moalem G, Monsonego A, Shani Y, Cohen IR, Schwartz M. Differential T cell response in central and peripheral nerve injury: connection with immune privilege. Faseb J. 1999;13:1207–1217. doi: 10.1096/fasebj.13.10.1207. [DOI] [PubMed] [Google Scholar]
- Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res Brain Res Rev. 2004;44:154–178. doi: 10.1016/j.brainresrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, Yofu S, Hashimoto H, Shintani N, Baba A, Kopf M, Iwakura Y, Matsuda K, Arimura A, Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Vigh S, Maderdrut JL, Arimura A. Neuroprotective effects of PACAP38 in a rat model of transient focal ischemia under various experimental conditions. Ann N Y Acad Sci. 2000;921:119–128. doi: 10.1111/j.1749-6632.2000.tb06958.x. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Somogyvari-Vigh A, Szanto Z, Kertes E, Lenard L, Arimura A, Lengvari I. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides. 2002;23:2227–2234. doi: 10.1016/s0196-9781(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Reimer M, Moller K, Sundler F, Hannibal J, Fahrenkrug J, Kanje M. Increased expression, axonal transport and release of pituitary adenylate cyclase-activating polypeptide in the cultured rat vagus nerve. Neuroscience. 1999;88:213–222. doi: 10.1016/s0306-4522(98)00240-1. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe CJ, Tetzlaff JE, Coers S, Sanders VM, Jones KJ. Functional recovery after facial nerve crush is delayed in severe combined immunodeficient mice. Brain Behav Immun. 2002;16:808–812. doi: 10.1016/s0889-1591(02)00017-x. [DOI] [PubMed] [Google Scholar]
- Suarez V, Guntinas-Lichius O, Streppel M, Ingorokva S, Grosheva M, Neiss WF, Angelov DN, Klimaschewski L. The axotomy-induced neuropeptides galanin and pituitary adenylate cyclase-activating peptide promote axonal sprouting of primary afferent and cranial motor neurones. Eur J Neurosci. 2006;24:1555–1564. doi: 10.1111/j.1460-9568.2006.05029.x. [DOI] [PubMed] [Google Scholar]
- Suh J, Lu N, Nicot A, Tatsuno I, DiCicco-Bloom E. PACAP is an anti-mitogenic signal in developing cerebral cortex. Nat Neurosci. 2001;4:123–124. doi: 10.1038/83936. [DOI] [PubMed] [Google Scholar]
- Takei N, Torres E, Yuhara A, Jongsma H, Otto C, Korhonen L, Abiru Y, Skoglosa Y, Schutz G, Hatanaka H, Sofroniew MV, Lindholm D. Pituitary adenylate cyclase-activating polypeptide promotes the survival of basal forebrain cholinergic neurons in vitro and in vivo: comparison with effects of nerve growth factor. Eur J Neurosci. 2000;12:2273–2280. doi: 10.1046/j.1460-9568.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Fournier A, Vaudry H. Neurotrophic activity of pituitary adenylate cyclase-activating polypeptide on rat cerebellar cortex during development. Proc Natl Acad Sci U S A. 1999;96:9415–9420. doi: 10.1073/pnas.96.16.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- Werner A, Willem M, Jones LL, Kreutzberg GW, Mayer U, Raivich G. Impaired axonal regeneration in alpha7 integrin-deficient mice. J Neurosci. 2000;20:1822–1830. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QL, Lin PX, Shi D, Xian H, Webster HD. Vasoactive intestinal peptide: mediator of laminin synthesis in cultured Schwann cells. J Neurosci Res. 1996;43:496–502. doi: 10.1002/(SICI)1097-4547(19960215)43:4<496::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Danielsen N, Sundler F, Mulder H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport. 1998;9:2833–2836. doi: 10.1097/00001756-199808240-00027. [DOI] [PubMed] [Google Scholar]
- Zhou X, Rodriguez WI, Casillas RA, Ma V, Tam J, Hu Z, Lelievre V, Chao A, Waschek JA. Axotomy-induced changes in pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP receptor gene expression in the adult rat facial motor nucleus. J Neurosci Res. 1999;57:953–961. [PubMed] [Google Scholar]