Abstract
The capsular polysaccharide of Neisseria meningitidis group B (MBPS) is a polymer of alpha (2 → 8) N-acetyl neuraminic acid, which is chemically identical to polysialic acid (PSA) expressed in human tissues. Antibodies from mice immunized with a MBPS-protein conjugate vaccine in which N-acetyl groups have been replaced by propionyl groups (N-Pr MBPS) can be bactericidal and show minimal or no cross-reactivity with human PSA. To investigate the molecular basis for antigen recognition, we cloned and sequenced the variable region (V) genes of five bactericidal anti-N-Pr MBPS murine mAbs and produced computer models of the combining sites. The results were compared to those reported in the literature for two autoreactive anti-MBPS. The V region genes of the anti-N-Pr MBPS mAbs and the anti-MBPS autoreactive mAbs are derived from a limited set of germline V, J, and D genes. However, the anti-N-Pr MBPS mAbs are more mutated than the anti-MBPS mAbs and the former use V–D–J editing that introduces arginine in H-CDR3. Models of the respective combining sites indicate that the anti-MBPS or anti-N-Pr MBPS mAbs that react with host PSA have relatively wide and shallow grooves with a high overall positive charge, consistent with recognition of extended helical polysaccharide structures recognized by the autoreactive mAbs. In contrast, anti-N-Pr MBPS mAbs that do not react with host PSA contain pockets and deep clefts that are consistent with recognition of discrete structural features of individual residues.
Keywords: Autoantibodies, Autoantigens, Meningococcal vaccine, Structural models, V region genes
1. Introduction
Pathogenic Neisseria meningitidis are a major cause of bacteremia and meningitis worldwide (Rosenstein et al., 2001). Safe and effective vaccines based on immunologically distinctive capsular polysaccharides have been developed for prevention of disease caused by strains with capsular groups A, C, Y, or W-135 (Granoff et al., 2004) but not for group B (Jodar et al., 2002; Morley and Pollard, 2001). The group B capsular polysaccharide (MBPS), alpha (2 → 8) N-acetyl neuraminic acid, is chemically identical to long chain polysialic acid that is abundantly expressed in brain, heart and kidney during fetal development (Finne et al., 1983). As a result, the group B capsular polysaccharide is poorly immunogenic, even when complexed with outer membrane proteins (Zollinger et al., 1979) or when conjugated to a carrier protein (Jennings and Lugowski, 1981) Monoclonal antibodies (mAbs) have been prepared by immunizing mice with group B bacteria (Mandrell and Zollinger, 1982; Moreno et al., 1983; Shin et al., 2001), and human MBPS-reactive IgM mAbs and paraproteins have been described (Azmi et al., 1994; Mandrell et al., 1995; Raff et al., 1991). However, IgG anti-MBPS have been produced only in New Zealand Black (NZB) mice that are hyper-responsive to many antigens and, in particular, make antibody responses to autoantigens (Frosch et al., 1985; Mellors, 1966; Tillman et al., 1992).
Jennings and coworkers prepared a MBPS derivative by removing the N-acetyl groups in aqueous NaOH and reacylating with propionic anhydride to produce N-propionyl (N-Pr) MBPS (Ashton et al., 1989; Jennings et al., 1987, 1986, 1985; Jennings and Lugowski, 1981; Pon et al., 1997).
Immunization of mice with N-Pr MBPS conjugated to a carrier protein elicited high titers of bactericidal IgG antibodies. A subset of the bactericidal antibodies did not cross-react with human polysialic acid antigens. Jennings and coworkers hypothesized that the N-Pr MBPS derivative mimics a conformational epitope present in the bacterial capsule but not in host PSA.
In a previous study, we prepared a panel of anti-N-Pr MBPS mAbs (Granoff et al., 1998) by immunizing mice with N-Pr MBPS conjugated to tetanus toxoid. The mAbs bound to the surface of live encapsulated group B bacteria but not to a capsular deficient mutant. A number of the mAbs elicited complement-mediated bactericidal activity in vitro, and conferred passive protection against bacteremia in an infant rat model. The bactericidal mAbs exhibited a range of different fine antigenic specificities and some showed minimal or no detectable autoreactivity with long chain polysialic acid expressed by the human cell line CHP-134 (Livingston et al., 1988) as measured by flow cytometry (Granoff et al., 1998). There also was no binding by immunohistochemical analysis with human fetal tissue (unpublished data). Recently we showed that one of the mAbs, SEAM 3, that has no detectable autoreactivity, binds to MBPS oliogosaccharides that contain de-N-acetyl residues. Further, MBPS derivatives containing de-N-Ac residues inhibited the bactericidal activity of SEAM 3. These results suggested that de-N-Ac residues are present in the capsule of live group B bacteria (Moe et al., 2005).
Achieving a better understanding of the molecular basis for antigen recognition by non-autoreactive anti-N-Pr MBPS mAbs will be important for furthering development of a safe and effective MBPS-based vaccine. In the present study, we cloned and sequenced the variable region (V) genes encoding five anti-N-Pr mAbs, and identified the putative germ line genes. We then used computer modeling to produce three-dimensional (3D) structural models of the Fv combining sites. The results were compared to similar analyses of the germline genes reported for two autoreactive anti-MBPS mAbs and, together, provide insights into structural differences between the paratopes of autoreactive and non-autoreactive mAbs that bind to group B capsular epitopes.
2. Materials and methods
2.1. mAbs
The anti-N-Pr MBPS murine mAbs SEAM 2 (IgG3), SEAM 3 (IgG2b), SEAM 12 (IgG2a), SEAM 18 (IgG2a) are representative of each of the four fine antigenic specificity groups described previously (Table 1) (Granoff et al., 1998). SEAM 35 (IgG2a, Table 1) also was included in our study because it exhibits greater cross-reactivity with polysialic acid antigens expressed by the human neuronal cell line CHP-134 than that of the other four SEAM mAbs (Granoff et al., 1998). The mAbs are bactericidal in the presence of complement and confer passive protection against meningococcal bacteremia in an infant rat model. SEAM 2 and SEAM 3 have no detectable autoreactive activity with host polysialic acid while SEAM 12 and SEAM 18 show minimal cross-reactivity.
Table 1.
Summary of fine antigenic specificities of the anti-N-Pr MBPS mAbsa
| mAb | ELISA reactivity to N-Ac MBPSb | ELISA inhibition of binding by N-Pr MBPS oligosaccharidesc | Autoreactivity with host polysialic acidd |
|---|---|---|---|
| SEAM 2 | 0 | 0 | 0 |
| SEAM 3 | 0 | +++ | 0 |
| SEAM 12 | ++ | 0 | + |
| SEAM 18 | +++ | +++ | + |
| SEAM 35 | +++ | + | ++ |
AII five mAbs are bactericidal in the presence of human and/or rabbit complement. Adapted from Granoff et al. (1998).
Ab binding to N-Ac MBPS: 0, OD <0.15; +, OD = 0.15–0.5; ++, OD = 0.5–1.0; +++, OD > 1.0 when tested at 5–25 μg/ml of Ab by ELISA.
Inhibition of antibody binding to N-Pr MBPS in an ELISA by soluble N-Pr MBPS (Dp < 6; average Dp = 3.8): 0, <20%; +, 21–48%; ++, 49–74%; +++, 75–100% inhibition when tested with an antibody concentration giving an OD = 0.5 after 30 min incubation with substrate.
Based on flow cytometry studies with the human cell line CHP-134 (Granoff et al., 1998), which expresses long chain polysialic acid (Livingston et al., 1988), and immuohistology with human fetal tissue (unpublished data): 0, no binding activity by flow to PSA when tested at 100 μg/ml of Ab; ++, binding activity detected at 10 μg/ml of Ab; +, binding activity detected at 100 μg/ml but not 10 μg/ml.
2.2. dsDNA ELISA
mAb binding to dsDNA was measured using methods described by Gilkeson et al. (1993). The positive control anti-DNA mAb was obtained from QED Biosciences (San Diego, CA) and isotype-matched irrelevant mAbs were obtained from Southern Biotech Inc. (Birmingham, AL).
2.3. V gene sequencing
Variable region genes of immunoglobulin heavy and light chains from mouse hybridoma cell lines were amplified by PCR using degenerate primers and cloned into the vector pGEM3zf− (Promega, Madison, WI) as described by Wang et al. (2000) using E. coli strain XL-2 Blue as a host. Plasmid DNA from individual transformants selected on LB-ampicillin plates was isolated using the Qiagen Mini Prep Kit (Qiagen) according to the manufacturers instructions. The cloned V genes were sequenced by BioNexus (Oakland, CA).
2.4. V gene sequence analysis
The mAb nucleotide sequences were analyzed using IGMT/V-QUEST and the mouse immunoglobulin nucleotide sequence data-base through the online web facilities of the international ImMunoGeneTics® information system (IMGT, http://imgt.cines.fr) that was initiated and coordinated by Marie-Paule Lefranc (Université Montpellier II, CNRS, LIGM, IGH, IFR3, Montpellier, France). Putative germ line genes were selected based on the closest match between germ line sequence in the database and cloned V gene sequence. Both amino acid and gene sequences were compared to respective sequences in the GenBank non-redundant sequence databases using BLAST (Altschul et al., 1997). In addition, we identified from the literature putative germ line genes used by a hybridoma clone expressing the anti-MBPS murine mAb, 735 (IgG2a). Since only the amino acid sequence of this mAb was available (Klebert et al., 1993; Vaesen et al., 1991), the predicted germline gene for this mAb is based on the closest amino acid sequence match in the IGMT/V-QUEST and GenBank/EMBL databases (Chenna et al., 2003). We also included in our comparative analysis the gene sequences and germline gene assignments for the anti-MBPS mAb 2-2-B (IgM (Mandrell and Zollinger, 1982)) reported by Berry et al. (2005).
2.5. 3D structure modeling of mAb combining sites
3D structure models were constructed using the online Web Antibody Modeling facility at the University of Bath, Great Britain (http://www.bath.ac.uk/cpad/). Modeling is based on the AbM package using a combination of established theoretical methods together with the latest antibody structural information (Martin et al., 1991). WAMpredict was used to assign canonical classes and H-CDR3 C-terminal conformation. Structure analysis, superposition, and graphical renderings were carried out using PyMOL (Delano Scientific, San Carlos, CA). Electrostatic surface potentials were calculated using APBS (Baker et al., 2001) as a plugin (developed by Michael G. Lerner, University of Michigan) in the Pymol Molecular Graphics System (Warren L. DeLano, DeLano Scientific, San Carlos, CA, http://www.pymol.org). All histidine residues were unprotonated. The solvent and protein dielectric constants were set at 80 and 20, respectively. The color scale shown in Fig. 2 ranges from −1 (red) to +5 (blue).
Fig. 2.
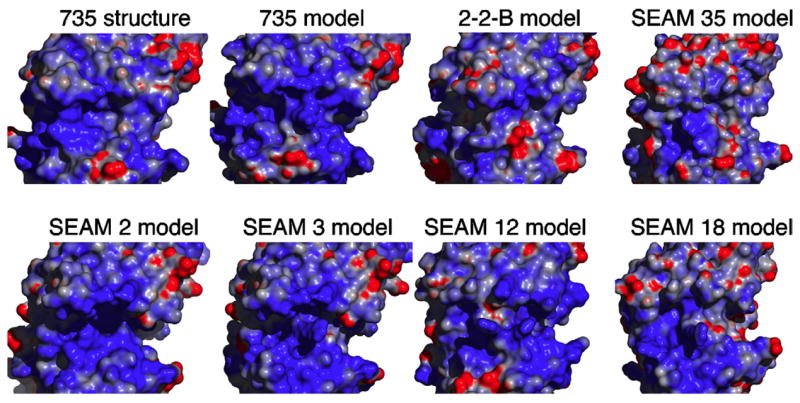
Combining site structure of mAb 735 and structural models of 2-2-B and anti-N-Pr MBPS mAbs SEAM 2, SEAM 3, SEAM 12, SEAM 18 and SEAM 35. The structures are shown as surface renderings and are arranged according to relative autoantibody activity from mAb 735 clockwise to SEAM 3 and SEAM 2, which have no autoantibody activity. The surface is colored according to electrostatic surface potential charge with dark blue corresponding to a charge of +5 and dark red a charge of −1. The surface potentials were calculated using APBS operating in Pymol. Note the dark shading between the light chain above and heavy chain below for the SEAM 2 and SEAM 3 models showing the presence of a deep cleft and pocket-like features, respectively.
3. Results
3.1. Variable region gene usage of murine anti-N-Pr MBPS mAbs
The germline gene usage for the anti-N-Pr MBPS and anti-MBPS mAbs are compared in Table 2. The respective amino acid sequences are shown in Fig. 1. The V region repertoire is restricted to a relatively few highly related VL or VH gene families. For example, SEAM 2 and SEAM 3, which have different fine antigenic specificities (Table 1), have identical VL amino acid sequences (Fig. 1), and the VL gene is from the same gene family (IgGKV1) as that encoding the autoreactive anti-MBPS mAbs, 2-2-B and 735 (Table 2). Similarly, the VL genes used by SEAM 12 and SEAM 18, which have different fine antigenic specificities, are from the same family (IgGKV4). The respective VH sequences of SEAM 3 and SEAM 18 are nearly identical to each other (96% identity), and are from the same germline gene family (IgGHV7S3) used for SEAM 35 VH (Table 2). The germline VH genes for SEAM 2 and SEAM 12 are different from each other and from the other three anti-N-Pr MBPS mAbs but the germline VH gene used for SEAM 2 is related to those used by the two autoreactive anti-MBPS mAbs, 2-2-B and 735 (both 72% identical; see Fig. 1).
Table 2.
Comparison of the genetic origin of variable regions of anti-MBPS and anti-N-Pr MBPS mAbsa
| Clone | Isotype | VH | DH | JH | VL | JK |
|---|---|---|---|---|---|---|
| 735 | IgG2a,κ | IGHV653*01 | IGHD-Q52*02 | IGHJ2*01 | IGKV1-110*02 | IGKJ5*01 |
| 2-2-B | IgM,κ | J558.2 | IGHD-SP2.7*01 | IGHJ2*01 | IGKV1-110*01 | IGKJ2*01 |
| SEAM 2 | IgG3,κ | IGHV1S4*01 | NDb | IGHJ2*01 | IGKV1-135*01 | IGKJ5*01 |
| SEAM 3 | IgG2b,κ | IGHV7S3*02 | IGHD-SP2.8*01/inv | IGHJ2*01 | IGKV1-135*01 | IGKJ5*01 |
| SEAM 12 | IgG2a,κ | IGHV13S1*01 | IGHD-ST4*01 | IGHJ2*01 | IGKV4-63*01 | IGKJ5*01 |
| SEAM 18 | IgG2b,κ | IGHV7S3*02 | IGHD-SP2.8*01/inv | IGHJ2*01 | IGKV4-74*01 | IGKJ2*01 |
| SEAM 35c | IgG2a,κ | IGHV7S3*02 | NDb | IGHJ4*01 | IGKV6-20*01 | IGKJ2*01 |
Closest matches from either the IGMT or GenBank/EMBL databases. MAb 735 is from a hybridoma produced from a NZB mouse immunized with viable N. meningitidis group B strain ATCC 13090 (Frosch et al., 1985). Since only the amino acid sequences were reported (Klebert et al., 1993; Vaesen et al., 1991), assignment of the germline genes for this mAb was based on the closest match (see Section 2). MAb 2-2-B was produced from a BALB/CJ mouse immunized with N. meningitidis group B strain P355. Germ line assignments were based on the published DNA sequences (Berry et al., 2005). The SEAM mAbs are from hybridomas prepared from CD1 mice immunized with N-Pr MBPS tetanus toxoid conjugate (Granoff et al., 1998).
ND, not determined since the segment was too short to enable identification of a specific gene.
Note that in our original paper this mAb was listed as being IgG2b, we have subsequently confirmed that it is lgG2a. The subclasses of all of the other anti-N-Pr MBPS mAbs used in this study were retested and confirmed as listed here and in (Granoff et al., 1998).
Fig. 1.
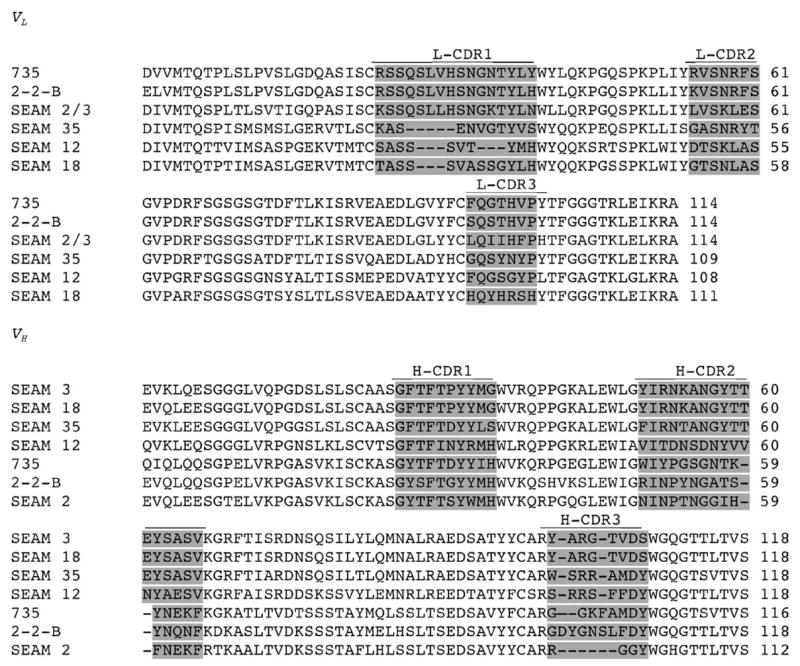
Comparison of VL and VH sequences for mAb 735, 2-2-B and SEAM mAbs. Shaded segments correspond to complementarity determining regions (CDR) loops. GenBank accession numbers: SEAM 2 VH, DQ113489; SEAM 2 VL, DQ113490; SEAM3VH, DQ113491; SEAM 3 VL, DQ113492; SEAM 12 VH, DQ113493; SEAM 12VL, DQ113494; SEAM 18 VH, DQ113495; SEAM 18 VH, DQ113496; SEAM 35 VH, DQ113497; SEAM 35 VL, DQ113498.
Anti-MBPS mAbs, 2-2-B and 735 are reactive with MBPS while anti-N-Pr MBPS SEAM 2 is not. The close homology of both the respective heavy and light chain V amino acid sequences between SEAM 2 and the autoreactive mAbs 2-2-B and 735 (VH 70%, VL 75%), is therefore of particular interest. The most striking difference between the two sequences is in the heavy chain (H-CDR3) where SEAM 2 consists of the minimal four amino acids, two of which are glycine, compared with a length of eight amino acids in mAb 735.
The VL and VH genes of anti-MBPS mAB 2-2-B are unmutated (100% identical) as compared with their putative germline genes (Table 3). Similarly, the amino acid sequence of the expressed VL of anti-MBPS mAb 735 is >99% identical to that of the assigned germline gene. In contrast, the anti-N-Pr MBPS mAbs have a greater percentage of mutations as compared with the respective germline sequences (the expressed genes are 89–95% identical to germline sequences, Table 3). Also, all five anti-N-Pr MBPS mAbs contain one or more arginine residues in H-CD3, which are encoded by editing at the D–J junction. Neither of the two anti-MBPS mAbs contain arginine in H-CDR3.
Table 3.
Comparison of assigned germline gene and amino acid sequences to respective expressed sequences for anti-MBPS and anti-N-Pr MBPS mAbsa
| mAb | VL
|
VH
|
||
|---|---|---|---|---|
| Gene | Amino acid | Gene | Amino acid | |
| 2-2-Bb | 100 | 100 | 100 | 100 |
| 735c | Unknownd | >99 | Unknownd | 94.9 |
| SEAM 2 | 94.7 | 90.5 | 95.4 | 89.4 |
| SEAM 3 | 95.4 | 90.5 | 96.5 | 92.6 |
| SEAM 12 | 97.4 | 94.3 | 96.5 | 91.6 |
| SEAM 18 | 96.7 | 93.3 | 96.5 | 92.6 |
| SEAM 35 | 97.4 | 97.7 | 96.1 | 92.6 |
Percent identity as calculated from pairwise CLUSTALW alignments. The sequence comparison does not include residues determined by the primers used to amplify and clone the gene sequences.
From (Berry et al., 2005).
From (Klebert et al., 1993; Vaesen et al., 1991).
The gene sequences are unknown. The identification of germline sequence is based on comparison of the amino acid sequence with the sequences in the IMGT/V-QUEST database.
3.2. Comparison of SEAM mAb structural models with the crystal structure of mAb 735
Structural models of the SEAM mAbs were obtained through the Web Antibody Modeling (WAM) services provided by the Centre for Protein Analysis and Design at the University of Bath (Martin et al., 1991). The region most likely to differ between the model and actual structure is in H-CDR3, which results from V–D–J recombination and is therefore, not as well represented by canonical structures used to model the initial structural configuration in the structure calculation. To evaluate the quality of computationally derived structural models for anti-MBPS mAbs, we compared the X-ray crystal structure of mAb 735 determined by Evans et al. (1995) with a 3D computer model constructed for the present study through WAM and the sequences of mAb 735 (Fig. 2). As expected, the structural model is very similar to the determined structure with the largest differences between the superimposed structures occurring in H-CDR3. The root mean square difference (RMSD) for VL is 0.053 and 0.225 Å for VH. The results show that computational models may be useful for inferring general features, such as size and shape of the antibody combining sites.
Fig. 2 shows surface renderings of the structures of the other mAb combining sites produced by computer modeling. The surfaces are colored by charge with dark blue representing a charge of +5 and dark red −1. The surface electrostatic potentials were calculated using APBS (Baker et al., 2001) operating in Pymol. The structures are arranged clockwise from mAbs reacting with human polysialic acid (mAb 735, 2-2-B, and SEAM 35) in the upper left side of the figure to those that are minimally cross-reactive mAbs (SEAM 18 and SEAM 12), to non-autoreactive mAbs SEAM 3 and SEAM 2. All the mAbs are shown from the same perspective with the light chain above the heavy chain.
The combining sites for all of the mAbs are characterized by having relatively large positive surface potentials (Fig. 2). This feature of the combing sites is likely to be important for binding polyanionic antigens. Qualitatively, the structural models of the combining sites of 2-2-B, SEAM 12, SEAM 18 and SEAM 35 are similar to the structure of mAb 735 in having a shallow wide groove. In contrast, the combining site of SEAM 2 is a narrow deep cleft (apparent from the dark shadowing in Fig. 2) that cuts across the entire surface, and that of SEAM 3 has a distinctive pocket feature (both left and right sides of the combining site). The deep cleft in the SEAM 2 model results from the unusually short length of H-CDR3 (four residues, see Fig. 1).
As described by Evans et al., the wide, shallow groove of 735 is consistent with the mAb specificity of binding to extended polysaccharide structures of at least eight residues. A combining site in the shape of a wide shallow groove may be a feature that is related to cross-reactivity with polysialic acid as the structure of 735 and the structural models of 2-2-B and the three anti-N-Pr MBPS mAbs all have similar combining site structures and are reactive with polysialic acid while SEAM 2 and SEAM 3, which have distinctively deep and narrow combining sites, show no reactivity with the host antigen or MBPS.
As shown in a previous study, SEAM 3 recognizes an MBPS oligosaccharide of two to three residues containing at least one de-N-acetyl residue (Moe et al., 2005). The structural model of SEAM 3 having pocket-like features is consistent with this observed antigenic specificity. The only negatively charged surface in the combining site model that could interact with the positively charged amino group of a de-N-acetylated residue is the gamma-carboxyl group of Glu60 in the light chain (located in the upper right side on the upper edge of a pocket in the combining site of SEAM 3 shown in Fig. 2). Although we did not identify specific molecules that bind to SEAM 2, the mAb has the same VL as SEAM 3 and may also have some specificity for de-N-acetyl residues.
4. Discussion
The reasons why a relatively minor chemical modification of replacing N-acetyl groups of MBPS with N-propionyl groups results in a remarkable change in immunogenicity and antibody specificity are unknown. In the present study, we cloned and sequenced the V-region genes of five bactericidal anti-N-Pr MBPS mAbs, and produced V-region structural models of antibodies with little or no cross-reactivity with host polysialic acid antigens. The results show that the genetics of the antibody response to immunization with an N-Pr MBPS-tetanus toxoid conjugate vaccine differ in several important respects from that elicited by MBPS or reported for the closely related meningococcal group C polysaccharide (MCPS, which has a alpha 2–9 linkage versus alpha 2–8 linkage in MBPS). The V region repertoire to MBPS or MCPS is restricted to a few germ line genes from the same gene family and the expressed sequences are either identical or nearly identical to germ line sequences. In contrast, the respective VH and VL genes used by the anti-N-Pr MBPS mAbs are limited to a larger number of gene families, appear to be more mutated ranging from 94% to 97% identical to the germ line sequences (amino acid sequences 89–96% identical). The results indicate that the antibody response to N-Pr MBPS is antigen driven and T-dependent with the expressed genes being the product of somatic mutation.
A BLAST search of the GenBankEMBL non-redundant protein sequence database for sequences homologous to the VL and VH sequences of the anti-MBPS and anti-N-Pr MBPS mAbs (H-CDR3 and L-CDR3 sequences included) identified closely related VH and VL antibody sequences for mAbs of known antigenic specificity. Of particular interest, the VH and VL sequences of mAb 735 are homologous to anti-DNA mAbs (Tillman et al., 1992) 17s-c2 (accession number PH0997) and 25.12 (accession number CAA80111), respectively, and the VH of SEAM 3 and SEAM 18 is homologous to VH of the anti-polydC mAb 9E10 (O’Connor et al., 2001) (accession number AAC04534.1). The VL of SEAM 2 and 3 is homologous to the anti-DNA mAb 17.3 ((Tillman et al., 1992), AAC04534.1). Also, several investigators have reported that anti-DNA antibodies produced in various mouse strains exhibit a statistical preference for arginine residues in H-CDR3 and that the arginine residues are often encoded by editing occurring at V–D or more particularly D–J junctions (Eilat et al., 1988; Krishnan et al., 1996; Krishnan and Marion, 1998; Shlomchik et al., 1990). Similar D–J editing was observed for the anti-N-Pr MBPS mAbs. However, none of the anti-N-Pr MBPS mAbs shows significant binding to dsDNA in an ELISA at concentrations of mAb up to 10 μg/ml (data not shown). Thus, even though the respective VH and VL sequences of the anti-N-Pr MBPS mAbs and anti-dsDNA antibodies are highly homologous, the small differences are sufficient to produce distinct specificities.
Overall, the picture that emerges from our molecular analysis of anti-N-Pr MBPS mAbs is that chemical modification of MBPS including de-N-acetyl and N-propionyl residues elicits antibodies that differ from autoantibodies in the germline genes used and in the percentage of mutations compared to the germline genes. The structural models of the autoreactive anti-MBPS mAbs and the three anti-N-Pr MBPS mAbs that react with host PSA are all characterized by shallow grooves. As described by Evans et al., these grooves are likely to be capable of binding to extended polysaccharide structures of at least eight residues (Evans et al., 1995). In contrast, anti-N-Pr MBPS mAbs SEAM 2 and SEAM 3, which do not exhibit autoreactivity with PSA, have distinctively deeper and narrower combining sites.
We conclude that antibody responses to group B polysaccharide residues that have been modified by de-N-acetylation or by propionylation are distinctively different than antibody responses to the homogeneous repeating polymeric capsular polysaccharide. This conclusion is supported by immunologic data from a previous study by Fusco et al. that an N-Pr MBPS conjugate vaccine elicited a population of capsular-specific antibody that was different from the existing (i.e. preimmune) PSA-reactive antibody (Fusco et al., 1997). Further, the pre-existing levels of PSA-reactive antibody were not increased or boosted by immunization with the N-Pr MBPS conjugate vaccine (Fusco et al., 1997). Thus, it is likely that antibodies elicited by residues modified during the preparation of the N-Pr MBPS react with similarly modified residues in the group B capsule, and that such residues are key to distinguishing specific reactivity to group B bacteria from host PSA autoreactivity.
Acknowledgments
We are grateful to Dr. Leyu Liu, Children’s Hospital Research Institute, Oakland, CA, for help with cloning the heavy chain gene for SEAM 12. This work was supported by grants RO1 AI45642, AI058122, and AI46464 from the National Institute of Allergy and Infectious Disease of the National Institutes of Health and was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number CO6 RR-16226 from the National Center for Research Resources, National Institutes of Health.
Abbreviations
- 3D
three dimensional
- CDR
complementarity determining region
- Dp
degree of polymerization
- Fv
antibody fragment variable
- MBPS
meningococcal group B polysaccharide
- MCPS
meningococcal group C polysaccharide
- N-Ac MBPS
N-acetyl MBPS
- N-Pr MBPS
N-propionyl MBPS
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton FE, Ryan JA, Michon F, Jennings HJ. Protective efficacy of mouse serum to the N-propionyl derivative of meningococcal group B polysaccharide. Microb Pathog. 1989;6:455–458. doi: 10.1016/0882-4010(89)90087-9. [DOI] [PubMed] [Google Scholar]
- Azmi FH, Lucas AH, Raff HV, Granoff DM. Variable region sequences and idiotypic expression of a protective human immunoglobulin M antibody to capsular polysaccharides of Neisseria meningitidis group B and Escherichia coli K1. Infect Immun. 1994;62:1776–1786. doi: 10.1128/iai.62.5.1776-1786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Hoist MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JD, Boese DJ, Law DK, Zollinger WD, Tsang RS. Molecular analysis of monoclonal antibodies to group variant capsular polysaccharide of Neisseria meningitidis: recurrent heavy chains and alternative light chain partners. Mol Immunol. 2005;42:335–344. doi: 10.1016/j.molimm.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucl Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D, Webster DM, Rees AR. V region sequences of anti-DNA and anti-RNA autoantibodies from NZB/NZW F1 mice. J Immunol. 1988;141:1745–1753. [PubMed] [Google Scholar]
- Evans SV, Sigurskjold BW, Jennings HJ, Brisson JR, To R, Tse WC, Altman E, Frosch M, Weisgerber C, Kratzin HD, et al. Evidence for the extended helical nature of polysaccharide epitopes. The 2.8 A resolution structure and thermodynamics of ligand binding of an antigen binding fragment specific for alpha-(2 → 8)-polysialic acid. Biochemistry. 1995;34:6737–6744. doi: 10.1021/bi00020a019. [DOI] [PubMed] [Google Scholar]
- Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Frosch M, Gorgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA. 1985;82:1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco PC, Michon F, Tai JY, Blake MS. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and nonhuman primates. J Infect Dis. 1997;175:364–372. doi: 10.1093/infdis/175.2.364. [DOI] [PubMed] [Google Scholar]
- Gilkeson GS, Bloom DD, Pisetsky DS, Clarke SH. Molecular characterization of anti-DNA antibodies induced in normal mice by immunization with bacterial DNA. Differences from spontaneous anti-DNA in the content and location of VH CDR3 arginines. J Immunol. 1993;151:1353–1364. [PubMed] [Google Scholar]
- Granoff DM, Bartoloni A, Ricci S, Gallo E, Rosa D, Ravenscroft N, Guarnieri V, Seid RC, Shan A, Usinger WR, Tan S, McHugh YE, Moe GR. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J Immunol. 1998;160:5028–5036. [PubMed] [Google Scholar]
- Granoff DM, Feavers IM, Borrow R. Meningococcal vaccines. In: Plotkin SAO, Walter A, editors. Vaccines. Saunders; Philadelphia, PA: 2004. pp. 959–988. [Google Scholar]
- Jennings HJ, Gamian A, Ashton FE. N-propionylated group B meningococcal polysaccharide mimics a unique epitope on group B Neisseria meningitidis. J Exp Med. 1987;165:1207–1211. doi: 10.1084/jem.165.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings HJ, Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981;127:1011–1018. [PubMed] [Google Scholar]
- Jennings HJ, Roy R, Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986;137:1708–1713. [PubMed] [Google Scholar]
- Jennings HJ, Roy R, Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol. 1985;134:2651–2657. [PubMed] [Google Scholar]
- Jodar L, Feavers I, Salisbury D, Granoff DM. Development of vaccines against meningococcal disease. Lancet. 2002;359:1499–1508. doi: 10.1016/S0140-6736(02)08416-7. [DOI] [PubMed] [Google Scholar]
- Klebert S, Kratzin HD, Zimmermann B, Vaesen M, Frosch M, Weisgerber C, Bitter-Suermann D, Hilschmann N. Primary structure of the murine monoclonal IgG2a antibody mAb735 against alpha (2–8) polysialic acid. 2 Amino acid sequence of the heavy (H-) chain Fd’ region. Biol Chem Hoppe Seyler. 1993;374:993–1000. doi: 10.1515/bchm3.1993.374.7-12.993. [DOI] [PubMed] [Google Scholar]
- Krishnan MR, Jou NT, Marion TN. Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J Immunol. 1996;157:2430–2439. [PubMed] [Google Scholar]
- Krishnan MR, Marion TN. Comparison of the frequencies of arginines in heavy chain CDR3 of antibodies expressed in the primary B-cell repertoires of autoimmune-prone and normal mice. Scand J Immunol. 1998;48:223–232. doi: 10.1046/j.1365-3083.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- Livingston BD, Jacobs JL, Glick MC, Troy FA. Extended polysialic acid chains (n greater than 55) in glycoproteins from human neuroblastoma cells. J Biol Chem. 1988;263:9443–9448. [PubMed] [Google Scholar]
- Mandrell RE, Azmi FH, Granoff DM. Complement-mediated bactericidal activity of human antibodies to poly alpha 2 → 8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- Mandrell RE, Zollinger WD. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J Immunol. 1982;129:2172–2178. [PubMed] [Google Scholar]
- Martin AC, Cheetham JC, Rees AR. Molecular modeling of antibody combining sites. Meth Enzymol. 1991;203:121–153. doi: 10.1016/0076-6879(91)03008-5. [DOI] [PubMed] [Google Scholar]
- Mellors RC. Autoimmune disease in NZB/BL mice. 3 Induction of membranous glomerulonephritis in young mice by the transplantation of spleen cells from old mice. J Exp Med. 1966;123:1025–1034. doi: 10.1084/jem.123.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe GR, Dave A, Granoff DM. Epitopes recognized by a nonautoreactive murine anti-N-propionyl meningococcal group B polysaccharide monoclonal antibody. Infect Immun. 2005;73:2123–2128. doi: 10.1128/IAI.73.4.2123-2128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C, Hewitt J, Hastings K, Brown D. Immunological properties of monoclonal antibodies specific for meningococcal polysaccharides: the protective capacity of IgM antibodies specific for polysaccharide group B. J Gen Microbiol. 1983;129 (Pt 8):2451–2456. doi: 10.1099/00221287-129-8-2451. [DOI] [PubMed] [Google Scholar]
- Morley SL, Pollard AJ. Vaccine prevention of meningococcal disease, coming soon? Vaccine. 2001;20:666–687. doi: 10.1016/s0264-410x(01)00410-8. [DOI] [PubMed] [Google Scholar]
- O’Connor KC, Nguyen K, Stollar BD. Recognition of DNA by VH and Fv domains of an IgG anti-poly(dC) antibody with a singly mutated VH domain. J Mol Recognit. 2001;14:18–28. doi: 10.1002/1099-1352(200101/02)14:1<18::AID-JMR515>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Pon RA, Lussier M, Yang QL, Jennings HJ. N-propionylated group B meningococcal polysaccharide mimics a unique bactericidal capsular epitope in group B Neisseria meningitides. J Exp Med. 1997;185:1929–1938. doi: 10.1084/jem.185.11.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff HV, Bradley C, Brady W, Donaldson K, Lipsich L, Maloney G, Shuford W, Walls M, Ward P, Wolff E, et al. Comparison of functional activities between IgG1 and IgM class-switched human monoclonal antibodies reactive with group B streptococci or Escherichia coli K1. J Infect Dis. 1991;163:346–354. doi: 10.1093/infdis/163.2.346. [DOI] [PubMed] [Google Scholar]
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–1388. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- Shin JS, Lin JS, Anderson PW, Insel RA, Nahm MH. Monoclonal antibodies specific for Neisseria meningitidis group B polysaccharide and their peptide mimotopes. Infect Immunol. 2001;69:3335–3342. doi: 10.1128/IAI.69.5.3335-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman DM, Jou NT, Hill RJ, Marion TN. Both IgM and IgG anti-DNA antibodies are the products of clonally selective B cell stimulation in (NZB × NZW)F1 mice. J Exp Med. 1992;176:761–779. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaesen M, Frosch M, Weisgerber C, Eckart K, Kratzin H, Bitter-Suermann D, Hilschmann N. Primary structure of the murine monoclonal IgG2a antibody mAb735 against alpha (2–8) polysialic acid. 1) Amino-acid sequence of the light (L-) chain, kappa-isotype. Biol Chem Hoppe Seyler. 1991;372:451–453. doi: 10.1515/bchm3.1991.372.1.451. [DOI] [PubMed] [Google Scholar]
- Wang Z, Raifu M, Howard M, Smith L, Hansen D, Goldsby R, Ratner D. Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3′–5′exonuclease activity. J Immunol Meth. 2000;233:167–177. doi: 10.1016/s0022-1759(99)00184-2. [DOI] [PubMed] [Google Scholar]
- Zollinger WD, Mandrell RE, Griffiss JM, Altieri P, Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979;63:836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]


