Abstract
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of ovine pulmonary adenocarcinoma, a transmissible lung cancer of sheep. The envelope of JSRV may have oncogenic properties, since it can morphologically transform mouse NIH 3T3 cells and other fibroblast lines. Recently, we found that the cytoplasmic tail of the envelope transmembrane (TM) protein is necessary for transformation, and in particular a consensus binding motif (YXXM) for phosphatidylinositol 3-kinase (PI3K) is important. Moreover, JSRV-transformed cells show phosphorylation (activation) of Akt/protein kinase B, a downstream target of PI3K. In these studies, we directly tested for the involvement of PI3K in transformation by JSRV. Contrary to expectations, four different experiments indicated that PI3K is not necessary for JSRV-induced transformation: (i) cotransfection with a dominant negative truncated form of the PI3K regulatory subunit (Δp85) did not affect transformation frequency, (ii) cells stably expressing Δp85 showed the same frequencies of transformation as parental NIH 3T3 cells, (iii) fibroblasts established from double-knockout mice lacking PI3K p85α and p85β could be transformed with JSRV envelope, and (iv) incubation of cells with the PI3K inhibitor LY294002 did not specifically inhibit transformation, nor did the drug reverse transformation of JSRV-transformed cells. One alternate explanation for the lack of transformation by YXXM mutants could be that they were defective in intracellular trafficking. However, confocal microscopy of epitope-tagged envelope proteins of both wild-type and nontransforming YXXM mutants showed a cell surface or plasma membrane localization. While PI3K is not required for JSRV-induced transformation of NIH 3T3 cells, the downstream target Akt kinase was found to be activated (phosphorylated) in JSRV-transformed PI3K-negative cells. Therefore, JSRV envelope can induce PI3K-independent phosphorylation of Akt.
Oncogenic retroviruses induce tumors in animals and humans, and they have been important models for understanding the molecular basis of oncogenesis. With respect to tumorigenicity, retroviruses can be divided into nonacute retroviruses and acute transforming retroviruses (reviewed in reference 26). Nonacute retroviruses have genome organizations typical of replication-competent retroviruses. They induce tumors relatively slowly, and a common mechanism is insertional activation of cellular proto-oncogenes (18, 26). Acute transforming retroviruses induce disease rapidly and can frequently transform cells in culture. The high oncogenicity of acute transforming retroviruses has been associated with the presence of viral oncogenes, transduced cellular proto-oncogenes, in their genomes.
Jaagsiekte sheep retrovirus (JSRV) is the etiological agent of ovine pulmonary adenocarcinoma, a contagious lung cancer of sheep (15, 23). The mechanism by which the virus causes oncogenic transformation of lung epithelial cells is still unclear. The genome sequence of JSRV reveals a typical betaretrovirus, with no evidence for a transduced cellular gene (35), which would suggest that JSRV is a nonacute retrovirus. However, in vivo JSRV induces tumors quite rapidly (ca. 6 weeks) in experimentally inoculated newborn animals, and the resulting disease is generally multifocal—properties associated with acute transforming retroviruses (10, 29). Members of our laboratory have shown that JSRV may have a novel mechanism for oncogenic transformation (20). Transfection of JSRV DNA into mouse NIH 3T3 fibroblasts yields foci of morphologically transformed cells, a common assay result for oncogenes. Surprisingly, the transforming potential was found to be in the JSRV envelope gene—the gene encoding proteins of the viral envelope. The envelope protein of the closely related ovine nasal adenocarcinoma virus has also been shown to transform NIH 3T3 cells (3, 13). In addition, avian hemangioma virus envelope protein has been shown to morphologically transform cells (4).
The finding that JSRV envelope protein causes transformation raised several possibilities for the mechanism. One possibility may be that the envelope protein surface (SU) protein binds to its normal receptor on the exterior of the cell, leading to stimulation through the receptor of positive growth signals or inhibition of growth-suppressive signals. In this regard, the JSRV receptor has been identified as hyaluronidase 2 (HYAL-2), a glycosylphosphatidylinositol-linked cell surface molecule (25). It is noteworthy that the human HYAL-2 gene is located on chromosome 3 in a region of common loss of heterozygosity in human lung cancer (3p21.3), so the HYAL-2 gene is a candidate tumor suppressor gene (24, 25). A second possibility may be that the SU protein may bind to another cell surface protein and stimulate growth through that protein. For instance, the transforming protein (gp55) of the spleen focus-forming component of the Friend murine erythroleukemia virus complex is an internally deleted version of a recombinant mink cell focus-inducing (MCF) envelope protein (14, 27). Transformation of erythroid cells by spleen focus-forming virus results from binding of gp55 to the erythropoietin receptor, resulting in constitutive activation of signaling (19). A third possibility may be that the cytoplasmic tail of the envelope TM protein binds to intracellular proteins, leading to growth stimulation. The cytoplasmic tail of JSRV TM is quite short (45 amino acids), and there are no discernible enzymatic domains such as protein kinases. However, JSRV TM protein contains one cytoplasmic tyrosine residue in a sequence motif (YRNM) that may potentially bind either phosphatidylinositol 3-kinase (PI3K) (YXXM) or growth factor receptor binding protein 2 (YXN) (22). Mutational analyses indicated that the amino acids Y590 and M593 are necessary for transformation, but N592 is dispensable (22). This suggested that JSRV envelope protein could transform cells by docking PI3K to the plasma membrane via SH2 domains in class IA PI3K regulatory subunits; in many systems, this leads to production of 3′-phosphorylated phosphatidylinositols (e.g., PIP3), followed by recruitment and phosphorylation of downstream signaling molecules (32). Consistent with this, JSRV-transformed cells showed phosphorylation (activation) of Akt/protein kinase B (Akt/PKB), a kinase that is downstream of PI3K (22). The PI3K/Akt pathway has been implicated in several cancers and oncogenic systems. Avian and murine acute transforming retroviruses have transduced both Akt (v-Akt in the AKT8 murine lymphoma virus [6]) and the p110 subunit of PI3K (v-P3k in avian S-7 virus [8]). Amplification and/or overexpression of the Akt gene has also been reported in a variety of human cancers (33).
In the results described here, we directly tested whether PI3K is involved in JSRV-induced transformation by using a variety of different approaches. We also characterized the subcellular localization of JSRV envelope in cells.
MATERIALS AND METHODS
Cells and transfection.
Mouse NIH 3T3 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum. Human 293T cells were grown in DMEM supplemented with 10% fetal bovine serum. Fibroblasts from embryos homozygous for null mutations in the PI3K regulatory subunit genes Pik3r1 (encoding p85α, p55α, and p50α) and Pik3r2 (encoding p85β) were grown in DMEM supplemented with 15% fetal bovine serum and 2 mM l-glutamine (Sigma). Cells used in this study were supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml).
Fibroblast lines were derived from embryos of p85α−/− p85β−/− and wild-type (129 × C57BL/6) mice. The establishment of these lines as well as embryonic fibroblasts from crosses of p85β+/− mice will be described in detail elsewhere. In brief, the embryos were transferred onto gelatinized tissue culture dishes and the fibroblasts were immortalized after a few passages by way of a retroviral vector expressing simian virus 40 large T antigen. The genotypes of the cells were determined by PCR analysis. The generation of the p85β−/− mice has been described previously (31). Restoration of p85α or p85β to the 785.2 cells was accomplished by infecting them with retroviral vectors (pBABE based) expressing either gene. One week after infection, cells were selected for 1 week by growth in medium containing puromycin.
Transfection was performed by using the CalPhos mammalian transfection kit (Clontech). For transient transfections, cells (4 × 106 per 10-cm-diameter dish) were transfected with 28 μg of DNA and cultured for 48 h after being washed with phosphate-buffered saline (PBS) and refed with growth medium. For indirect immunofluorescence assays (IFA), cells were seeded in 10-cm-diameter culture dishes with sterilized glass coverslips on the bottom and transfected as described above. The cells on the coverslips were used for IFA, and the remaining cells were subjected to Western blot analysis. For transformation assays, cells were cultured for 4 weeks after transfection with medium changes every 3 days, as described previously (20). To inhibit focus formation in NIH 3T3 cells, thePI3K-specific inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one(LY294002; Calbiochem) was added at a final concentration of 10 μM at every medium change.
To establish NIH 3T3 cells stably expressing the dominant negative mutant of p85 (Δp85) (17), Δp85 cloned into pcDNA3 (Invitrogen) was transfected into NIH 3T3 cells. The cells were cultured with 400 μg of G418 (Sigma)/ml, and individual G418-resistant clones were picked and grown up. The clones were maintained in medium containing 200 μg of G418/ml.
Plasmid constructs.
The different JSRV envelope expression constructs used are shown in Fig. 1. The pCMV3ΔGP expression plasmid for JSRV envelope was described previously (20). pCMV3ΔGP(AgeI) contained an AgeI site inserted at the env gene stop codon by site-directed mutagenesis. To make pCMV3ΔGP(HA), sense and antisense oligomers encoding the influenza hemagglutinin (HA) peptide (YPYDVPDYA) were synthesized, annealed, and inserted into the AgeI site of pCMV3ΔGP(AgeI). Three pCMV3ΔGP(HA) mutants, pCMV3ΔGP(Y590F-HA), pCMV3ΔGP(N592T-HA), and pCMV3ΔGP(M593T-HA), were made by site-directed mutagenesis starting with pCMV3ΔGP(HA). To make pCMV3ΔGP(NruIendogenous), pCMV3ΔGP(AgeI) was digested with AgeI and NruI. Nucleotides corresponding to amino acids 577 to 611 of the endogenous molecular clone, pCMV2en56A1 (21), were amplified by PCR and inserted into the AgeI- and NruI-digested pCMV3ΔGP(AgeI). pCMV3ΔGP(NruIendogenous+Y) was made by site-directed mutagenesis of pCMV3ΔGP(NruIendogenous)—the endogenous Env H586KNM in the cytoplasmic tail was mutated to Y586KNM.
FIG. 1.
JSRV envelope expression constructs. Different JSRV envelope expression constructs used in the experiments are shown. They are based on pCMV3ΔGP, a cytomegalovirus (CMV)-driven expression plasmid for JSRV envelope (20). Addition of an influenza virus HA epitope at the carboxy terminus is indicated by stippled boxes. In chimeras in which corresponding sequences from an endogenous JSRV-related virus were substituted into the C termini of the cytoplasmic tails, the endogenous virus sequences are indicated by striped boxes. Individual amino acid substitutions are also indicated. R and U5, subregions of the long terminal repeat; LTR, long terminal repeat.
Cell labeling and immunoprecipitation.
Cells transfected with pCMV3ΔGP(HA) were labeled by [35S]methionine and [35S]cysteine (40 uCi/ml in 10 ml of cysteine- and methionine-free DMEM; NEN) for 16 h and lysed with Triton lysis buffer (50 mM Tris-Cl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 10% glycerol, and phosphatase inhibitor cocktail 2 [Sigma]) for 30 min. Lysates were incubated by rocking with 100 ng of anti-HA antibody (Clontech) at 4°C for 2 h followed by incubation and rocking with 100 μl of 10% (vol/vol) protein agarose A at 4°C for 1 h. Proteins bound to protein agarose A were recovered by centrifugation for 15 min at 4°C in a microfuge, and the agarose beads were washed three times with Triton lysis buffer and once with PBS, resuspended in sodium dodecyl sulfate (SDS) sample buffer (0.35 M Tris-HCl [pH 6.8], 10.28% [wt/vol] SDS, 36% [vol/vol] glycerol, 5% β-mercaptoethanol, 0.012% [wt/vol] bromophenol blue), and boiled for 3 min. The supernatant was collected after centrifugation for 1 min in a microfuge and used for SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
PDGF treatment.
Recombinant human platelet-derived growth factor AA (PDGF-AA; R&D Systems) was used to stimulate NIH 3T3 cells. Cells were cultured in serum-free DMEM for 18 to 24 h and pretreated with or without Wortmannin (200 nM) or LY294002 (10 μM) for 30 min followed by PDGF-AA (50 ng/ml) stimulation for 30 min. Cell lysates were subjected to immunoblot analysis.
Immunoblotting.
Protein samples (10 to 30 μg per sample) were subjected to SDS-PAGE and immunoblot analysis. To detect an HA epitope, HA tag polyclonal antibody (Clontech) was used as a first antibody (0.1 μg/ml). To detect Akt/PKB and phosphorylated Akt, anti-Akt/PKB and anti-phospho-Akt1/PKBα(Ser473) or anti-phospho-Akt1/PKBα(Thr308) (Upstate Biotechnology) were used as primary antibodies, respectively. Secondary antibodies appropriate for the species of primary antibodies were then used. We used rabbit anti-sheep (DAKO), goat anti-mouse (Caltag Laboratories), or goat anti-rabbit (Pierce) immunoglobulin G conjugated with horseradish peroxidase as a secondary antibody. Blots were visualized by SuperSignal West Pico chemiluminescent substrate (Pierce).
IFA.
To analyze the localization of Env in the transfected cells, confocal IFA was performed. Cells on coverslips were washed with PBS, dried, and fixed with acetone. Fixed cells were incubated with polyclonal antibody to HA tag (50 ng/ml; BD Biosciences Clontech) for 30 min at 37°C. After being washed with PBS, cells were incubated with goat anti-rabbit immunoglobulin G conjugated to fluorescein isothiocyanate (Santa Cruz Biotechnology, Santa Cruz, Calif.) for 30 min at 37°C and then observed by using a fluorescence microscope or a confocal microscope system MRC1024 (Bio-Rad, Hercules, Calif.).
RESULTS
Testing the role of PI3K in JSRV-induced transformation.
While previous studies indicated that the putative PI3K docking site in JSRV TM is necessary for transformation, we wished to test the role of PI3K in JSRV transformation more directly. However, an appropriate antibody for JSRV TM protein is not yet available, so we could not test whether PI3K physically interacts with JSRV TM protein in transformed cells. We performed several experiments to directly test whether PI3K is involved in JSRV-induced transformation. First, we tested whether cotransfection with the Δp85 dominant negative expression plasmid could inhibit JSRV transformation (17). Δp85 retains the ability to bind to phosphotyrosine residues in the YXXM consensus but does not form a dimer with class IA PI3K catalytic subunits. NIH 3T3 cells were cotransfected with pCMV3ΔGP and either pΔp85 or pcDNA3.1(−) (the empty expression plasmid lacking the p85 sequences, as a negative control). After 4 weeks of culture, transformed foci were counted (Table 1). The numbers of foci in cells cotransfected with pCMV3ΔGP and pΔp85 (at three different concentrations) were essentially the same as those in cells cotransfected with pCMV3ΔGP and pcDNA3.1(−). These results were surprising, since they suggested that class IA PI3K is not necessary for JSRV-induced transformation.
TABLE 1.
Transient cotransfections with Δp85a
| Amt of:
|
No. of foci
|
|||
|---|---|---|---|---|
| pcDNA3.1 (μg) | pCMV3ΔGP (μg) | pΔp85 (μg) | Expt 1 | Expt 2 |
| 0 | 0 | |||
| 5 | 0 | 0 | ||
| 5 | 32 | 38 | ||
| 5 | 0 | 0 | ||
| 5 | 5 | 27 | 19 | |
| 5 | 10 | 3 | 18 | |
| 5 | 20 | 11 | 18 | |
| 5 | 5 | 20 | 19 | |
| 10 | 5 | 14 | 17 | |
| 20 | 5 | 18 | 17 | |
NIH 3T3 cells were cotransfected with pCMV3ΔGP and the Δp85 expression plasmid or with the pcDNA3.1 empty expression plasmid, and foci of transformed cells per 10-cm-diameter dish were scored after 4 weeks as described in Materials and Methods. The results from two experiments are shown.
One potential explanation for the results in Table 1 may have been that efficiency of cotransfection in the cultures was not high—i.e., that a significant percentage of the cells taking up and expressing pCMV3ΔGP did not also take up and express Δp85. Therefore, transfected NIH 3T3 cells that stably expressed the Δp85 expression vector were prepared as described in Materials and Methods. The efficiency of JSRV envelope transformation of several different Δp85-expressing clones is compared to that of equivalent pcDNA3.1-containing clones in Table 2. While there was variation in transformation efficiencies between the different clones, there was no systematic decrease in transformation frequency for the Δp85-containing clones. To verify that expression of Δp85 in the stable transfectants led to inhibition of PI3K activity, clones were treated with PDGF, a known activator of PI3K, and phosphorylation of Akt was measured by Western blot analysis with an antibody specific for phosphorylated Akt (Fig. 2A). As expected, PDGF treatment resulted in the appearance of phosphorylated Akt in the parental NIH 3T3 cells. Two stable Δp85 transfectants (1 and 2) showed no phosphorylated Akt after PDGF treatment, indicating a lack of functional PI3K, as predicted.
TABLE 2.
JSRV transformation of NIH 3T3 cells stably expressing Δp85a
| Cell clone
|
No. of foci
|
||
|---|---|---|---|
| pcDNA3.1 | pΔp85 | Expt 1 | Expt 2 |
| 1 | 76 | >100 | |
| 2 | >100 | >100 | |
| 3 | 45 | 61 | |
| 4 | >100 | >100 | |
| 5 | 52 | 72 | |
| 1 | 86 | 86 | |
| 2 | >100 | 55 | |
| 3 | 79 | >100 | |
| 4 | >100 | >100 | |
| 5 | >100 | >100 | |
NIH 3T3 cell clones stably transformed with pcDNA3.1 or pΔp85 were isolated as described in Materials and Methods. Focus formation assays with pCMV3ΔGP under standard conditions were carried out, and the number of foci per 10-cm-diameter dish at 4 weeks was tabulated. The results from two independent experiments are shown.
FIG. 2.
Inhibition of PI3K activity. (A) NIH 3T3 cells were serum starved overnight and then stimulated for 30 min with PDGF in the presence or absence of the PI3K inhibitor LY294002 or Wortmannin as described in Materials and Methods. Cell lysates were then analyzed by immunoblotting with an antibody specific for phosphorylated Akt (upper panel) or for total Akt (lower panel). Two NIH 3T3 clones (1 and 2) stably transfected with the Δp85 expression plasmid were serum starved overnight and then stimulated (or not stimulated) with PDGF. Immunoblotting for total and phospho-Akt was carried out in parallel with the NIH 3T3 cell extracts. (B) The Δp85 stable transfectant clones 1 and 2 were transfected with pCMV3ΔGP, and a transformed focus from each clone was picked. Serum-starved JSRV transformants of clones 1 and 2 (lanes c and d) showed phosphorylated Akt (upper panel) after immunoblotting. As expected, serum-starved clones 1 and 2 (lanes a and b) did not show phosphorylated Akt. Results of immunoblotting for total Akt are shown in the lower panels.
Interestingly, when transformed foci from stable Δp85 transfectants were picked, they showed phosphorylated Akt in the absence of PDGF induction (Fig. 2B). Thus, JSRV envelope-induced transformation of NIH 3T3 cells can apparently lead to PI3K-independent activation of Akt.
Another approach to eliminating PI3K from cells was also employed. Five isoforms of the regulatory subunit of PI3K have been identified, p85α, p85β, p55α, p55γ, and p50α (31). p85α, p55α, and p50α are alternative transcripts from a single gene. In fibroblasts, p85α and p85β are the predominant isoforms. Mouse embryo fibroblasts in which both the alpha and beta isoforms of the p85 subunit were eliminated by gene targeting were prepared (785.2 and 785.9 cells). The generation of these fibroblasts will be described in detail elsewhere. Transfection of 785.2 and 785.9 cells with pCMV3ΔGP resulted in the appearance of transformed foci, although at a lower frequency than in NIH 3T3 cells (Table 3). It has also been found that other rodent fibroblast lines (e.g., Rat 6) show lower frequencies of transformation than NIH 3T3 cells (20). The transformation was JSRV dependent, since parallel transfection with the nontransforming mutant pCMV3ΔGP(Y590F) resulted in no transformed foci. Moreover, when transformed foci were picked and cloned, they contained JSRV DNA. Restoration of p85α or p85β activity to 785.2 cells by infection with retroviral expression vectors did not increase the efficiency of transformation (Table 3). The morphologies of the transformed foci in these cells were the same regardless of whether p85β was expressed or not (Fig. 3), and they were identical to the morphology of foci induced in NIH 3T3 cells previously described (20). These results also suggested that JSRV-induced transformation of mouse fibroblasts does not require PI3K activity. However, these experiments did not rule out the possibility that PI3K activity resulting from the p55γ regulatory subunit could be mediating transformation of the p85α-p85β null cell lines by JSRV. These cells express p55γ, and they do show weak but detectable PIP3 formation or Akt phosphorylation upon PDGF treatment (unpublished data).
TABLE 3.
Transformation of fibroblasts from p85 null micea
| Cell lineb | No. of foci after transfection with pCMV3ΔGP |
|---|---|
| 785.2 | 3 |
| 785.6 | 1 |
| 785.9 | 4 |
| 15 | 2 |
| 25 | 0 |
| 35 | 1 |
| 45 | 4 |
Fibroblast lines from different mouse embryos were tested for transformation by pCMV3ΔGP, pCMV3ΔGP(Y590F), or no DNA under standard conditions. The number of foci per 10-cm-diameter dish at 4 weeks is shown. No foci were observed after transfection with pCMVΔGP(Y590F) or no DNA.
785.2 and 785.9, fibroblasts from p85α−/− p85β−/− embryos; 785.6, fibroblasts from p85α+/− p85β−/− embryo; 15, 785.2 cells transfected with empty plasmid vector; 25 and 45, 785.2 cells with restoration of p85α expression; 35, 785.2 cells with restoration of p85β expression.
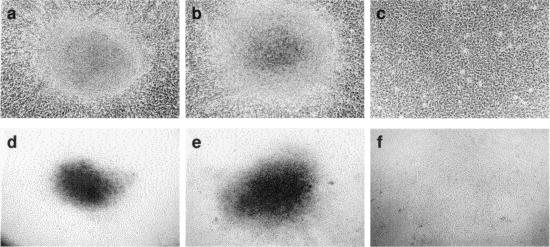
FIG. 3.
Focus formation in PI3K knockout cells. Transformed foci induced by pCMV3ΔGP in 785.2 cells (p85α−/− p85β−/−) (panels a and d) and in clone 25 cells (785.2 cells expressing p85α) (panels b and e) are shown. The morphologies of these foci are the same as those previously reported for JSRV-transformed NIH 3T3 fibroblasts (20). Mock-transfected 785.2 cells are also shown at the same magnifications (c and f). Panels a to c are shown at higher magnification, and panels d to f are shown at lower magnification.
We also tested whether an inhibitor of PI3K activity, LY294002 (34), could inhibit JSRV-induced transformation. Long-term treatment with LY294002 has been used to inhibit transformation and focus formation by the v-crk oncogene in chicken embryo fibroblasts (2). To determine an appropriate dose, viability of NIH 3T3 cells was measured in the presence of different concentrations of LY294002. At 20 μM, cell viability was 40%, while at 10 μM viability was not affected. As also shown in Fig. 2, treatment of NIH 3T3 cells with this concentration of inhibitor resulted in substantial inhibition of Akt phosphorylation after PDGF treatment. Transformation assays of NIH 3T3 cells with pCMV3ΔGP in the presence or absence of 10 and 20 μM LY294002 were performed, and transformed foci were scored after 3 weeks (Table 4, experiment 1). There was a dose-dependent inhibition of focus formation, which could indicate PI3K-dependent transformation. However, it was also possible that LY290042 treatment might generally suppress cell transformation. Therefore, we also tested the effects of LY290042 on transformation by an oncogene that transforms independently of PI3K—v-mos (Table 4, experiment 2). LY290042 treatment showed equivalent inhibition of transformation by JSRV envelope and v-mos. Thus, the inhibition of focus formation in JSRV-transformed cells was most likely due to a nonspecific effect of LY290042 on cell transformation in NIH 3T3 cells.
TABLE 4.
Effect of LY294002 on transformationa
| DNA | Concn of LY294002 (μM) | No. of foci
|
|
|---|---|---|---|
| Expt 1 | Expt 2 | ||
| None | 0 | 0 | |
| pcDNA3.1 | 0 | 0 | |
| pCMV3ΔGP | 66 | 42 | |
| pCMV3ΔGP | 10 | 29 | 7 |
| pCMV3ΔGP | 20 | 19 | |
| v-mos | 21 | ||
| v-mos | 10 | 5 | |
NIH 3T3 cells were tested under standard conditions for transformation by pCMV3ΔGP or v-mos expression plasmid. In some cultures, LY294002 was added to the growth medium at the concentrations indicated; for these cultures, medium with fresh LY294002 was changed daily. The numbers of foci appearing after 4 weeks are given; the results of two experiments are shown.
LY294002 was also tested for the ability to reverse transformation in NIH 3T3 or Rat 6 cells that were already transformed by JSRV. Treatment with the inhibitor did not result in morphological reversion of the transformed cells, nor did it reduce the ability of the transformed cells to grow in agar suspension (data not shown).
In summary, three different approaches all indicated that PI3K is not required for JSRV transformation of NIH 3T3 cells, even though the critical residues in the PI3K docking site in the cytoplasmic tail of TM are indispensable.
Transformation studies with a chimeric JSRV envelope protein.
Normal uninfected sheep cells contain multiple copies of endogenous JSRV-related proviruses that are quite closely related to exogenous oncogenic JSRV (11). We previously molecularly cloned several endogenous JSRV-related proviruses and found high sequence homology between exogenous and endogenous viruses, with the exception of three regions of variability: VR1, VR2, and VR3 (21). VR1 and VR2 are located in the gag gene, while VR3 maps to the section of the env gene corresponding to the C terminus—i.e., the membrane-spanning and cytoplasmic domains of TM protein. We previously generated chimeras with the endogenous (nontransforming) and exogenous (transforming) env genes and showed that exchange of only the C-terminal 35 amino acids from an endogenous virus TM protein (from the NruI site) with 39 amino acids of the exogenous JSRV TM protein led to loss of transformation (22). This chimera, pCMV3ΔGP(NruIend), lacked the YXXM motif of JSRV, but it also contained additional amino acid differences. If the only residues downstream from the NruI site responsible for JSRV transformation were the PI3K docking site, then restoration of the YXXM motif into the chimera would be expected to lead to transformation. We generated an expression plasmid based on pCMV3ΔGP(NruIend) in which mutation of the histidine at 586 to tyrosine resulted in restoration of both the YXXM and YXNX motifs (YKNM), pCMV3ΔGP(NruIend +Y). However, when this plasmid was tested in a NIH 3T3 transformation assay, no foci were observed (data not shown). This suggests that other residues in the cytoplasmic tail of JSRV TM protein besides the putative PI3K docking site are also important for transformation.
Generation and analysis of epitope-tagged JSRV TM protein.
As mentioned above, no antibodies to JSRV TM protein are available. Therefore, we generated a new JSRV envelope expression construct, pCMV3ΔGP(HA), in which an influenza virus HA epitope was fused to the C terminus of TM (Fig. 1). When pCMV3ΔGP(HA) was transfected into 293T cells, immunoprecipitation or Western blot analysis with an anti-HA antibody resulted in detection of a protein of ca. 37 kDa, as would be predicted for TM protein (Fig. 4). SDS-PAGE on a high-concentration gel did not reveal smaller (alternate) cleavage products (data not shown). HA-tagged versions of several mutant JSRV Env proteins were also generated (Fig. 1). These included versions of two nontransforming mutants (Y590F and M593T) and one mutant that actually showed higher efficiency of transformation (N592T).
FIG. 4.
Detection of epitope-tagged TM protein. Cell lysates from mock-transfected human 293T cells (lane a) and 293T cells transiently transfected with pCMV3ΔGP(HA) (lane b) were subjected to immunoblotting with an anti-HA monoclonal antibody. A specific band at 37 kDa (the predicted size for JSRV TM) was detected (arrow). In the right panel (taken from the same gel), lysates from NIH 3T3 cells (lane c) and pCMV3ΔGP(HA)- and pCMV3ΔGP(N592T-HA)-induced transformants (lanes d and e, respectively) were analyzed. The same 37-kDa specific band was detected. The relative mobilities of prestained molecular mass markers on the gel are indicated.
The HA-tagged versions of the JSRV env expression plasmids were tested for transformation in NIH 3T3 cells (Table 5). pCMV3ΔGP(HA) induced foci of transformation, indicating that the epitope-tagged JSRV envelope retained transforming ability. However, the efficiency of transformation was reduced approximately fivefold in comparison to that of the wild-type envelope protein. As expected, HA-tagged versions of the nontransforming mutants (Y590F-HA and M593T-HA) also did not transform NIH 3T3 cells. Interestingly, the HA-tagged version of the N592T mutant (N592T-HA) showed transformation efficiency almost equivalent to that of the wild-type JSRV envelope. Foci from pCMV3ΔGP(HA)- and pCMV3ΔGP(N592T-HA)-transformed cells were picked and grown up. Western blot analysis with an anti-HA antibody revealed the expected 37-kDa TM protein (data not shown).
TABLE 5.
Transformation by epitope-tagged JSRV envelope proteina
| Tranfected DNA | No. of foci
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| None | 0 | 0 |
| pcDNA3.1 | 0 | 0 |
| pCMV3ΔGP | 32 | 38 |
| pCMV3ΔGP(HA) | 6 | 7 |
| pCMV3ΔGP(Y590F-HA) | 0 | 0 |
| pCMV3ΔGP(N592T-HA) | 26 | 29 |
| pCMV3ΔGP(M593T-HA) | 0 | 0 |
NIH 3T3 cells were transfected with different DNAs under standard conditions. Transformed foci appearing at 4 weeks were scored; the results of two experiments are shown.
One potential concern about previous studies with the different JSRV env mutants was that the nontransforming mutants might have expressed less-stable envelope protein. The availability of the epitope-tagged versions of these mutants allowed us to address this possibility. Transient transfection of the different epitope-tagged expression plasmids into 293T cells, followed by Western blot analysis with an anti-HA antibody, indicated that all of the mutant proteins had similar efficiencies of expression (data not shown).
Intracellular localization of JSRV envelope protein.
Another explanation for the inability of some mutant JSRV envelope proteins to induce transformation may be that the mutations altered the intracellular localization of the proteins. Based on the topology of the envelope protein in the virion, we previously hypothesized that the protein would assume the same topology in the transformed cell—i.e., with the TM protein spanning the plasma membrane and SU protein being extracellular. However, it was possible that mutations could have affected the intracellular localization. Indeed, a motif for trafficking of proteins to the plasma membrane is YXXL (12), quite similar to the critical YXXM in the cytoplasmic tail of JSRV TM. Therefore, we tested the intracellular localization of the epitope-tagged wild-type and mutant JSRV envelope proteins by transient transfection of the various expression plasmids into 293T cells followed by confocal immunofluorescent microscopy with anti-HA antibody. As expected, wild-type envelope protein [from pCMV3ΔGP(HA)-transfected cells] could be detected and showed a predominantly cell surface localization (Fig. 5b). When pCMV3ΔGP(Y590F-HA)- and pCMV3ΔGP(M593T-HA)-transfected 293T cells were studied, they showed equivalent cell surface localizations of envelope protein. Thus, the lack of transformation by these mutants did not reflect altered intracellular localization.
FIG. 5.
Intracellular localization of JSRV TM protein. 293T cells growing on coverslips were transiently transfected with pCMV3ΔGP(HA) (wild type) or derivatives containing the indicated amino acid substitutions. After 60 h, the samples were fixed and stained with the anti-HA antibody as described in Materials and Methods. The samples were then subjected to confocal immunofluorescence microscopy with an HA-specific monoclonal antibody. 293T cells were transfected with pCMV3ΔGP(HA) (a), pCMV3ΔGP(Y590F-HA) (b), pCMV3ΔGP(N592T-HA) (c), or pCMV3ΔGP(M593T-HA) (d) or were mock transfected (e). Both the wild-type and mutant envelope proteins showed localization at the plasma membrane. (f) NIH 3T3 cells stably transformed with pCMV3ΔGP(HA) (from an isolated focus). (g) Parental NIH 3T3 cells.
We also studied the intracellular localization of JSRV envelope protein in NIH 3T3 cells transformed with pCMV3ΔGP(HA). In these cells, the distribution of envelope protein was more generally cytoplasmic (Fig. 5f). NIH 3T3 cells transiently transfected with pCMV3ΔGP(HA) also showed a general cytoplasmic staining pattern, as did cells transfected with mutations in the YXXM motif (data not shown). Thus, while there was a difference in the intracellular staining patterns in 293T versus NIH 3T3 cells, mutations in the YXXM motif did not change the localizations for either cell type.
DISCUSSION
In the results described here, we directly tested the hypothesis that PI3K is necessary for transformation by JSRV envelope protein. This hypothesis was based on previous mutational analyses indicating that a YXXM motif in the cytoplasmic tail of the TM protein is necessary for transformation and the fact that JSRV-transformed cells show activation of the downstream Akt kinase (22). However, results of four different experiments all indicated that PI3K is not necessary for JSRV transformation of NIH 3T3 cells. The dominant negative truncated Δp85 subunit of PI3K did not interfere with JSRV-induced transformation, either in cotransfection assays or in stably expressing cells. In addition, fibroblasts derived from p85α/p85β double-knockout mice could still form foci after transfection with JSRV envelope. Finally, treatment of cells with the PI3K inhibitor LY294002 did not specifically inhibit focus formation, nor did it cause phenotypic reversion of JSRV-transformed fibroblasts. Therefore, PI3K is not necessary for establishment (or maintenance) of JSRV envelope-induced transformation, despite the requirement of the YXXM motif.
Results of other experiments also were not consistent with PI3K docking as the mechanism for JSRV transformation. PI3K docking would require that the tyrosine in the YXXM motif be phosphorylated. However, Western blotting of extracts from JSRV-transformed cells with an anti-phosphotyrosine antibody did not identify JSRV TM protein (data not shown). Pull-down assays with epitope-tagged JSRV envelope protein and PI3K p85 subunit also failed to demonstrate interaction between the two proteins (data not shown). Although these results were negative (and possibly simply represented technical difficulties), they also suggested that JSRV transformation does not involve PI3K.
While these experiments indicated that PI3K is not necessary for JSRV-induced transformation in fibroblasts, the downstream Akt kinase is indeed phosphorylated in JSRV-transformed cells, and it remains possible that Akt could be important for transformation. Other signaling pathways also lead to Akt phosphorylation (activation) (7, 16, 28), and it is possible that JSRV envelope could activate one of those pathways as well. In fact, as shown in Fig. 2B, Akt is activated in JSRV-transformed NIH 3T3 cells lacking functional PI3K (the stable Δp85 transfectants). It remains to be determined whether the PI3K-independent activation of Akt is occurring from the cytoplasmic tail of JSRV TM or from other envelope protein domains.
These results indicate that JSRV envelope-mediated transformation involves interaction of the cytoplasmic tail with other signaling molecules besides PI3K. The cytoplasmic tail of TM protein is important for transformation, since a chimera exchanging the cytoplasmic tail of exogenous JSRV with that of endogenous (and presumably nontransforming) JSRV was nontransforming (22). Thus, it will be interesting to search for relevant interacting molecules. To this end, we have initiated a yeast two-hybrid screen with the cytoplasmic tail of JSRV envelope as bait. It is also interesting that mutation of a valine residue in the cytoplasmic tail near the membrane-spanning region also abolishes transformation (N. Maeda and H. Fan, unpublished data). This could reflect another domain of the cytoplasmic tail in addition to the YXXM motif that is important for interaction with cellular proteins involved in transformation. The fact that restoration of the YXXM motif to the cytoplasmic tail of endogenous JSRV-related TM protein did not result in transformation also supports the notion that sequences in addition to this motif are important.
These experiments also raise the question of why mutations in either the tyrosine or methionine of the YXXM motif abolished transformation. These effects were relatively specific, since mutation of an adjacent asparagine residue (which eliminated a YXN binding motif for growth factor receptor binding protein 2) actually increased transformation efficiency. We considered the possibility that the nontransforming mutants were defective because of improper intracellular location of the envelope protein. However, confocal microscopy indicated that both wild-type and mutant envelope proteins showed a cell surface or plasma membrane localization in 293T cells, as expected for an envelope protein. It is possible that some other protein besides PI3K binds to the YXXM motif (perhaps even independently of tyrosine phosphorylation), resulting in transformation.
It should be emphasized that previous mutational analyses have demonstrated that the cytoplasmic tail of JSRV TM protein is necessary for transformation, but they have not shown that it is sufficient. It is possible that extracellular domains of TM or domains of SU are also important for transformation. To this end, we are studying a series of overlapping N-terminal deletion mutants of SU. Preliminary results indicate that elimination of the SU sequences, leaving only TM, eliminates or greatly reduces transformation in NIH 3T3 cells (A. Hofacre and H. Fan, unpublished data). Thus, domains of SU may be important for transformation as well.
Given the multiple signal transduction pathways that can affect transformation, different pathways may be important for JSRV-induced transformation of different cells or cell types. In the case of the v-src oncogene of avian sarcoma virus (encoding the prototypical tyrosine-specific protein kinase), transformation in NIH 3T3 cells is dependent on signaling through c-ras (30). On the other hand, in rat fibroblasts, v-src transformation is not exclusively dependent on c-ras (1). Recently, Allen et al. (5) have studied JSRV envelope-induced transformation in the avian DF-1 fibroblast cell line. They also found a requirement for the cytoplasmic tail of TM protein; however, mutation of the YXXM motif did not inhibit transformation, indicating signaling through different motifs in these cells. Other investigators have concluded that the YXXM motif is not necessary for transformation of rat 208F fibroblasts as well (A. D. Miller, personal communication). Most interestingly, it has recently been reported that JSRV envelope transformation of a human lung epithelial cell line involves interactions of the SU domain with the HYAL-2 receptor, with resulting activation of a transmembrane receptor tyrosine kinase (Stk/RON) (9). Thus, both the SU and TM domains of JSRV envelope protein may be important for transformation, and the importance of different domains may differ depending on the cell types and lines studied.
In summary, these experiments indicate that while the cytoplasmic tail of JSRV envelope protein is necessary for transformation of NIH 3T3 cells, docking of PI3K is not essential—even though activation of Akt can be demonstrated in cells transformed by JSRV envelope. Binding of some other signaling protein(s) is presumably important for transformation, which may or may not involve Akt. Ultimately, the question is whether the ability of JSRV envelope to transform NIH 3T3 cells is related to oncogenicity in vivo. It will be important to build nontransforming envelope mutants back into replication-competent JSRV and test whether they cause lung tumors in sheep. Some of the transformation-negative envelope mutants yield infectious virus in tissue culture (e.g., Y590F and Y590D), and in vivo experiments with them are planned.
Acknowledgments
This work was supported by NIH grants CA82564 and CA094188 to H.F. and AI50831 to D.F. Y.I. is the recipient of a JSPS Overseas Research Fellowship. S.M.B. was sponsored by the Boehringer Ingelheim Fonds.
REFERENCES
- 1.Aftab, D. T., J. Kwan, and G. S. Martin. 1997. Ras-independent transformation by v-Src. Proc. Natl. Acad. Sci. USA 94:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akagi, T., T. Shishido, K. Murata, and H. Hanafusa. 2000. v-Crk activates the phosphoinositide 3-kinase/AKT pathway in transformation. Proc. Natl. Acad. Sci. USA 97:7290-7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti, A., C. Murgia, S. L. Liu, M. Mura, C. Cousens, M. Sharp, A. D. Miller, and M. Palmarini. 2002. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol. 76:5387-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alian, A., D. Sela-Donenfeld, A. Panet, and A. Eldor. 2000. Avian hemangioma retrovirus induces cell proliferation via the envelope (env) gene. Virology 276:161-168. [DOI] [PubMed] [Google Scholar]
- 5.Allen, T. E., K. J. Sherrill, S. M. Crispell, M. R. Perrott, J. O. Carlson, and J. C. DeMartini. 2002. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 83:2733-2742. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa, A., J. R. Testa, S. P. Staal, and P. N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:274-277. [DOI] [PubMed] [Google Scholar]
- 7.Chan, T. O., S. E. Rittenhouse, and P. N. Tsichlis. 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68:965-1014. [DOI] [PubMed] [Google Scholar]
- 8.Chang, H. W., M. Aoki, D. Fruman, K. R. Auger, A. Bellacosa, P. N. Tsichlis, L. C. Cantley, T. M. Roberts, and P. K. Vogt. 1997. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 276:1848-1850. [DOI] [PubMed] [Google Scholar]
- 9.Danilkovitch-Miagkova, A., F. M. Duh, I. Kuzmin, D. Angeloni, S. L. Liu, A. D. Miller, and M. I. Lerman. 2003. Hyaluronidase 2 negatively regulates RON receptor tyrosine kinase and mediates transformation of epithelial cells by jaagsiekte sheep retrovirus. Proc. Natl. Acad. Sci. USA 100:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De las Heras, M., L. Gonzalez, and J. M. Sharp. 2003. Pathology of ovine pulmonary adenocarcinoma. Curr. Top. Microbiol. Immunol. 275:25-54. [DOI] [PubMed] [Google Scholar]
- 11.DeMartini, J. C., J. O. Carlson, C. Leroux, T. Spencer, and M. Palmarini. 2003. Endogenous retroviruses related to jaagsiekte sheep retrovirus. Curr. Top. Microbiol. Immunol. 275:117-137. [DOI] [PubMed] [Google Scholar]
- 12.Deschambeault, J., J. P. Lalonde, G. Cervantes-Acosta, R. Lodge, E. A. Cohen, and G. Lemay. 1999. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J. Virol. 73:5010-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirks, C., F. M. Duh, S. K. Rai, M. I. Lerman, and A. D. Miller. 2002. Mechanism of cell entry and transformation by enzootic nasal tumor virus. J. Virol. 76:2141-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dresler, S., M. Ruta, M. J. Murray, and D. Kabat. 1979. Glycoprotein encoded by the Friend spleen focus-forming virus. J. Virol. 30:564-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan, H. 2003. Jaagsiekte sheep retrovirus and lung cancer, vol. 275. Springer, Berlin, Germany.
- 16.Filippa, N., C. L. Sable, C. Filloux, B. Hemmings, and E. Van Obberghen. 1999. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 19:4989-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara, K., K. Yonezawa, H. Sakaue, A. Ando, K. Kotani, T. Kitamura, Y. Kitamura, H. Ueda, L. Stephens, T. R. Jackson, et al. 1994. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl. Acad. Sci. USA 91:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayward, W. S., B. G. Neel, and S. M. Astrin. 1981. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature 290:475-480. [DOI] [PubMed] [Google Scholar]
- 19.Li, J. P., A. D. D'Andrea, H. F. Lodish, and D. Baltimore. 1990. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature 343:762-764. [DOI] [PubMed] [Google Scholar]
- 20.Maeda, N., M. Palmarini, C. Murgia, and H. Fan. 2001. Direct transformation of rodent fibroblasts by jaasiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA 98:4449-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmarini, M., C. Hallwirth, D. York, C. Murgia, T. de Oliveira, T. Spencer, and H. Fan. 2000. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveal a different cell tropism from that of the highly related exogenous jaagsiekte sheep retrovirus. J. Virol. 74:8065-8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmarini, M., N. Maeda, C. Murgia, C. De-Fraja, A. Hofacre, and H. Fan. 2001. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J. Virol. 75:11002-11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmarini, M., J. M. Sharp, M. de las Heras, and H. Fan. 1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 73:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rai, S. K., J. C. DeMartini, and A. D. Miller. 2000. Retrovirus vectors bearing jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J. Virol. 74:4698-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rai, S. K., F. M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller. 2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 98:4443-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg, N., and P. Jolicoeur. 1997. Retroviral pathogenesis, p. 475-585. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Press, Cold Spring Harbor, N.Y. [PubMed]
- 27.Ruscetti, S. K., D. Linemeyer, J. Feild, D. Troxler, and E. M. Scolnick. 1979. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J. Virol. 30:787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sable, C. L., N. Filippa, B. Hemmings, and E. Van Obberghen. 1997. cAMP stimulates protein kinase B in a Wortmannin-insensitive manner. FEBS Lett. 409:253-257. [DOI] [PubMed] [Google Scholar]
- 29.Sharp, J. M., and J. C. DeMartini. 2003. Natural history of JSRV in sheep. Curr. Top. Microbiol. Immunol. 275:55-79. [DOI] [PubMed] [Google Scholar]
- 30.Smith, M. R., S. J. DeGudicibus, and D. W. Stacey. 1986. Requirement for c-ras proteins during viral oncogene transformation. Nature 320:540-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueki, K., C. M. Yballe, S. M. Brachmann, D. Vicent, J. M. Watt, C. R. Kahn, and L. C. Cantley. 2002. Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA 99:419-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanhaesebroeck, B., S. J. Leevers, K. Ahmadi, J. Timms, R. Katso, P. C. Driscoll, R. Woscholski, P. J. Parker, and M. D. Waterfield. 2001. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70:535-602. [DOI] [PubMed] [Google Scholar]
- 33.Vivanco, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2:489-501. [DOI] [PubMed] [Google Scholar]
- 34.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241-5248. [PubMed] [Google Scholar]
- 35.York, D. F., R. Vigne, D. W. Verwoerd, and G. Querat. 1992. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J. Virol. 66:4930-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]