Abstract
Individual cardiac progenitors emerge at defined positions within each segment in the trunk mesoderm. Their specification depends on segmental information from the pre-patterned ectoderm, which provides positional information to the underlying cardiac mesoderm via inductive signals. This pattern is further reinforced by repressive interactions between transcription factors that are expressed in neighboring sets of cardiac progenitors. For example, even-skipped (eve) and ladybird early (lbe) gene products mark adjacent cardiac cell clusters within a segment, and their antagonistic interaction results in mutually exclusive expression domains. Lbe acts directly on the eve mesodermal enhancer (eme) to participate in restricting its expression anteriorly. We hypothesized that additional repressive activities must regulate the precise pattern of eve expression in the cardiac mesoderm via this enhancer. In this study, we identified two additional repressor motifs: 4 copies of an “AT”-rich motif (M1a–d) and 2 copies of an “GC”-rich motif (M2a,b), which when mutated cause expansion of eme-dependent reporter gene expression. We have also examined potential negative regulators of eve and found that their overexpression is sufficient to repress eve as well as the eme enhancer via these sites. Our data suggests that a combination of factors is likely to interact with multiple essential repressor sites to confer precise spatial specificity of eve expression in the cardiac mesoderm.
Keywords: Drosophila, heart, Cardiogenesis, cell specification, transcriptional regulation, epicardium
INTRODUCTION
A fundamental question in organogenesis is how the spatially patterned cellular identities are generated in precise positions within a primordial field. Over the years, it has become clear that during animal development spatially localized inductive and/or repressive signals are essential for the subdivision and prepatterning of the undifferentiated field that leads to the specification and positioning of organ and tissue primordia. As a consequence, distinct types of progenitor cells emerge from the anlagen of these organs. The genetic requirements for the specification of organ progenitors have been studied in some detail, but the transcriptional basis for precise localization and positioning of gene expression and thus cell type specification within an organ primordium is largely unknown.
The Drosophila heart is a highly ordered linear tube structure with only a few defined cell types arranged in a stereotyped metameric pattern. Thus, the Drosophila heart provides an attractive model for studying the genetic and molecular mechanisms governing the specification and positioning of the progenitor cells (Bodmer and Frasch, 1999; Zaffran and Frasch, 2002). The Drosophila heart is a mesodermal derived organ and the cardiac primordium is specified and positioned along the dorsal-most margin of the trunk mesoderm by the combined activities of two secreted molecules Wg (Wnt) and Dpp (TGF-β) from the overlaying ectoderm (Frasch, 1995; Lockwood and Bodmer, 2002; Park et al., 1996; Wu et al., 1995).
As a first step in the progressive specification of mesodermal cell fates, an inductive signal (Dpp), which is initially expressed in a broad domain in the dorsal ectoderm, subdivides the mesoderm into the ventral and dorsal portions by maintaining the expression of the homeobox gene tinman (tin) in the dorsal mesoderm (Frasch, 1995; Staehling-Hampton et al., 1994). Both dpp and tin are essential for establishing dorsal fates (Azpiazu and Frasch, 1993; Bodmer, 1993; Lockwood and Bodmer, 2002; Yin and Frasch, 1998). Superimposed on this initial subdivision, the dorsal mesodermal fates are further distinguished along the anteroposterior axis by inductive factors encoded by wg and hh. Both of these factors are expressed in striped patterns along the anterior-posterior axis in the overlying ectoderm, thus providing cues for subdivision of the dorsal mesoderm within each parasegment in a metameric fashion. As a result, the domain underlying the ectodermal hh stripes is fated to become visceral mesoderm, whereas the domain receiving the Wg signal develops into dorsal somatic muscles or cardiac mesoderm (Azpiazu et al., 1996; Park et al., 1996; Riechmann et al., 1997; Wu et al., 1995). As a consequence of refinement of dpp expression to the dorsal ectodermal edge, tinman expression is further restricted to the cardiac mesoderm along with GATA and T-box factors, encoded by pannier (pnr) and dorsocross (doc), respectively. This combination of factors provides the correct mesodermal context for cardiac differentiation (Klinedinst and Bodmer, 2003; Lockwood and Bodmer, 2002; Reim and Frasch, 2005).
The expression of additional transcription factors in the cardiogenic region results in the identity specification of subsets of cardiac progenitors. As a consequence, distinct types of cardiac progenitors emerge at discrete locations along the anteroposterior axis within each segment. Expression of the transcription factors ladybird early (lbe), even-skipped (eve) or seven-up (svp) (Han et al., 2002; Jagla et al., 1997; Lo and Frasch, 2001; Su et al., 1999) mark essentially non-overlapping subpopulations of cardiac progenitors along the anterior-posterior axis within each segment. The generation of this segmentally repeated pattern of identity gene expression depends on a (wg-independent) role of hh in the ectoderm. hh is required for the formation of two clusters of cardiac cells adjacent to its ectodermal stripes: the anterior eve cells and the posterior svp cells (Liu et al., 2006; Ponzielli et al., 2002). The formation of the eve cells also relies on the activation of Ras signaling in the dorsal mesoderm (Buff et al., 1998; Carmena et al., 2002; Halfon et al., 2000), which is also activated by hh signaling (Carmena et al., 2002; Halfon et al., 2000; Liu et al., 2006). In addition, hh signaling can also repress lbe expression in the mesoderm independent of ras signaling (Jagla et al., 1997; Liu et al., 2006). As a consequence, lbe-expressing cell clusters are located some distance away from the hh stripes. Further refinement of this pattern is achieved, in part, by mutual repression between these identity genes, generating mutually exclusive expression domains. This cross-repressive interaction is best exemplified by the antagonistic relationship between lbe and eve (Han et al., 2002; Jagla et al., 2002).
Previous studies suggest that at least five different genetic inputs are necessary for the activation of eve in the mesoderm, based on mutagenesis of conserved sites for Twist, Tinman, dTCF (Wg signaling), ETS (Ras signaling) and Mad (Dpp signaling) in the mesodermal eve enhancer (Halfon et al., 2000; Han et al., 2002; Knirr and Frasch, 2001). In addition to these eve activating inputs, eve expression is likely restricted to a small group of cells by mechanisms involving transcriptional repression. One such repressor is Lbe, expressed anteriorly to the Eve cells. Lbe overexpression abolishes mesodermal eve expression, and this repression depends on a Lb consensus binding site in the eve mesodermal enhancer (eme; see Fig. 1; Han et al., 2002; Jagla et al., 1997; Jagla et al., 2002). Mutating this Lb site results in a dramatic expansion of reporter gene expression within cardiogenic region (Han et al., 2002). However, the expansion is not only anterior, but also posterior to the eve clusters. Thus, additional repressors are likely to be involved. Of potential candidates, Svp is unlikely to be involved since overexpression has no effect (Han et al., 2002).
Figure 1.
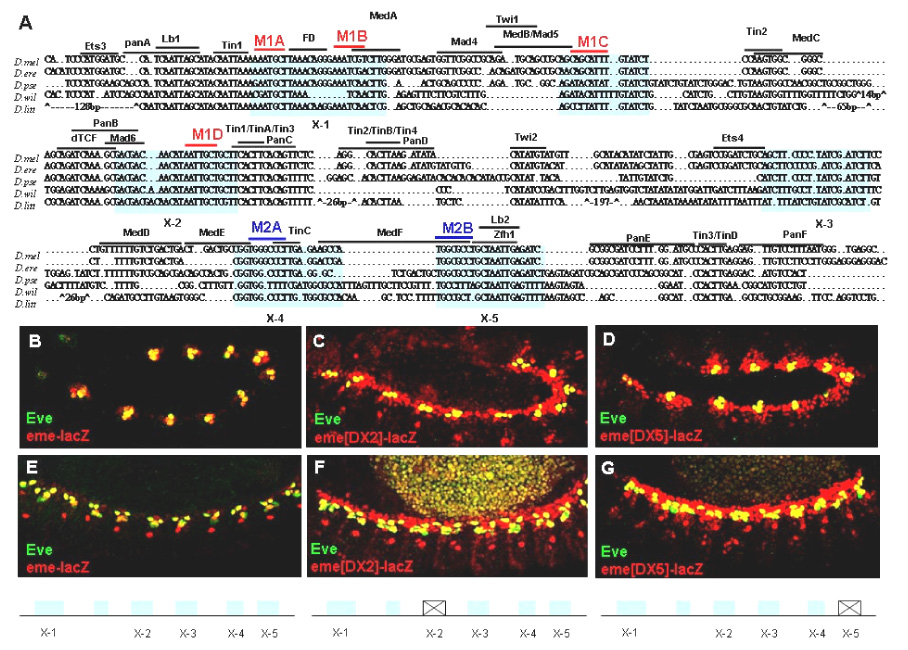
eme contains multiple essential repressor sites. (A) eme sequence alignment among five Drosophila species uncovered several new regions with conserved sequences. Two repeated sets of sequences are highlighted in Red (M1 motifs) and Blue (M2 motifs), respectively. (B–D) Stage 11/12 wildtype embryos. (E–G) Stage 14 wildtype embryos. (B,E) eme-mediated lacZ expression (red) and nuclear Eve protein (green) overlap. Note lacZ but not eve expression in the Eve progenitor-derived DO2 founder (red nuclei in E). (C–G) eme with X-2 (C,F) or X-5 (D,G) region deleted, respectively. Note that these deletions expand eme enhancer activity into the entire cardiac mesoderm (see Han et al., 2002).
Here, we studied the mechanisms of eve repression by first comparing the eme sequences between Drosophila melanogaster and four other Drosophila species. We identified additional essential repressor motifs in eme, an “AT”-rich M1 motif (M1a–d) and a “GC”-rich M2 motif (M2a,b), that are required for restricting expression along the anterior-posterior axis within the cardiac mesoderm. In vivo functional analysis demonstrated that both motifs and the previously identified Lbe binding site are necessary, but individually not sufficient, for confining the enhancer activity to a small cluster of cells. In addition to Lbe, three other homeodomain transcription factors (Msh, Lim3 and C15) are expressed in the vicinity of the eve expressing clusters in the dorsal mesoderm, and thus were considered potential negative regulators of eve expression. Ectopic activity of either factor is capable of repressing eve (and eme) in the mesoderm. Mutating the “AT”-rich site M1b renders eme insensitive to repression by lim3 or lbe overexpression, which suggests that their (ectopic) transcriptional repression activity on eme is mediated by “AT”-rich M1 sites. In addition, transcriptional repression of eme by Lbe is also mediated through the “GC”-rich M2a motif. Together, our data suggest that the restriction of eve expression to a small cluster of cells in the dorsal mesoderm is achieved by the combined and cooperative actions of multiple repressor sites.
MATERIALS AND METHODS
Drosophila strains
The following Drosophila strains were used in this study: lim3¹, lim3², c15², c15³, msh-lacZ line rH96, UAS-c15, UAS-lim3, UAS-lbe, UAS-msh (Jagla et al., 1997); (Campbell, 2005; Nose et al., 1998; Thor et al., 1999). Ectopic transgenes expression was induced using the Gal4/UAS system (Brand and Perrimon, 1993). The following Gal4 driver lines were used: twi-Gal4 (Greig and Akam, 1993), 24B-Gal4 (Brand and Perrimon, 1993), and the double combination twi-Gal4;24B-Gal4 (Lockwood and Bodmer, 2002). twi-Gal4 drives expression in all mesoderm cells from stage 9 through stage 12. 24B-Gal4 initiates expression in the cardiac and somatic mesoderm at stage 10/11 and continues throughout development and adult life (Bidet et al., 2003).
In vivo analysis of eme lacZ transgenes
For in vivo enhancer functional analysis, the various mutated eme enhancer fragments were generated by site directed mutagenesis (QuickChange Site-Directed Mutagenesis Kit, Stratagene). The wildtype eme fragment derived from eme900 was amplified by PCR, cloned into the pGEMT- easy vector (Promega) and subjected to mutagenesis. The following underlined base pair changes were made: M1a*, AAATGCT; M1b*, AAATCGT; M1c*, AGCATTT; M1d*, AATTGCT; M2a*, TGGGCCCT or TGGGCCCT; M2b*, TGGCGCCT; Lb2*, GCCTGCTGCCTGAGA. Subsequently, the mutated eme fragments were individually subcloned into the EcoRI site of P[lacZ,w+] of C4pLZ (Wharton and Crews, 1993) and the orientation of the eme fragments in the vector was confirmed by restriction enzyme digestion and sequencing. Constructs were introduced into the Drosophila germline according to standard microinjection procedures (Spradling and Rubin, 1982). At least four independent transgenic lines were analyzed by anti-β-galactosidase (LacZ) staining for each construct.
Immunohistochemistry and in situ hybridization
Immunostaining and fluorescent in situ hybridization/antibody double labeling were performed as described previously (Liu et al., 2006; Qian et al., 2005). The indirect TSA System (Perkin Elmer) was used to enhance the signal for Lbe staining and c15 fluorescent in situ hybridization followed by a 30-minute incubation period with the Streptavidin–Fluoresceine DTAF (1:300; The Jackson Laboratory). Embryos were mounted in VectaShield (Vector Laboratories). Fluorescent whole-mount embryo staining was analyzed by a Biorad (MRC-1024MP) confocal microscope. Primary antibodies were used at the following dilutions: rabbit anti-Eve, 1:300 (Frasch et al., 1987); mouse anti-Eve, 1:50 (Developmental studies hybridoma bank, Univ. Iowa); mouse anti-Lbe 1:40 (Jagla et al., 1997); rabbit anti-Tinman 1:1000 (Venkatesh et al., 2000); rabbit anti-β-gal 1:2000 (Cappel); mouse anti-β-gal 1:500 (Sigma). Cy3- or FITC-conjugated secondary antibodies were used at 1:200 (Jackson Laboratory). The c15 probe was amplified from first strand cDNA using the following primer pair: forward, TCCAGCCACGAGGAGGATGACC; reverse, GCAGCCCGCCGCATGCGACGCC.
Yeast one-hybrid screens
Four tandem copies of X2 or X5 fragment (about 20 bp each) were inserted into the yeast-1 hybrid pHISi reporter vector. The target reporter constructs were subjected to Xba I digestion and transformed into the yeast strain (YM4271). The resulting yeast colonies were then tested for background expression individually and the one with optimal background expression was used for transfection with an λACT2 library of Drosophila embryonic cDNAs. Approximately 3 × 106 transformants were screened for activation of the reporter genes HIS3 for growth on his−/leu− minimal medium containing 30 mM 3-aminotriazol (Andrioli et al., 2002; Kumar et al., 1996; Li and Herskowitz, 1993).
RESULTS
Identification of novel potential DNA binding motifs in eme
The 210 bp eme element is capable of conferring precise transcriptional information with respect to position and timing within the developing embryo. Previous studies have identified a number of binding sites for genetically defined regulators within the eme (Halfon et al., 2000; Han et al., 2002; Knirr and Frasch, 2001). With the exception of Lbe and Msh, which function as transcriptional repressors for mesodermal eve expression, all other known inputs activate eve expression (Han et al., 2002; Jagla et al., 2002). In order to identify novel sequences important for the regulation of eve expression, we compared the eme sequences from D. melanogaster with four other Drosophila species since functionally important cis-regulatory elements may be evolutionary conserved. Indeed, our sequence comparison suggests that most of the known functional binding sites are conserved, including the Tin, dTCF (Pangolin) and Lbe binding sites. Utilizing these sites as anchoring points, we aligned the intervening regions among the sequences. Interestingly, this comparison unveiled five genomic regions that apparently harbor additional blocks with considerable sequence conservation (X-1 to X-5; Fig. 1A).
As a first step to test for a possible function of these conserved regions in regulating eme, we deleted each individually and examined its enhancer activity in transgenic flies. The X-3 deletion resulted in a slight reduction in expression compared to the wildtype (data not shown). However, all the other deletions caused a dramatic de-repression of eme enhancer activity within the cardiac mesoderm (Fig. 1B–G). Thus, these regions are likely to contain binding sites for repressive activities that prevent ectopic eme expression.
Essential AT-rich sites confer repression anterior and posterior to eve
We identified multiple occurrences of two motifs within the conserved regions (Fig. 1A), a common feature of transcriptional enhancer regions (Berman et al., 2002; Stanojevic et al., 1991). Thus, these motifs may represent novel binding sites for transcriptional repressors. An AT-rich motif (M1) is repeated 4-fold (in X-1, X-2 and one other site), and a GC-rich motif (M2) occurs in X-4 & -5 (Fig. 1A and 2A). Since a 20 base pair (bp) deletion may alter enhancer activity in unpredictable ways, we mutagenized sites individually or in combination by making 2–4 bp changes per site (Fig. 3). Mutating M1a* or M1d* only slightly expands eme enhancer activity (Fig. 2B,C). However, when M1b* or M1c* sites are mutated, we observed dramatic expansion of reporter gene expression within the entire Tinman-positive cardiogenic region, both anteriorly and posteriorly to the endogenous Eve cells (Fig. 2D). Later, the cells that express eme-lacZ migrate dorsally (as previously reported for the Lbe positive heart cells; Jagla et al., 1997) to form a continuous row of myocardial cells dorsal to the eve positive cells (Fig. 2E). Triple or quadruple M1 site mutants exhibit a similar or only slightly more severe phenotype (Fig. 2F & Fig. 3, and data not shown). This suggests that the M1b and M1c sites are each necessary to confer repression, but within the context of eme are not sufficient. Thus, when either site is mutated dramatic, nearly complete de-repression within the cardiogenic region ensues.
Figure 2.
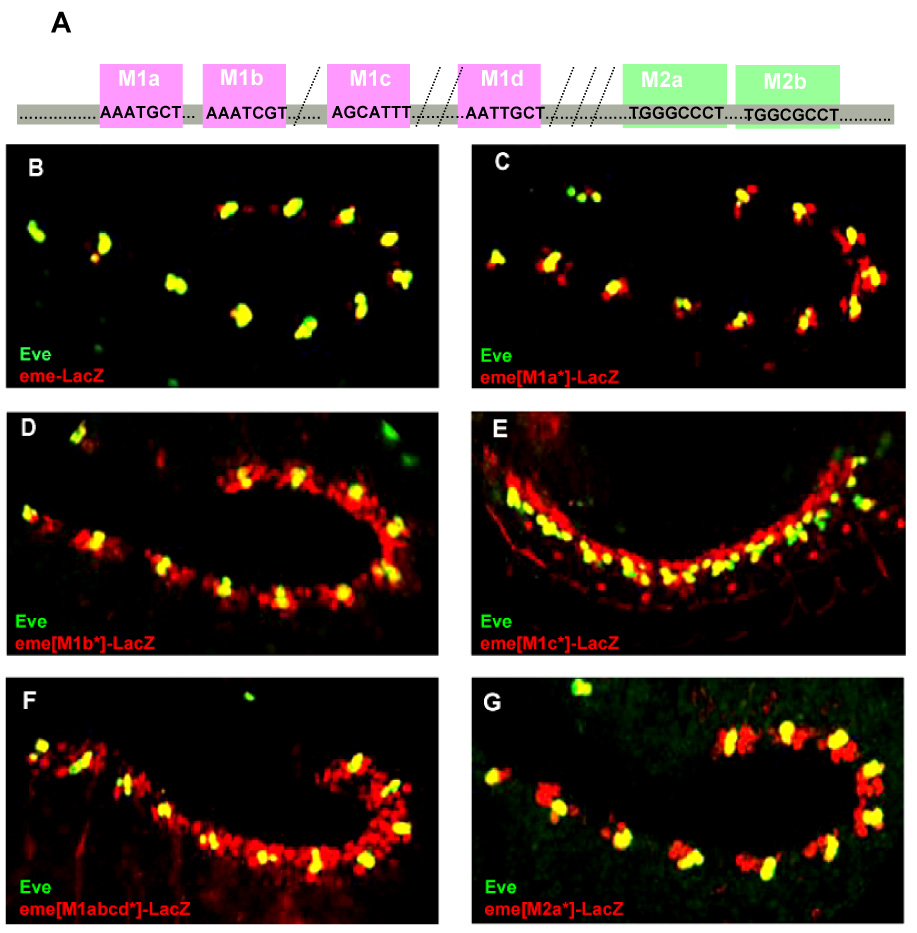
Characterization of new eme enhancer motifs M1 and M2. (A) Schematic representation of the M1 and M2 sites in eme. (B–G) Wildtype stage 11/12 embryos (except for stage 14 in E) labeled for LacZ reporter (red) and Eve protein (green). (B) Wildtype eme. (C–G) Mutant eme with and M1a*, M1b*, M1c*, M1abcd* or M2a* site mutated. Note the dramatic expansion of reporter gene expression to both sides (C–F) of Eve or only anterior (G) of Eve.
Figure 3.
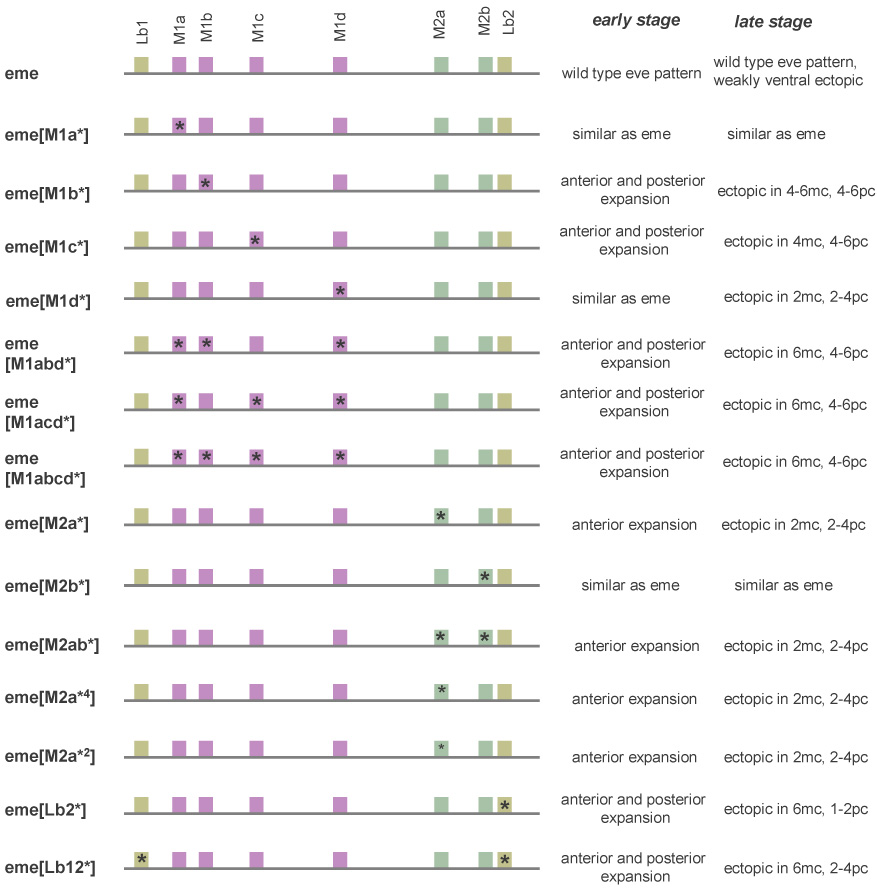
Summary of mutated eme enhancer and the corresponding activity in transgenic embryos of the present study
Essential GC-rich site confer repression anterior to the Eve clusters
We then mutated the M2 sites and found that mutation of the M2a* but not the M2b* site also results in expansion of reporter gene expression. This expansion, however, is restricted to the anterior portion of the Eve cluster (Fig. 2G & Fig. 3). As the germband retracts, cells expressing eme(M2a*)-lacZ migrate dorsally to the eve expressing clusters to form a subset of Dmef2-expressing myocardial cells that largely overlaps with lbe expression, as revealed by triple labeling for Eve, Lbe and LacZ (Fig. 4A,B). Thus, mutating the M2a site causes ectopic expression specifically in the lbe expressing territory. We conclude that the M2a site is essential to mediate repression anterior to the mesodermal Eve cluster.
Figure 4.
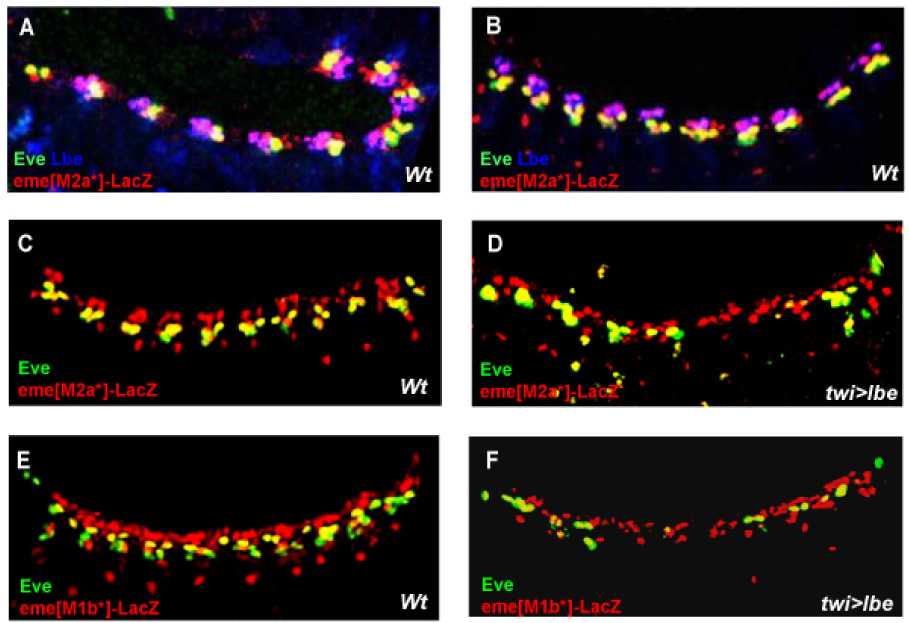
M2a and M1b sites mediate transcriptional repression by Lbe. (A,B) Triple labeling showing localization of LacZ reporter (red), Eve (green) and Lbe (blue) at stage 12 (A) and stage 14 (B). Note the overlap of Lbe and LacZ expansion in purple. (C–F) Mesodermal reporter gene expression is less sensitive to repression by lbe overexpression following mutation of the M2a site (D) or M1b site (F) (by twi>: twist-Gal4; see Materials and Methods). Note that Eve protein is much reduced whereas LacZ is not. To quantify the repressive activity of Lbe on endogenous Eve, eme-LacZ, and eme[m1b*]-LacZ expression, the number of Eve or β-Gal positive cells from T3 to A5 were counted. (Data is expressed as mean number of cells per hemisegment +/− Standard Deviation). In 4E, the number of LacZ (β-Gal) and Eve positive cells per hemisegment is 8.6 (+/−0.5) and 3.4 (+/−0.4) (n=9 embryos) respectively, while in 4F the number of β-Gal and Eve positive cells is 6.3 (+/−0.9) and 1.3 (+/−0.7) (n=11 embryos), respectively.
Lbe mediated repression of eme requires M1b and M2a sites
Since a mutant M2a site causes an anterior expansion into the Lbe domain, it raises the question of whether the M2a site also participates in the transcriptional repression by Lbe (in addition to the Lb2 site; Han et al., 2002). To test this idea, we overexpressed lbe in embryos containing the eme reporter with the M2a site mutated. In contrast to embryos with wildtype eme, in which reporter or eve expression is dramatically diminished by lbe (Han et al., 2002), embryos with a mutated M2a* site showed no significant reduction in reporter gene expression (Fig. 4C,D). Thus, efficient repression of eme by Lbe overexpression requires the M2a site, in addition to the Lb2 site.
Similar experiments were conducted in embryos containing eme with the M1b site mutated. As for the M2a site, mutations in the M1b* eme enhancer also result in a reduced sensitivity to repression by Lbe. We observed only 25% reduction in eme-LacZ expression by lbe overexpression, compared to the reduction of endogenous eve expression, which is reduced by more than 50% as compared to wildtype levels (Fig. 4F). Thus, M1b site mutation renders eme-LacZ also less sensitive to repression by Lbe, similar to mutating the M2a or Lb2 sites (Fig. 4D; Han et al., 2002). Taken together, these data suggest that the transcriptional repression of Lbe on eme is mediated by multiple sites, including Lb2, M1b and M2a, each of which appears to be necessary and must be intact in the eme enhancer to confer complete repression.
Evaluating new candidate repressors of mesodermal eve expression
The identification of new, required sites for eve repression posterior to the Eve clusters, where Lbe is not expressed, suggests that there may be other factors contributing to eve repression posteriorly. In an attempt to identify additional potential repressors, we performed yeast 1-hybrid and expression library screens using four tandem copies of repressor sites-containing eme regions as bait (see Materials and Methods). From these screens, 35 independent clones were obtained, most of which encode homeodomain transcription factors. This is consistent with the known propensity of homeodomain proteins to bind to AT-rich sequences, which include the Lb2 and M1 motifs (Table 1 and 2).
Table 1.
Yeast One-hybrid candidates using X-5 eme region as bait
| Gene | Hits | Molecular function | Expression domain | Protein domain |
|---|---|---|---|---|
| slouch | 8 | Transcription factor (repressor) | mesoderm | helix-turn-helix and homeobox domains |
| C15 | 5 | Transcription factor | mesoderm | homeodomain |
| unplugged | 6 | Transcription factor | ectoderm | helix-turn-helix and homeobox domains |
| suppressor of sable | 1 | Transcription factor (repressor) | salivary gland | |
| msh | 10 | Transcription factor (repressor) | mesoderm | helix-turn-helix and homeobox domains |
| engrailed | 3 | Transcription factor | ectoderm | homeodomain |
| invected | 1 | Transcription factor | hindgut | helix-turn-helix and homeobox domains |
| lolal | 1 | Transcription factor | everywhere |
Table 2.
Yeast One-hybrid candidates using X-2 eme region as bait
| Gene | Hits | Molecular function | Expression domain | Protein domain |
|---|---|---|---|---|
| caudal | 1 | Transcription factor | ectoderm, endoderm | helix-turn-helix and homeobox domains |
| slouch | 2 | Transcription factor (repressor) | mesoderm | helix-turn-helix and homeobox domains |
| exex | 2 | Transcription factor | CNS | homeodomain |
| unplugged | 1 | Transcription factor (repressor) | ectoderm | helix-turn-helix and homeobox domains |
| lmpt | 1 | Transcription factor | mesoderm | lim-domain |
| CG1841 | 1 | unknow | unknow | unknow |
| CG5677 | 1 | unknow | unknow | unknow |
One of the factors identified in this screen was the homeodomain protein Msh, which is known to act as a ventral repressor of cardiogenesis and is also required for the specification of two dorsal muscles (Jagla et al., 2002). The temporal and spatial expression patterns of msh are consistent with a role for this transcription factor in the ventral repression of mesodermal eve expression (see Fig. 5A). Embryonic overexpression of msh results in a dramatic reduction in mesodermal eve expression is dramatically reduced, whereas in msh mutants eve expression is expanded ventrally (Jagla et al., 2002). Thus, at least one of the 1-hybrid repressor candidates seems to be a bona fide repressor of eme.
Figure 5.
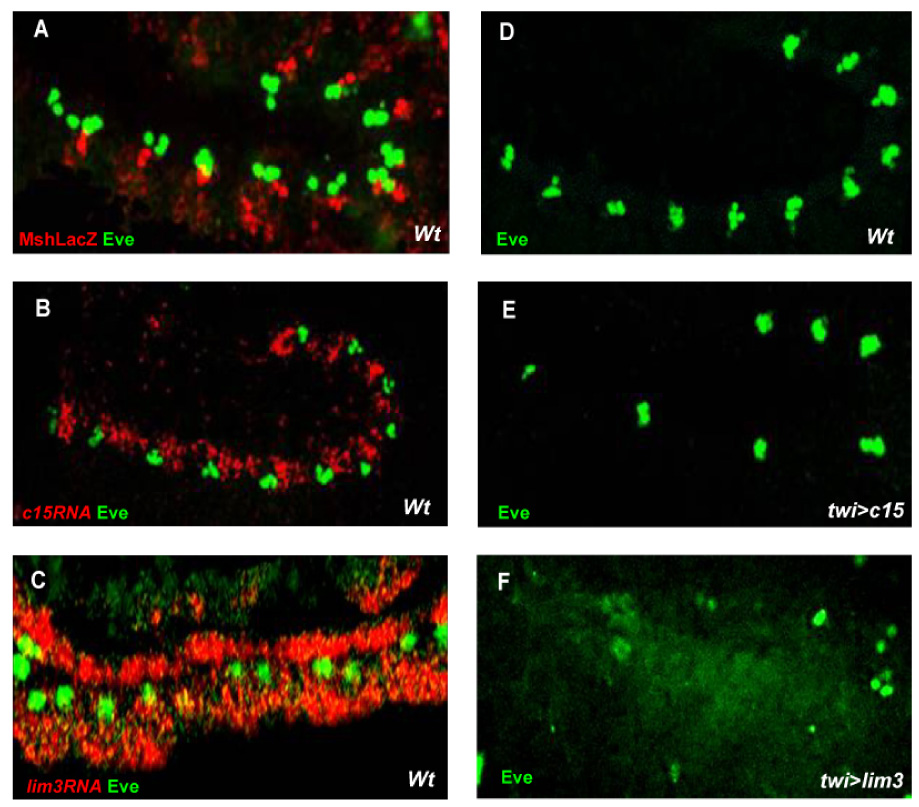
eve expression in the mesoderm is negatively regulated by ectopic Msh, C15 and Lim3. (A) Msh progenitors marked by Msh-LacZ are located ventrally adjacent to the Eve progenitors. (B) c15 is expressed posterior to Eve. (C) lim3 expression marks dorsal cardiac and muscle primodria but does not normally overlap with Eve-positive cells in the developing heart. (D) Mesodermal eve expression in wildtype embryos. Overexpression of c15 (E) or lim3 (F) represses mesodermal eve expression by 50% or more, especially lim3.
Another factor that was identified by in the 1-hybrid screens was the homeobox gene c15, which encodes the Drosophila homologue of vertebrate Hox11. c15 is highly expressed in the cardiac mesoderm and seems to be enriched in cells posterior to the Eve clusters (Fig. 5B), consistent with a possible role in repression of eve in the cardiac mesoderm. We then overexpressed c15 throughout the mesoderm and found that eve expression was dramatically reduced (Fig. 5D,E). Thus, c15 is expressed in the cardiogenic mesoderm and is capable of repressing eve. Next, we examined whether c15 is required for restricting mesodermal eve expression. We examined the phenotype of two c15 null alleles, c15² and c15³ (Campbell, 2005), but did not detect any significant change in mesodermal expression of eve or of the eme-lacZ reporter (data not shown). This suggests that c15 either is not absolutely required for restricting eve expression, acts redundantly with other repressors, or perhaps plays no role.
Since the 1-hybrid screen also identified LIM proteins, we also used a candidate gene approach and found that mesodermal overexpression of the Drosophila LIM-homeodomain protein Lim3 (Thor et al., 1999) caused a dramatic reduction of mesodermal eve expression (Fig. 5D,F) with little effect on the cardiac expression of tinman (data not shown). Thus, the ectopic activity of Lim3 seems to specifically repress eve expression. lim3 is expressed in the nervous system, alary muscles and the heart (Thor et al., 1999). The heart expression includes both the cardiac and dorsal muscle primordia but apparently does not overlap with Eve (Fig. 5C). To assay the effect of loss of lim3 activity on mesodermal eve expression, we monitored eve expression in lim3 mutants (Thor et al., 1999). As with c15, lim3 mutations did not seem to affect eve expression, nor did lim3,c15 double mutants (data not shown). Thus, we were unable to demonstrate a requirement for c15 and lim3 in regulating eve/eme, despite their suggestive expression in the cardiac region and their ability to repress eve/eme when overexpressed. We conclude that c15 and lim3 either have no role in the mesodermal control of eve/eme or they act redundantly with other repressors.
The inhibition of mesodermal eve expression by Lim3 prompted us to examine whether the repressive effect of Lim3 requires the identified repressor sites in eme, Lb2, M1b,c and M2a. For this purpose, we overexpressed lim3 in embryos containing the eme reporter gene with mutated Lb2, M1b, M1c or M2a sites. Ectopic activity of Lim3 no longer represses mutant M1b*, M1c* or Lb2* sites in eme-lacZ (Fig. 6A,C,D,E), which is similar lbe overexpression. Unlike lbe however, eme repression by lim3 overexpression is undiminished when the M2a* site is mutated (Fig. 6B,F). This suggests that the ectopic Lim3 repressor activity does not require or act via the GC-rich M2a site.
Figure 6.
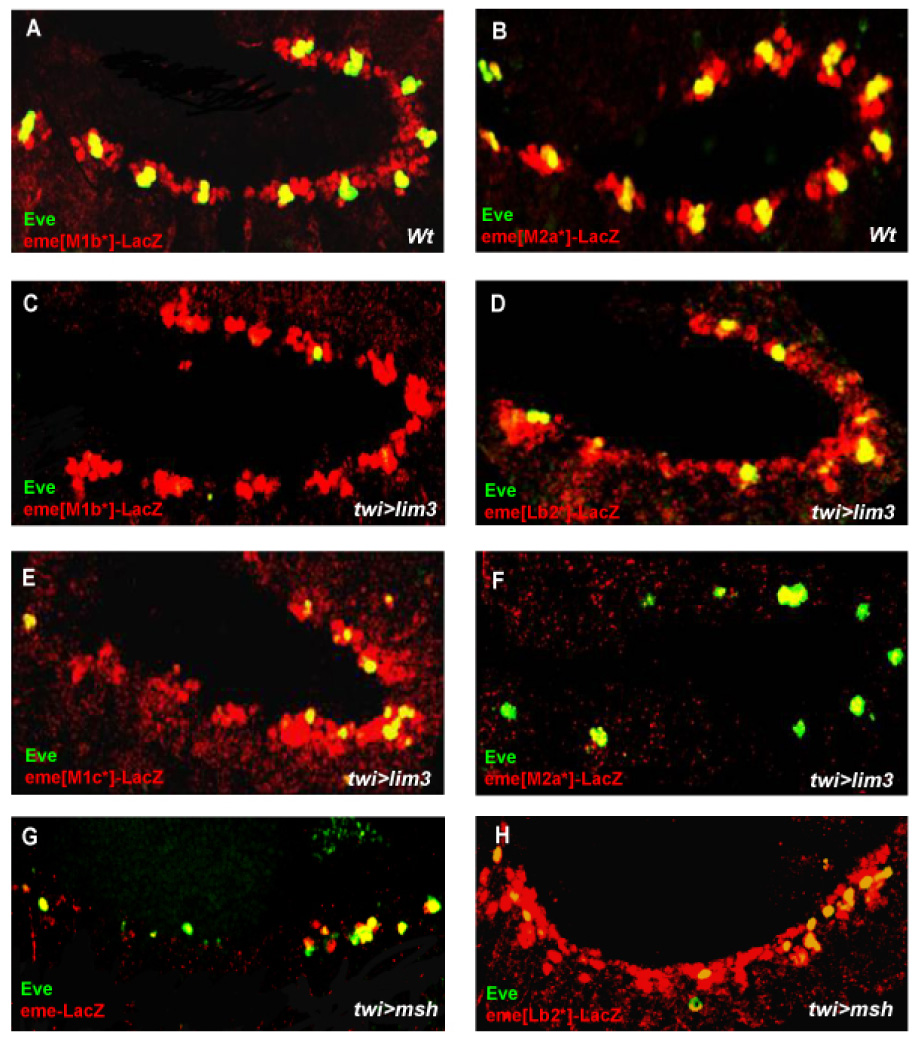
Effective repression of eme by Lim3 requires the M1b, M1c and Lb2 sites, but not the M2a site. (A,B) eme with M1b (A) or M2a (B) site mutated. (C–F) Overexpression of lim3 in embryos which carry eme reporter with M1b site (C), Lb2 site (D), M1c site (E) or M2a site (F) mutated. Note the lack of repression of the reporter in the absence of a M1b, M1c or Lbe site and almost complete repression of reporter in the absence of M2a site. (G,H) Mesodermal msh overexpression strongly represses Eve and wildtype eme reporter expression (G) but not Lb2* mutant eme-lacZ expression (H).
We also examined whether overexpression of msh and c15 repressed eme. We observed a dramatic inhibition of reporter gene expression of eme in wildtype flies, but this repression was diminished when the Lb2 site was mutated (Fig. 6G,H; data not shown). Thus, as is the case for Lbe and Lim3, Msh- and C15-mediated repression of eve expression act via a mechanism that requires an intact Lb2 site.
DISCUSSION
We have identified two essential repressor motifs, the AT-rich M1 and the GC-rich M2 motif, which act in concert with the previously identified Lb2 binding site and are required to achieve the spatial specificity of mesodermal eve expression. Although each site is necessary, none are individually sufficient for restricting the eme enhancer activity to the eve expression domain. We have also demonstrated that several additional homeodomain proteins, including Msh, C15 and Lim3, are capable of repressing mesodermal eve expression by interacting with specific sites within the enhancer element. While the repression of mesodermal eve expression by Msh, C15 and Lim3 is likely mediated by the AT-rich M1 sites and the Lb2 site, the repression of eve expression by Lbe requires both the AT-rich M1 and Lb2 sites as well as the GC-rich M2a site. Therefore, each of the four repressor sites apparently is required on order to confer sensitivity to repression by Lbe. This raises the possibility that repression is the result of a complex in which the cooperation of all four repressor elements is required for successful repression.
Repressive mechanisms in cellular diversification of the developing heart
A prominent feature of the Drosophila is its segmental polarity that includes distinct cardiac cell types that are precisely positioned within each segment. These cardiac progenitors are specified along the anterior-posterior axis during development and are marked by Lbe, Eve or Svp. As the embryo develops, a linear heart tube is formed and this metameric arrangement of cardiac cells types continues to be maintained. Within each hemi-segment, the anterior two pairs form the tinman-expressing ‘working myocardium’, while the posterior pair that expresses svp and the T-box transcriptional factor Doc form the ostia (Molina and Cripps, 2001; Ponzielli et al., 2002; Reim and Frasch, 2005; Reim et al., 2003; Zaffran et al., 2006). Previous studies suggested that repressive interactions between cardiac factors expressed in non-overlapping subtypes of cardiac cells likely contribute to the diversification and maintenance of cellular identities. Svp and Tin have been shown to repress each other’s expression during heart tube formation (Lo and Frasch, 2001) and our data suggests that antagonistic interactions between Lbe and Eve are also a part of this mutual repression network. In addition, our data show that eve expression within the cardiac mesoderm is negatively regulated by multiple repressor sites, thus further supporting the idea that transcriptional repression mechanisms play a prominent role in the generation of cellular diversity in the developing heart. We also demonstrate roles for two potential repressors, C15 and Lim3. Although they do not seem to be essential for patterning mesodermal eve expression they are normally expressed in the cardiac mesoderm in the vicinity of the Eve cells and they do repress the eme enhancer via the identified repressor sites when ectopically expressed. Therefore, it is also possible that they function redundantly with other negative regulators yet to be identified.
Repression of mesodermal eve by multiple repressor sites
Default repression is a common mechanism utilized by major signaling pathways, including Wnt, Shh and Notch pathways, to restrict target gene expression (Barolo et al., 2002). In the absence of signaling, signal-regulated transcription factors function mainly as transcriptional repressors, thus preventing low levels of target gene expression that might be activated by weak, local activators (‘default repression’, see Barolo et al., 2002). In response to signals, some transcription factors are then converted into transcriptional activators to promote target gene expression. Thus, transcriptional repression and activation can be mediated by the same binding sites. Default repression mechanisms may also contribute to the restricted mesodermal eve pattern. It has been reported that mesodermal eve expression is under the direct transcriptional control of Wg signaling (Halfon et al., 2000; Han et al., 2002; Knirr and Frasch, 2001). Mutating several putative binding sites for dTCF, the transcriptional mediator of Wg signaling, results in an expansion of low level reporter gene expression within the cardiac mesoderm that is unaffected by reduced wg activity (Knirr and Frasch, 2001). Thus, dTCF may serve as a default signal to restrict mesodermal eve expression in the absence of wg signaling.
It has previously been shown that Hh signaling promotes eve and svp, but also inhibits lbe expression in the dorsal mesoderm (Liu et al., 2006; Ponzielli et al., 2002). One mechanism for Hh signaling may be via inhibition of Cubitus interruptus (Ci) mediated repression. Interestingly, there is some similarity between the M2a sequence examined here (TGGGCCCT) and the consensus sequence for Ci (TGGGTGGTC). This raises the interesting possibility that M2a site may be a putative Ci binding site in eme. Thus, mutations of M2a site, which result in the anterior expansion of eme activity into Lbe expressing cells, may reflect a lack of repression by Ci. Alternatively, the M2a site may mediate transcriptional repression by Lbe or its potential cofactors. The latter hypothesis is more consistent with the observation that reporter gene expression is rendered insensitive to inhibition by Lbe overexpression when the M2a site is mutated in eme. As the M2a site does not resemble the Lbe consensus sequence, we favor the idea that another factor binds to the M2a site, which then cooperates with Lbe in repressing mesodermal eve expression. This interaction may be facilitated by the close proximity of the two sites.
In sum, our in vivo functional dissection of eme has revealed that each of two AT-rich sites, M1b or M1c and the previous studied Lb2 site, when mutated, causes reporter gene expansion that encompasses the entire cardiac mesoderm, overlapping with Tinman protein at late stage 12. In addition, the GC-rich site M2a is required for repression anterior to the Eve cluster. The absolute requirement of each repressor site for successful restriction of eve expression within the cardiac mesoderm is in striking contrast to the mechanism of incremental activation of this enhancer in the cardiac mesoderm by activators such as Tinman, dTCF, Mad, E-box and ETS sites. Repression through these repressor sites may require cooperation between the sites, perhaps via a repressor complex. Thus, eliminating the function of any of these sites will disrupt interactions with the complex causing de-repression within the ‘activator’-dependent cardiac mesoderm.
Acknowledgements
We thank John Thomas, Gerard Campbell, Krzysztof Jagla and the Bloomington Stock Center and the Developmental Studies Hybridoma Bank at the University of Iowa for fly stocks and antibodies. J. L. was supported by a predoctoral fellowship from the American Heart Association. This work was supported by grants to R.B. from NHLBI of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrioli LP, Vasisht V, Theodosopoulou E, Oberstein A, Small S. Anterior repression of a Drosophila stripe enhancer requires three position-specific mechanisms. Development. 2002;129:4931–4940. doi: 10.1242/dev.129.21.4931. [DOI] [PubMed] [Google Scholar]
- Azpiazu N, Frasch M. tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993;7:1325–1340. doi: 10.1101/gad.7.7b.1325. [DOI] [PubMed] [Google Scholar]
- Azpiazu N, Lawrence PA, Vincent JP, Frasch M. Segmentation and specification of the Drosophila mesoderm. Genes Dev. 1996;10:3183–3194. doi: 10.1101/gad.10.24.3183. [DOI] [PubMed] [Google Scholar]
- Barolo S, Stone T, Bang AG, Posakony JW. Default repression and Notch signaling: Hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev. 2002;16:1964–1976. doi: 10.1101/gad.987402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman BP, Nibu Y, Pfeiffer BD, Tomancak P, Celniker SE, Levine M, Rubin GM, Eisen MB. Exploiting transcription factor binding site clustering to identify cis-regulatory modules involved in pattern formation in the Drosophila genome. Proc Natl Acad Sci U S A. 2002;99:757–762. doi: 10.1073/pnas.231608898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet Y, Jagla T, Da Ponte JP, Dastugue B, Jagla K. Modifiers of muscle and heart cell fate specification identified by gain-of-function screen in Drosophila. Mech Dev. 2003;120:991–1007. doi: 10.1016/s0925-4773(03)00182-5. [DOI] [PubMed] [Google Scholar]
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Frasch M. Heart Development. San Diego, London, New York: Academic Press; 1999. Genetic determination of Drosophila heart development. [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Campbell G. Regulation of gene expression in the distal region of the Drosophila leg by the Hox11 homolog, C15. Dev Biol. 2005;278:607–618. doi: 10.1016/j.ydbio.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Carmena A, Buff E, Halfon MS, Gisselbrecht S, Jimenez F, Baylies MK, Michelson AM. Reciprocal regulatory interactions between the Notch and Ras signaling pathways in the Drosophila embryonic mesoderm. Dev Biol. 2002;244:226–242. doi: 10.1006/dbio.2002.0606. [DOI] [PubMed] [Google Scholar]
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–467. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- Frasch M, Hoey T, Rushlow C, Doyle H, Levine M. Characterization and localization of the even-skipped protein of Drosophila. Embo J. 1987;6:749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig S, Akam M. Homeotic genes autonomously specify one aspect of pattern in the Drosophila mesoderm. Nature. 1993;362:630–632. doi: 10.1038/362630a0. [DOI] [PubMed] [Google Scholar]
- Halfon MS, Carmena A, Gisselbrecht S, Sackerson CM, Jimenez F, Baylies MK, Michelson AM. Ras pathway specificity is determined by the integration of multiple signal-activated and tissue-restricted transcription factors. Cell. 2000;103:63–74. doi: 10.1016/s0092-8674(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Han Z, Fujioka M, Su M, Liu M, Jaynes JB, Bodmer R. Transcriptional integration of competence modulated by mutual repression generates cell-type specificity within the cardiogenic mesoderm. Dev Biol. 2002;252:225–240. doi: 10.1006/dbio.2002.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagla K, Frasch M, Jagla T, Dretzen G, Bellard F, Bellard M. ladybird, a new component of the cardiogenic pathway in Drosophila required for diversification of heart precursors. Development. 1997;124:3471–3479. doi: 10.1242/dev.124.18.3471. [DOI] [PubMed] [Google Scholar]
- Jagla T, Bidet Y, Da Ponte JP, Dastugue B, Jagla K. Cross-repressive interactions of identity genes are essential for proper specification of cardiac and muscular fates in Drosophila. Development. 2002;129:1037–1047. doi: 10.1242/dev.129.4.1037. [DOI] [PubMed] [Google Scholar]
- Klinedinst SL, Bodmer R. Gata factor Pannier is required to establish competence for heart progenitor formation. Development. 2003;130:3027–3038. doi: 10.1242/dev.00517. [DOI] [PubMed] [Google Scholar]
- Knirr S, Frasch M. Molecular integration of inductive and mesoderm-intrinsic inputs governs even-skipped enhancer activity in a subset of pericardial and dorsal muscle progenitors. Dev Biol. 2001;238:13–26. doi: 10.1006/dbio.2001.0397. [DOI] [PubMed] [Google Scholar]
- Kumar R, Chen S, Scheurer D, Wang QL, Duh E, Sung CH, Rehemtulla A, Swaroop A, Adler R, Zack DJ. The bZIP transcription factor Nrl stimulates rhodopsin promoter activity in primary retinal cell cultures. J Biol Chem. 1996;271:29612–29618. doi: 10.1074/jbc.271.47.29612. [DOI] [PubMed] [Google Scholar]
- Li JJ, Herskowitz I. Isolation of ORC6, a component of the yeast origin recognition complex by a one-hybrid system. Science. 1993;262:1870–1874. doi: 10.1126/science.8266075. [DOI] [PubMed] [Google Scholar]
- Liu J, Qian L, Wessells RJ, Bidet Y, Jagla K, Bodmer R. Hedgehog and RAS pathways cooperate in the anterior-posterior specification and positioning of cardiac progenitor cells. Dev Biol. 2006;290:373–385. doi: 10.1016/j.ydbio.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Lo PC, Frasch M. A role for the COUP-TF-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech Dev. 2001;104:49–60. doi: 10.1016/s0925-4773(01)00361-6. [DOI] [PubMed] [Google Scholar]
- Lockwood WK, Bodmer R. The patterns of wingless, decapentaplegic, and tinman position the Drosophila heart. Mechanisms of Development. 2002;114:13–26. doi: 10.1016/s0925-4773(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Molina MR, Cripps RM. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mechanisms of Development. 2001;109:51–59. doi: 10.1016/s0925-4773(01)00509-3. [DOI] [PubMed] [Google Scholar]
- Nose A, Isshiki T, Takeichi M. Regional specification of muscle progenitors in Drosophila: the role of the msh homeobox gene. Development. 1998;125:215–223. doi: 10.1242/dev.125.2.215. [DOI] [PubMed] [Google Scholar]
- Park M, Wu X, Golden K, Axelrod JD, Bodmer R. The wingless signaling pathway is directly involved in Drosophila heart development. Dev Biol. 1996;177:104–116. doi: 10.1006/dbio.1996.0149. [DOI] [PubMed] [Google Scholar]
- Ponzielli R, Astier M, Chartier A, Gallet A, Therond P, Semeriva M. Heart tube patterning in Drosophila requires integration of axial and segmental information provided by the Bithorax Complex genes and hedgehog signaling. Development. 2002;129:4509–4521. doi: 10.1242/dev.129.19.4509. [DOI] [PubMed] [Google Scholar]
- Qian L, Liu J, Bodmer R. Neuromancer Tbx20-related genes (H15/midline) promote cell fate specification and morphogenesis of the Drosophila heart. Dev Biol. 2005;279:509–524. doi: 10.1016/j.ydbio.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Reim I, Frasch M. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development. 2005;132:4911–4925. doi: 10.1242/dev.02077. [DOI] [PubMed] [Google Scholar]
- Reim I, Lee HH, Frasch M. The T-box-encoding Dorsocross genes function in amnioserosa development and the patterning of the dorsolateral germ band downstream of Dpp. Development. 2003;130:3187–3204. doi: 10.1242/dev.00548. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Irion U, Wilson R, Grosskortenhaus R, Leptin M. Control of cell fates and segmentation in the Drosophila mesoderm. Development. 1997;124:2915–2922. doi: 10.1242/dev.124.15.2915. [DOI] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, Hoffmann FM, Baylies MK, Rushton E, Bate M. dpp induces mesodermal gene expression in Drosophila. Nature. 1994;372:783–786. doi: 10.1038/372783a0. [DOI] [PubMed] [Google Scholar]
- Stanojevic D, Small S, Levine M. Regulation of a segmentation stripe by overlapping activators and repressors in the Drosophila embryo. Science. 1991;254:1385–1387. doi: 10.1126/science.1683715. [DOI] [PubMed] [Google Scholar]
- Su MT, Fujioka M, Goto T, Bodmer R. The Drosophila homeobox genes zfh-1 and even-skipped are required for cardiac-specific differentiation of a numb-dependent lineage decision. Development. 1999;126:3241–3251. doi: 10.1242/dev.126.14.3241. [DOI] [PubMed] [Google Scholar]
- Thor S, Andersson SG, Tomlinson A, Thomas JB. A LIM-homeodomain combinatorial code for motor-neuron pathway selection. Nature. 1999;397:76–80. doi: 10.1038/16275. [DOI] [PubMed] [Google Scholar]
- Venkatesh TV, Park M, Ocorr K, Nemaceck J, Golden K, Wemple M, Bodmer R. Cardiac enhancer activity of the homeobox gene tinman depends on CREB consensus binding sites in Drosophila. Genesis. 2000;26:55–66. [PubMed] [Google Scholar]
- Wharton KA, Jr, Crews ST. CNS midline enhancers of the Drosophila slit and Toll genes. Mech Dev. 1993;40:141–154. doi: 10.1016/0925-4773(93)90072-6. [DOI] [PubMed] [Google Scholar]
- Wu X, Golden K, Bodmer R. Heart development in Drosophila requires the segment polarity gene wingless. Dev Biol. 1995;169:619–628. doi: 10.1006/dbio.1995.1174. [DOI] [PubMed] [Google Scholar]
- Yin Z, Frasch M. Regulation and function of tinman during dorsal mesoderm induction and heart specification in Drosophila. Dev Genet. 1998;22:187–200. doi: 10.1002/(SICI)1520-6408(1998)22:3<187::AID-DVG2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91:457–469. doi: 10.1161/01.res.0000034152.74523.a8. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Reim I, Qian L, Lo PC, Bodmer R, Frasch M. Cardioblast-intrinsic Tinman activity controls proper diversification and differentiation of myocardial cells in Drosophila. Development. 2006;133:4073–4083. doi: 10.1242/dev.02586. [DOI] [PubMed] [Google Scholar]


