Abstract
We report four young women who developed acute psychiatric symptoms, seizures, memory deficits, decreased level of consciousness, and central hypoventilation associated with ovarian teratoma (OT) and cerebrospinal fluid (CSF) inflammatory abnormalities. Three patients recovered with treatment of the tumor or immunosuppression and one died of the disorder. Five other OT patients with a similar syndrome and response to treatment have been reported. Our patients' serum or CSF showed immunolabeling of antigens that were expressed at the cytoplasmic membrane of hippocampal neurons and processes and readily accessed by antibodies in live neurons. Immunoprobing of a hippocampal-expression library resulted in the isolation of EFA6A, a protein that interacts with a member of the two-pore-domain potassium channel family and is involved in the regulation of the dendritic development of hippocampal neurons. EFA6A-purified antibodies reproduced the hippocampal immunolabeling of all patients' antibodies and colocalized with them at the plasma membrane. These findings indicate that in a young woman with acute psychiatric symptoms, seizures, and central hypoventilation, a paraneoplastic immune-mediated syndrome should be considered. Recognition of this disorder is important because despite the severity of the symptoms, patients usually recover. The location and function of the isolated antigen suggest that the disorder is directly mediated by antibodies.
Paraneoplastic limbic encephalitis (LE) often associates with brainstem dysfunction and predominantly affects older individuals with lung cancer.1 A review of 137 patients with LE showed that young individuals with germ-cell tumors of the testis or ovarian teratoma (OT) were more frequently affected than patients of any age with more prevalent tumors such as cancer of the breast, prostate, or colon.2 Subsequent studies defined the LE associated with germ-cell tumors as a syndrome with dominant limbic, diencephalic and upper brainstem dysfunction, and the Ma proteins as the main autoantigens.3 Because germ-cell tumors often contain teratoma elements, we reasoned that a similar disorder may occur in women with OT. This led us to investigate the neurological and immunological features of four women with OT and encephalitis examined by us, and to review the clinical features of similar cases in the literature.4-12 None of our four patients had antibodies to Ma proteins, but we were impressed by the similarity and severity of the neurological symptoms, which often resembled an acute psychotic episode, malingering, or drug abuse. These patients often had cerebrospinal fluid (CSF) inflammatory abnormalities and the neurological syndrome improved after tumor resection, immunotherapy, or both. On the basis of these observations, we postulated that teratoma-associated encephalitis is an immune-mediated disorder and that if antibodies are involved they are not detected by conventional testing. We report the clinical features of this disorder along with the associated antibodies and preliminary characterization of one of the antigens.
Patients and Methods
Four patients were examined by the authors, and sera or CSF was obtained at symptom presentation (three cases) or recurrence (one case) and kept frozen at −80°C until use. A brief description of Patient 1 has been reported previously (Case 4 in Ances and colleagues12); this patient and Patient 2 are fully reported here (see online supplementary information). The clinical features of Patients 3 and 4 have been previously reported.9-11
Immunohistochemistry and Immunocompetition Assays
Rats were anesthetized and euthanized by decapitation and the brain removed and processed as reported.12 Frozen 7μm-thick sections were directly mounted on slides and the patients' sera (diluted 1:250) or CSF (1:10) were tested using the avidin-biotin-peroxidase technique.12 To determine whether patients' antibodies targeted the same epitopes, we used immunocompetition assays between IgG biotinylated from one patient's serum and whole serum of other patients.13
Distribution of Immunolabeling in Hippocampal Neuronal Cultures
Rat hippocampal neuronal cultures were prepared as reported.14 Neurons were grown on coverslides, fixed with paraformaldehyde (PFA), and serially incubated with patients' sera (1:250) for 1 hour and fluorescein-labeled goat anti–human IgG for 30 minutes. After washing, slides were incubated with biotinylated IgG from control patients with voltage-gated potassium channels (VGKCs) or normal individuals or mouse antibodies to the VGKC Kv1.2 (1:50; Upstate Biotechnology, Lake Placid, NY) or ARF6 (1:25; Chemicon International, Temecula, CA). The reactivity of biotinylated human IgG was developed with avidin-rhodamine (1:2000; Vector, Burlingame, CA) and the reactivity of mouse antibodies was developed with Alexa Fluor rhodamine-labeled goat anti–mouse IgG (1:2000; Molecular Probes, Eugene, OR).
Expression of Antigens in Live Hippocampal Neurons
To determine whether the target antigens were accessible in live neurons, we added patients' antibodies to the neuronal cell cultures for 30 minutes. Afterward, the media was removed and the neurons were gently washed in phosphate-buffered saline and fixed in PFA, and the bound antibodies were revealed with Alexa Fluor fluorescein-labeled anti–human IgG (1:2,000). Live neurons similarly incubated with normal human IgG and anti–Hu IgG served as controls. All human IgGs were used at concentration 0.37μg/ml obtained from patients' CSF.
Isolation of cDNA Clones and Affinity Purification of Antibodies
A uni-ZAP-XR rat hippocampal library (Stratagene, La Jolla, CA) was probed with sera from Patients 1, 2, and 3, as reported.15 Positive clones were purified with several rounds of antibody screening and subcloned into pBluescript using the in vivo phage rescue protocol (Stratagene) and sequenced. Filters with purified relevant or irrelevant phage plaques were incubated with patients' or control sera for 12 hours at 4°C. After acid elution and neutralization, antibodies were concentrated with a column of protein A–Sepharose and used in immunohistochemical studies as indicated above.
Results
Clinical Findings
The four patients had in common the development of acute behavioral changes and prominent psychiatric symptoms that were followed by rapid neurological deterioration, seizures (in three cases), decrease of level of consciousness or hypersomnia, and central hypoventilation, requiring admission to an intensive care unit and extended ventilatory support. This neurological syndrome is similar in many respects to that of five previously reported patients with OT (Table 1). When considered together, the clinical picture of these nine patients was dominated by severe behavioral and personality changes (eight patients), short-term memory deficits (six at presentation, and two noted after recovering from seizures or decreased level of consciousness), central hypoventilation (five), seizures (five), and hallucinations (three). The neurological disorder preceded the tumor diagnosis in seven patients (median, 1 month; range, 1–18 months) and developed shortly after the tumor diagnosis in two patients. The associated OT was benign or mature in four patients (one diagnosed radiologically as a dermoid cyst), immature in four, and mixed in one.
Table 1.
Clinical Features in Patients with Teratoma-Associated Encephalitis
| Case No. |
Sex/Age, yr (teratoma histology) |
Time from TE to Tumor Diagnosis |
Prodrome | Main Symptoms | Other Symptoms |
|---|---|---|---|---|---|
| 112 | F/26 (dermoid cyst)a | 3 weeks | Decrease appetite and insomnia (3 weeks) | Psychiatric syndrome,b generalized seizures, CHV | Incomprehensive speech, decrease of level of consciousness, STMD |
| 2 | F/40 (mature) | 3 weeks | — | Secondary generalized seizures, psychiatric syndrome,b CHV | Decrease of level of consciousness, STMD |
| 311 | F/14 (immature) | 2 mo | Rhinorrhea, cough, fever (2 days) | Psychiatric syndrome (hallucinations, extreme panic), generalized seizures, CHV | Incomprehensive speech, choreoathetotic movements, hypersomnia, autonomic instability |
| 49,10 | F/28 (mature) | 1 mo after tumor | — | First episode: psychiatric syndrome (delusional thinking, personality change), auditory hallucinations, STMD, dysphagia, horizontal nystagmus, vertical gaze paresis, CHV | First episode: hypersomnia, comatose, flaccid paraplegia |
| Second episode: dysarthria. Third episode: diplopia, facial numbness, dysphagia, ataxia. | |||||
| 54 | F/19 (immature) | 3 mo | Headache, nausea, fever, insomnia (2 mo) | STMD, psychiatric syndrome (depression, “schizophrenia”), myoclonic seizures, decrease consciousness, CHV | Hypersomnia, catalepsy-like features, decrease level of consciousness |
| 65 | F/15 (immature) | 1 mo | — | STMD, psychiatric syndrome (acutely confused, incoherent thoughts) | — |
| 76 | F/39 (immature) | 1 mo after tumor | — | STMD, psychiatric syndrome (depression, delusions of persecution, feeling of impending doom), secondary generalized seizures | Wernicke's-like aphasia, disinhibited, Kluver–Bucy syndrome |
| 88 | F/15 (mixed) | 18 mo | Failure at school, STMD (18 mo), headache insomnia, (2 days) | Psychiatric syndrome (behavioral change, acute confusion), auditory hallucinations | Incomprehensive speech (expressive aphasia), hypersomnia, tremor, rigidity |
| 97 | F/33 (mature) | 3 mo | — | STMD | — |
Radiological diagnosis.
Described in supplementary material available online. TE = teratoma-associated encephalitis.”
STMD = short-term memory deficit; CHV = central hypoventilation.
In six of nine patients, brain magnetic resonance imaging (MRI) was abnormal and in three normal (Fig 1, Table 2). Abnormalities usually were seen with fluid-attenuated inversion recovery (FLAIR) sequences and involved several brain regions (three patients), brainstem (two), cerebellum (one), pituitary gland and infundibulum (one), and spinal cord (one). Brain 18Ffluorodeoxyglucose (FDG) positron emission tomography (PET) or single-photon emission CT (SPECT) obtained in four patients demonstrated abnormalities not seen with MRI in temporal lobes (three patients), basal ganglia (one patient), and brainstem (one patient; see Fig 1). The CSF was abnormal in seven patients: four with pleocytosis and increased protein concentration, two with pleocytosis, one with increased protein concentration. Electroencephalogram was abnormal in all eight patients examined: seven had diffuse or focal slow wave activity, and one had bilateral temporalparietal epileptic activity (Patient 3).
Fig 1.
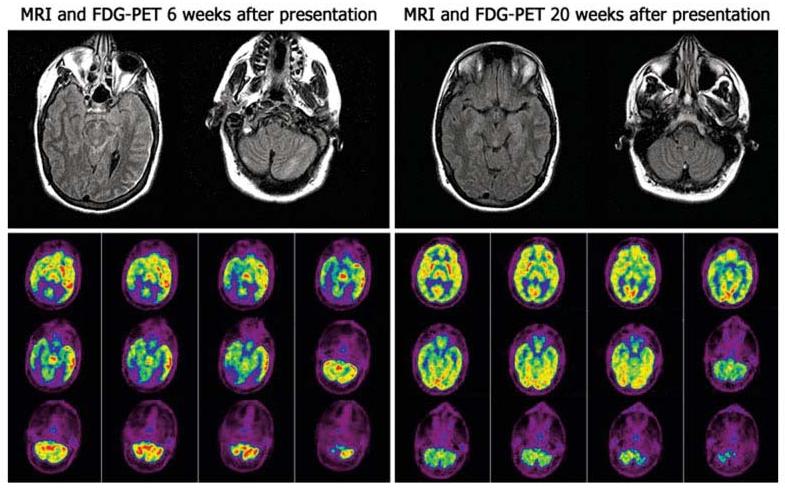
Brain magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) of Patient 1. A brain MRI obtained 6 weeks after symptom presentation showed fluid-attenuated inversion recovery and T2 hyperintensities over the left cerebral cortex and cerebellum; mild contrast enhancement was noted in the cerebellum (data not shown). FDG-PET demonstrated increased FDG activity in the left temporal lobe, brainstem and cerebellum, and hypoactivity in the occipital lobes. A follow-up MRI obtained 14 weeks later was normal; the FDG-PET showed minimal decreased activity in the left temporoparietal region.
Table 2.
Diagnostic Tests, Treatment, and Outcome
| Case | Neuroimaging | CSF | Treatment | Interval First Symptom to Initial Improvement |
Outcome (duration follow-up) |
|---|---|---|---|---|---|
| 1 | MRI: FLAIR/T2 hyperintensities in cerebral cortex and cerebellum; mild cortical cerebellar enhancement FDG-PET: increased activity in the left temporal lobe, brainstem and cerebellum; decreased in occipital lobes | 49 WBC; protein 67; glucose 66 | Corticosteroids | Approximately 7 weeks (49 days of ventilatory support) | Complete recovery (14 mo) |
| 2 | MRI: FLAIR abnormalities involving the cingulum and gray matter of the frontal lobes | 9 WBC; glucose 3.3; protein 40 | Surgery | Approximately 16 weeks (90 days of ventilatory support) | Residual cognitive dysfunction and memory problem (5 yr) |
| 3 | MRI: small enhancing suprasellar mass with an enhancing and enlarged pituitary and infundibular tubercinereum; SPECT: normal | 115 WBC; protein 92; glucose 71 | Surgery, IVIg, plasma exchange | No improvement (ventilatory support until death approximately 90 days) | Died 6 mo after symptom presentation |
| 4 | MRI first episode: T2 hyperintensity in the dorsal aspect of the medulla and three similar areas in the spinal cord. Second episode: T2 hyperintensity in the dorsal aspect of the medulla. Third episode: FLAIR/T2 hyperintensity in dorsal pons | First episode: WBC 23; protein 61; glucose 4.2 Second, third episodes: normal | Each episode: surgery, IVIg, corticosteroids | First episode: approximately 8 weeks (30 days of ventilatory support) Second episode: 6 weeks Third episode: 2 weeks | First episode: recovered memory and cognitive function; residual mild truncal dysesthesias (6 mo) Second, third episodes: complete recovery (10 mo) |
| 5 | MRI: small hyperintensity in the right medial temporal lobe and pons. SPECT: increased perfusion in the frontotemporal cortex | WBC 34; protein 19; glucose 63 | Surgery, corticosteroids | Approximately 11 weeks (39 days of ventilatory support) | Partial improvement; residual short-term memory (6 mo) |
| 6 | MRI: normal | Normal | Surgery | Approximately 14 weeks | Complete recovery (32 mo) |
| 7 | MRI: normal | WBC 65; protein 64; glucose 50 | Surgery, chemotherapy | Approximately 12 weeks | Residual short-term memory and cognitive deficit; brain MRI normal (21 mo) |
| 8 | MRI: normal; SPECT: decreased perfusion in anterior pole of temporal lobes and right basal ganglia | No pleocytosis, protein 54 | Surgery, corticosteroids | “After corticosteroids” | Partial improvement |
| 9 | — | Normal | Surgery | >3 mo | Complete recovery (12 mo) |
CSF = cerebrospinal fluid; MRI = magnetic resonance imaging; FLAIR = fluid-attenuated inversion recovery; FDG = 18F-fluorodeoxyglucose; PET = positron emission tomography; WBC = white blood cell; IVIg = intravenous immunoglobulin; SPECT = single-photon emission computed tomography.
All patients except Patient 1 had surgery and tumor resection; four patients received corticosteroids, two received intravenous immunoglobulins (IVIg), one received plasma exchange, and one received chemotherapy (see Table 2). Eight patients had neurological improvement (four full recovery) and one died as a result of the neurological disorder. In six patients with available information, the median time from development of the main neurological syndrome until initial evidence of improvement was 12 weeks (range, 7–16 weeks), and for those requiring ventilatory support the median time on a ventilator was 49 days (range, 30–90 days).
Teratoma-Associated Encephalitis Antibodies Target Antigens Enriched in the Neuropil of Hippocampus and Readily Accessed in Live Neurons
The sera of the four patients showed identical reactivity with the neuropil of hippocampus (Fig 2). The CSF available from two patients showed more intense and hippocampal-specific reactivity than the sera; minimal or barely visible reactivity was seen in other areas of the brain or cerebellum. In the hippocampus, the most intense immunolabeling localized in the inner aspect of the molecular layer adjacent to the granular neurons of the dentate gyrus, sparing the cell bodies (Figs 2 and 3A). The stratum lacunosum moleculare of the cornu ammonis 1 region of the hippocampus adjacent to the hippocampal fissure was spared by the antibody reactivity of the four patients.
Fig 2.
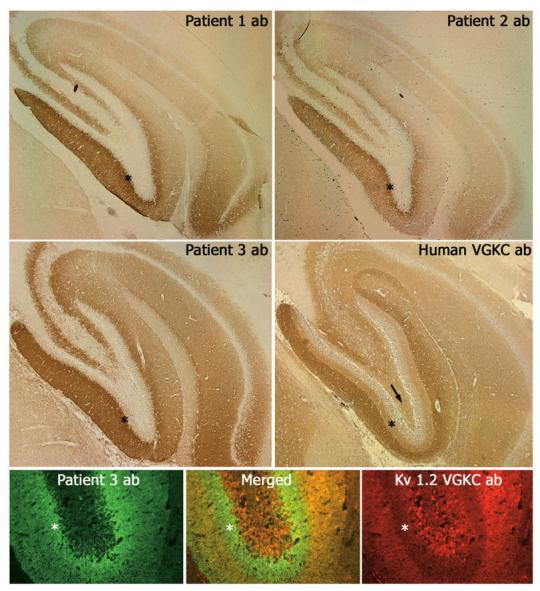
Sera and cerebrospinal fluid (CSF) of patients with teratoma-associated encephalitis react with hippocampal antigens. Top two rows of panels demonstrate the reactivity of serum antibodies of Patient 1 (Patient 1 ab) and CSF antibodies of Patients 2 and 3 (Patient 2 ab and Patient 3 ab) with rat hippocampus. The pattern of reactivity is identical for the three patients' antibodies, but different from that seen in a patient with voltage-gated potassium channel (VGKC) antibodies (Human VGKC ab). Note that the antibodies of the three patients with teratoma-associated encephalitis predominantly react with the inner aspect of the molecular layer adjacent to the dentate gyrus; in contrast, the serum of the patient with VGKC antibodies predominantly reacts with the outer aspect of the molecular layer. These differences are emphasized in a double immunolabeling assay shown in the bottom row panels, and in the higher magnifications shown in Figure 3. Asterisks are shown in the inner aspect of the molecular layer to allow comparison among all panels. The arrow points to the hilum of hippocampus, which is spared by the three patients' antibodies but is immunolabeled by VGKC antibodies. Bottom row panels compare the reactivity of antibodies from Patient 3 (Patient 3 ab; green) with that of a monoclonal antibody to the Kv1.2 VGKC (red). Note that the immunolabeling of Patient 3 antibodies predominates in the inner aspect of the molecular layer (asterisks), whereas the immunolabeling of the Kv1.2 VGKC antibody predominates in the outer aspect of the molecular layer. Of note, this monoclonal antibody also reacts with some neurons of the dentate gyrus, a feature not seen with human antibodies to the Kv1.2 VGKC (see magnifications using human antibodies in Fig 3). Magnfication: top two row panels ×50; bottom panels ×200.
Fig 3.
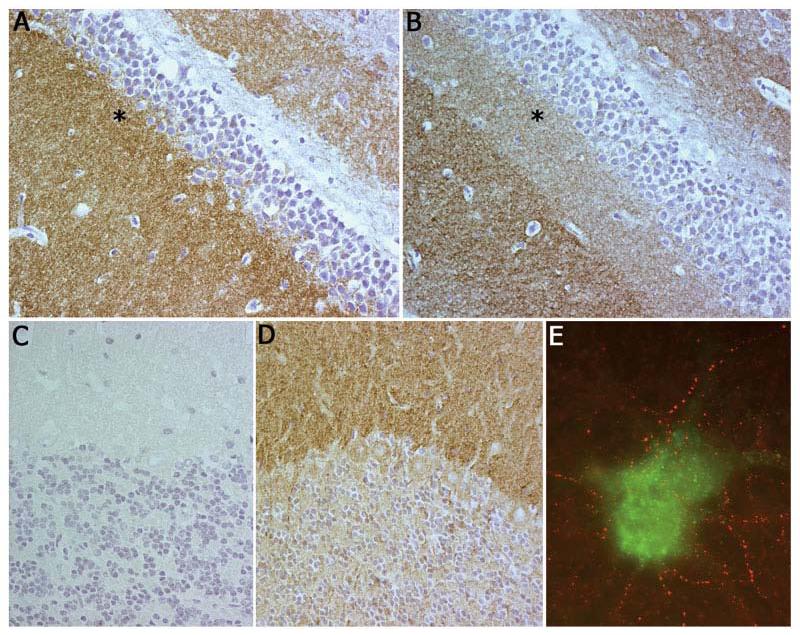
Pattern of reactivity of teratoma-associated encephalitis antibodies compared with voltage-gated potassium channel (VGKC) antibodies. Panel A shows that antibodies from Patient 3 predominantly react with the inner aspect of the molecular layer of hippocampus, whereas human VGKC antibodies (B) predominantly react with the outer aspect (asterisk shown in the same area as in Fig 2). Note that for both antibodies the reactivity spares cytoplasm and nuclei of neurons. Panel C shows that antibodies from Patient 3 (as well as from Patients 1 and 2, not shown here) have minimal reactivity with cerebellum, whereas human VGKC antibodies (D) have intense reactivity with the molecular layer. Panel E shows double immunolabeling of neuronal cultures with teratoma-associated encephalitis antibodies (red) and Kv1.2 VGKC antibodies (green); note the lack of colocalization. Magnification: panels A–D ×400; panel E ×800 oil objective.
Because some patients with LE have antibodies to VGKCs,16 we compared the reactivity of our patients' antibodies with that from patients with VGKC antibodies. This demonstrated clear differences; sera of patients with VGKC antibodies predominantly react with the outer aspect of the molecular layer of the hippocampus without sparing the stratum lacunosum moleculare, and also react with the molecular layer of the cerebellum (see Figs 2 and 3A-D). Double immunolabeling with human VGKC antibodies or Kv1.2 antibodies in neuronal cultures did not show colocalization with the teratoma-associated encephalitis antibodies (see Fig 3E).
The appearance of cell surface immunolabeling lead us to investigate whether the antigens of teratoma-associated encephalitis were readily accessed by antibodies in live neurons. For these studies, live neuronal cultures were exposed for 30 minutes to antibodies added to the media followed by extensive washing and then fixation in PFA. These studies showed intense immunolabeling of the neuronal cell membrane by patients' antibodies, but not by normal IgG or antibodies to intracellular antigens (Hu), strongly suggesting that the autoantigens are exposed on the cell surface (Fig 4).
Fig 4.
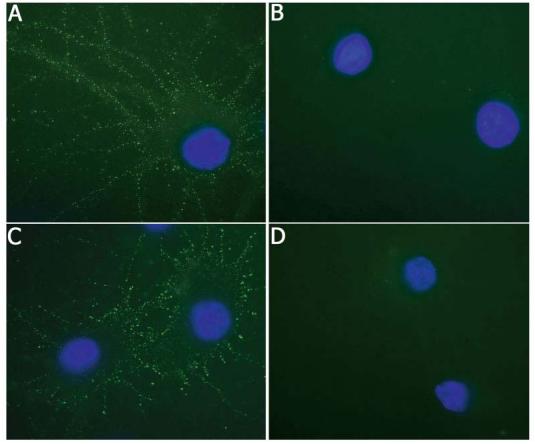
Teratoma-associated encephalitis antibodies readily recognize membrane antigens in live neuronal cultures. Panels A and C show neuronal cultures that have been incubated for 30 minutes with media containing antibodies from Patients 1 (A) and 3 (C) followed by fixation with paraformaldehyde. Neurons similarly incubated with anti–Hu antibodies (B) and normal IgG (D) serve as controls. Note that the antibodies of patients with teratoma-associated encephalitis produce intense immunolabeling of the neuronal cell membrane and processes, whereas antibodies to intracellular antigens or normal IgG do not bind to the cell surface. To visualize neurons, we labeled nuclei with 4,6-diamidino-2-phenylindole-2-HCl. All panels ×800 oil objective.
Immunocompetition assays between patients' antibodies showed that they partially blocked the hippocampal reactivity of each other, indicating that some, but not all epitopes are common targets (data not shown).
Initial serum antibody titers were 1 to 2,000 for Patient 3, 1 to 1,000 for Patients 1 and 2, and 1 to 100 for Patient 4. Follow-up titers, after neurological improvement, were obtained in Patients 1, 2, and 4; no antibody reactivity was detected in any of the patients.
EFA6A, a Brain-Specific Protein, Colocalizes with the Antibody Immunolabeling of Teratoma-Associated Encephalitis Patients
To further determine the target antigens of teratoma-associated encephalitis antibodies, we probed a rat hippocampal expression library combining sera of three patients (Patients 1–3). This resulted in the isolation of two overlapping clones. A homology search of Gen-Bank databases demonstrated that the consensus sequence formed by the two clones corresponded to a 330–amino acid portion of EFA6A (exchange factor for ARF6) and included part of the Sec7 domain, the entirety of the PH (pleckstrin homology) domain, and the coiled-coil motifs.17,18
When tested individually against EFA6A-expressing phage plagues, one of the four patient's sera reacted with EFA6A (Fig 5A). We then used these phage plaques to purify EFA6A-specific antibodies from the patient's serum, and the reactivity of these antibodies was compared with that of the other three patients' sera or CSF. These studies demonstrated that affinity-purified EFA6A antibodies had an identical pattern of hippocampal reactivity to that of the other three patients' antibodies (see Fig 5B). Furthermore, in a double immunolabeling assay with hippocampal neurons, the reactivity of all patients' antibodies colocalized with EFA6A (see Fig 5C), indicating that the patients' antibodies targeted several epitopes contained in EFA6A or proteins interacting with EFA6A at the plasma membrane. Among these interacting proteins, we explored whether ARF6 (ADP-ribosylation factor 6) was a target antigen; no significant colocalization of reactivities was identified between patients' antibodies and ARF6 antibodies (the latter mainly identifying intracytoplasmic ARF6; data not shown).
Fig 5.
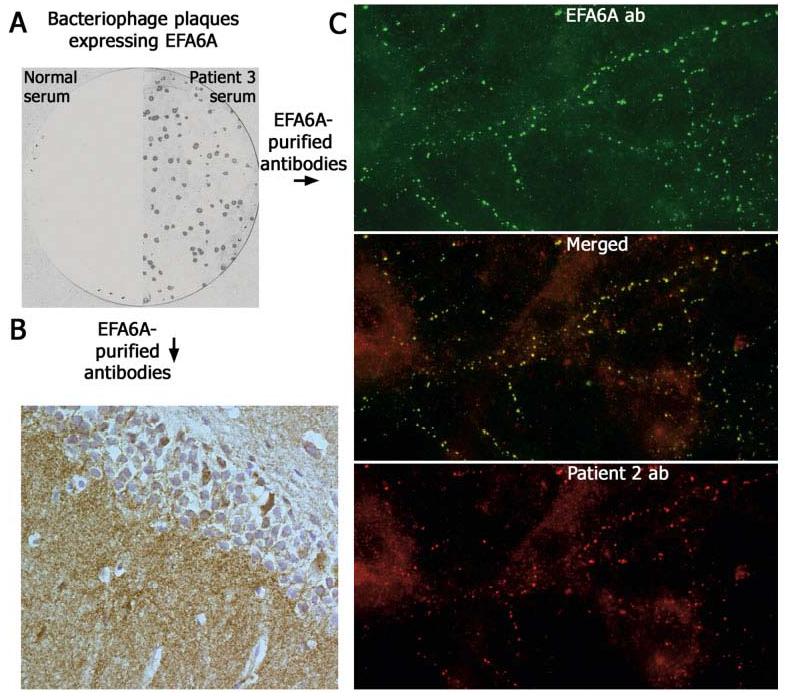
EFA6A, a brain-specific protein, colocalizes with the target antigen of teratoma-associated encephalitis. Immunoprobing a cDNA hippocampus expression library with patients' sera resulted in the isolation of EFA6A. (A) nitrocellulose filter with EFA6A-expressing bacteriophage plaques incubated with serum from Patient 3 and normal human serum (sera from Patients 1, 2, and 4 did not react with EFA6A protein expressed in plaques). Panel B shows that affinity-purified antibodies eluted from EFA6A reproduce the same pattern of hippocampal immunolabeling as that of all patients' antibodies. Panel C confirms that EFA6A colocalizes with the antigen targeted by patients' antibodies (Patient 2 shown in the figure). All panels ×800 oil objective.
In addition to the hippocampal immunolabeling, highly concentrated EFA6A affinity-purified antibodies also reacted with the neuropil and cytoplasm of neurons of the cerebral cortex, brainstem, and cerebellum, indicating that the expression of EFA6A predominates but is not restricted to the hippocampus (data not shown).
Discussion
We report the clinical and neuroimaging features of a disorder that occurs in women with OT and associates with subacute psychiatric symptoms, seizures, decrease level of consciousness, and frequent central hypoventilation. CSF findings suggest an encephalitic process that in prior accounts was suspected to be immune mediated. This hypothesis is now strongly supported by the detection in patients' sera and CSF of an immune response to antigens that colocalize with EFA6A, a brain-specific protein involved in the regulation of dendritic development of hippocampal neurons.19
Clinical recognition of this syndrome is important for two reasons: (1) the subacute presentation of the indicated symptoms in a young woman often leads to an initial diagnosis of acute psychosis, malingering, or drug abuse, and (2) despite the severity of symptoms patients usually recover. Because the symptom presentation is usually acute with prominent confusion, delusional thinking, agitation, and low level of consciousness, the recognition of the short-term memory deficits characteristic of limbic dysfunction can be difficult. Furthermore, the neuroimaging findings may differ from classic LE: combining MRI, FDG-PET, and SPECT only one third of the patients had involvement of the limbic system (cingulum, orbitofrontal region, temporal lobes), and none had the characteristic FLAIR or T2 medial temporal abnormalities seen in LE; the other two thirds of the patients had abnormalities in other areas of the central nervous system (CNS) or normal MRI.
Central hypoventilation has been reported in a few patients with paraneoplastic disorders involving brainstem, hypothalamus, or both.20-22 The high frequency of this complication in our group of patients (young women without a history of respiratory problems) is remarkable and suggests a pathogenic mechanism in common with the associated encephalitis. The development of hypoventilation was unrelated to seizures or antiepileptic medication; all patients required prolonged ventilatory support (median, 49 days) until recovery or in one case until death (Patient 3). The autopsy of this patient showed nonspecific microglial activation throughout gray and white matter of the brain, brainstem, and cerebellum, with scattered monocytes in perivascular spaces.11 Involvement of the brainstem was also shown by FDG-PET or MRI in three other patients (Patients 1, 4, and 5). Overall, these findings and other transient symptoms in some patients (ie, aphasia, diplopia, myelopathy) suggest that teratoma-associated encephalitis is an extensive or multifocal encephalitis, in which brainstem involvement is frequent and may be the cause of hypoventilation.
Despite the severity of the clinical features, three of our four patients and five patients reported by others survived, four with total recovery and four with substantial improvement. All but one patient had tumor resection, and six received immunosuppressants or chemotherapy. Because this is a small series, the contribution of each therapy to the neurological improvement is unclear. The close temporal association between presentation of teratoma-associated encephalitis and tumor diagnosis (median, 3 months) and the striking correlation in one case (Patient 4) between three episodes of neurological relapse and tumor recurrence suggest that the tumor plays a role in triggering the immune response. Frozen tumor tissue (not available from our patients) is needed to determine whether immunological activation depends on the tumor expression of neuronal proteins.
What is the cause of teratoma-associated encephalitis? The identification in four of four patients of antibodies with a unique pattern of reactivity with the CNS provides strong evidence that the disorder is immune mediated. Several features put these antibodies in a recently reported category12 that is different from the group of classic paraneoplastic antibodies to intracellular neuronal antigens (ie, HuD, CRMP5, or Ma2): (1) the immunohistochemical reactivity is highly restricted to the neuropil of rat hippocampus, sparing neuronal cell bodies; (2) the reactivity is missed by immunoblot analysis (data not shown) or if rat brain tissue is fixed with acetone or methanol-acetone; (3) studies with hippocampal neurons demonstrate that the immunolabeling occurs at the plasma membrane and dendritic processes suggesting that the antigens are on the cell surface; and (4) serum antibody titers become undetectable after neurological improvement.
To identify the autoantigens of the disorder, we probed a cDNA library of rat hippocampus with patients' sera, resulting in the isolation of EFA6A. When each individual serum was reacted with EFA6A expressed in bacteriophage plaques, only one recognized the protein, but affinity-purified EFA6A antibodies reproduced the same unique pattern of hippocampal reactivity as that of all patients' antibodies, with striking colocalization of neuronal immunolabeling. This finding indicates that the disorder's autoantigen is EFA6A itself but some epitopes are conformational or absent in the isolated partial sequence or that there is an additional coantigen that forms part of the complex of proteins that bind EFA6A. When EFA6A interacts with ARF6 at the plasma membrane,23 it binds to TWIK-1,24 a member of a new family of K(+) channels that is composed at least of 14 related subunits, each characterized by two-pore domains.25,26 In preliminary studies, we have found that ARF6 is not a coautoantigen, but the immunolabeling pattern of patients' antibodies resembles that of commercially available antibodies to some members of the two-pore-domain K(+) channels (ie, TASK-3; data not shown). These channels (also known as “leak K+ channels”) differ in structure and function from the shaker channels recently reported as autoantigens of nonparaneo-plastic LE.16,27 A general property of the two-pore K+ channels is that they show little time and voltage dependence and play critical roles in controlling the resting membrane potential.
The ARF6/EFA6A/TWIK-1 complex is important for endocytosis, channel internalization, and recycling.17,24 Because the reactivity of patients' antibodies colocalizes with EFA6A, it is reasonable to speculate that an immune-mediated disruption of the ARF6/EFA6A/TWIK-1 complex could result in severe deficits, similar to those of our patients. These would have broad effects on the limbic system where EFA6A is enriched and other areas of the CNS where at least three EFA6 isoforms (B, C, D) are also expressed.18,24,28 Pertinent to the frequent hypoventilation and low level of consciousness of our patients, the function of at least two-pore channels (TASK-1 and TASK-3) is important for control of breathing and arousal.29,30 A next step to determine other autoantigens is to precipitate EFA6A-interacting proteins using patients' antibodies.
On the clinical side, this study has the following implications: (1) when confronted with a young woman with a new-onset psychiatric syndrome associated with seizures, memory loss, decreased level of consciousness or central hypoventilation physicians should suspect a paraneoplastic disorder; (2) the tumor search should focus in the ovaries, and if a tumor is detected (even with a benign appearance) removal is recommended; (3) clinical improvement is slow; the development of central hypoventilation does not necessarily imply an ominous prognosis, but patients usually require extended ventilatory support; (4) based in a recently reported animal model demonstrating CNS dysfunction caused by antibodies to membrane-associated antigens,31 the finding that the antigens of teratoma-associated encephalitis are readily accessed by antibodies in live neurons suggests that early use of corticosteroids and IgG-depleting strategies (IVIg or plasma exchange) may improve outcome. This could not be determined in our patients because immunotherapies were used empirically when the patients were critically ill. The hippocampal antibodies were retrospectively identified in archived sera and CSF examined after recovery in three patients, and after death in one case.
Analysis for EFA6A-specific antibodies by immuno-precipitation or affinity purification from patient's serum is complex, and we consider the use of these methods as a diagnostic test premature. However, immunohistochemical analysis for the antibodies reported here (anti-EFA6A or anti-EFA6A–interacting proteins) is simple, and the pattern of reactivity characteristic enough to allow its use to identify the disorder. Analysis of the functional effects of the antibodies in live neurons is in progress.
Supplementary Material
Acknowledgments
This work was supported by the NIH (National Cancer Institute, RO1CA89054 and RO1CA107192, J.D.).
We thank A. Zupa-Fernandez and M. Maronski for their excellent technical assistance. The authors are indebted to Drs R. H. Muni and D. S. Liebeskind for providing information and serum, and Drs F. Graus, L. Bataller, and M. R. Rosenfeld for critical review of the manuscript.
References
- 1.Alamowitch S, Graus F, Uchuya M, et al. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120:923–928. doi: 10.1093/brain/120.6.923. [DOI] [PubMed] [Google Scholar]
- 2.Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–1494. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 3.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 4.Nokura K, Yamamoto H, Okawara Y, et al. Reversible limbic encephalitis caused by ovarian teratoma. Acta Neurol Scand. 1997;95:367–373. doi: 10.1111/j.1600-0404.1997.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 5.Okamura H, Oomori N, Uchitomi Y. An acutely confused 15-year-old girl. Lancet. 1997;350:488. doi: 10.1016/S0140-6736(97)06208-9. [DOI] [PubMed] [Google Scholar]
- 6.Aydiner A, Gurvit H, Baral I. Paraneoplastic limbic encephalitis with immature ovarian teratoma—a case report. J Neurooncol. 1998;37:63–66. doi: 10.1023/a:1005875822605. [DOI] [PubMed] [Google Scholar]
- 7.Fadare O, Hart HJ. Anti-Ri antibodies associated with short-term memory deficits and a mature cystic teratoma of the ovary. Int Semin Surg Oncol. 2004;1:11. doi: 10.1186/1477-7800-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee AC, Ou Y, Lee WK, Wong YC. Paraneoplastic limbic encephalitis masquerading as chronic behavioural disturbance in an adolescent girl. Acta Paediatr. 2003;92:506–509. doi: 10.1111/j.1651-2227.2003.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RB, Mason W, Kong K, Wennberg R. Reversible paraneoplastic encephalomyelitis associated with a benign ovarian teratoma. Can J Neurol Sci. 1999;26:317–320. doi: 10.1017/s0317167100000469. [DOI] [PubMed] [Google Scholar]
- 10.Muni RH, Wennberg R, Mikulis DJ, Wong AM. Bilateral horizontal gaze palsy in presumed paraneoplastic brainstem encephalitis associated with a benign ovarian teratoma. J Neuroophthalmol. 2004;24:114–118. doi: 10.1097/00041327-200406000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Stein-Wexler R, Wootton-Gorges SL, Greco CM, Brunberg JA. Paraneoplastic limbic encephalitis in a teenage girl with an immature ovarian teratoma. Pediatr Radiol. 2005;35:694–697. doi: 10.1007/s00247-005-1402-1. [DOI] [PubMed] [Google Scholar]
- 12.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881–886. [PMC free article] [PubMed] [Google Scholar]
- 14.Buchhalter JR, Dichter MA. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991;26:333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 15.Bataller L, Rosenfeld MR, Graus F, et al. Autoantigen diversity in the opsoclonus-myoclonus syndrome. Ann Neurol. 2003;53:347–353. doi: 10.1002/ana.10462. [DOI] [PubMed] [Google Scholar]
- 16.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 17.Franco M, Peters PJ, Boretto J, et al. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derrien V, Couillault C, Franco M, et al. A conserved C-terminal domain of EFA6-family ARF6-guanine nucleotide exchange factors induces lengthening of microvilli-like membrane protrusions. J Cell Sci. 2002;115:2867–2879. doi: 10.1242/jcs.115.14.2867. [DOI] [PubMed] [Google Scholar]
- 19.Sakagami H, Matsuya S, Nishimura H, et al. Somatodendritic localization of the mRNA for EFA6A, a guanine nucleotide exchange protein for ARF6, in rat hippocampus and its involvement in dendritic formation. Eur J Neurosci. 2004;19:863–870. doi: 10.1111/j.0953-816x.2004.03195.x. [DOI] [PubMed] [Google Scholar]
- 20.Dietl HW, Pulst SM, Engelhardt P, Mehraein P. Paraneoplastic brainstem encephalitis with acute dystonia and central hypoventilation. J Neurol. 1982;227:229–238. doi: 10.1007/BF00313390. [DOI] [PubMed] [Google Scholar]
- 21.Nunn K, Ouvrier R, Sprague T, et al. Idiopathic hypothalamic dysfunction: a paraneoplastic syndrome? J Child Neurol. 1997;12:276–281. doi: 10.1177/088307389701200412. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan AM, Itabashi HH. Encephalitis associated with carcinoma. Central hypoventilation syndrome and cytoplasmic inclusion bodies. J Neurol Neurosurg Psychiatry. 1974;37:1166–1176. doi: 10.1136/jnnp.37.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macia E, Luton F, Partisani M, et al. The GDP-bound form of Arf6 is located at the plasma membrane. J Cell Sci. 2004;117:2389–2398. doi: 10.1242/jcs.01090. [DOI] [PubMed] [Google Scholar]
- 24.Decressac S, Franco M, Bendahhou S, et al. ARF6-dependent interaction of the TWIK1 K+ channel with EFA6, a GDP/GTP exchange factor for ARF6. EMBO Rep. 2004;5:1171–1175. doi: 10.1038/sj.embor.7400292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talley EM, Solorzano G, Lei Q, et al. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duprat F, Lesage F, Fink M, et al. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thieben MJ, Lennon VA, Boeve BF, et al. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology. 2004;62:1177–1182. doi: 10.1212/01.wnl.0000122648.19196.02. [DOI] [PubMed] [Google Scholar]
- 28.Matsuya S, Sakagami H, Tohgo A, et al. Cellular and subcellular localization of EFA6C, a third member of the EFA6 family, in adult mouse Purkinje cells. J Neurochem. 2005;93:674–685. doi: 10.1111/j.1471-4159.2005.03072.x. [DOI] [PubMed] [Google Scholar]
- 29.Washburn CP, Bayliss DA, Guyenet PG. Cardiorespiratory neurons of the rat ventrolateral medulla contain TASK-1 and TASK-3 channel mRNA. Respir Physiol Neurobiol. 2003;138:19–35. doi: 10.1016/s1569-9048(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 30.Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive “leak” K(+) channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- 31.Sommer C, Weishaupt A, Brinkhoff J, et al. Paraneoplastic stiff-person syndrome: passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet. 2005;365:1406–1411. doi: 10.1016/S0140-6736(05)66376-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


