Abstract
Maleimides are often used for biomolecular conjugation with thiols. An underappreciated aspect of the imido group in a maleimide conjugate is its susceptibility to spontaneous hydrolysis, resulting in undesirable heterogeneity. Here, a chromophoric maleimide is used to demonstrate that both molybdate and chromate catalyze the hydrolysis of an imido group near neutral pH. Tungstate and 4-(dimethylamino)pyridine are less effective as catalysts. This work reveals a new mode of chemical reactivity for molybdate and chromate, and provides a strategy for decreasing the heterogeneity of bioconjugates derived from maleimides.
Chemoselective bioconjugation has revolutionized functional genomics, proteomics, and glycomics1. Reactions, such as the Staudinger ligation,2 the Huisgen 1,3-dipolar cycloaddition,3 carbonyl group condensation reactions,4 and maleimide conjugation5 enable the site-specific labeling of biomolecules, and their uniform immobilization to generate microarrays.
Bioconjugation via maleimides is especially common. This reaction involves the Michael addition of a biomolecular thiolate, often from the side chain of a cysteine residue, to a maleimide to form a succinimidyl thioether.6, 7 The reaction proceeds rapidly and in high yield in neutral aqueous solutions at room temperature, making it ideal for biological applications.
An underappreciated aspect of maleimides and succinimides is the tendency of their imido groups to undergo spontaneous hydrolysis.8, 9 As water can attack either of the two carbons of the imido group, hydrolysis of a succinimidyl thioether produces isomeric succinamic acid thioethers. Accordingly, a thiol-conjugated maleimide actually consists of a mixture of many species, some having an additional carboxylic acid that can ionize near neutral pH. As hydrolysis proceeds over time, the concentrations of these species change, introducing yet another complexity.
The heterogeneity of succinimidyl thioethers can alter the activity of bioconjugates and convolute the interpretation of experimental data. For example, an N-(1-pyrene)maleimide-conjugate of α,α-tropomyosin was observed to hydrolyze in aqueous solution, causing a time-dependent increase in fluorescence.10 The cellular internalization of some peptides and proteins relies on their cationic charge,11 and the presence of a hydrolyzed succinimidyl thioether could complicate data analysis by reducing that charge. Surface-based assays can be especially sensitive to heterogeneity within the immobilized species. For example, the orientation of liquid crystals near a surface is likely to highly sensitive to non-uniformity.12 Heterogeneity can also affect data analysis in experiments that are independent of molecular charge. For example, assays based on mass spectrometry can be confounded by the additional 18 amu introduced upon hydrolysis of a succinimidyl thioether.13
We reasoned that the heterogeneity of succinimidyl thioethers could be reduced by intentionally increasing their rate of hydrolysis. The hydrolysis of maleimides and succinimides and its catalysis has been studied previously. In 1955, Gregory reported that the rate constant for maleimide hydrolysis is considerably less than that for its reaction with a thiol.7 Subsequently, the hydrolysis of both maleimides and succinimides was found to be catalyzed by base.14 In general, however, bioconjugates are not stable at elevated pH.
Here, we report on catalysts of imido group hydrolysis that are effective near neutral pH. As a model system that enables facile quantitation of the rate of imido group hydrolysis, we chose a maleimide that is activated for hydrolysis and that undergoes a spectral change upon hydrolysis: N-(p-nitrophenyl)maleimide (1). We synthesized a model succinimidyl thioether by reacting maleimide 1 with ethanethiol to give succinimide 2, as shown in Scheme 1.15 This conjugate has an absorbance maximum at 271 nm before hydrolysis that shifts to 319 nm after hydrolysis, enabling a continuous spectrophotometric assay of hydrolysis.
Scheme 1.
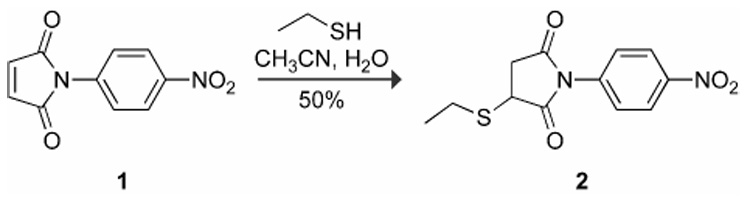
Route for the synthesis of succinimide 2.
The molybdate anion is known to act as a nucleophilic catalyst for the hydrolysis of oxoesters,16, 17 thioesters,16 phosphoesters,18 2,4-dinitrofluorobenzene,17 and methyl iodide.17 The nucleophilicity of molybdate is remarkable, given its low basicity (pKa 4.1).19 To determine if molybdate could also act as a catalyst of imido-group hydrolysis, we incubated succinimide 2 with varying concentrations of Na2MoO4 in 0.50 M HEPES–NaOH buffer at pH 7.5, containing NaCl (0.10 M).20 The resulting kinetic traces were indicative of substantial catalysis (Figure 1A). Similar kinetic traces were obtained with maleimide 1. First-order rate constants were derived from exponential fits of the kinetic traces obtained with different concentrations of molybdate, and were plotted versus the molybdate concentration (Figure 1B). The ensuing second-order rate constant for the hydrolysis of maleimide 1 (k2 = 2.5 M−1 min−1) was 9-fold greater than that for succinimide 2 (k2 = 0.28 M−1 min−1).
Figure 1.
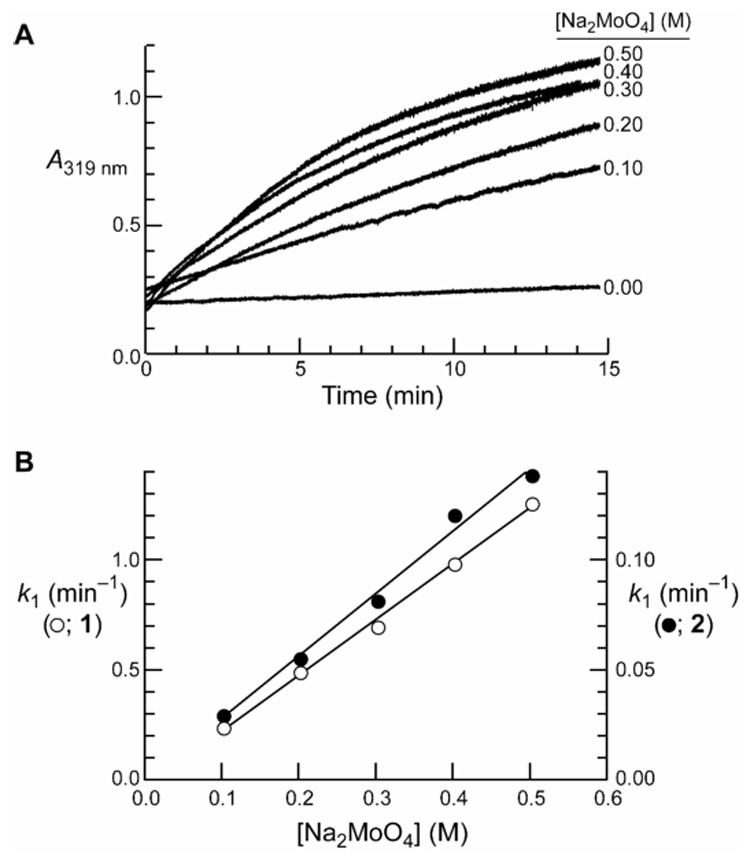
Molybdate-catalyzed hydrolysis of imido groups. (A) Raw data for catalysis of the hydrolysis of succinimide 2. (B) Transformed data for the hydrolysis of maleimide 1 (k2 = 2.5 M−1 min−1) and succinimide 2 (k2 = 0.28 M−1 min−1).
Kinetic analyses were also performed with other group VI oxometallates, namely, those of chromium and tungsten. Chromate has a high absorbance at 271 and 319 nm, obviating the use of the spectrophotometric assay. To circumvent this obstacle, an HPLC-based assay was developed to monitor catalysis by chromate.21 An aqueous solution of succinimide 2 was incubated with Na2CrO4 (0.10 M) for 2 h. The reaction was quenched by extraction, and analyzed by using analytical HPLC and monitoring at 271 nm. Unhydrolyzed succinimide 2 elutes at ~45 min (Figure 2); hydrolyzed succinimide 2 elutes in two closely spaced peaks at ~40 min and was characterized by performing hydrolysis on a larger scale and isolating the hydrolysis products by semi-preparative HPLC.22 A solution of Na2SO4 (0.10 M) was used to report on the uncatalyzed rate of hydrolysis. The data indicated that chromate is a comparable catalyst to molybdate, whereas tungstate is a poor catalyst (Figure 2).
Figure 2.
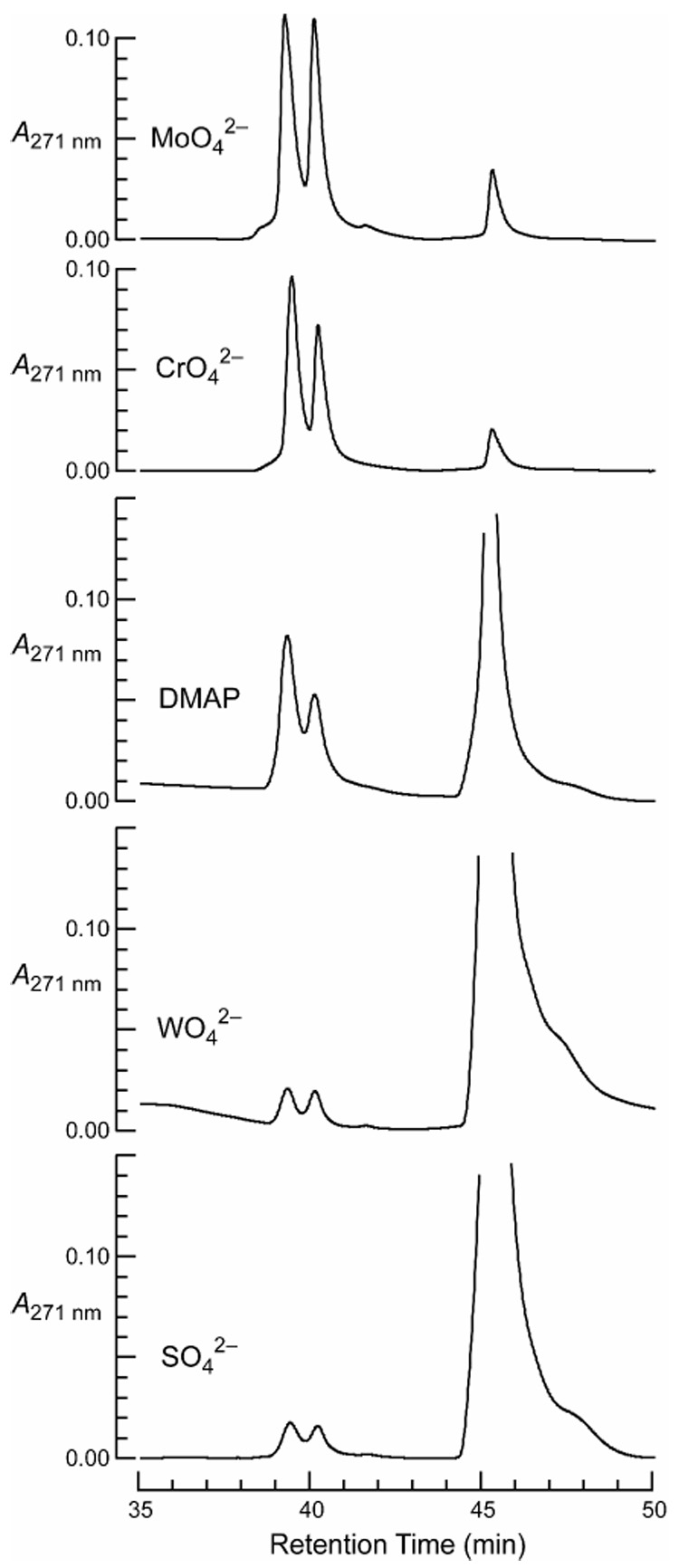
HPLC analysis of succinimide 2 after incubation for 2 h at room temperature with a putative catalyst (0.10 M) of hydrolysis. The twin peaks at ~40 min correspond to the hydrolysis products; the peak at ~45 min corresponds to intact succinimide 2.
4-(Dimethylamino)pyridine (DMAP), a common catalyst for acylation reactions,23 accelerated the hydrolysis to a significantly lesser extent than did molybdate or chromate (Figure 2). To compare catalysis by molybdate and chromate directly, succinimide 2 was incubated with molybdate or chromate (0.10 M) for different time periods, and its hydrolysis was quantitated by peak integration of HPLC traces. The results indicate that the apparent first-order rate constant in the presence of molybdate was 1.3-fold higher than that in the presence of chromate (Figure 3).
Figure 3.
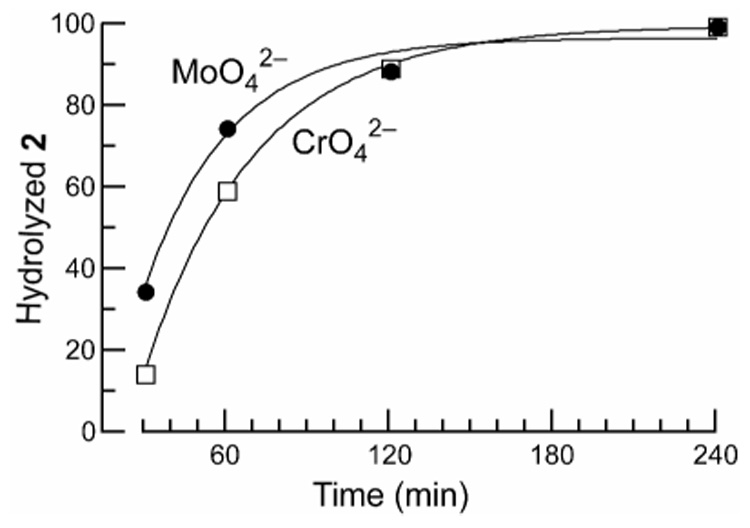
Comparison of molybdate and chromate as catalysts for the hydrolysis of succinimide 2. Assays were performed by HPLC, as shown in Figure 2. Data were fitted to the equation: [hydrolyzed 2]t = [hydrolyzed 2]t=∞(1 − e−kt), where k is the apparent first-order rate constant.
The mechanism of molybdate-catalyzed ester hydrolysis has been proposed to proceed via the nucleophilic attack of a molybdate oxygen on the ester carbon, followed by the spontaneous hydrolysis of the resulting acid molybdate intermediate.16, 17 An analogous mechanism for the molybdate- and chromate-catalyzed hydrolysis of an imido group is shown in Scheme 2. Why are these two group VI oxometallates effective catalysts? To search for a correlation between reactivity and basicity, Um and coworkers subjected the reaction of p-nitrophenylacetate with group VI oxometallates to a Brϕnsted analysis.18 Although tungstate conformed to the expected trend, molybdate and chromate showed significant positive deviations. The anomalous nucleophilicity of molybdate has been attributed to its poor solvation.16 In addition, the high reactivity of molybdate with oxoesters and thioesters has been attributed to Lewis acid catalysis via formation of a transient Mo–O bond with the nonbridging ester oxygen in a tetrahedral intermediate.16, 17
Scheme 2.
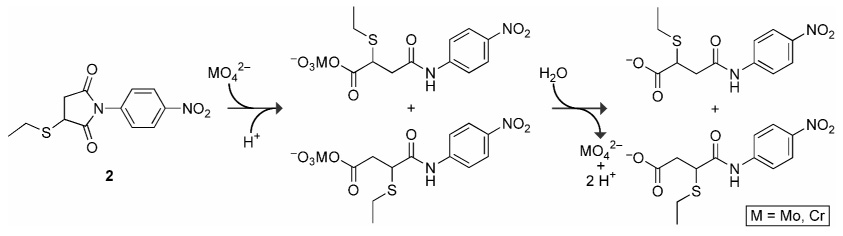
Putative mechanism for the molybdate- and chromate-catalyzed hydrolysis of succinimide 2.
In conclusion, we have discovered that molybdate and chromate catalyze the hydrolysis of the imido group of maleimide conjugates. This discovery adds to the unexpected nucleophilic reactivity of molybdate and chromate, and provides a potential strategy for hydrolyzing the unactivated succinimidyl thioethers found in bioconjugation reactions.1 These catalysts contain but five atoms, and are readily separable from bioconjugates by size-exclusion chromatography, gel-filtration chromatography, or dialysis. Although chromate reacts with thiols to form chromium(VI) thioesters,24 maleimide conjugates would presumably be devoid of thiol groups. Finally, we anticipate that our work will increase awareness of the heterogeneity of maleimide conjugates and inspire means to diminish that heterogeneity.
Acknowledgments
We are grateful to Dr. L. E. Strong for contributive discussions. This work was supported by grant GM44783 (NIH) and the Materials Research Science and Engineering Center at the University of Wisconsin–Madison (NSF DMR-0520527).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Aslam M, Dent A, editors. Bioconjugation: Protein Coupling Techniques for the Biomedical Sciences. London: Macmillan Reference Ltd.; 1998. [Google Scholar]; (b) Lundblad RL. Chemical Reagents for Protein Modification. 3rd ed. Boca Raton, FL: CRC Press; 2005. [Google Scholar]; (c) Prescher JA, Bertozzi CR. Nat. Chem. Biol. 2005;1:13. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 2.(a) Köhn M, Breinbauer R. Angew. Chem. Int. Ed. Engl. 2004;43:3106. doi: 10.1002/anie.200401744. [DOI] [PubMed] [Google Scholar]; (b) Nilsson BL, Soellner MB, Raines RT. Annu. Rev. Biophys. Biomolec. Struct. 2005;34:91. doi: 10.1146/annurev.biophys.34.040204.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kalia J, Abbott NL, Raines RT. Bioconjug. Chem. 2007;18:1064. doi: 10.1021/bc0603034. [DOI] [PubMed] [Google Scholar]
- 3.(a) Huisgen R. Angew. Chem. Int. Ed. Engl. 1963;2:565. [Google Scholar]; (b) Kolb HC, Sharpless KB. Drug Discov. Today. 2003;15:1128. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]; (c) Kalia J, Raines RT. Chembiochem. 2006;7:1375. doi: 10.1002/cbic.200600150. [DOI] [PubMed] [Google Scholar]
- 4.(a) Shao J, Tam JP. J. Am. Chem. Soc. 1995;117:3893. [Google Scholar]; (b) Grandjean C, Rommens C, Gras-Masse H, Melnyk O. Angew. Chem. Int. Ed. 2000;39:1068. [PubMed] [Google Scholar]; (c) Zatsepin TS, Stetsenko DA, Gait MJ, Oretskaya TS. Bioconjug. Chem. 2005;16:471. doi: 10.1021/bc049712v. [DOI] [PubMed] [Google Scholar]; (d) Tully SE, Rawat M, Hsieh-Wilson LC. J. Am. Chem. Soc. 2006;128:7740. doi: 10.1021/ja061906t. [DOI] [PubMed] [Google Scholar]
- 5.(a) Houseman BT, Gawalt ES, Mrksich M. Langmuir. 2002;19:1522. [Google Scholar]; (b) Lavis LD, Chao TY, Raines RT. ACS Chem. Biol. 2006;1:252. doi: 10.1021/cb600132m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Watzke A, Gutierrez-Rodriguez M, Kohn M, Wacker R, Schroeder H, Breinbauer R, Kuhlmann J, Alexandrov K, Niemeyer CM, Goody RS, Waldmann H. Bioorg. Med. Chem. 2006;14:6288. doi: 10.1016/j.bmc.2006.05.006. [DOI] [PubMed] [Google Scholar]; (d) Slavica A, Dib I, Nidetzky B. Biotechnol. Bioeng. 2007;96:9. doi: 10.1002/bit.21181. [DOI] [PubMed] [Google Scholar]
- 6.(a) Friedmann E. Biochim. et Biophys. Acta. 1952;9:65. doi: 10.1016/0006-3002(52)90121-2. [DOI] [PubMed] [Google Scholar]; (b) Bednar RA. Biochemistry. 1990;29:3684. doi: 10.1021/bi00467a014. [DOI] [PubMed] [Google Scholar]
- 7.Gregory JD. J. Am. Chem. Soc. 1955;77:3922. [Google Scholar]
- 8.Nektar Therapeutics AL, Corp. 2004/06095. World Patent WO. 2004
- 9.Succinimides that form in polypeptide chains by the cyclization of asparagine residues are known to undergo spontaneous hydrolysis to form isoasparte residues. For a review, see:Clark S. Ageing Res. Rev. 2003;2:263–285. doi: 10.1016/s1568-1637(03)00011-4.
- 10.Ishii Y, Lehrer SS. Biophys. J. 1986;50:75. doi: 10.1016/S0006-3495(86)83440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Fuchs SM, Raines RT. Cell. Mol. Life Sci. 2006;63:1819. doi: 10.1007/s00018-006-6170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Johnson RJ, Chao T-Y, Lavis LD, Raines RT. Biochemistry. 2007;46 doi: 10.1021/bi700857u. xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luk Y-Y, Tingey ML, Dickson KA, Raines RT, Abbott NL. J. Am. Chem. Soc. 2004;126:9024. doi: 10.1021/ja0398565. [DOI] [PubMed] [Google Scholar]
- 13.Rutkoski TJ, Raines RT, Underbakke ES, Kiessling LL. unpublished results. personal communication.
- 14.(a) Niyaz Khan N, Aziz Khan A. J. Org. Chem. 1975;140:1793. [Google Scholar]; (b) Matsui S, Aida H. J. Chem. Soc. Perkin II. 1978:1277. [Google Scholar]
- 15.N-(p-Nitrophenyl)maleimide (2.0 g, 9.17 mmol) was dissolved in acetonitrile (100 mL) and water (20 mL). Ethanethiol (5.5 mL, 74.25 mmol) was added, and the resulting solution was stirred for 2.5 h. Solvent and unreacted ethanethiol were removed under reduced pressure, and the residue was purified by flash chromatography (silica gel, 60% v/v hexanes in ethyl acetate) to give the desired product as an off-white solid (1.29 g, 50%). ¹H NMR (400 MHz, CD3CN) δ = 8.35 (d, J = 9.2 Hz, 2H), 7.60 (d, J = 9.2 Hz, 2H), 4.05 (dd, J = 9.3, 4.3 Hz, 1H), 3.37 (dd, J = 18.7, 9.3 Hz, 1H), 2.87 (m, 2H), 2.72 (dd, J = 18.7, 4.6 Hz, 1H), 1.32 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ = 174.8, 173.0, 147.2, 137.3, 127.0, 124.5, 38.9, 36.2, 26.4, 14.2; anal. calcd. for C12H12N2SO4: C 51.41, H 4.32, N 10.00, S 11.44, O 22.83; found: C 51.76, H 4.26, N 9.96, S 11.81, O 22.21.
- 16.Wikjord B, Byers LD. J. Am. Chem. Soc. 1992;114:5553. [Google Scholar]
- 17.Wikjord B, Byers LD. J. Org. Chem. 1992;57:6814. [Google Scholar]
- 18.Ahn B-T, Park H-S, Lee E-J, Um I-H. Bull. Korean Chem. Soc. 2000;21:905. [Google Scholar]
- 19.Sasaki Y, Lindqvist I, Sillen LG. J. Inorg. Nuc. Chem. 1959;9:93. [Google Scholar]
- 20.Putative catalysts were dissolved in 0.50 M HEPES–NaOH buffer at pH 7.5 containing NaCl (0.10 M). Na2SO4 was added to maintain a constant ionic strength of I = 1.6. The catalyst solution (157 µL) was added to a cuvette, and the absorbance at 319 nm was adjusted to zero. Hydrolysis was initiated by adding maleimide 1 or succinimide 2 (3 µL of a 1.65 mM solution in acetonitrile) to the cuvette, and the absorbance at 319 nm was monitored with time. Kinetic traces were fitted to the equation: (A319 nm)t = (A319 nm)t=∞(1 − e−kt) to give the rate constant, k. The uncatalyzed rate constant for hydrolysis was subtracted from the catalyzed rate constant to yield the first-order rate constant, k1. The k1 values were plotted versus catalyst concentration, and the data were fitted to the equation: k1 = k2[catalyst], where k2 is the second-order rate constant.
- 21.Succinimide 2 (382 µL of a 1.65 mM solution in acetonitrile) was added to a solution (20 mL) of 50 mM HEPES–NaOH buffer at pH 7.5 containing catalyst (0.10 M) and NaCl (0.10 M). The reaction mixture was stirred for 2 h, and extracted with ethyl acetate (2 × 20 mL). The organic layer was concentrated under reduced pressure, and the residue was analyzed by C18 HPLC using a linear gradient of water (80–20% v/v) in acetonitrile containing TFA (0.1% v/v) over 50 min. Hydrolyzed succinimide 2 eluted in two peaks at 40 min; intact succinimide 2 eluted at 45 min. To compare molybdate and chromate as catalysts, reactions were performed on 90-mL scale, and 20-mL aliquots were removed at four time points and subjected to HPLC analysis.
- 22.Characterization data for hydrolyzed succininimide 2: ¹H NMR (400 MHz, CD3CN, 2 isomers) δ = 9.1 and 8.9 (s, 1H), 8.2 (m, 2H), 7.8 (m, 2H), 3.7 (m, 1H), 3.11–2.96 (m, 1H), 2.78–2.62 (m, 3H), 1.2 (m, 3H); ESI (M+Na)+ calcd. for C12H14SN2O5, 321.06; found, 321.00.
- 23.Fu GC. Acc. Chem. Res. 2004;37:542. doi: 10.1021/ar030051b. [DOI] [PubMed] [Google Scholar]
- 24.(a) Connett PH, Wetterhahn KE. J. Am. Chem. Soc. 1985;107:4282. [Google Scholar]; (b) Levina A, Lay PA. Inorg. Chem. 2004;43:324. doi: 10.1021/ic034901v. [DOI] [PubMed] [Google Scholar]


