Abstract
Objective
To provide data on the short-term effect of intravitreal bevacizumab for diabetic macular edema (DME).
Design
Randomized phase 2 clinical trial.
Participants
121 eyes of 121 subjects (109 eligible for analysis) with DME and Snellen acuity equivalent ranging from 20/32-20/320.
Interventions
Random assignment to one of five groups: focal photocoagulation at baseline (N=19, Group A), intravitreal injection of 1.25mg bevacizumab at baseline and 6 weeks (N=22, Group B), intravitreal injection of 2.5mg bevacizumab at baseline and 6 weeks (N=24, Group C), intravitreal injection of 1.25mg bevacizumab at baseline and sham injection at 6 weeks (N=22, Group D), or intravitreal injection of 1.25mg bevacizumab at baseline and 6 weeks with photocoagulation at 3 weeks (N=22, Group E).
Main Outcome Measures
Central subfield thickness (CST) on optical coherence tomography and best-corrected visual acuity (VA) were measured at baseline and after 3, 6, 9, 12, 18, and 24 weeks.
Results
At baseline, median CST was 411 microns and median Snellen VA equivalent was 20/50. Compared with Group A, Groups B and C had a greater reduction in CST at 3 weeks and about one line better median visual acuity over 12 weeks. There were no meaningful differences between Groups B and C in CST reduction or VA improvement. A CST reduction >11% (the reliability limit) was present at 3 weeks in 36/84 (43%) bevacizumab-treated eyes and in 5/18 (28%) eyes treated with laser alone, and at 6 weeks in 31/84 (37%) and 9/18 (50%) eyes, respectively. Combining focal photocoagulation with bevacizumab resulted in no apparent short-term benefit or adverse outcomes. Endophthalmitis developed in one eye. The following events occurred during the first 24 weeks in subjects treated with bevacizumab without attributing cause to the drug: myocardial infarction (N=2), congestive heart failure (N=1), elevated blood pressure (N=3), and worsened renal function (N=3).
Conclusion
These results demonstrate that intravitreal bevacizumab can reduce DME in some eyes, but the study was not designed to determine whether treatment is beneficial. A phase 3 trial would be needed for that purpose.
Introduction
Macular edema is a major cause of central vision impairment in patients with diabetic retinopathy. To date, demonstrated means to reduce the risk of vision loss from diabetic macular edema (DME) include focal laser photocoagulation,1, 2 intensive glycemic control,3 and blood pressure control.4 In the Early Treatment Diabetic Retinopathy Study (ETDRS), focal photocoagulation of eyes with macular edema reduced the risk of moderate visual acuity loss (defined as a loss of 15 or more letters) by approximately 50% (from 24% to 12%) three years after randomization.1 Among eyes with center-involved macular edema and baseline acuity worse than a Snellen equivalent of 20/40 that were treated with focal photocoagulation, the 15-letter improvement rate at 1 year was 11% and at 3 years was 16% (computed from ETDRS dataset by the authors).
The low frequency of improvement following focal laser photocoagulation for DME has prompted interest in other treatment modalities, including intravitreal triamcinolone acetonide,5 oral protein kinase C beta inhibitors,6, 7 pars plana vitrectomy,8 and intravitreal aptamers9 or antibodies directed against vascular endothelial growth factor (VEGF).10, 11
Bevacizumab is a humanized monoclonal antibody that competitively inhibits all isoforms of the VEGF-A family in the extracellular space. While bevacizumab is currently approved by the Food and Drug Administration (FDA) for the treatment of metastatic colorectal cancer, metastatic breast cancer, and non-small cell lung cancer, it is widely used as an off-label treatment for neovascular age-related macular degeneration and retinal vascular disorders including retinal vein occlusion and diabetic macular edema (Practices and Trends survey data from the American Society of Retina Specialists regarding treatment methods for vitreoretinal disorders, 2006). Other anti-VEGF drugs, pegaptanib and ranibizumab, are currently approved by the FDA for the treatment of age-related macular degeneration.12, 13 DME improvement has been reported with intravitreal pegaptanib in a 36-week phase 2 randomized trial9 and with intravitreal ranibizumab in two case series.14 10
We conducted a pilot study to evaluate the short-term safety and effect of intravitreal bevacizumab, either alone or in combination with focal photocoagulation, in the treatment of DME.
Study Participants and Methods
This phase 2 randomized, multi-center clinical trial was conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net) at 36 clinical sites in the United States. The protocol and Health Insurance Portability and Accountability Act (HIPAA)-compliant informed consent forms were approved by multiple institutional review boards. An investigational new drug application number (100,050) was obtained from the FDA for the protocol. Study oversight was provided by an independent data and safety monitoring committee. The study is listed on www.clinicaltrials.gov (NCT00336323). The protocol, which is available on the DRCR.net website (www.drcr.net), is summarized below.
Study Objectives
The overall study objective was to provide pilot data on the short-term effects of intravitreal injection(s) of bevacizumab, alone or with focal photocoagulation, for DME. Specific study questions included: 1) Does 1.25 mg intravitreal bevacizumab reduce optical coherence tomography (OCT)-measured retinal thickening in DME? 2) Does 2.5 mg intravitreal bevacizumab reduce OCT-measured retinal thickening in DME? 3) Does 2.5 mg intravitreal bevacizumab produce a greater shorter-term reduction in OCT-measured retinal thickening from DME than 1.25 mg intravitreal bevacizumab? 4) What is the duration of reduction in OCT-measured retinal thickening following the initial injection of intravitreal bevacizumab? 5) What is the duration of reduction in OCT-measured retinal thickening following the second injection of intravitreal bevacizumab? 6) Is there a greater reduction in OCT-measured retinal thickening using intravitreal bevacizumab followed by focal photocoagulation compared with intravitreal bevacizumab alone?
Study Population
Eligible subjects were at least 18 years old with type 1 or type 2 diabetes. The major eligibility criteria for the study eye included: (1) best corrected electronic-ETDRS15 (E-ETDRS) visual acuity letter score ≥ 24 (20/320 or better) and ≤ 78 (20/32 or worse), (2) definite retinal thickening due to DME involving the center of the macula based on clinical exam, (3) OCT central subfield thickness ≥ 275 microns, and (4) no history of treatment for DME at any time within the prior 3 months. A subject could have only one study eye; if both eyes were eligible at the time of study entry, the study eye was selected by the investigator and subject. Additional eligibility and exclusion criteria are listed in Table 1 (available at http://aaojournal.org).
Table 1.
Eligibility and Exclusion Criteria
| Subject-level Inclusion Criteria |
|
| Subject-level Exclusion Criteria |
|
| Study Eye Inclusion Criteria |
|
| Study Eye Exclusion Criteria |
|
| Fellow Eye Inclusion Criteria |
|
Synopsis of Study Design
After eligibility was confirmed and informed consent was obtained, each study eye was randomly assigned with equal probability to one of five treatment groups on the DRCR.net website:
Treatment Group A. Focal photocoagulation at baseline
Treatment Group B. Intravitreal injection of 1.25 mg bevacizumab (Avastin, Genentech, Inc.) at baseline and 6 weeks
Treatment Group C. Intravitreal injection of 2.5 mg bevacizumab at baseline and 6 weeks
Treatment Group D. Intravitreal injection of 1.25 mg bevacizumab at baseline and sham injection at 6 weeks
Treatment Group E. Intravitreal injection of 1.25 mg bevacizumab at baseline, focal photocoagulation at 3 weeks, and intravitreal injection of 1.25 mg bevacizumab at 6 weeks (referred to as the bevacizumab+laser group)
Subjects in Groups B, C, and D were masked to bevacizumab dose and were also masked to whether the injection at 6 weeks was bevacizumab or sham. Subjects were not masked as to whether focal photocoagulation was being received. Investigators were not masked but in most cases the visual acuity testers, OCT technicians, and photographers were masked. OCT graders were masked.
The trial consisted of two phases. Efficacy was assessed over a 12-week period and safety over a 70 week period (only the first 24 weeks of follow up are presented in this report). Follow-up visits were performed at 3, 6, 9, 12, 18, 24, 41, and 70 weeks. The primary outcome variables were OCT-measured retinal thickening in the central subfield and best-corrected electronic ETDRS visual acuity.
During the first 12 weeks of the study, treatment was administered as listed above by treatment group; no other treatment for DME was permitted in the study eye. At 12 weeks, additional treatment was deferred in eyes in which the central subfield was < 250 microns or if ≥ 250 microns, the central subfield thickening had decreased by at least 50% from baseline. At 18 weeks, additional treatment was again deferred if either the central subfield was < 250 microns or if central subfield was ≥ 250 microns and there was at least an additional 20% decrease in central subfield thickening from baseline. Eyes not meeting these criteria at 12 or 18 weeks were treated at investigator discretion. Eyes in treatment group A (focal photocoagulation at baseline) not meeting the deferral criteria could receive an intravitreal injection of 1.25 mg bevacizumab at 12 and at 18 weeks. After 24 weeks, treatment was at investigator discretion for all groups.
Treatment Protocols
The bevacizumab injection technique was standardized, based on investigator usual practices. Topical antibiotic drops, which could be used at the discretion of the investigator, were administered prior to 61% of injections (sham and true) on the same day as the injection. A previously unopened 4 ml (25mg/ml) vial of bevacizumab was used for each injection, which was given within 6 hours of opening the vial. Using a sterile eyelid speculum and topical anesthesia, followed by a prep with povidone iodine, bevacizumab in doses of 1.25 mg in 0.05 cc or 2.5 mg in 0.1 cc was injected using a 30-gauge needle on a 1 cc syringe into the vitreous cavity through the pars plana 3.0-4.0 mm posterior to the limbus. At the discretion of the investigator, topical antibiotic eye drops were prescribed to be used for up to three days (this was employed following 82% of injections, sham and true). The sham injection technique included the same preparation as for an intravitreal injection and utilization of a syringe without a needle; the syringe hub was pressed against the conjunctival surface to simulate the force of an actual injection.
The focal photocoagulation technique was modified from the original ETDRS protocol as described previously and used in prior protocols.16 Laser burns were less intense (gray) and were smaller (50 microns) than in the original ETDRS protocol (50-200 microns).17 A fluorescein angiogram was used to guide treatment at the investigator’s discretion in 51% of cases.
Examination Procedures
At baseline and at each follow-up visit, best-corrected visual acuity was measured at 3 meters by a certified tester using an electronic procedure based on the ETDRS method (E-ETDRS).15 A standardized refraction was performed at baseline and 9 weeks, and at other visits if there was a 10 or more letter decrease from baseline. At each visit, the subject was queried about adverse events and a clinical exam was performed by a certified investigator, including dilated slit lamp exam, fundus exam, and intraocular pressure measurement. Standard ETDRS 7-field color stereoscopic fundus photographs were obtained at baseline and sent to the DRCR.net Reading Center at the University of Wisconsin-Madison for grading. HemoglobinA1c (HbA1c) was measured at baseline. Any untoward medical occurrence in a study subject, irrespective of whether the event was considered treatment-related, was considered an adverse event and recorded.
OCT images were obtained at each visit following pupil dilation by a certified operator using the Zeiss Stratus OCT (OCT3) machine. Scans were 6mm length and included the 6 radial line pattern (fast macular scan option with OCT3) for quantitative measures and the cross hair pattern (6-12 to 9-3 o’clock) for qualitative assessment of retinal morphology. The OCT scans were sent to the DRCR.net Reading Center for grading. For 10% of the 109 baseline scans and 12% of the 612 follow-up scans, the automated thickness measurements were judged by the Reading Center to be inaccurate and center point thickness (usually manually measured) was used to impute a value for the central subfield (based on a correlation of the two measures of 0.98 as published previously16). Retinal morphology was assessed at baseline from OCT images for cystoid abnormalities and subretinal fluid.
Statistical Methods
Statistical principles were not used to estimate the sample size, which was planned to be about 20 eyes per treatment group. Since the study was designed to generate hypotheses rather than test hypotheses, it was decided a priori to exclude from the efficacy analyses ineligible subjects (N=9, see Figure 1 [available at http://aaojournal.org] for reasons), subjects with no follow up (N=2) and subjects who developed a major complication of the intravitreal injection affecting visual acuity (N=1, endophthalmitis). All subjects receiving study treatment were included in the safety analysis. The primary time point for analysis varied according to the objective of each analysis.
Figure 1. Patient Outcome Visit Follow-Up Flow Chart.
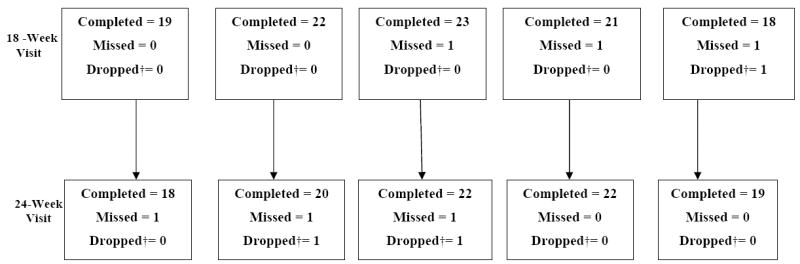
*Nine subjects/eyes were excluded due to ineligibility: 1 received laser treatment within 3 months prior to randomization, 5 had baseline central subfield thickness < 275 microns, 1 had a baseline optical coherence tomography image that could not be graded due to low signal strength and therefore was unable to confirm central subfield thickness for eligibility, 2 had choroidal neovascularization first identified by the Reading Center and subsequently confirmed by the enrolling ophthalmologist after randomization. Two subjects with no follow-up visits and 1 subject with endophthalmitis after the initial injection also were excluded.
†Dropped includes deaths, withdrawals, lost to follow up occurring since the last visit
Normality of distributions was evaluated and parametric tests were deemed appropriate; therefore, continuous central subfield thickness and visual acuity outcome measures were assessed using least squares regression models adjusted for baseline values. Results did not differ substantially when models included adjustments for other baseline characteristics. Medians and interquartile ranges have been reported to provide information on the distribution of the data. The bevacizumab groups were pooled to assess the effect of various baseline characteristics (central subfield thickness, visual acuity, age, gender, prior treatment, retinopathy severity, clinician classification of DME, and subretinal fluid) on retinal thickness and visual acuity at 3 weeks using least squares regression models adjusted for baseline values.
All p-values are 2-tailed. SAS version 9.0 was used for all analyses.
Results
Between June 5, 2006 and August 4, 2006, 121 subjects were randomized to the five treatment groups (one eye per subject) at 36 clinical sites. Of these 121 subjects, 109 met criteria for inclusion in the analyses (19-24 per group, exclusions detailed in Figure 1 [available at http://aaojournal.org]). Median age was 65 years and 39% were women. The racial/ethnicity distribution was 76% White, 16% African-American, 6% Hispanic, 1% Asian, and 1% other. Type 2 diabetes was present in 93% and type 1 diabetes in 7%. Median Snellen equivalent visual acuity in the 109 eyes was 20/50 (letter score 64; range, 26 to 78) and median OCT central subfield thickness was 411 microns (range, 275 to 785 microns); 75 eyes (69%) had received prior treatment for DME. Additional baseline characteristics by treatment group are provided in Table 2 (available at http://aaojournal.org).
Table 2.
Baseline Subject Data According to Treatment Group
| All
N = 109 |
A
Laser at Baseline N = 19 |
B
1.25mg at 0+6w N = 22 |
C
2.5mg at 0+6w N = 24 |
D
1.25mg at Baseline Only N = 22 |
E
1.25mg at 0+6w/ Laser at 3w N = 22 |
|
|---|---|---|---|---|---|---|
| Gender: Women - N(%) | 43 (39%) | 9 (47%) | 6 (27%) | 9 (38%) | 9 (41%) | 10 (45%) |
| Age Median (quartiles)-years | 65 (57, 73) | 64 (57, 72) | 63 (54, 73) | 68 (59, 75) | 60 (54, 75) | 67 (60, 71) |
| Race- N(%) | ||||||
| White | 83 (76%) | 10 (53%) | 16 (73%) | 20 (83%) | 18 (82%) | 19 (86%) |
| African-American | 17 (16%) | 7 (37%) | 3 (14%) | 2 (8%) | 3 (14%) | 2 (9%) |
| Hispanic or Latino | 7 (6%) | 2 (11%) | 2 (9%) | 2 (8%) | 0 | 1(5%) |
| Asian | 1 (1%) | 0 | 0 | 0 | 1 (5%) | 0 |
| Unknown/ not reported | 1 (1%) | 0 | 1 (5%) | 0 | 0 | 0 |
| Diabetes Type- N(%) | ||||||
| Type 1 | 8 (7%) | 1 (5%) | 1 (5%) | 3 (13%) | 2 (9%) | 1 (5%) |
| Type 2 | 101 (93%) | 18 (95%) | 21 (95%) | 21 (88%) | 20 (91%) | 21 (95%) |
| Duration of Diabetes Median (quartiles)-years | 17 (11, 23) | 17 (13, 22) | 15 (8, 22) | 18 (12, 22) | 17 (11, 25) | 20 (7, 30) |
| Hemoglobin A1c Median (quartiles)* | 6.9 (6.3, 8.1) | 7.0 (6.5, 8.2) | 7.4 (5.9, 7.8) | 7.3 (6.4, 8.4) | 6.7 (6.3, 7.4) | 7.1 (6.2, 7.7) |
| Prior Treatment for Diabetic Macular Edema (DME) in Study Eye– N(%) | ||||||
| None | 34 (31%) | 7 (37%) | 5 (23%) | 10 (42%) | 5 (23%) | 7 (32%) |
| Focal photocoagulation alone | 39 (36%) | 4 (21%) | 11 (50%) | 9 (38%) | 6 (27%) | 9 (41%) |
| Focal photocoagulation plus other treatment | 31 (28%) | 8 (42%) | 3 (14%) | 3 (13%) | 11 (50%) | 6 (27%) |
| Other treatment without focal photocoagulation | 5 (5%) | 0 | 3 (14%) | 2 (8%) | 0 | 0 |
| Prior Panretinal Scatter Photocoagulation –N(%) | 13 (12%) | 3 (16%) | 2 (9%) | 3 (13%) | 1 (5%) | 4 (18%) |
| Baseline Visual Acuity | ||||||
| Median (quartiles) -letter score | 64 (56, 71) | 64 (50, 70) | 65 (60, 70) | 63 (57, 71) | 64 (52, 68) | 66 (57, 72) |
| Approx. Snellen score | 20/50-1 | 20/50-1 | 20/50 | 20/50-2 | 20/50-1 | 20/50+1 |
| Lens Status: Phakic N (%) | 66 (61%) | 12 (63%) | 15 (68%) | 14 (58%) | 12 (55%) | 13 (59%) |
| Retinopathy Severity** – N(%) (Early Treatment Diabetic Retinopathy Study Severity Scale) | ||||||
| Mild nonproliferative diabetic retinopathy (NPDR, level 35) | 14 (14%) | 1 (6%) | 6 (29%) | 3 (14%) | 0 | 4 (18%) |
| Moderate NPDR (level 43) | 15 (15%) | 5 (28%) | 3 (14%) | 1 (5%) | 3 (15%) | 3 (14%) |
| Moderately severe NPDR (level 47) | 36 (35%) | 5 (28%) | 6 (29%) | 8 (36%) | 8 (40%) | 9 (41%) |
| Severe NPDR (level 53) | 8 (8%) | 0 | 1 (5%) | 2 (9%) | 4 (20%) | 1 (5%) |
| Mild proliferative diabetic retinopathy (PDR, levels 60 and 61) | 24 (23%) | 4 (22%) | 5 (24%) | 6 (27%) | 5 (25%) | 4 (18%) |
| Moderate PDR (level 65) | 4 (4%) | 1 (6%) | 0 | 2 (9%) | 0 | 1 (5%) |
| High Risk PDR (levels 71 and 75) | 2 (2%) | 2 (11%) | 0 | 0 | 0 | 0 |
| Character of Diabetic Macular Edema (DME)† | ||||||
| Typical/Predominantly Focal | 20 (18%) | 6 (32%) | 5 (23%) | 2 (8%) | 3 (14%) | 4 (18%) |
| Neither Predominantly Focal or Diffuse | 26 (24%) | 4 (21%) | 5 (23%) | 5 (21%) | 6 (27%) | 6 (27%) |
| Typical/Predominantly Diffuse | 63 (58%) | 9 (47%) | 12 (55%) | 17 (71%) | 13 (59%) | 12 (55%) |
| Optical Coherence Tomography (OCT) Central Subfield Thickness Median (quartiles) microns | 411 (334, 505) | 441 (354, 512) | 397 (320, 538) | 446 (342, 543) | 406 (353, 520) | 389 (308, 452) |
| OCT Retinal Volume§ Median (quartiles)– mm3 | 8.6 (7.8, 10.1) | 8.3 (7.3, 10.2) | 9.5 (8.0, 10.3) | 9.1 (8.0, 10.0) | 8.9 (7.7, 10.0) | 8.6 (7.7, 9.2) |
| Cystoid Abnormalities on OCT∥– N(%) | 106 (98%) | 18 (100%) | 22 (100%) | 24 (100%) | 21 (95%) | 21 (95%) |
| Subretinal Fluid on OCT¶ N(%) | ||||||
| Definite, center | 17 (16%) | 2 (11%) | 2 (9%) | 7 (29%) | 2 (9%) | 4 (18%) |
| Definite, not center | 3 (3%) | 0 | 1 (5%) | 1 (4%) | 0 | 1 (5%) |
| Questionable | 3 (3%) | 0 | 1 (5%) | 0 | 2 (9%) | 0 |
| No evidence | 85 (79%) | 16 (89%) | 18 (82%) | 16 (67%) | 18 (82%) | 17 (77%) |
Missing for 8 subjects.
Missing for 6 subjects.
Question on form: Indicate how you would characterize type– focal versus diffuse–in your own daily practice. You are free to use, or not use, OCT, fluorescein angiography, and/or fundus photos in addition to your clinical examination.
Missing for 19 subjects.
Missing for 1 subject.
Missing for 1 subject.
Follow-up and Treatment
Two subjects were dropped from the study before completing 12 weeks of follow up. The overall visit completion rate was 93%, ranging from 83% to 98% in the five groups (Figure 1, available at http://aaojournal.org).
Deviations from the treatment protocol are indicated in Figure 1 (available at http://aaojournal.org). No treatment for DME other than the randomized treatment was administered to any eye prior to the 12-week visit.
Effect of Treatment on Retinal Thickening and Visual Acuity During First 12 Weeks
Central subfield retinal thickness during the first 12 weeks is presented according to treatment group in Table 3. Compared with the laser alone group, Group B and Group C both demonstrated a greater reduction in central subfield thickness at 3 weeks (P=0.009 and <0.001, respectively) but only a trend towards a greater reduction at 6, 9, and 12 weeks (Table 3). For visual acuity, Groups B and C both had about a median one line improvement at the 3-week visit which was sustained through 12 weeks and was greater than the change in visual acuity in Group A (P=0.01 and 0.003, respectively, Table 4). Over the 12-week period, no meaningful differences were found comparing Groups B and C with each other in reduction in central subfield thickening or improvement in visual acuity (for change in central subfield thickening, P=0.66, 0.49, 0.45, and 0.90, respectively, at 3, 6, 9, and 12 weeks and for change in visual acuity, P= 0.42, 0.67, 0.48, and 0.82, respectively). At the 12-week visit, comparing Groups B and E, there were no meaningful differences in central subfield thickening or visual acuity identified.
Table 3.
Central Subfield Retinal Thickness During First 12 Weeks According to Treatment Group*
| A
Laser at Baseline N = 19 |
B
1.25mg at 0+6w N = 22 |
C
2.5mg at 0+6w N = 24 |
D
1.25mg at Baseline Only N = 22 |
E
1.25mg at 0+6w /Laser at 3w N = 22 |
|
|---|---|---|---|---|---|
| Baseline -Median (quartiles) microns | 441 (354, 512) | 397 (320, 538) | 446 (342, 543) | 406 (353, 520) | 389 (308, 452) |
| Change from Baseline – Median (quartiles) microns | |||||
| 3 Weeks | +21 (-62, +79) | -35 (-155, +6) | -86 (-131, -11) | -3 (-49, +7) | -13 (-104, +26) |
| 6 Weeks | -40 (-105, +73) | -35 (-112, +6) | -42 (-127, -10) | -17 (-58, +25) | -20 (-73, +35) |
| 9 Weeks | -53 (-115, +53) | -74 (-113, -31) | -56 (-127, -20) | +5 (-34, +53) | -48 (-128, +33) |
| 12 Weeks | -40 (-146, +85) | -56 (-120, -6) | -47 (-125, -16) | -5 (-41, +53) | -40 (-103, +33) |
| <250 Microns or ≥50% Reduction in Retinal Thickening – (%) | |||||
| 3 Weeks | 11% | 37% | 38% | 10% | 25% |
| 6 Weeks | 17% | 30% | 22% | 19% | 25% |
| 9 Weeks | 19% | 38% | 22% | 10% | 37% |
| 12 Weeks | 21% | 33% | 33% | 14% | 25% |
Medians and interquartile ranges are reported rather than means and standard deviations because of the small sample size per group in order to present a better perspective on the distribution of the data and to minimize the effect of extreme values. The Ns completing each visit are given in Figure 1. In addition to the missed visits: 10 Optical Coherence Tomography measurements were not done at completed visits (Group A: 2 at 9wk; Group B: 2 at 3wk, 2 at 6wk; Group C: 1 at 6wk; Group D: 1 at 3wk, 1 at 6wk, 1 at 9wk).
Table 4.
Visual Acuity During First 12 Weeks According to Treatment Group*
| A
Laser at Baseline N = 19 |
B
1.25mg at 0+6w N = 22 |
C
2.5mg at 0+6w N = 24 |
D
1.25mg at Baseline Only N = 22 |
E
1.25mg at 0+6w /Laser at 3w N = 22 |
|
|---|---|---|---|---|---|
| Baseline Letter Score - median (quartiles) | 64 (50, 70) | 65 (60,70) | 63 (57, 71) | 64 (52, 68) | 66 (57, 72) |
| Change from Baseline – median (quartiles) letters | |||||
| 3 Weeks | -2 (-7, +3) | +5 (-1,+8) | +6 (+1,+9) | +2 (0, +7) | 0 (-6, +6) |
| 6 Weeks | +1 (-6, +6) | +5 (-2,+12) | +6 (+2,+11) | +3 (-2, +6) | 0 (-4, +6) |
| 9 Weeks | +3 (-5, +6) | +7 (+2,+10) | +8 (+3,+12) | +1 (-3, +5) | -2 (-5, +11) |
| 12 Weeks | -1 (-6, +5) | +5 (+1,+12) | +7 (+4,+11) | +4 (-3, +7) | 0 (-5, +8) |
| Distribution of Change –N(%) | |||||
| 3 Weeks | |||||
| ≥15 letter improvement | 1 (6%) | 1 (5%) | 0 | 2 (9%) | 1 (5%) |
| ≥ 10 letter improvement | 1 (6%) | 4 (19%) | 4 (17%) | 2 (9%) | 2 (10%) |
| within ± 9 letters | 16 (89%) | 16 (76%) | 20 (83%) | 19 (86%) | 18 (90%) |
| ≥ 10 letters worse | 1 (6%) | 1 (5%) | 0 | 1 (5%) | 0 |
| 6 Weeks | |||||
| ≥15 letter improvement | 1 (6%) | 2 (9%) | 1 (4%) | 1 (5%) | 1 (5%) |
| ≥ 10 letter improvement | 2 (11%) | 7 (32%) | 7 (29%) | 3 (14%) | 3 (15%) |
| within ± 9 letters | 14 (78%) | 15 (68%) | 16 (67%) | 18 (82%) | 13 (65%) |
| ≥ 10 letters worse | 2 (11%) | 0 | 1 (4%) | 1 (5%) | 4 (20%) |
| 9 Weeks | |||||
| ≥15 letter improvement | 1 (6%) | 3 (14%) | 3 (13%) | 3 (14%) | 3 (16%) |
| ≥ 10 letter improvement | 3 (18%) | 6 (29%) | 9 (39%) | 3 (14%) | 5 (26%) |
| within ± 9 letters | 13 (76%) | 14 (67%) | 14 (61%) | 18 (86%) | 12 (63%) |
| ≥ 10 letters worse | 1 (6%) | 1 (5%) | 0 | 0 | 2 (11%) |
| 12 Weeks | |||||
| ≥15 letter improvement | 1 (5%) | 3 (14%) | 3 (13%) | 2 (9%) | 3 (15%) |
| ≥ 10 letter improvement | 3 (16%) | 7 (33%) | 6 (25%) | 2 (9%) | 4 (20%) |
| within ± 9 letters | 15 (79%) | 13 (62%) | 18 (75%) | 18 (82%) | 14 (70%) |
| ≥ 10 letters worse | 1 (5%) | 1 (5%) | 0 | 2 (9%) | 2 (10%) |
Medians and interquartile ranges are reported rather than means and standard deviations because of the small sample size per group in order to present a better perspective on the distribution of the data and to minimize the effect of extreme values. The Ns for each visit are given in Figure 1. In addition to the missed visits, 1 Group A subject did not complete visual acuity testing at the 9 week visit.
A reduction in central subfield thickness exceeding 11% (the reliability limit for real change determined in another DRCR.net study16) was present at 3 weeks in 23 of 60 (38%) 1.25 mg bevacizumab-treated eyes (pooling Groups B, D, and E), in 13 of 24 (54%) 2.5 mg bevacizumab-treated eyes, and in 5 of 18 (28%) eyes treated with laser alone. The respective proportions at 6 weeks were: 22 of 61 (36%) 1.25 mg bevacizumab-treated eyes (pooling Groups B, D, and E), 9 of 23 (39%) 2.5 mg bevacizumab-treated eyes, and 9 of 18 (50%) eyes treated with laser alone. Twenty-five of 57 (44%) 1.25 mg bevacizumab-treated eyes, 14 of 23 (61%) 2.5 mg bevacizumab-treated eyes and 9 of 17 (53%) eyes treated with laser alone had a reduction in central subfield thickness exceeding 11% at one or both of the visits. As seen in Table 2 (available at http://aaojournal.org), at 12 weeks, no more than one-third of the eyes in each group met the protocol-specified criteria to defer further treatment (central subfield thickness < 250 microns or a reduction from baseline in central subfield thickening by at least 50%). Among eyes meeting the deferral criteria at 12 weeks, the deferral criteria were also met at 18 weeks in 2 of 4 eyes in Group A, 5 of 7 eyes in Group B, 1 of 8 eyes in Group C, 2 of 3 eyes in Group D, and 3 of 5 eyes in Group E.
As seen in Tables 3, 4, and 5 (Table 5 available at http://aaojournal.org), the data do not suggest continued reduction in central subfield thickening or visual acuity improvement in most eyes between 3 and 6 weeks or a more prolonged effect with the 2.5 mg dose than with the 1.25 mg dose. Among the 14 eyes in the 1.25 mg dose groups (B and D) and 13 eyes in the 2.5 mg dose group (C) that experienced a decrease from baseline to 3 weeks in central subfield thickness exceeding 11%, there were more eyes in each group in which central subfield thickness increased (>11%) between 3 and 6 weeks than decreased further. Among the 7 eyes in Group B and 3 eyes in Group C which experienced a decrease from 6 to 9 weeks in central subfield thickness exceeding 11%, there were no eyes with a further decrease (>11%) between 9 and 12 weeks.
Table 5.
Duration of Bevacizumab Effect Based on Change in Central Subfield Thickness†
| 4a. Duration of Initial Injection – 1.25 mg groups* | |||
| Change from Baseline to 3 Weeks | |||
| > 11%
decrease (N=14) |
Within
± 11% (N=22) |
> 11%
increase (N=2) |
|
|
| |||
| Change from 3 Weeks to 6 Weeks | |||
| > 11%decrease | 1 | 2 | 1 |
| within ± 11% | 11 | 16 | 1 |
| > 11% Increase | 2 | 4 | 0 |
|
| |||
| * Groups B and D pooled. N=38 eyes with data at baseline, 3 wks, and 6 wks | |||
| 4b. Duration of Initial Injection – 2.5 mg group * | |||
| Change from Baseline to 3 Weeks | |||
| > 11%
decrease (N=13) |
Within
± 11% (N=10) |
> 11%
increase (N=0) |
|
|
| |||
| Change from 3 Weeks to 6 Weeks | |||
| > 11% decrease | 0 | 0 | 0 |
| within ± 11% | 9 | 9 | 0 |
| > 11% increase | 4 | 1 | 0 |
|
| |||
| * Group C. N=23 eyes with data at baseline, 3 wks, and 6 wks | |||
| 4c. Duration of Second Injection – 1.25 mg group * | |||
| Change from 6 Weeks to 9 Weeks | |||
| > 11%
decrease (N=7) |
Within
± 11% (N=9) |
> 11%
increase (N=2) |
|
|
| |||
| Change from 9 Weeks to 12 Weeks | |||
| > 11% decrease | 0 | 1 | 0 |
| within ± 11% | 4 | 6 | 2 |
| > 11% increase | 3 | 2 | 0 |
|
| |||
| * Group B. N=18 eyes with data at 6 wks, 9 wks, and 12 wks | |||
| 4d. Duration of Second Injection – 2.5 mg group* | |||
| Change from 6 Weeks to 9 Weeks | |||
| > 11%
decrease (N=3) |
Within
± 11% (N=17) |
> 11%
increase (N=2) |
|
|
| |||
| Change from 9 Weeks to 12 Weeks | |||
| > 11% decrease | 0 | 2 | 1 |
| within ± 11% | 2 | 14 | 1 |
| > 11% increase | 1 | 1 | 0 |
|
| |||
| * Group C. N=22 eyes with data at 6 wks, 9 wks, and 12 wks | |||
Change in central subfield thickness was categorized according to whether it exceeded 11%, the reliability limit for real change determined in another Diabetic Retinopathy Clinical Research Network study16
Subgroup Analyses of Pooled Bevacizumab Groups at 3 Weeks
The 4 bevacizumab groups (Groups B, C, D, and E; N=87) were pooled to compare differences in response at 3 weeks among subgroups of interest (Table 6, available at http://aaojournal.org). Eyes with thicker retinas at baseline experienced a greater absolute reduction in central subfield thickening (P<0.0001) but the association was less pronounced for a relative reduction in thickening (change in thickening relative to baseline thickening) (P=0.12). Likewise, with visual acuity, eyes with worse baseline visual acuity showed greater improvement in visual acuity at 3 weeks (P=0.006) but the percent reduction in the visual acuity deficit did not differ according to baseline acuity (P=0.40). Change in central subfield thickening and change in visual acuity from baseline to 3 weeks did not vary substantially according to subject age (P=0.44 and 0.23, respectively), gender (P=0.55 and 0.37, respectively), retinopathy severity (P=0.53 and 0.38, respectively), or clinician categorization of DME as focal or diffuse (P=0.93 and 0.45, respectively). There was a suggestion of greater effect on visual acuity in eyes that had not been treated previously for DME compared with previously treated eyes (P=0.04) but less so for central subfield thickening (P=0.16). In eyes with subretinal fluid compared with eyes with no evidence of subretinal fluid, there was a suggestion of greater effect on change in visual acuity (P=0.06) but not on change in central subfield thickening (P=0.52).
Table 6.
3 Week Outcomes in Bevacizumab Groups* According to Patient Characteristics-website
| Change in Central Subfield thickness (microns) from baseline to 3 weeks | Change in visual acuity (letters) from baseline to 3 weeks | |||||
|---|---|---|---|---|---|---|
| N | Median (quartiles) | P-value† | N | Median (quartiles) | P-value† | |
| Baseline central subfield thickness ‡ | <0.0001§ | 0.22 | ||||
| <400 microns | 43 | -3 (-49, +13) | 44 | +1 (-2, +8) | ||
| >=400 microns | 41 | -102 (-145, -6) | 43 | +5 (0, +8) | ||
| Baseline visual acuity‡ | 0.31 | 0.006∥ | ||||
| <65 letters | 41 | -35 (-130, +5) | 44 | +3 (0, +9) | ||
| >=65 letters | 43 | -28 (-102, +13) | 43 | +1 (-3, +7) | ||
| Age‡ | 0.44 | 0.23 | ||||
| <=66 years | 41 | -45 (-131, +5) | 42 | +4 (-1, +8) | ||
| >66 years | 43 | -16 (-107, +6) | 45 | +2 (0, +8) | ||
| Gender | 0.55 | 0.37 | ||||
| F | 33 | -28 (-115, +6) | 33 | +3 (0, +8) | ||
| M | 51 | -35 (-105, +5) | 54 | +3 (-1, +7) | ||
| Prior treatment for diabetic macular edema (DME) | 0.16 | 0.04 | ||||
| No | 26 | -40 (-141, -3) | 26 | +5 (-1, +8) | ||
| Yes | 58 | -29 (-102, +7) | 61 | +2 (-1, +8) | ||
| Baseline retinopathy severity¶ | 0.53 | 0.38 | ||||
| <Severe nonproliferative diabetic retinopathy (NPDR) | 52 | -31 (-115, +6) | 53 | +3 (0, +8) | ||
| Proliferative diabetic retinopathy or severe NPDR | 27 | -31 (-105, +13) | 29 | +1 (-3, +7) | ||
| Baseline clinical DME characterization** | 0.93 | 0.45 | ||||
| Typical/Predominantly Focal | 14 | -13 (-67, -2) | 14 | +7 (0, +9) | ||
| Neither Predominantly Focal or Diffuse | 20 | -8 (-32, +20) | 21 | 0 (-5, +5) | ||
| Typical/Predominantly Diffuse | 50 | -82 (-139, +5) | 52 | +3 (0, +8) | ||
| Baseline subretinal fluid | 0.52 | 0.06 | ||||
| Definite/Questionable | 21 | -35 (-131, +24) | 21 | +6 (+3, +11) | ||
| No Evidence | 63 | -29 (-102, +3) | 66 | +1 (-1, +7) | ||
Groups B, C, D and E pooled. Optical coherence tomography was not done at the 3 week visit by 3 subjects.
P-values from least squares regression model, adjusted by baseline score. Medians and interquartile ranges are reported rather than means and standard deviations because of the small sample size per group in order to present a better perspective on the distribution of the data and to minimize the effect of extreme values.
Continuous factor used for calculating p-value
Median (quartiles) percent change in central subfield thickening at 3 weeks for baseline thickness <400 microns = -2% (-37%, +9%) and for baseline thickness ≥400 microns = -26% (-56%, -2%); P-value for continuous measure of baseline thickness from a least squares regression model = 0.12
Median (quartiles) percent reduction in visual acuity deficit at 3 weeks for baseline acuity <65 letters = +11% (0%, +25%) and for baseline acuity ≥65 letters = +7% (-12%, +33%); P-value for continuous measure of baseline acuity from a least squares regression model = 0.40
Excludes 5 missing baseline retinopathy severity (photographs lost or ruined for 3 subjects, and cannot grade for 2 subjects)
P-value for typical/predominantly focal vs typical/predominantly diffuse
Adverse Effects
Endophthalmitis (due to coagulase-negative staphylococcus) developed in one subject following an intravitreal bevacizumab injection. A transient increase in intraocular pressure occurred in one subject 6 weeks following an initial 1.25 mg bevacizumab injection. There were no other cases of consequential treatment-related ocular adverse events, including no reported cases of uveitis.
Among the 107 subjects who received at least one bevacizumab injection, a myocardial infarction occurred in two and congestive heart failure in one. One fatal myocardial infarction occurred in a 78 year old man 73 days following the second injection of 1.25 mg bevacizumab and a nonfatal myocardial infarction occurred in a 69 year old man 5 days following an initial injection of 2.5 mg bevacizumab; both men had a history of prior coronary artery bypass surgery. The episode of congestive heart failure occurred in a 56 year old woman who had a history of 3 prior similar episodes, 40 days following the second injection of 1.25 mg bevacizumab.
Three bevacizumab-treated subjects experienced elevation of blood pressure (Groups C, D and E); 1 of these subjects had a history of hypertension. There were no significant differences in mean blood pressure comparing the focal photocoagulation group with the bevacizumab groups (pooled) at the 3, 6, 9 or 12 week visits. Other reported adverse events in bevacizumab-treated subjects included: death due to pancreatic cancer (N=1, Group B), peripheral vascular disease (N=1, Group C), syncope (N=1, Group B), worsening of renal function (N=3, Groups C, D and E), and anemia (N=4, Groups B, C, D and E). In the 12 subjects who received only focal photocoagulation, there were no thromboembolic cardiovascular events; one case of anemia, 2 cases of peripheral vascular disease, 1 case of hypertension, and 1 case of worsening of renal function were reported.
Discussion
To assist in the development of a phase 3 randomized trial protocol, this study was designed to address six questions related to the short-term effect of intravitreal bevacizumab for DME plus provide preliminary ocular and systemic safety data.
1) Does 1.25 mg intravitreal bevacizumab reduce OCT-measured retinal thickening in DME?
2) Does 2.5 mg intravitreal bevacizumab reduce OCT-measured retinal thickening in DME?
Compared with a control group receiving focal photocoagulation, both the 1.25 mg and 2.5 mg bevacizumab-treated eyes had a greater reduction in central retinal thickness at 3 weeks. Eyes in the photocoagulation group demonstrated improvement in these parameters with longer follow up. As a result, there were no meaningful differences in central subfield thickness observed for bevacizumab relative to photocoagulation after the 3-week time point. Only about half of the eyes showed what was judged to be a response to intravitreal bevacizumab (exceeding an 11% reduction in retinal thickness compared with baseline) at either the 3-week or 6-week visit. For visual acuity, with both bevacizumab doses, on average there was about one line greater improvement relative to photocoagulation throughout the 12 weeks.
3) Does 2.5 mg intravitreal bevacizumab produce a greater shorter-term reduction in OCT-measured retinal thickening from DME than 1.25 mg intravitreal bevacizumab?
Comparisons of the 2.5 mg and 1.25 mg doses suggest that there is not likely a large difference in short-term effect between the two doses. However, no conclusions should be drawn about the long-term comparative effect of the two doses.
4) What is the duration of reduction in OCT-measured retinal thickening following the initial injection of intravitreal bevacizumab?
5) What is the duration of reduction in OCT-measured retinal thickening following the second injection of intravitreal bevacizumab?
The reduction in retinal thickness associated with bevacizumab at 3 weeks appeared to plateau or decrease in most eyes between the 3-week and 6-week visits, suggesting that 6 weeks may be too long for an optimal initial injection interval. Four of 61 bevacizumab only-treated eyes (7%) showed a reduction in central subfield thickness between 3 and 6 weeks whereas 11 of the 61 eyes (18%) showed an increase in thickness between 3 and 6 weeks. Following the second injection, 4 of 40 eyes (10%) had a decrease in thickness between the 9 and 12 week visits while 7 of 40 (18%) had an increase, again suggesting that 6 weeks may be too long for an optimal second injection interval.
6) Is there a greater reduction in OCT-measured retinal thickening using intravitreal bevacizumab followed by focal photocoagulation compared with intravitreal bevacizumab alone?
Combining photocoagulation with bevacizumab resulted in no apparent short-term benefit or adverse outcomes. Although this study demonstrated the feasibility for future protocols of including a group that receives intravitreal bevacizumab followed by focal photocoagulation at 3 weeks, the follow up was too short to determine if combination therapy would be beneficial in either improving visual outcome or reducing the number of intravitreal injections required. A beneficial effect of focal photocoagulation could occur over a longer time period than the duration of this study.
Comparison with Literature
While reports in the literature note individual cases of short-term improvement in visual acuity and reduction in OCT-measured retinal thickening following intravitreal injection of an anti-VEGF drug (bevacizumab, pegaptnaib, or ranibizumab), none of these reports included subjects concurrently randomized to focal photocoagulation.11, 18
Safety
The systemic use of bevacizumab has been associated with an increased risk of cerebrovascular and cardiovascular thromboembolic events (bevacizumab package insert). Although the intravitreal dose is 1/400 or less of the usual systemic dose, the possibility of a systemic adverse effect after an intravitreal bevacizumab injection nevertheless exists. According to the ranibizumab package insert, intravitreal ranibizumab has a “theoretical risk of arterial thromboembolic events” and, in an ongoing study, Safety Assessment of Intravitreal Lucentis for Age-Related Macular Degeneration (SAILOR), of ranibizumab delivered intravitreally to patients with neovascular age-related macular degeneration, a planned interim safety analysis performed on data from Cohort 1, patients with an average follow up of 230 days demonstrated a higher incidence of strokes in the 0.5 mg dose group compared with the 0.3 mg dose group (1.2% versus 0.3%, p=0.02; letter dated January 24, 2007 from Hal Barron, MD, Chief Medical Officer of Genentech, Inc to health care providers [www.gene.com/gene/products/information/pdf/healthcare-provider-letter.pdf. Accessed May 30, 2007]).
Systemic safety evaluation of bevacizumab in the current study is limited by the small sample size and short follow up. There were several cases of systemic cardiovascular or renal adverse effects, all of which occurred in subjects with related pre-existing medical conditions, including two cases of myocardial infarction. In this study, there was one case of injection-related endophthalmitis in 185 injections, an uncommon but well recognized complication of intravitreal injection, but no important ocular complications attributable to the drug. The follow up of patients in published reports of the use of intravitreal bevacizumab in humans is too short to be conclusive regarding the ocular safety of intravitreal bevacizumab.19-21
Conclusions
This study was conducted to provide data to assist in the development of a phase 3 randomized clinical trial protocol and, by design, had a short follow-up period and a modest sample size. Therefore, definitive safety and effectiveness conclusions are limited. Although about half of eyes demonstrated an initial positive response to intravitreal bevacizumab (exceeding an 11% reduction in retinal thickness compared with baseline at either the 3-week or 6-week visit), this response was similar to that observed in the laser group after more than 3 weeks. In addition, the magnitude of the response was not large for most subjects. Thus, these short-term results of the current study should not be generalized to conclude that there is a clinically meaningful benefit in treating DME with intravitreal bevacizumab or other anti-VEGF drugs. This determination of clinical benefit will require the conduct of a large phase 3 randomized clinical trial.
Acknowledgments
Financial Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases EY14231, EY14269, EY14229 and a grant from the Juvenile Diabetes Research Foundation
Writing Committee
Lead authors: Ingrid U. Scott, Allison R. Edwards, Roy W. Beck. Additional writing committee members (alphabetical): Neil M. Bressler, Clement K. Chan, Michael J. Elman, Scott M. Friedman, Craig Michael Greven, Raj K. Maturi, Dante J. Pieramici, Michel Shami, Lawrence J. Singerman, Cynthia R. Stockdale
The Diabetic Retinopathy Clinical Research Network*
*A complete list of the members of the Diabetic Retinopathy Clinical Research Network participating in the trial is available at http://aaojournal.org.
Clinical Sites that Participated in this Protocol
Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Investigators are listed for each site.
Baltimore, MD Elman Retina Group, P.A. (15): Michael J. Elman, M.D.; Indianapolis, IN Raj K. Maturi, M.D., P.C. (8): Raj K. Maturi; Portland, OR Casey Eye Institute (8): Andreas K. Lauer; Christina J Flaxel; Lakeland, FL Central Florida Retina Institute (7): Scott M. Friedman; Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (6): David J. Browning; Andrew N. Antoszyk; Ft. Lauderdale, FL Retina Vitreous Consultants (6): Ronald J. Glatzer; W. Scott Thompson; Scott Anagnoste; Palm Springs, CA Southern California Desert Retina Consultants, MC (5): Clement K Chan; Steven G Lin; Asha S.D. Nuthi; David M. Salib; Cleveland, OH Retina Associates of Cleveland, Inc. (4): Lawrence J. Singerman; David G. Miller; Michael A. Novak; Columbia, SC Palmetto Retina Center (4): W. Lloyd Clark; John A. Wells; Lake Mary, FL Central Florida Retina (4): Preston P. Richmond; Saad A. Shaikh; C. Durham Barnes; John C. Olson; Lubbock, TX Texas Retina Associates (4): Michel Shami; Winston-Salem, NC Wake Forest University Eye Center (4): Craig Michael Greven; M. Madison Slusher; Bangor, ME Maine Vitreoretinal Consultants (3): Deborah Hoffert; Thomas E. Flynn; Joliet, IL Illinois Retina Associates (3): John S. Pollack; Joseph M. Civantos; Knoxville, TN Southeastern Retina Associates, P.C. (3): Joseph Googe; John C. Hoskins; Nicholas G. Anderson; Lexington, KY Retina and Vitreous Associates of Kentucky (3): Thomas W Stone; Rick D. Isernhagen; William J. Wood; John W. Kitchens; Loma Linda, CA Loma Linda University Health Care, Department of Ophthalmology (3): Joseph T. Fan; Michael E. Rauser; Paducah, KY Paducah Retinal Center (3): Carl W. Baker; Walnut Creek, CA Bay Area Retina Associates (3): Stewart A. Daniels; T. Daniel Ting; Craig J. Leong; Subhransu K Ray; Arlington, TX Texas Retina Associates (2): David G. Callanan; Wayne A. Solley; Augusta, GA Southeast Retina Center, P.C. (2): Dennis M. Marcus; Harinderjit Singh; Austin, TX Retina Research Center (2): Brian B. Berger; Boston, MA Ophthalmic Consultants of Boston (2): Trexler M Topping; Jeffrey S Heier; Hershey, PA Penn State College of Medicine (2): Ingrid U. Scott; David A. Quillen; Thomas W. Gardner; Kimberly A. Neely; Houston, TX Charles A. Garcia, P.A and Associates (2): Charles A. Garcia; John McCrary; Ricardo Stevenson; Providence, RI Retina Consultants (2): Robert H. Janigian; Harold A. Woodcome; Santa Barbara, CA California Retina Consultants (2): Dante J. Pieramici; Alessandro Castellarin; Abilene, TX West Texas Retina Consultants P.A. (1): Sunil S. Patel; Boston, MA Joslin Diabetes Center (1): Jennifer K. Sun; Paul G. Arrigg; George S. Sharuk; Columbia, SC Carolina Retina Center (1): Jeffrey G. Gross; Dallas, TX Texas Retina Associates (1): Gary E. Fish; Robert C. Wang; Minneapolis, MN Retina Center, PA (1): Abdhish R. Bhavsar; New Albany, IN American Eye Institute (1): Howard S. Lazarus; Portland, OR Retina Northwest, PC (1): Mark A. Peters; Irvin L. Handelman; Michael S. Lee; Salisbury, MD Retina Consultants of Delmarva, P.A. (1): Jeffrey D. Benner; Seattle, WA University of Washington Medical Center (1): James L. Kinyoun.
Coordinating Center
Jaeb Center for Health Reserch, Tampa, Florida. Investigators: Roy W. Beck (Director), Adam R. Glassman, Cynthia R. Stockdale, Allison R. Edwards, Crag Kollman.
DRCR.net Chairman’s Office
Johns Hopkins University, Baltimore, MD. Neil M. Bressler (Network Chair).
Fundus Photograph Reading Center
University of Wisconsin– Madison, Madison, WI: Matthew D. Davis (Director Emeritus), Ronald P. Danis (Director).
The Diabetic Retinopathy Clinical Research Network Clinical Sites that Participated in this Protocol
Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Investigator, (C) for Coordinator, (V) for Visual Acuity Tester, and (P) for Photographer.
Baltimore, MD Elman Retina Group, P.A. (15): Michael J. Elman, M.D.(I); Michelle D. Sloan (C); JoAnn Starr (C,V); Tammy M. Butcher (C); Teresa Coffey (V); Nancy Gore (V); Pamela V. Singletary (V); Giorya Andreani (P); Terri Cain (P); Peter Sotirakos (P); Indianapolis, IN Raj K. Maturi, M.D., P.C. (8): Raj K. Maturi (I); Laura A. Bleau (C,P,V); Jama L. Poston (V); Michelle Storie (V); Thomas Steele (P); Abby Maple (P); Portland, OR Casey Eye Institute (8): Andreas K. Lauer (I); Christina J Flaxel (I); Susan I. Pope (C,V); Susan K. Nolte (V); Patrick B. Rice (P); Chris S Howell (P); Patrick R. Wallace (P); Lakeland, FL Central Florida Retina Institute (7): Scott M. Friedman (I); Kelly A. Blackmer (C); Virginia Gregory (C,P,V); Steve Carlton (C,P,V); Damanda A. Fagan (V); Jolleen S. Key (P,V); Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (6): David J. Browning (I); Andrew N. Antoszyk (I); Danielle R. Brooks (C,V); Melissa K. Cowen (C,V); Angela K. Price (C); Jennifer V. Helms (C,V); Roderick O. Walker (V); Rachel E. Pierce (V); Heather L. Murphy (V); Linda M Davis (P); Karen A. Ruiz (P); Michael D. McOwen (P); Loraine M. Clark (P); Donna McClain (P); Ft. Lauderdale, FL Retina Vitreous Consultants (6): Ronald J. Glatzer (I); W. Scott Thompson (I); Scott Anagnoste (I); Jaclyn A. Brady-Lopez (C); Clifford M. Sherley (V); Antonio Bolet (V); Michelle Earl (P); Brian M. Fernandez (P); Palm Springs, CA Southern California Desert Retina Consultants, MC (5): Clement K Chan (I); Steven G Lin (I); Asha S.D. Nuthi (I); David M. Salib (I); Teri A. Andresen (C); Eric D. Dickerson (C); Kelly E. Sage (C); Trina L. Keith (C); Kara Rollins (V); Sara Warren (V); Kenneth M Huff (P); Sabrina E. Bretz (P); Cleveland, OH Retina Associates of Cleveland, Inc. (4): Lawrence J. Singerman (I); David G. Miller (I); Michael A. Novak (I); Trina M. Nitzsche (C,V); Vivian Tanner (V); Elizabeth McNamara (V); Kimberly A. Dubois (V); John C. DuBois (P); Sheila K. Smith-Brewer (P); Gregg A. Greanoff (P); Columbia, SC Palmetto Retina Center (4): W. Lloyd Clark (I); John A. Wells (I); Marcia D. Gridine (C,V); Robbin Spivey (V); Amy B. Hickman (P); Lake Mary, FL Central Florida Retina (4): Preston P. Richmond (I); Saad A. Shaikh (I); C. Durham Barnes (I); John C. Olson (I); Laverne Denise Davila (C); Joyce A. Treutel (V); Ginny Bell (P); Trudy E. Thornton (P); Lubbock, TX Texas Retina Associates (4): Michel Shami (I); Phyllis Pusser (C); Carrie L. Tarter (C,V); Linda Squires (V); Thom F. Wentlandt (P); Winston-Salem, NC Wake Forest University Eye Center (4): Craig Michael Greven (I); M. Madison Slusher (I); Joan Fish (C,V); Frances Ledbetter (C,V); David T Miller (P); Marshall Tyler (P); Bangor, ME Maine Vitreoretinal Consultants (3): Deborah Hoffert (I); Thomas E. Flynn (I); Dawn Sutherland (C,P,V); Tara Forni (V); Pru Betterley (P); Joliet, IL Illinois Retina Associates (3): John S. Pollack (I); Joseph M. Civantos (I); Barbara J. Ciscato (C); Robin A. Mikota (V); Daniel W. Muir (P); Knoxville, TN Southeastern Retina Associates, P.C. (3): Joseph Googe (I); John C. Hoskins (I); Nicholas G. Anderson (I); Tina T. Higdon (C,V); Stephanie Evans (C); Charity D. Morris (C); David J. Cimino (V); Cecile Hunt (V); Paul A. Blais (P); Lexington, KY Retina and Vitreous Associates of Kentucky (3): Thomas W Stone (I); Rick D. Isernhagen (I); William J. Wood (I); John W. Kitchens (I); Wanda R. Heath (C); Diana Holcomb (C); Michelle Buck (V); Judith L Cruz (V); Catherine Millett (V); Edward A. Slade (P); S.Todd Blevins (P); Loma Linda, CA Loma Linda University Health Care, Department of Ophthalmology (3): Joseph T. Fan (I); Michael E. Rauser (I); Carrousel J. Corliss (C,P,V); Sarina L. Osuna (C); Alice M. Ortega (V); William H. Kiernan (V); Gene Saldana (P); Paducah, KY Paducah Retinal Center (3): Carl W. Baker (I); Tracey M. Caldwell (C); Lynnette F. Lambert (V); Mary J. Palmer (V); Dawn D. Smith (P); Walnut Creek, CA Bay Area Retina Associates (3): Stewart A. Daniels (I); T. Daniel Ting (I); Craig J. Leong (I); Subhransu K Ray (I); Cindy M. Moreci (C); Sumi Takahara (V); Rouella J. Tejada (V); Fred Hanamoto (P); Arlington, TX Texas Retina Associates (2): David G. Callanan (I); Wayne A. Solley (I); Cheryl A. Grimes (C); Yolanda Garcia (V); Jodi Creighton (V); Bob Boleman (P); Augusta, GA Southeast Retina Center, P.C. (2): Dennis M. Marcus (I); Harinderjit Singh (I); Graciela R. Zapata (C); Kimbi Y. Overton (C); Mari C. McAteer (C); Ken Ivey (V); Austin, TX Retina Research Center (2): Brian B. Berger (I); Barbara Pereira (C); Linda N. Nguyen (C,V); Erin N. Scrivner (C,V); Elisabeth A. Durham (C); Melissa A. Talbert (V); Ben Ostrander (P); Boston, MA Ophthalmic Consultants of Boston (2): Trexler M Topping (I); Jeffrey S Heier (I); Victoria M. Hurley (C); Taneika N. Howard (V); Heather L. Davis (V); Margie Graham (P); Mike Jones (P); Hershey, PA Penn State College of Medicine (2): Ingrid U. Scott (I); David A. Quillen (I); Thomas W. Gardner (I); Kimberly A. Neely (I); Susan M. Chobanoff (C,V); Mary Wilmarth (C,V); Mary L. Frawley (V); Timothy J. Bennett (P); James D. Strong (P); Houston, TX Charles A. Garcia, P.A and Associates (2): Charles A. Garcia (I); John McCrary (I); Ricardo Stevenson (I); Elizabeth Garibay (C); Emma M. Lessieur (C,V); Cecilia Vi Nguyen (V,P); Juan P. Montoya (V); Luis R. Salinas (P); Providence, RI Retina Consultants (2): Robert H. Janigian (I); Harold A. Woodcome (I); Sylvia Varadian (C); Collin L. DuCoty (C); Erika Banalewicz (V); Claudia Salinas (V); Mark Hamel (P); Alex L. Nagle (P); Santa Barbara, CA California Retina Consultants (2): Dante J. Pieramici (I); Tamara A. Norton (C,V); Sarah K. Davies (C,V);Alessandro Castellarin (I); Liz Tramel (V,P); Kelly Avery (V) ;Matthew Giust (P);Karen Boyer (P); Abilene, TX West Texas Retina Consultants P.A. (1): Sunil S. Patel (I); Brandi L. Dunn (C,P); Kristen L. Garcia (C,P,V); Brenda K. Arrington (C,P,V); Leah D. Adams (P); Boston, MA Joslin Diabetes Center (1): Jennifer K. Sun (I); Paul G. Arrigg (I); George S. Sharuk (I); Margaret E Stockman (C,V); Ann Kopple (C); Leila Bestourous (V); Jerry D. Cavallerano (V); Robert W. Cavicchi (P); Ellen L. Casazza (P); Columbia, SC Carolina Retina Center (1): Jeffrey G. Gross (I); Amy M. Flowers (C); Heidi K. Lovit (V); Regina A. Gabriel (V); Randall L. Price (P); Dallas, TX Texas Retina Associates (1): Gary E. Fish (I); Robert C. Wang (I); Jean Arnwine (C); Brenda Sanchez (V); Diana Jaramillo (P); Kimberly Cummings (P); Hank Aguado (P); Minneapolis, MN Retina Center, PA (1): Abdhish R. Bhavsar (I); Tanya M Olson (C); Carmen Chan (C,P); William B. Carli (V); Craig H. Hager (V); Melinda Spike-Kivel (V); Laura Taylor-Reetz (P); New Albany, IN American Eye Institute (1): Howard S. Lazarus (I); Debra Paige Bunch (C,V); Liana C. Davis (V); Jay Moore (P); Margaret Trimble (P); Portland, OR Retina Northwest, PC (1): Mark A. Peters (I); Irvin L. Handelman (I); Michael S. Lee (I); Stephen Hobbs (C,P,V); Sennie M. Kramer (V); Marcia Kopfer (V); Joe Logan (P); Salisbury, MD Retina Consultants of Delmarva, P.A. (1): Jeffrey D. Benner (I); Hannah Scott (C,V); Cristy Carbaugh (P); Robin L. Hurley (P); Seattle, WA University of Washington Medical Center (1): James L. Kinyoun (I); Susan A. Rath (C,V); Patricia K. Ernst (V); Brad C. Clifton (P); Chuck Stephens (P); James D. Leslie (P)
DRCR.net Coordinating Center – Jaeb Center for Health Research, Tampa, FL
Roy W. Beck (Director), Adam R. Glassman (Assistant Director), Joy Barros, Brian B. Dale, Sharon R. Constantine, Simone S. Dupre, Allison R. Edwards, Charissa L. Felgemacher, Karalyn L. Grant, Nicola B. Hill, Paula A. Johnson, Craig Kollman, Lee Anne Lester, Brenda L. Loggins, Shannon L. McClellan, Pamela S. Moke, Ana C. Perez, Haijing Qin, Apryl C. Quillen, Rosa Pritchard, Cynthia R. Stockdale, Samara F. Strauber
DRCR.net Chairman’s Office – Johns Hopkins University, Baltimore, MD
Neil M. Bressler – Baltimore, MD (Network Chair)
Fundus Photograph Reading Center – University of Wisconsin- Madison, Madison, WI
Matthew D. Davis (Director Emeritus), Ronald P. Danis (Director), Larry Hubbard (Associate Director), James Reimers (Lead Color Photography Evaluator), Pamela Vargo (Lead Photographer), Ericka Lambert (Digital Imaging Specialist), Dawn Myers (Lead OCT Evaluator), Brian Hong (Project Manager)
Data and Safety Monitoring Committee
John Connett (Chair), Deborah R. Barnbaum (2006-present), Harry W. Flynn, Jr., Robert N. Frank, Saul Genuth, Lee Jampol, Jeanette Resnick (2002-2005), Stephen Wisniewski
Steering Committee
Ingrid U. Scott (Protocol Chair), Lloyd P. Aiello, Roy W. Beck, Abdhish Bhavsar, Neil M. Bressler, David J. Browning, Alexander J. Brucker, Peter Campochiaro, Ronald P. Danis, Michael J. Elman Frederick L. Ferris, Joan Fish, Scott M. Friedman, Adam R. Glassman, Mary Elizabeth R. Hartnett, Päivi H. Miskala
DRCR.net Executive Committee
Neil M. Bressler (Chair), Lloyd P. Aiello, Roy W. Beck, Abdhish Bhavsar, David M. Brown, David J. Browning, Ronald P. Danis, Matthew D. Davis, Michael J. Elman, Frederick L. Ferris, Adam R. Glassman, Cynthia J. Grinnell, Päivi H. Miskala
National Eye Institute
Päivi H. Miskala, Donald F. Everett (2002 – 2004)
Footnotes
Conflict of Interest: Writing committee financial interest disclosure forms are attached to the transmittal letter. For a listing of financial disclosures for all Network investigators as of date of submission go to the DRCR.net website (www.drcr.net).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 2.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Ophthalmology. 1991;98(suppl):766–85. [PubMed] [Google Scholar]
- 3.Diabetes Control and Complication Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122:1631–40. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 5.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–7. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 6.PKC-DRS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54:2188–97. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 7.PKC-DRS2 Group. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113:2221–30. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Pendergast SD, Hassan TS, Williams GA, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178–86. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- 9.Macugen Diabetic Retinopathy Study Group. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112:1747–57. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142:961–9. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 14.Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology. 2006;113:1706–12. doi: 10.1016/j.ophtha.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 16.Diabetic Retinopathy Clinical Research Network. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. doi: 10.1016/j.ophtha.2006.10.055. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 2. Ophthalmology. 1987;94:761–74. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 18.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–50. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006;47:4569–78. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- 20.Grover S, Fishman GA, Birch DG, et al. Variability of full-field electroretinogram responses in subjects without diffuse photoreceptor cell disease. Ophthalmology. 2003;110:1159–63. doi: 10.1016/S0161-6420(03)00253-7. [DOI] [PubMed] [Google Scholar]
- 21.Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:270–4. doi: 10.1097/00006982-200603000-00003. [DOI] [PubMed] [Google Scholar]