Abstract
The inflammatory response to acute ocular herpes simplex virus type 1 (HSV-1) infection in mice involves the innate and adaptive immune response, with an associated increase in the secretion of chemokines, including CXCL10 (interferon-inducible protein 10 kDa [IP-10]). Neutralizing antibodies to mouse CXCL10 were used to determine the role of CXCL10 during the acute phase of HSV-1 ocular infection. Treatment of HSV-1-infected mice with antibody to CXCL10 significantly reduced CXCL10 levels in the eye and trigeminal ganglion and reduced mononuclear cell infiltration into the corneal stroma. These results coincided with reduced ICAM-1 and CXCR3 transcript expression, macrophage inflammatory protein-1α and CXCL10 levels, and corneal pathology but increased viral titers in the stroma and trigeminal ganglion. Progression of the virus from the corneal stroma to the retina during acute infection was significantly hindered in anti-CXCL10-treated mice. In addition, colocalization of viral antigen with infiltrating leukocytes in the iris and retina during acute infection suggests that one means by which HSV-1 traffics to the retina involves inflammatory cells (primarily CD11b+ cells). Collectively, the results suggest that CXCL10 expression in the eye initially orchestrates the inflammatory response to acute HSV-1 infection, which facilitates the spread of the virus to other restricted sites within the eye.
Corneal herpes simplex virus type 1 (HSV-1) infection results in an explosive host response initiated by production of chemokines, including CXCL10, KC (murine CXCL1), macrophage inflammatory protein (MIP)-2, monocyte chemoattractant protein 1, MIP-1β, and RANTES (50, 58), as well as proinflammatory cytokines, including interleukin 6 (IL-6) (21). A variety of cells within or proximal to the cornea are a likely source of these molecules, including resident Langerhans cells (24), keratocytes (35), and macrophages (6). Neutrophils respond to MIP-2 and KC (15), resulting in their infiltration (56, 57) and subsequent secretion of inflammatory molecules, including inducible nitric oxide synthase, tumor necrosis factor alpha, IL-12, and gamma interferon (IFN-γ) (13, 18). These soluble mediators elicit the expression or up-regulation of adhesion molecules, including CD31 (platelet endothelial cell adhesion molecule 1 [PECAM-1]) and CD54 (ICAM-1) (53), and the costimulatory molecule CD80 on resident Langerhans cells and keratocytes (12, 44), resulting in the infiltration of CD4+ T cells (37, 42). It is the infiltration of these CD4+ T lymphocytes and the subsequent secretion of cytokines, including IL-2 and IFN-γ along with other mediators independent of T cells (e.g., IL-12), that lead to the pathological manifestations of herpetic eye disease referred to as herpetic stromal keratitis (4, 10, 25, 42, 52). By antagonizing the activation cascade of the immune system elicited by replicating HSV-1, it may be possible to reduce the collateral damage of the ensuing inflammatory response, thereby saving the visual axis. In support of this hypothesis, the absence of MIP-1α renders mice less susceptible to the development of severe stromal keratitis without hindering the capacity to clear infectious virus from the eye (58).
CXCL10 is a CXC chemokine with potent chemoattractant properties for activated T cells and NK cells (3, 20, 39), monocytes (55), and neutrophils (34). This activity is mediated through the receptor CXCR3 (31) and other unknown mechanisms (5, 48). The expression of CXCL10 has been implicated with tissue accumulation of T cells in Toxoplasma gondii infection (27) as well as pathogenesis in a number of diseases, including Alzheimer's disease (60), human immunodeficiency virus type 1 infection (28), and multiple sclerosis (47). Experimentally, neutralization of CXCL10 reduces demyelination as a result of central nervous system infection with mouse hepatitis virus (MHV) (30). However, the expression of CXCL10 is essential in controlling MHV replication through the infiltration of CD4+ and CD8+ T lymphocytes and production of IFN-γ in the central nervous system (16, 29). Similar to MHV infection, ocular HSV-1 infection up-regulates the expression of CXCL10 within the cornea (50). Since CXCL10 has previously been found to elicit a protective state against vaccinia virus in athymic mice (33), it was predicted that the expression of CXCL10 is protective against ocular HSV-1 infection by facilitating the adaptive immune response against ocular HSV-1 infection. The present study suggests that neutralizing CXCL10 reduces the initial inflammatory response within the cornea. Coincidently with reduced inflammatory response, we observed reduced HSV-1 trafficking to the retina, implying that leukocytes promote the dispersion of HSV-1 from the anterior to the posterior region of the eye.
MATERIALS AND METHODS
Virus and cells.
Propagation of and plaque assays using green monkey kidney fibroblasts (Vero cells, ATCC CCL-81, American Type Culture Collection, Manassas, Va.) were carried out in RPMI-1640 medium supplemented with 10% fetal bovine serum, gentamicin (Invitrogen, Carlsbad, Calif.) and antibiotic-antimycotic solution (Invitrogen) at 37°C, 5% CO2, and 95% humidity (culture medium). HSV-1 stocks (McKrae and KOS strains) were prepared as previously described (22). The anti-CXCL10 mouse monoclonal antibody (Ab) (clone IR7C6) was generated by immunizing BALB/c mice with a peptide corresponding to an epitope of CXCL10 (CIHIDDGPVRMRAIGK) previously shown to produce Abs that effectively neutralize CXCL10 function in vivo (30). Spleens from immunized mice were removed and fused with SP2/0 myeloma cells with polyethylene glycol. Hybridoma cell lines that produced Abs against CXCL10 were selected by enzyme-linked immunosorbent assay (ELISA) and cloned twice by limiting dilution. Anti-CXCL10 hybridoma clones were selected based on their ability to recognize full-length CXCL10 protein via ELISA and their viability in culture. Clone IP6C7 was chosen and produces a monoclonal Ab that is an immunoglobulin G2 (IgG2) isotype, κ light chain. The hybridoma was grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, gentamicin (Invitrogen), and antibiotic-antimycotic solution (Invitrogen) at 37°C, 5% CO2, and 95% humidity. Supernatant was collected from the hybridoma cultures, and the IgG fraction was purified by using protein G columns according to the manufacturer's suggestions (Pierce, Rockford, Ill.). IP6C7 effectively blocks T-cell chemotaxis in vitro and inhibits Ca2+ mobilization (T. Lane, unpublished observation). Polyclonal murine IgG was purified from mouse serum by using protein G columns.
Infection of mice.
Female ICR mice (25 to 30 g, approximately 6 to 7 weeks of age; Harlan Sprague-Dawley, Indianapolis, Ind.) or Tie2 transgenic mice (6 weeks of age) (43) were anesthetized by intraperitoneal injection with 0.1 ml of phosphate-buffered saline (PBS) containing xylazine (2 mg/ml; 6.6. mg/kg of body weight) and ketamine (30 mg/ml; 100 mg/kg). Corneas were scarified with a 25-gauge needle, and tear film was blotted with tissue before inoculation with 300 PFU of HSV-1/eye. At the time of infection and 2 and 5 days postinfection (p.i.), mice received 100 μg of anti-CXCL10 IgG or control IgG intraperitoneally. Animals were handled in accordance with the National Institutes of Health guidelines on the Care and Use of Laboratory Animals (Publication 85-23, revised 1996). All procedures were approved by the University of Oklahoma Health Sciences Center institutional animal care and use committee. Mice were either monitored for cumulative survival over 30 days p.i. or euthanized at indicated times for viral titers, detection of viral antigen expression of infected tissue, or visualization for neovascularization.
Clinical scoring.
The corneas of mice were observed in a blind manner for pathology by an ophthalmologist using a Kowa portable slit lamp (Kowa Optimed Inc., Torrance, Calif.). Corneal pathology assessed with a slit lamp was keyed as follows: 0, no pathology; 1, injected eye, no opacity; 2, focal opacity; 3, hazy opacity over entire cornea; 4, dense opacity in central cornea with remainder hazy; 5, same as rating 4 but with ulcer; 6, corneal perforation.
Plaque assay.
At day 3, 5, or 7 p.i., virally infected mice were euthanized, and the corneal buttons, iris, retina, and trigeminal ganglion (TG) were isolated and homogenized in 1.0 ml of RPMI 1640. The clarified supernatant (1,000 × g, 1 min) from the homogenized tissue was serially diluted and placed (100 μl) onto Vero cell monolayers in 96-well cultured plates. After a 1-h incubation at 37°C, 5% CO2, and 95% humidity, the supernatants were discarded, and 75 μl of an overlay solution (0.5% methylcellulose in culture medium) was added on top of the monolayers. The cultures were incubated at 37°C in 5% CO2 and 95% humidity for 24 to 28 h to observe plaque formation, and the amount of infectious virus was reported as PFU per ml. In another set of experiments, CD11b+- or CD11b−-enriched cells (60 to 2,500 cells/well) were added to Vero cell monolayers and observed for plaque formation 28 h after initiation of culture.
Immunohistochemical staining for HSV-1 antigens and leukocyte infiltration.
Ocular tissue expression of HSV-1 antigen and detection of cellular infiltration in the cornea and retina of infected eyes were carried out as previously described (38).
Whole-mount staining for HSV-1 antigen expression.
Whole eyes were fixed in 4% paraformaldehyde overnight. The retinas were then dissected away from the sclera and choroid and placed in separate tubes. Tissues were then washed in PBS-T (PBS containing 1% Triton X-100) and then blocked in PBS-T containing 10% horse serum for 30 min at room temperature (25°C). Tissues were then incubated with a fluorescein isothiocyanate (FITC)-conjugated anti-HSV-1 Ab (F0318, DAKO, Carpinteria, Calif.), diluted 1:200 in PBS-T containing 10% horse serum for 4 h at room temperature. Tissues were then washed with PBS-T three times for 15 min each wash and then flat mounted in 50% glycerol in PBS onto microscope slides. Tissues were then imaged on a Nikon fluorescence microscope equipped with a high-resolution digital camera.
Detection of LacZ expression in the anterior segment of Tie2 mice.
Tie2 transgenic mice were infected with HSV-1 (300 PFU/eye) and treated with 100 μg of control or anti-CXCL10 IgG at 0, 2, and 5 days p.i. Mice were euthanized at day 6 p.i., and the eyes were removed and enucleated. To isolate anterior segments, enucleated eyes were dissected in ice-cold PBS to remove posterior segment and lens and fixed in ice-cold buffer consisting of 2% paraformaldehyde, 2 mM MgCl2, 2 mM EGTA, and 0.1 M piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 6.9) for 45 min. The fixed anterior segments were rinsed with PBS three times for 5 min each. The LacZ expression was detected by room temperature overnight incubation in a mixture of 0.1% X-Gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mM magnesium chloride, 0.002% NP-40, 0.01% sodium deoxycholate, and PBS (pH 7.0). After staining, the anterior segments were rinsed in PBS and postfixed overnight at 4°C in 4% paraformaldehyde-PBS, pH 7.0. For whole-mount photography, the postfixed eyes were rinsed in PBS and equilibrated in 50% glycerol-PBS. Images were captured on an Olympus SZX12 stereo microscope.
Reverse transcriptase real-time PCR.
Total RNA was extracted from corneal buttons in Ultraspect RNA isolation reagent (Biotecx Inc., Houston, Tex.) according to the manufacturer's protocol. First-strand cDNA was synthesized by using avian myeloblastosis virus reverse transcriptase and an oligonucleotide dT primer (Promega, Madison, Wis.). Semiquantitative real-time PCR was carried out in 96-well PCR plates (Bio-Rad, Hercules, Calif.) using a Bio-Rad iCycler. Real-time PCR conditions for ICAM-1 included an initial ramp of 50°C for 2 min, followed by a denaturing step for 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and annealing and elongation at 62°C for 1 min. Each reaction contained 25 μl of Bio-Rad Supermix, 22.5 μl of filtered water, and 2.5 μl of cDNA sample. Oligonucleotide sequences for ICAM-1 include the forward primer, 5′-AGGTATCCATCCATCCCAGAGA-3′, and reverse primer, 5′-GAGCTCATCTTTCAGCCACTGA-3′. The specificity of the primer pair has previously been described (36). The conditions and oligonucleotide sequences used to detect the housekeeping gene for glyceraldehyde 3-phosphate dehydrogenase have been previously described (23). Murine IL-12p40 and CXCR3 mRNA levels were measured by using Taqman probes labeled with fluourescent Ab to membrane antigen and oligonucleotide sequences according to the manufacturer's instructions (Roche, Branchburg, N.J.). The PCR results were analyzed on the iCycler software (version 3.0), and threshold cycles were determined as previously described (23).
IFN-γ and chemokine quantitation.
In vivo levels of CXCL10, monokine induced by IFN-γ (MIG or CXCL9), vascular endothelial growth factor (VEGF), IFN-γ, MIP-1α, MIP-2, and RANTES were measured in the cornea, iris, retina, and/or TG of mice infected with HSV-1 at days 1 to 7 p.i. with commercially available ELISA kits (R&D Systems, Minneapolis, Minn.). Mice were treated with control polyclonal IgG or anti-CXCL10 IgG as described above and infected with HSV-1 (300 PFU/eye). At day 3 to 7 p.i., the mice were euthanized, and the corneal buttons, iris, retina, and/or TG were removed and placed in PBS (pH 7.4) supplemented with a cocktail of protease inhibitors (Calbiochem, San Diego, Calif.). Following homogenization, the samples were clarified by centrifugation (10,000 × g, 2 min), and the clarified supernatant was assayed for chemokine and IFN-γ content in duplicate according to the manufacturer's instructions. Noninfected eyes served as the negative control.
Enrichment of CD11b+ and CD11b−cells.
Five days following ocular infection with HSV-1 (300 PFU/eye), the eyes were removed and the vitreous was collected. The contents of the vitreous were washed in PBS (pH 7.4), and the resulting cells were placed in 500 μl of degassed PBS containing 2 mM EDTA and 0.5% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.). The cells were then positively selected for CD11b+ cells by using LS+ separating columns and CD11b magnetic microbeads according to the manufacturer's instructions (Miltenyi Biotech, Auburn, Calif.). Following the separation of CD11b+ and CD11b− cells, the recovered cells were counted and assayed for HSV-1 content by plaque assay. In order to obtain enough cells to conduct a plaque assay, the vitreous from 20 eyes/experiment was employed, recovering approximately 30,000 total mononuclear cells.
Statistics.
One-way analysis of variance (ANOVA) and Tukey's test were used to determine significance (P < 0.05) of differences between the viral titers, clinical scores, and relative values for targeted gene expression recovered from the corneal buttons, iris, retina, and TG of control (IgG-treated) and anti-CXCL10-treated mice. All statistical analysis was performed with the GBSTAT program (Dynamic Microsystems, Silver Spring, Md.).
RESULTS
Anti-CXCL10 Ab reduces ocular pathology and leukocyte infiltration in the cornea and stroma of virus-infected eyes.
Initially, HSV-1-infected mice treated with the anti-CXCL10 or control IgG were inspected for gross pathology by physical exam with a slit lamp at a time reflecting maximum inflammation but prior to the initiation of mortality (day 5 to 6 p.i.). Control-treated mice (n = 10) presented with clinical scores ranging from 2.1 to 2.2 ± 0.3 in either the left or right eye. In contrast, the anti-CXCL10-treated mice (n = 10) presented with clinical scores ranging from 0.5 to 0.8 ± 0.3 in either the left or right eye (P < 0.01 when comparing the control- to anti-CXCL10 Ab-treated groups). Histological assessment of the eye at 6 days p.i. confirmed that the majority (five of eight) of anti-CXCL10 Ab-treated mice infected with HSV-1 showed modest inflammation in the cornea, proximal to the iris and ciliary body (Fig. 1A). In contrast, a majority (six of eight) of control-treated, HSV-1-infected mice showed an impressive cellular infiltrate in the iris and ciliary body as well as the stroma, proximal to the ciliary body (Fig. 1B). To further define the inflammatory process within the stroma, corneal buttons were removed from the treated mice at 3 or 6 days p.i., and CXCR3, ICAM-1, and IL-12p40 transcript levels were determined by real-time PCR. The results show a reduction in CXCR3 and ICAM-1, but not IL-12p40, transcript expression recovered from HSV-1-infected mice treated with the anti-CXCL10 Ab day 3 p.i. (Table 1). By day 6 p.i., only ICAM-1 mRNA levels were reduced in the corneal buttons from the anti-CXCL10 Ab-treated mice (Table 1).
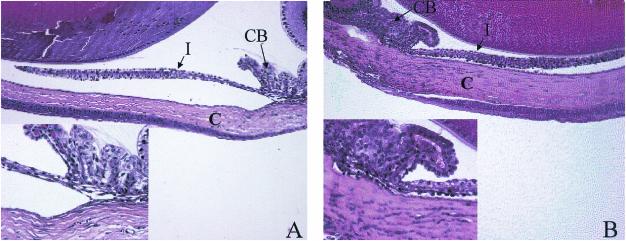
FIG. 1.
Anti-CXCL10 Ab treatment reduces infiltrating leukocytes into the corneal stroma, ciliary body, and iris of HSV-1-infected mice. Mice (n = 8/group) were infected with HSV-1 (300 PFU/eye) and inoculated with 100 μg of anti-CXCL10 IgG or control IgG at time zero and 2 and 5 days p.i. At day 6 p.i., the mice were euthanized, and the eyes were removed and processed for hematoxylin-eosin staining. (A) Anti-CXCL10 Ab-treated mouse cornea (C), iris (I), and ciliary body (CB) at an original magnification of 40×. (B) Control Ab-treated mouse cornea, iris, and ciliary body at an original magnification of 40×. The insets depict ciliary body and stroma at an original magnification of 400×. This figure is representative of the results from two experiments with n = 4 mice/group/experiment.
TABLE 1.
Expression of genes associated with ocular inflammation during HSV-1 infectiona
| Day p.i. | Transcript | Transcript level for treatment with:
|
|
|---|---|---|---|
| Anti-CXCL10 Ab | Control Ab | ||
| 3 | CXCR3 | 18.4 ± 5.7* | 43.2 ± 9.3 |
| ICAM-1 | 2.4 ± 0.9* | 6.3 ± 1.1 | |
| IL-12 | 4.9 ± 2.3 | 6.4 ± 3.7 | |
| 6 | CXCR3 | 6.5 ± 0.5 | 10.9 ± 2.4 |
| ICAM-1 | 1.8 ± 0.4* | 4.0 ± 1.1 | |
| IL-12 | 2.2 ± 0.4 | 2.3 ± 0.6 | |
Mice (n = 8/group) were infected with HSV-1 (300 PFU/eye) and inoculated with 100 μg of anti-CXCL10 IgG1 or control IgG at time 0 and 2 and 5 days p.i. At day 3 or 6 p.i., the mice were euthanized, the corneal buttons were removed, and the RNA extracted from the tissue was processed for real-time PCR analysis. Numbers are relative values ± standard errors of the mean *, P < 0.05 for comparison of the anti-CXCL10- to control antibody-treated groups. This table is a summary of three experiments with n = 2 to 3 mice/group/experiment.
Anti-CXCL10 Ab suppresses local production of selective chemokines following ocular HSV-1 infection.
Since previous results have shown a correlation between chemokine expression and corneal pathology during acute HSV-1 infection (50, 58) and anti-CXCL10-treated mice displayed reduced inflammatory cell infiltrate in the anterior segment of the eye, selective chemokine protein levels were assessed during the initial stage of infection. To verify that treatment of mice with the neutralizing Ab reduced tissue levels of CXCL10, CXCL10 protein was measured at times post-HSV-1 infection. During the early period of acute infection (i.e., day 1 to 3 p.i.), mice receiving anti-CXCL10 showed a significant reduction in CXCL10 expression in the eye compared to control IgG-treated, HSV-1 infected mice (Table 2). The chemokine MIG, induced by IFN-γ and involved in Th1-directed inflammatory responses, was not significantly reduced in the eye of anti-CXCL10 Ab-treated mice (Table 2). Another difference worth noting in ocular levels of CXCL10 and MIG is that there are detectable levels of CXCL10 in uninfected mice, whereas MIG levels are detected only in the eye of mice infected with HSV-1 (Table 2). Whereas CXCL10 and MIG levels were not significantly different in the TG during the early course of infection (days 1 to 3), by day 6 p.i., anti-CXCL10 Ab-treated mice showed a significant reduction of both chemokines in the TG compared to control IgG-treated animals (Table 2).
TABLE 2.
Treatment of mice with anti-CXCL10 antibody suppresses tissue levels of CXCL10 during HSV-1 infectiona
| Day p.i. | Tissue | Chemokine | CXCL10 levels (pg/tissue ± SEM) for treatment with:
|
|
|---|---|---|---|---|
| Anti-CXCL10 Ab | Control Ab | |||
| 1 | Eye | CXCL10 | 0.0 ± 0.0* | 72 ± 26 |
| 3 | Eye | CXCL10 | 28 ± 19* | 110 ± 32 |
| 6 | Eye | CXCL10 | 532 ± 224 | 934 ± 321 |
| 1 | Eye | MIG | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 3 | Eye | MIG | 34 ± 14 | 82 ± 23 |
| 6 | Eye | MIG | 1331 ± 323 | 1,128 ± 342 |
| 1 | Eye | VEGF | 7.4 ± 3.3 | 8.0 ± 1.6 |
| 3 | Eye | VEGF | 5.7 ± 1.2* | 10.1 ± 1.6 |
| 6 | Eye | VEGF | 4.2 ± 2.0 | 5.6 ± 2.2 |
| 1 | TG | CXCL10 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 3 | TG | CXCL10 | 111 ± 55 | 287 ± 158 |
| 6 | TG | CXCL10 | 807 ± 225* | 1,466 ± 131 |
| 1 | TG | MIG | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 3 | TG | MIG | 128 ± 67 | 77 ± 29 |
| 6 | TG | MIG | 681 ± 164* | 1,520 ± 180 |
Female ICR mice (n = 6/group) were infected with HSV-1 (300 PFU/eye) and inoculated with 100 μg of anti-CXCL10 IgG1 or control IgG at time 0 and 2 and 5 days p.i. At day 1, 3, or 6 p.i., the mice were euthanized and the eyes and TG were removed and assayed for chemokine content by ELISA. Numbers are in pg/tissue ± standard error of the mean. *, P < 0.05 for comparison of the anti-CXCL10- to control antibody-treated groups. This table is a summary of two experiments with n = 3 mice/group/experiment. It should be noted that neither VEGF nor MIG was detected in the eye or TG of uninfected mice. In contrast, CXCL10 was detected in the eye of uninfected mice at 20 ± 3 pg/eye but not in the TG.
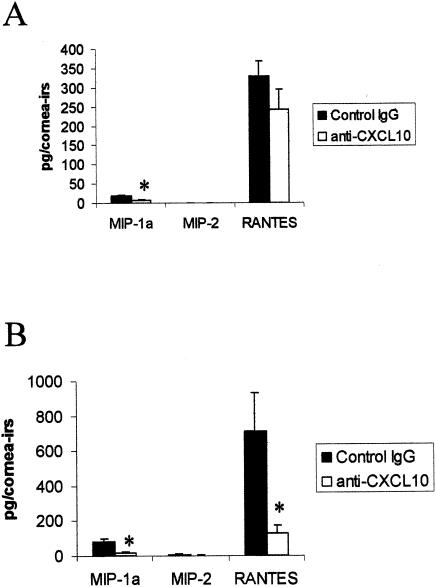
Additional chemokines were measured during the latter time points of the acute infection, as these levels are reportedly not detected until day 5 p.i. (58). On day 5 p.i., MIP-1α levels in the cornea and iris were reduced threefold in the anti-CXCL10-treated group in comparison to the control-treated mice (Fig. 2A). The prominent expression of RANTES, while reduced in the anti-CXCL10-treated mice, was not significantly different in the control-treated mice at day 5 p.i. (Fig. 2A). MIP-2 levels were below the level of detection (<15.6 pg) in most animals (7 of 10) of either group of treated mice at this time point. By day 7 p.i., both RANTES and MIP-1α, but not MIP-2, levels were significantly reduced in the cornea and iris of the anti-CXCL10-treated, HSV-1-infected mice in comparison to the control-treated group (Fig. 2B). There was no measurable MIP-1α, MIP-2, or RANTES in noninfected ocular tissue.
FIG. 2.
Anti-CXCL10 Ab treatment reduces MIP-1α and RANTES levels in the cornea and iris of HSV-1-infected mice. Mice (n = 5/group) were infected with HSV-1 (300 PFU/eye) and inoculated with 100 μg of anti-CXCL10 IgG or control IgG at time zero and 2 and 5 days p.i. At day 5 (A) or 7 (B) p.i., the mice were euthanized, and the corneal buttons and iris from each eye were removed and homogenized. The clarified supernatant from each sample (n = 10/group [day 5 p.i.] or n = 4/group [day 7 p.i.]) was then assayed for chemokine content by ELISA. *, P < 0.05 for comparison of the anti-CXCL10-treated group to the corresponding control-treated group, as determined by ANOVA and Tukey's post hoc t test. This figure is a summary of two experiments with n = 2 or n = 5 mice/group/time point.
Neutralization of CXCL10 enhances HSV-1 replication but hinders trafficking to the retina.
Previous results suggest that neutrophil depletion of mice during acute ocular HSV-1 infection results in an increase in and prolonged presence of infectious virus recovered in the cornea (56, 57). Since the anti-CXCL10 Ab-treated mice showed a reduction in leukocyte infiltration in the stroma, ciliary body, and iris following corneal HSV-1 infection, viral titers were determined in ocular tissue of the eye of anti-CXCL10- and control IgG-treated mice during the acute viral infection. At day 3 p.i., viral titers were elevated in the corneal button taken from anti-CXCL10 Ab-treated mice (Table 3). However, by day 5 p.i., there was no significant difference in viral titers recovered from the cornea (Table 3). By comparison, HSV-1 was initially recovered in the iris of control- but not anti-CXCL10 Ab-treated mice as early as day 3 p.i. (Table 3). By day 5 p.i., there was a significant increase in the amount of HSV-1 recovered in the iris of the control-treated mice compared to the anti-CXCL10 Ab-treated group. By day 7 p.i., both groups of animals had significant amounts of infectious virus in the iris that were clearly above the amount recovered in the corneal buttons. Retinal tissue was employed as a negative control, since earlier work suggested that the ipsilateral retina is spared from HSV-1 following anterior segment infection (41). Although no virus was recovered early during infection (i.e., 0 of 8 per group at day 3 p.i.), by day 5 p.i., 12 of 20 retinas surveyed from control Ab-treated mice were positive for infectious virus compared to 1 of 12 retinas from the anti-CXCL10 Ab-treated group (Table 3). By day 7 p.i., there was a significant increase in viral titers recovered from retina of the control-treated group in comparison to the anti-CXCL10-treated mice (Table 3).
TABLE 3.
Anti-CXCL10 treatment hinders HSV-1 spread to the retinaa
| Day p.i. | Tissue | Titer for treatment with:
|
|
|---|---|---|---|
| Anti-CXCL10 Ab | Control Ab | ||
| 3 | Cornea | 4,054 ± 932* | 1,359 ± 321 |
| 5 | Cornea | 17,567 ± 11,611 | 99,430 ± 41,585 |
| 7 | Cornea | 736 ± 289 | 1,974 ± 898 |
| 3 | Iris | 0 ± 0 | 50 ± 25 |
| 5 | Iris | 17,254 ± 7,475* | 144,607 ± 56,698 |
| 7 | Iris | 91,938 ± 56,693 | 672,055 ± 408,184 |
| 3 | Retina | 0 ± 0 | 0 ± 0 |
| 5 | Retina | 10 ± 10 | 61 ± 27 |
| 7 | Retina | 119 ± 91* | 5,318 ± 2,000 |
| 3 | TG | 3,716 ± 2,322 | 5,421 ± 1,521 |
| 5 | TG | 21,696 ± 4,008* | 9,030 ± 2,456 |
Mice (n = 6 to 10/group) were infected with HSV-1 (300 PFU/eye) and inoculated with 100 μg of anti-CXCL10 IgG or control IgG at time 0 and 2 and 5 days p.i. At the indicated time p.i., the designated tissue was removed using a dissecting microscrope, homogenized, and assayed for infectious virus by plaque assay. Numbers indicate viral titer in PFU per corneal button, iris, retina, or TG ± standard error of the mean. *, P < 0.05 for comparison of the control to the anti-CXCL10 Ab-treated groups for each tissue at each time point. This table is a summary of two to three experiments with n = 3 to 4 mice/group/experiment.
Immunohistochemical staining of HSV-1 antigen in the eye.
Ocular tissue was assessed for viral antigen expression and location by comparing tissue of the control IgG-treated mice to that of the anti-CXCL10-treated group. Both groups of mice expressed HSV-1 antigen in the cornea and iris, with more viral antigen expression in the iris of control-treated mice (Fig. 3), consistent with viral titers recovered from this tissue (Table 3). Likewise, viral antigen was expressed in select areas adjacent to or around arterioles and within the choroid and in the retina, typically, the ganglion cell layer, and in the inner plexiform layer of the retina (Fig. 4). In addition, viral antigen expression was detected in the ciliary nerve of HSV-1-infected mice 6 days p.i. (Fig. 5). Neovascularization was also evident in the stromal layer of the anti-CXCL10 Ab-treated, HSV-1 infected mice (Fig. 3). To further explore the possible evidence for increased neovascularization in the anti-CXCL10 Ab-treated, HSV-1 infected mice, VEGF levels were measured in HSV-1 infected mice treated with the anti-CXCL10 IgG. The results show a transient but significant reduction in the VEGF level in the eye of anti-CXCL10 Ab-treated mice compared to the control IgG-treated group 3 days p.i. (Table 2). However, to more fully appreciate the relationship between vascular development in the eye of HSV-1 infected mice treated with anti-CXCL10 versus control IgG, transgenic mice expressing the LacZ gene under the vascular endothelial-specific promoter, Tie2, would allow us to visualize in no uncertain terms corneal neovascularization. The majority (6 of 9) of HSV-1- infected, Tie2-LacZ transgenic mice treated with control Ab showed neovascularization 6 days p.i., compared to 40% (4 of 10) of the anti-CXCL10-treated mice (Fig. 6). Collectively, while HSV-1-infected mice treated with the anti-CXCL10 Ab appear to show a modest reduction in the incidence or level of neovascularization, the results are not striking and involve a number of factors, some of which may be modified by local levels of CXCL10.
FIG. 3.
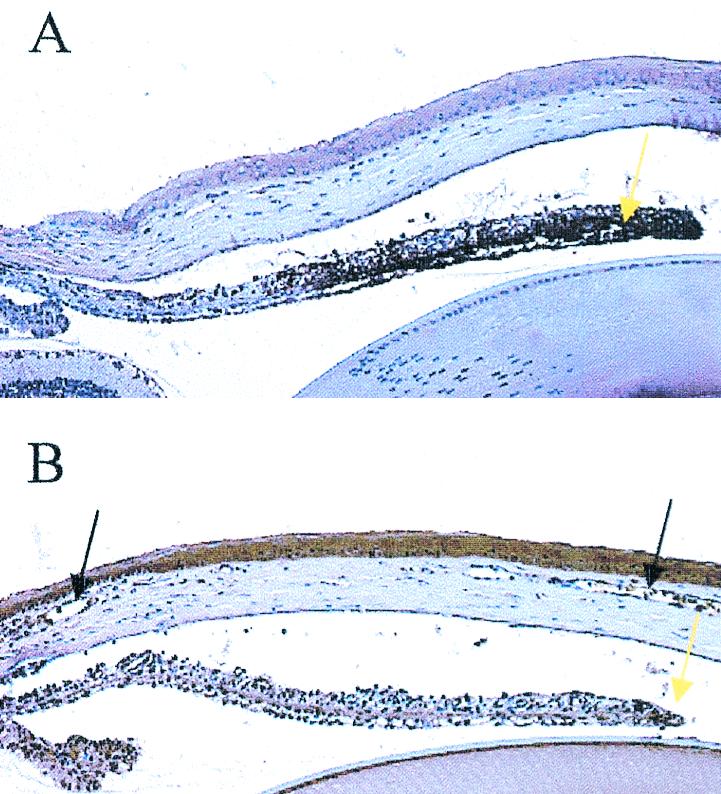
HSV-1 antigen expression in the iris is reduced in anti-CXCL10 Ab-treated, HSV-1-infected mice. Mice (n = 4/group) were infected with HSV-1 (300 PFU/eye) and inoculated with 100 μg of anti-CXCL10 IgG or control IgG at time zero and 2 and 5 days p.i. At day 6 p.i., the mice were euthanized and the eyes were removed and processed for HSV-1 antigen expression. The experiment was repeated twice. (A) IgG-treated mouse iris at a magnification of 100×. (B) Anti-CXCL10 Ab-treated mouse iris at a magnification of 100×. Note the dark brown-stained tissue indicative of HSV-1 antigen (yellow arrows) and the edematous presentation of the corneal stroma of the control IgG-treated mice relative to that of the anti-CXCL10 IgG-treated animals. Neovascularization in the anti-CXCL10 Ab-treated mouse cornea is noted by black arrows.
FIG. 4.
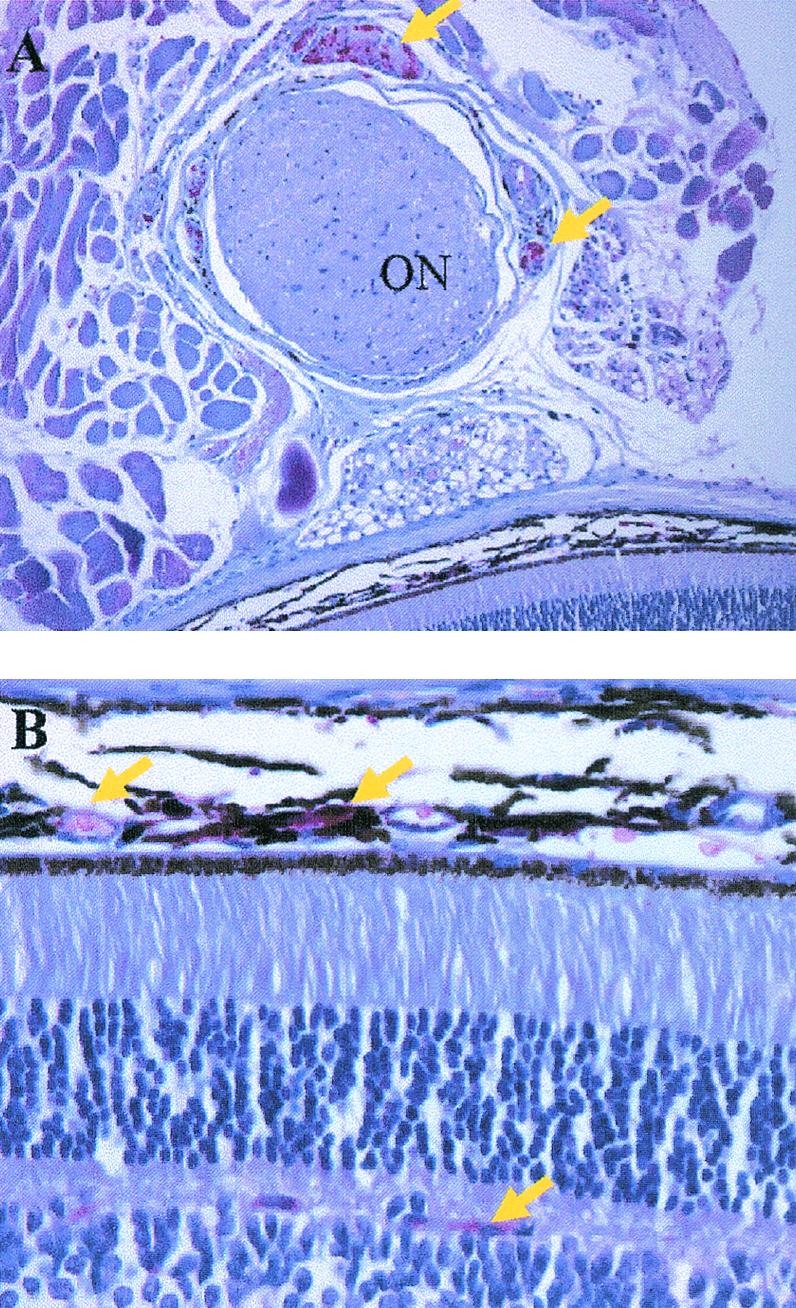
Viral antigen expression in the retina of mice infected with HSV-1. Mice (n = 4/group) were infected with HSV-1 and euthanized 6 days p.i., and the eyes were removed and processed for HSV-1 antigen expression. (A) HSV-1 antigen expression (in red, indicated by yellow arrows) in tissue surrounding the optic nerve (ON). (B) HSV-1 antigen expression (in red, indicated by yellow arrows) in the choroid and photoreceptor layer of the retina.
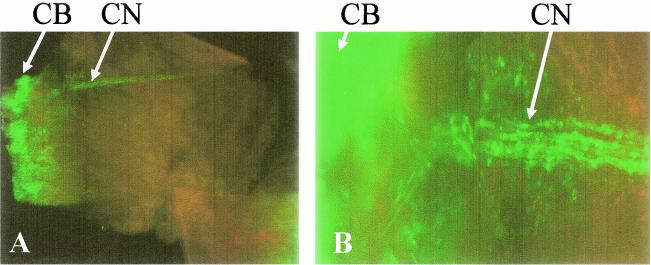
FIG. 5.
Expression of HSV-1 antigen in the ciliary body and nerve of mice 6 days p.i. Whole mounts of ocular tissue were incubated with rabbit anti-HSV-1 polyclonal Ab. After excessive washing, the tissue was viewed under a fluorescence microscope. Shown are the ciliary body (CB) and nerve (CN) labeled with HSV-1 antigen at an original magnification of 40× (A) and an identical panel at an original magnification of 200× (B).
FIG. 6.
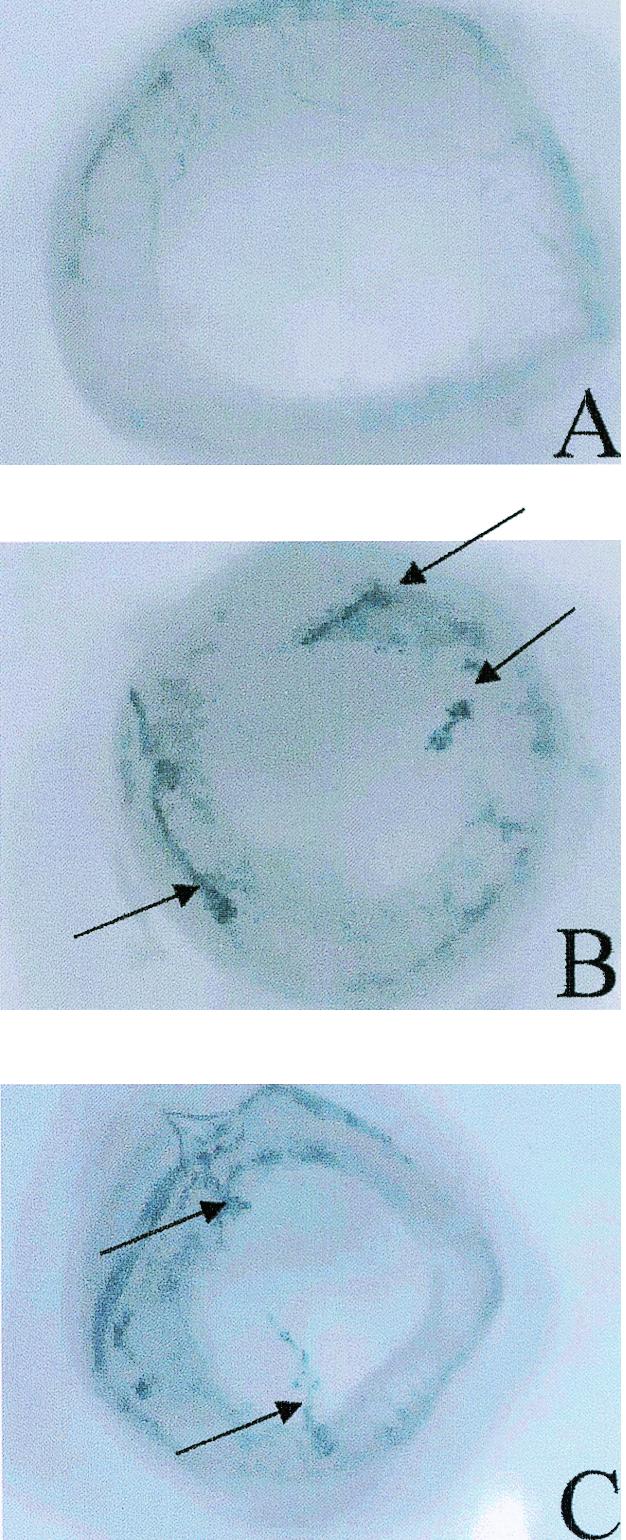
Neovascularization of the cornea in HSV-1-infected mice treated with anti-CXCL10 or control IgG. Tie2-LacZ transgenic mice were infected with HSV-1 (300 PFU/eye) and inoculated with 100 μg of anti-CXCL10 IgG (n = 10) or control IgG (n = 9) at time zero and 2 and 5 days p.i. At day 6 p.i., the mice were euthanized and the eyes were removed, fixed in 4% paraformaldehyde for 2 h, and placed in X-Gal substrate. Following an overnight incubation, the corneal button was removed and flat mounts of the cornea were prepared for visualization of vascularization (noted in dark blue). Vascular beds in the iris can be visualized peripheral to the cornea. Arrows indicate sites of neovascularization in the cornea. Shown are results for anti-CXCL10 IgG-treated, HSV-1-infected mouse showing no neovascularization (A), control IgG-treated, HSV-1-infected mouse showing neovascularization in the cornea (B), and an anti-CXCL10 IgG-treated, HSV-1-infected mouse also showing neovascularization in the cornea (C).
Cells infiltrating the vitreous of the eye of HSV-1-infected mice harbor infectious HSV-1.
Since anti-CXCL10 Ab-treated mice presented with a reduced cellular infiltrate within the corneal stroma proximal to the iris and ciliary body along with a delay in infectious virus recovered in the retina compare to control-treated, HSV-1-infected mice, it was anticipated that inflammatory cells might contribute to the infection of the retina. In fact, occasional cellular infiltrate in the vitreous of HSV-1-infected mice expressed HSV-1 antigen (data not shown). To determine if such cells harbored infectious virus, cells isolated from the vitreous humor were enriched for the CD11b+ phenotype (targeting macrophages, neutrophils, NK cells, and some dendritic cells) and assayed for viral content by plaque assay. More than 50 PFU were obtained from 62 CD11b+ cells, compared to 15 ± 12 PFU from 62 CD11b− cells, suggesting that the CD11b+ cells harbor the majority of infectious HSV-1 in the vitreous. There was no significant difference in the number of cells recovered in the vitreous of HSV-1-infected mice treated with control Ab [(4.3 ± 0.9) × 104 cells] compared to mice treated with the anti-CXCL10 Ab [(3.3 ± 1.6) × 104 cells], suggesting that the virus may infect the retina by additional routes independent of inflammatory cells (e.g., the ciliary nerve).
Corneal scarification is required for HSV-1 presence in the retina.
Since HSV-1 in the retina has not been previously reported following corneal infection, an additional experiment was undertaken to determine the requirement for HSV-1 access to the retina. Mice were infected with the McKrae strain of HSV-1 either with (200 PFU/eye) or without (70,000 PFU/eye) scarification and assessed for viral titers in the corneal buttons, iris, and retina at day 5 p.i. The results show that only when the cornea was scarified was virus recovered in the retina, even when over 100-fold more virus was applied to the nonscarified cornea compared to the scarified cornea (Table 4). In addition, there was a significant reduction in the viral load in the iris from mice in which virus was applied to the nonscarified cornea, suggesting that scarification augments the access to internal sites within the anterior segment of the eye (Table 4). HSV-1 infection of the retina was not strain dependent, as the KOS strain of HSV-1 could also readily infect retinal tissue when applied topically to scarified, but not nonscarified, cornea (Table 4).
TABLE 4.
Corneal scarification is required for HSV-1 infection of retinaa
| Tissue | Titer for the indicated strain and treatment group
|
|||
|---|---|---|---|---|
| McKrae
|
KOS
|
|||
| Scarified | Nonscarified | Scarified | Nonscarified | |
| Cornea | 4,430 ± 1,190 | 70,830 ± 37,030 | 8,000 ± 4,910 | 1,700 ± 870 |
| Iris | 53,750 ± 17,330 | 450 ± 450* | 117,500 ± 40,430 | 50 ± 30* |
| Retina | 75 ± 48 | 0 ± 0 | 830 ± 700 | 0 ± 0 |
Mice (n = 4/group) were infected with the McKrae (scarified, 200 PFU/eye; nonscarified, 70,000 PFU/eye) or KOS (scarified or non-scarified, 70,000 PFU/eye) strain of HSV-1. Five days p.i., the mice were euthanized and the designated tissue was removed using a dissecting microscrope, homogenized, and assayed for infectious virus by plaque assay. Numbers indicate viral titer in PFU per corneal button, iris, or retina ± standard error of the mean. *, P < 0.05 for comparison of the scarified to nonscarified groups for each strain in the iris. This table is representative of two experiments with similar outcomes.
Inflammation in HSV-1-infected TG is reduced in mice treated with anti-CXCL10 Ab.
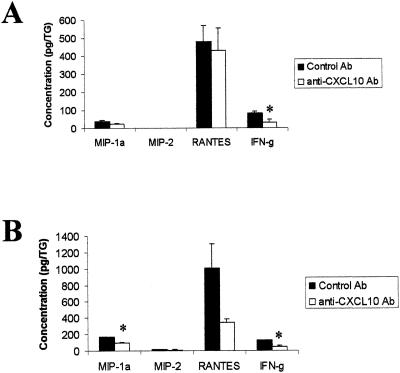
Following ocular HSV-1 infection, the virus traffics back into the sensory ganglion, where it establishes a latent infection. To determine whether mice treated with neutralizing Ab to CXCL10 showed a change in HSV-1 infection or inflammation in response to infection within the TG, the sensory ganglia of HSV-1-infected mice treated with control IgG or anti-CXCL10 IgG were removed during acute infection and assayed for viral titer and chemokine and IFN-γ protein levels. At day 3 p.i., there was no significant difference in viral loads between the control and anti-CXCL10 Ab-treated groups (Table 3). In contrast, at day 5 p.i., the anti-CXCL10 Ab-treated group showed a significant increase in viral load compared to the control-treated group (Table 3). Even though the viral yield was increased in the anti-CXCL10 Ab-treated group at day 5 p.i., IFN-γ levels in the TG at this time point were reduced (Fig. 7). MIP-1α, MIP-2, and RANTES levels were not significantly modified in the TG day 5 p.i. (Fig. 7A). By day 7 p.i., MIP-1α and the proinflammatory cytokine IFN-γ were reduced in the TG of mice treated with the anti-CXCL10 Ab (Fig. 7B). Taken together, the results suggest that similar to ocular tissue, HSV-1-infected TG taken from mice treated with the neutralizing Ab to CXCL10 show a reduced level of inflammation as measured by chemokine and IFN-γ protein concentration.
FIG. 7.
MIP-1α and IFN-γ levels are reduced in the TG of anti-CXCL10 Ab-treated, HSV-1-infected mice. Mice (n = 5/group) were infected with HSV-1 (300 PFU/eye) and inoculated with 100 μg of anti-CXCL10 IgG or control IgG at time zero and 2 and 5 days p.i. At day 5 (A) or 7 (B) p.i., the mice were euthanized and the corneal buttons and iris from each eye were removed and homogenized. The clarified supernatant from each sample (n = 10/group [day 5 p.i.] or n = 4/group [day 7 p.i.]) was then assayed for chemokine and IFN-γ content by ELISA. *, P < 0.05 for comparison of the anti-CXCL10-treated group to the corresponding control-treated group, as determined by ANOVA and Tukey's post hoc t test. This figure is a summary of two experiments with n = 2 or n = 5 mice/group/time point.
CXCL10 has no direct anti-viral effect against HSV-1.
Since viral yields in the eye and TG of anti-CXCL10 Ab-treated mice were greater than those found in the control Ab-treated animals at days 3 and 5 p.i., the direct effect of CXCL10 on HSV-1 replication was tested by using primary murine spleen cells or the fibroblast cell line, L929. Preincubating spleen or L929 cells with CXCL10 prior to infection with HSV-1 (McKrae strain) had no direct effect on viral replication as measured by viral titer (Table 5).
TABLE 5.
CXCL10 has no direct effect on HSV-1 replicationa
| Cell type | CXCL10 titer | Vehicle titer |
|---|---|---|
| L929 | 3,471,875 ± 234,375 | 2,968,750 ± 156,250 |
| Spleen | 17,188 ± 1,563 | 18,750 ± 3,125 |
Spleen cells (1 × 107 cells/culture) or L929 (1.6 × 106 cells/culture) cells were pretreated with CXCL10 (0.5 to 1.0 μg/culture) or vehicle (RPMI-1640) for 24 h prior to the addition of HSV-1 (multiplicity of infection, 0.5 for spleen cells and 1.0 for L929 cells). Twenty-four hours postinfection, the cultures were freeze-thawed and the cell-free supernatants were assayed for viral titer using Vero cells. This table is representative of two experiments; n = 2 cultures/treatment/experiment. Numbers are mean PFU/ml ± standard deviation.
DISCUSSION
CXCL10 is thought to direct migration of CXCR3-bearing cells, including NK cells and activated T cells, preferentially of the Th1 phenotype (31, 40). NK cells and T cells facilitate the clearance of HSV-1 either by direct lysis of virally infected cells or by inhibition of viral replication through the release of soluble mediators such as IFN-γ (8, 11, 19). In the present study, the administration of Ab to CXCL10 reduced the inflammatory response to ocular infection as measured by cellular infiltration and suppressed selective chemokine and ICAM-1 expression in the anterior segment of the eye and IFN-γ, MIG, and MIP-1α expression in the TG. Ocular levels of CXCL10 were significantly reduced very early during acute infection by the neutralizing Ab, suggesting that CXCL10 may be a key chemokine orchestrating the secretion of other chemokines, either directly by influencing the local release of other chemokines or indirectly through the recruitment of chemokine-secreting inflammatory cells into the stroma, ciliary body, and iris of the eye. Which cells within the eye are generating CXCL10 is currently unknown. The level of inflammation in the eye as well as the TG inversely correlated with viral titers recovered in the tissue of the control and anti-CXCL10 Ab-treated mice. The data suggest that the production of soluble mediators and the infiltration of immune cells either directly or indirectly antagonize viral replication proximal to the entry site as well as within the peripheral nervous system. Our data further demonstrate that this effect is greatly reduced in the anti-CXCL10 Ab-treated mice. It is evident that in the context of the present experimental model, viral titers recovered in the eye and TG do not reflect the outcome of survival of HSV-1-infected mice, since the anti-CXCL10 Ab-treated mice showed prolonged survival even though viral yields were significantly greater in the infected tissue surveyed. Such an outcome is consistent with a previous report showing that while cumulative survival of mice deficient in the IFN-γ receptor was less than that of wild-type mice, viral titers recovered in the TG were not different (9).
The reduced inflammatory profile in the cornea of HSV-1-infected mice treated with anti-CXCL10 Ab was most closely associated with a reduction in the chemokines MIP-1α and CXCL10 and the expression of ICAM-1 mRNA. These results are consistent with previously reported observations (58, 59) highlighting the key importance of MIP-1α in the inflammatory process associated with ocular HSV-1 infection. Another chemokine, RANTES, was reduced in the anti-CXCL10 Ab-treated mice specifically at the latter sampling point (day 7 p.i.) in both the cornea-iris and TG. The amount of RANTES recovered in the cornea and iris of HSV-1-infected mice was nearly 10-fold greater than that of MIP-1α, implicating this chemokine in the pathological manifestations of ocular herpes infection that typically occur after the initial clearing of virus from the eye at 14 to 21 days p.i. (58). RANTES is chemoattractant for monocytes as well as T cells (41), implicating this CC chemokine as another instigator, along with CD4+ T cells, in herpes keratitis. Although CXCL10, MIP-2, and RANTES mRNA are constitutively expressed in the cornea of mice (50), only CXCL10 was detectable by ELISA. Similar to the effects on MIP-1α and RANTES, treatment of HSV-1-infected mice with anti-CXCL10 Ab also reduced expression of mRNA from ICAM-1, which is another molecule associated with inflammation following ocular HSV-1 infection (14). Consequently, it may be that the additive effect of suppressing the expression of both adhesion molecules and chemokines ultimately reduces the marked inflammatory process normally observed during acute HSV-1 disease.
Part of the inflammatory process associated with ocular HSV-1 infection includes angiogenesis of the avascular cornea. Replicating HSV-1 has been found to induce VEGF, a critical angiogenic factor, in the infected corneal epithelium and to infiltrate inflammatory cells (61). While the mechanism of induction is not fully understood, CpG motifs found within HSV DNA may elicit VEGF secretion, as recently described (62). In the present study, HSV-1-infected mice inoculated with neutralizing Ab to CXCL10 exhibited some corneal neovascularization evident within 6 days p.i. These results are consistent with the angiostatic properties of CXCL10 (45), suggesting that local production by ocular tissue in response to HSV-1 may hinder blood vessel development, a response that is exaggerated in the presence of anti-CXCL10 Ab. Upon further analysis, VEGF levels were transiently suppressed in HSV-1-infected mice treated with the anti-CXCL10 Ab. It is probable that multiple sources of VEGF are responsible for neovascularization, including local cells within the anterior segment of the eye as well as infiltrating neutrophils during HSV-1 infection (62). Visualization of neovascularization with HSV-1 infected, anti-CXCL10 Ab-treated transgenic mice showed only a modest reduction in new vessel growth in the avascular cornea in comparison to control Ab-treated, HSV-1-infected mice. Since CXCL10 ocular levels were not completely abolished in the mice treated with anti-CXCL10 Abs, it is difficult to firmly establish the relationship between ocular CXCL10 levels and the incidence of corneal neovascularization. Only when mice deficient in CXCL10 or expression of the CXCL10 receptor, CXCR3, are employed will a more convincing picture develop.
Besides the findings implicating CXCL10 involvement in infiltration and inflammation in ocular HSV-1 infection, it was also found that HSV-1 traffics to the retina upon topical application onto the scarified cornea. Elegant studies have demonstrated the retrograde transport of HSV-1 following introduction of the virus onto scarified corneal epithelium, but targeted tissue has focused on cells within the peripheral and central nervous systems excluding retinal tissue (46). Other investigators have shown the zosteriform spread of HSV-1 during acute ocular infection involves the TG, requiring replication in the nervous system rather than corneal tissue (51). Clinically, HSV-1 infection of the retina, known as acute retinal necrosis syndrome, is a relatively rare disease presenting with vitritis and progressive retinal necrosis (17, 32). Experimentally, HSV-1 has been reported to infect the contralateral retina via the brain following anterior chamber inoculation (1). In this experimental model, the ipsilateral retina is protected by T cells and NK cells (2, 26, 54). With findings similar to ours, another group reported the presence of an HSV-1 ribonucleotide reductase mutant in the retina following the application of the virus onto the scarified cornea of a mouse (49). Although there was no indication by what mechanism the virus applied to the scarified cornea spread to the retina, the present study found CD11b+ and CD11b− cells within the vitreous to harbor infectious virus. In addition to inflammatory cells, another conduit by which the virus reaches the retina may be the ciliary nerve, which was found to stain positive for HSV-1 antigen in mice infected 6 days earlier. In humans, the ciliary ganglia has also been found to possess HSV-1 DNA, implicating this tissue in addition to the more traditional TG as a site of HSV-1 latency (7). The consequence of HSV-1 in the retina of mice was not the subject of the present study. Unfortunately, the majority of HSV-1-naive mice succumb to the infection. However, in two out of four mice inspected at day 13 p.i., there was massive cellular infiltrate in the retina, with destruction of the photoreceptor layer colocalizing with HSV-1 antigen (data not shown). Consequently, future work is warranted to address the mechanisms by which HSV-1 spreads to the retina as well as to characterize the local immune response to the infection in the hopes of identifying those pathways manifesting tissue pathology.
Acknowledgments
We thank Paula Pierce for her excellent immunohistochemical skills.
This work was supported by U.S.P.H.S. NIH grants AI053108 (D.J.J.C.) and NS41249 (T.E.L.), a Jules and Doris Stein RPB Research Professorship (D.J.J.C.), an RPB Lew R. Wasserman Merit Award (J.C.), Presbyterian Health Foundation (D.J.J.C.), an unrestricted grant from Research to Prevent Blindness, NIH COBRE-P20 RR017703 (J.A.), NEI EY14206-01 (J.A.), State of Oklahoma OCAST-HR02140RS (J.A.), and NEI core grant P30 EY12190.
REFERENCES
- 1.Atherton, S. S., and J. W. Streilein. 1987. Two waves of virus following anterior chamber inoculation of HSV-1. Investig. Ophthalmol. Vis. Sci. 28:571-579. [PubMed] [Google Scholar]
- 2.Azumi, A., and S. Atherton. 1994. Sparing of the ipsilateral retina after anterior chamber inoculation of HSV-1: requirement for either CD4+ or CD8+ T cells. Investig. Ophthalmol. Vis. Sci. 35:3251-3259. [PubMed] [Google Scholar]
- 3.Biddison, W. E., W. W. Cruikshank, D. M. Center, C. M. Pelfrey, D. D. Taub, and R. V. Turner. 1998. CD8+ myelin peptide-specific T cells can chemoattract CD4+ myelin peptide-specific T cells: importance of IFN-inducible protein 10. J. Immunol. 160:444-448. [PubMed] [Google Scholar]
- 4.Bouley, D. M., S. Kanangat, W. Wire, and B. T. Rouse. 1995. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-γ knockout mice. J. Immunol. 155:3964-3971. [PubMed] [Google Scholar]
- 5.Boztug, K., M. J. Carson, N. Pham-Mitchell, V. C. Asensio, J. DeMartino, and I. L. Campbell. 2002. Leukocyte infiltration, but not neurodegeneration, in the CNS of transgenic mice with astrocyte production of the CXC chemokine ligand 10. J. Immunol. 169:1505-1515. [DOI] [PubMed] [Google Scholar]
- 6.Brissette-Storkus, C. S., S. M. Reynolds, A. J. Lepisto, and R. L. Hendricks. 2002. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 43:2264-2271. [PMC free article] [PubMed] [Google Scholar]
- 7.Bustos, D. E., and S. S. Atherton. 2002. Detection of herpes simplex virus type 1 in human ciliary ganglia. Investig. Ophthalmol. Vis. Sci. 43:2244-2249. [PubMed] [Google Scholar]
- 8.Cantin, E. M., D. R. Hinton, J. Chen, and H. Openshaw. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantin, E., B. Tanamachi, and H. Openshaw. 1999. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J. Virol. 73:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr, D. J. J., J. Ash, K. Al-khatib, and I. L. Campbell. 2002. Unforeseen consequences of IL-12 expression in the eye of GFAP-IL12 transgenic mice following herpes simplex virus type 1 infection. DNA Cell Biol. 21:467-474. [DOI] [PubMed] [Google Scholar]
- 11.Carr, D. J. J., and S. Noisakran. 2002. The antiviral efficacy of the murine alpha-1 interferon transgene against ocular herpes simplex virus type 1 requires the presence of CD4+, α/β T-cell receptor-positive T lymphocytes with the capacity to produce gamma interferon. J. Virol. 76:9398-9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, H., and R. L. Hendricks. 1998. B7 costimulatory requirements of T cells at an inflammatory site. J. Immunol. 160:5045-5052. [PubMed] [Google Scholar]
- 13.Daheshia, M., S. Kanangat, and B. T. Rouse. 1998. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp. Eye Res. 67:619-624. [DOI] [PubMed] [Google Scholar]
- 14.Dennis, R. F., K. F. Siemasko, Q. Tang, R. L. Hendricks, and A. Finnegan. 1995. Involvement of LFA-1 and ICAM-1 in the herpetic disease resulting from HSV-1 corneal infection. Curr. Eye Res. 14:55-62. [DOI] [PubMed] [Google Scholar]
- 15.Diab, A., H. Abdalla, H. L. Li, F. D. Shi, J. Zhu, B. Hojberg, L. Lindquist, B. Wretlind, M. Bakhiet, and H. Link. 1999. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1α attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect. Immun. 67:2590-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 17.Duker, J. S., J. C. Nielsen, R. C. Eagle, Jr., T. M. Bosley, R. Granadier, and W. E. Benson. 1990. Rapidly progressive acute retinal necrosis syndrome secondary to herpes simplex virus type 1. Ophthalmology 97:1638-1643. [DOI] [PubMed] [Google Scholar]
- 18.Ellis, T. N., and B. L. Beaman. 2002. Murine polymorphonuclear neutrophils produce interferon-gamma in response to pulmonary infection with Nocardia asteroides. J. Leukoc. Biol. 72:373-381. [PubMed] [Google Scholar]
- 19.Engler, H., R. Zawatzky, A. Goldbach, C. H. Schroder, C. Weyand, G. J. Hammerling, and H. Kirchner. 1981. Experimental infection of inbred mice with herpes simplex virus. II. Interferon production and activation of natural killer cells in the peritoneal exudate. J. Gen. Virol. 55:25-30. [DOI] [PubMed] [Google Scholar]
- 20.Farber, J. M. 1997. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol. 61:246-257. [PubMed] [Google Scholar]
- 21.Fenton, R. R., S. Molesworth-Kenyon, J. E. Oakes, and R. N. Lausch. 2001. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Investig. Ophthalmol. Vis. Sci. 43:737-743. [PubMed] [Google Scholar]
- 22.Halford, W. P., B. M. Gebhardt, and D. J. J. Carr. 1996. Persistent cytokine expression in the trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 157:3542-3549. [PubMed] [Google Scholar]
- 23.Härle, P., V. Cull, M.-P. Agbaga, R. Silverman, B. R. G. Williams, C. James, and D. J. J. Carr. 2002. Differential effect of murine alpha/beta interferon transgenes on antagonization of herpes simplex virus type 1 replication. J. Virol. 76:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendricks, R. L., M. Janowicz, and T. M. Tumpey. 1992. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immuopathology in herpes simplex virus-1-infected mouse corneas. J. Immunol. 148:2522-2529. [PubMed] [Google Scholar]
- 25.Hendricks, R. L., T. M. Tumpey, and A. Finnegan. 1992. IFN-γ and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J. Immunol. 149:3023-3028. [PubMed] [Google Scholar]
- 26.Igietseme, J. U., J. W. Streilein, F. Miranda, S. J. Feinerman, and S. S. Atherton. 1991. Mechanisms of protection against herpes simplex virus type 1-induced retinal necrosis by in vitro-activated T lymphocytes. J. Virol. 65:763-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan, I., J. A. MacLean, F. Lee, L. Casciotti, E. DeHaan, J. Schwartzman, and A. D. Luster. 2000. The IP-10 chemokine is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity 12:483-494. [DOI] [PubMed] [Google Scholar]
- 28.Kolb, S. A., B. Sporer, F. Lahrtz, U. Koedel, H.-W. Pfister, and A. Fontana. 1999. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1 infected individuals as interferon-γ inducible protein 10. J. Neuroimmunol. 93:172-181. [DOI] [PubMed] [Google Scholar]
- 29.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 30.Liu, M. T., H. S. Keirstead, and T. E. Lane. 2001. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J. Immunol. 167:4091-4097. [DOI] [PubMed] [Google Scholar]
- 31.Loetscher, M., B. Gerber, P. Loetscher, S. A. Jones, L. Piali, I. C. Lewis, M. Baggiolini, and B. Moser. 1996. Chemokine receptor specific for IP-10 and Mig: structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 184:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludwig, I. H., H. Zegarra, and Z. N. Zakov. 1984. The acute retinal necrosis syndrome. Possible herpes simplex retinitis. Ophthalmology 91:1659-1664. [DOI] [PubMed] [Google Scholar]
- 33.Mahalingam, S., J. M. Farber, and G. Karupiah. 1999. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity in vivo. J. Virol. 73:1479-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalec, L., B. K. Choudhury, E. Postlewait, J. S. Wild, R. Alam, M. Lett-Brown, and S. Sur. 2002. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J. Immunol. 168:846-852. [DOI] [PubMed] [Google Scholar]
- 35.Mikloska, Z., V. A. Danis, S. Adams, A. R. Lloyd, D. L. Adrian, and A. L. Cunningham. 1997. In vivo production of cytokines and β (C-C) chemokines in human recurrent herpes simplex lesions—do herpes simplex virus-infected keratinocytes contribute to their production? J. Infect. Dis. 177:827-838. [DOI] [PubMed] [Google Scholar]
- 36.Moore, J. E., T. C. McMullen, I. L. Campbell, R. Rohan, Y. Kaji, N. A. Afshari, T. Usui, D. B. Archer, and A. P. Adamis. 2002. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Investig. Ophthalmol. Vis. Sci. 43:2905-2915. [PubMed] [Google Scholar]
- 37.Niemialtowski, M. G., and B. T. Rouse. 1992. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J. Immunol. 148:1864-1870. [PubMed] [Google Scholar]
- 38.Noisakran, S., and D. J. J. Carr. 2000. Plasmid DNA encoding IFN-α1 antagonizes herpes simplex virus type 1 ocular infection through CD4+ and CD8+ T lymphocytes. J. Immunol. 164:6435-6443. [DOI] [PubMed] [Google Scholar]
- 39.Piali, L., C. Weber, G. LaRosa, C. R. Mackay, T. A. Springer, I. Clark-Lewis, and B. Moser. 1998. The chemokine receptor CXCR3 mediates rapid and shearing resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur. J. Immunol. 28:961-972. [DOI] [PubMed] [Google Scholar]
- 40.Qin, S., J. B. Rottman, P. Myers, N. Kassam, M. Weinblatt, M. Leotscher, A. E. Koch, B. Moser, and C. R. Mackay. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Investig. 101:746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 42.Russell, R. G., M. P. Nasisse, H. S. Larsen, and B. T. Rouse. 1984. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Investig. Ophthalmol. Vis. Sci. 25:938-944. [PubMed] [Google Scholar]
- 43.Schlaeger, T. M., S. Bartunkova, J. A. Lawitts, G. Teichmann, W. Risau, U. Deutsch, and T. N. Sato. 1997. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. USA 94:3058-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo, S. K., B. M. Gebhardt, H. Y. Lim, S. W. Kang, S. Higaki, E. D. Varnell, J. M. Hill, H. E. Kaufman, and B. S. Kwon. 2001. Murine keratocytes function as antigen-presenting cells. Eur. J. Immunol. 31:3318-3328. [DOI] [PubMed] [Google Scholar]
- 45.Sgadari, C. A., L. Angiolillo, and G. Tosato. 1996. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 87:3877-3882. [PubMed] [Google Scholar]
- 46.Shimeld, C., S. Efstathiou, and T. Hill. 2001. Tracking the spread of a lacZ-tagged herpes simplex virus type 1 between the eye and the nervous system of the mouse: comparison of primary and recurrent infection. J. Virol. 75:5252-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson, J. E., J. Newcombe, M. L. Cuzner, and M. N. Woodroofe. 2000. Expression of the interferon-γ-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathol. Appl. Neurobiol. 26:133-142. [DOI] [PubMed] [Google Scholar]
- 48.Soejima, K., and B. J. Rollins. 2001. A functional IFN-γ-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J. Immunol. 167:6576-6582. [DOI] [PubMed] [Google Scholar]
- 49.Spencer, B., S. Agarwala, M. Miskulin, M. Smith, and C. R. Brandt. 2000. Herpes simplex virus-mediated gene delivery to the rodent visual system. Investig. Ophthalmol. Vis. Sci. 41:1392-1401. [PubMed] [Google Scholar]
- 50.Su, Y.-S., X.-T. Yan, J. E. Oakes, and R. N. Lausch. 1996. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J. Virol. 70:1277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Summers, B. C., T. P. Margolis, and D. A. Leib. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J. Virol. 75:5069-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang, Q., W. Chen, and R. L. Hendricks. 1997. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J. Immunol. 158:1275-1283. [PubMed] [Google Scholar]
- 53.Tang, Q., and R. L. Hendricks. 1996. Interferon γ regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J. Exp. Med. 184:1435-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanigawa, M., J. E. Bigger, M. Y. Kanter, and S. S. Atherton. 2000. Natural killer cells prevent direct anterior-to-posterior spread of herpes simplex virus type 1 in the eye. Investig. Ophthalmol. Vis. Sci. 41:132-137. [PubMed] [Google Scholar]
- 55.Taub, D. D., A. R. Lloyd, K. Conlon, J. M. Wang, J. R. Ortaldo, A. Harada, K. Matsushima, D. J. Kelvin, and J. J. Oppenheim. 1993. Recombinant human-interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J. Exp. Med. 177:1809-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas, J., S. Gangappa, S. Kanangat, and B. T. Rouse. 1997. On the essential involvement of neutrophils in the immunopathological disease. Herpetic stromal keratitis. J. Immunol. 158:1383-1391. [PubMed] [Google Scholar]
- 57.Tumpey, T. M., S.-H. Chen, J. E. Oakes, and R. N. Lausch. 1996. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 70:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tumpey, T. M., H. Cheng, D. N. Cook, O. Smithies, J. E. Oakes, and R. N. Lausch. 1998. Absence of macrophage inflammatory protein-1α prevents the development of blinding herpes stromal keratitis. J. Virol. 72:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tumpey, T. M., H. Cheng, X.-T. Yan, J. E. Oakes, and R. N. Lausch. 1998. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J. Leukoc. Biol. 63:486-492. [DOI] [PubMed] [Google Scholar]
- 60.Xia, M. Q., B. J. Baeski, R. B. Knowles, S. X. Qin, and B. T. Hyman. 2000. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. J. Neuroimmunol. 108:227-235. [DOI] [PubMed] [Google Scholar]
- 61.Zheng, M., S. Deshpande, S. Lee, N. Ferrara, and B. T. Rouse. 2001. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 75:9828-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng, M., D. M. Klinman, M. Gierynska, and B. T. Rouse. 2002. DNA containing CpG motifs induces angiogenesis. Proc. Natl. Acad. Sci. USA 99:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]