Abstract
Interleukin-18 (IL-18) is a proinflammatory cytokine that promotes natural killer (NK) and T-cell activation. Several poxviruses, including vaccinia virus (VV), encode a soluble IL-18-binding protein (IL-18bp). The role of the VV IL-18bp (gene C12L) in vivo was studied with wild-type (vC12L), deletion mutant (vΔC12L), and revertant (vC12L-rev) viruses in a murine intranasal model of infection. The data show that vΔC12L was markedly attenuated, characterized by a mild weight loss and reduced virus titers in lungs, brain, and spleen. Three days after infection, NK cytotoxic activity was augmented in the lung, spleen, and mediastinal lymph nodes (MLNs) of vΔC12L-infected mice compared to controls. Seven days after infection, vΔC12L-infected mice displayed heightened VV-specific cytotoxic T-lymphocyte (CTL) responses in the lungs, spleen, and MLNs. Gamma interferon (IFN-γ) levels were also dramatically elevated in lavage fluids and cells from lungs of mice infected with vΔC12L. Finally, we demonstrate that IL-18 is produced in vitro and in vivo after VV infection. Taken together, these data demonstrate a role for the vIL-18bp in counteracting IL-18 in both the innate and the specific immune response to VV infection and indicate that the ability of IL-18 to promote vigorous T-cell responses (cytotoxic activity and IFN-γ production) is a critical factor in the accelerated clearance of the vΔC12L mutant.
Interleukin-18 (IL-18), originally designated gamma interferon (IFN-γ)-inducing factor, has important roles in the regulation of both innate and specific immune responses. IL-18 mediates its activity via the induction of IFN-γ production in natural killer (NK) and T cells and the stimulation of lytic activity of NK cells, T-cell proliferation, and upregulation of Fas ligand by NK cells (reviewed in references 15 and 39). Furthermore, IL-12 and IL-18 act synergistically to promote Th1-mediated immune responses, which play a critical role in defense against intracellular microbes through the production of IFN-γ.
IL-18 is related structurally to IL-1 and is produced as an inactive precursor, which is processed and activated by caspase 1 (IL-1β-converting enzyme) (20, 21). IL-18 is produced by activated macrophages/monocytes and binds to the IL-18 receptor (IL-18R), which is composed of two subunits: IL-18Rα is the ligand-binding receptor chain, and IL-18Rβ is involved in signal transduction. The extracellular regions of IL-18Rα consist of three immunoglobulin (Ig)-like domains and shares amino acid similarity with the IL-1R family (reviewed in reference 49). Recently, soluble proteins from humans and mice have been described that can bind and neutralize IL-18 (40) and may play an important regulatory role. Surprisingly, these IL-18-binding proteins (IL-18bps) are unrelated to either subunit of the IL-18R.
IL-18 is important in protective immunity against viral infections. Depletion of IL-18 by antibody or in IL-18−/− mice demonstrated a role in protection against herpes simplex virus type 2 (HSV-2) infection (22) and in modulation of the IFN-γ response to infection with adenovirus (62), murine cytomegalovirus (CMV) (41), and influenza virus (36). Administration of IL-18 also protected mice from acute infection with HSV-1 (19) and encephalomyocarditis virus (26, 57). Furthermore, treatment with IL-18 suppressed pock formation on the tails of mice inoculated intravenously with vaccinia virus (VV) and augmented NK and cytotoxic T-lymphocyte (CTL) activity (56).
The importance of IL-18 in virus infections is underscored by the finding that poxviruses and papillomaviruses suppress IL-18 production or activity. Papillomavirus oncoproteins E6 and E7 reduce IL-18-induced IFN-γ production in primary human peripheral blood mononuclear cells (PBMCs) and the NK0 cell line (32). Poxviruses secrete a soluble IL-18bp (see below), inhibit signaling via the IL-18R (9), and encode an inhibitor of caspase 1 (30) that cleaves pro-IL-1β and pro-IL-18 to IL-1β and IL-18, respectively. Functional poxvirus IL-18bps (vIL-18bps) related to mammalian IL-18bps are encoded by molluscum contagiosum virus (MC54L) (61), ectromelia virus (EV), cowpox virus, and VV (8, 51). Other poxviruses, including variola virus (35), Yaba-like disease virus (31), monkeypox virus (48), and swinepox virus (1), are predicted to encode vIL-18bps. Deletion of the IL-18bp (gene C12L) from VV strain Western Reserve (WR) caused virus attenuation (54), and deletion of the corresponding gene from EV was associated with elevated local NK cell function in mice infected intraperitoneally (8).
Here we have studied the mechanism by which the VV soluble vIL-18bp contributes to VV virulence by using wild-type VV WR (vC12L), a deletion mutant lacking the vIL-18bp (vΔC12L), and a revertant virus (vC12L-rev) (54). Mice infected with vΔC12L showed greatly enhanced IFN-γ levels in lung cells and bronchoalveolar lavage (BAL) fluids and heightened NK and CD8+ T-cell cytotoxicity. IL-18 was also detected in lavage fluids from VV-infected mice and in culture supernatants from murine macrophages infected with VV in vitro. These results indicate the importance of vIL-18bp in counteracting the role of IL-18 in both the innate and the specific immune responses to VV infection.
MATERIALS AND METHODS
Cells and viruses.
Human TK−143B and D98OR cells and monkey BS-C-1 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (DMEM10). The origins of the orthopoxviruses used here and their growth, titration, and purification were described previously (5).
Assay for virus virulence.
Groups of female BALB/c mice between 6 and 8 weeks of age were anesthetized and infected intranasally with 104 PFU of VV in 20 μl of phosphate-buffered saline (PBS). Each day, mice were weighed individually and monitored for signs of illness (2), and those suffering a severe infection or having lost >25% of their original body weight were sacrificed. To determine virus titers in organs, mice were sacrificed and their lungs, brains, and spleens were removed, Dounce homogenized, frozen and thawed three times, and sonicated. The titer of infectious virus was determined by plaquing on BS-C-1 cells.
Recovery of immune cells for flow cytometry and cytotoxic assays.
BAL fluid and lung, spleen, and mediastinal lymph nodes (MLNs) were obtained from mock-infected and VV-infected mice 3 and 7 days postinfection (p.i.). To obtain BAL, mice were sacrificed, and the lungs of each mouse were inflated five times with a 1-ml volume of PBS containing 10 U of heparin per ml through a blunted 23-gauge needle inserted into the trachea. BAL was centrifuged at 3,000 rpm for 10 min in a Beckman bench-top centrifuge, and the supernatant was removed and frozen at −20°C for analysis of cytokines by enzyme-linked immunosorbent assay (ELISA). BAL cells were resuspended in Tris-NH4Cl (0.14 M NH4Cl in 17 mM Tris, adjusted to pH 7.2) to lyse erythrocytes, washed and resuspended in cold RPMI 1640 medium containing 10% fetal bovine serum. Single-cell suspensions of lung tissue, spleen, and MLN were prepared by sieving through a 100-μm-pore-diameter nylon mesh followed by hypotonic shock to lyse erythrocytes. Cell viability in all samples was assessed by trypan blue exclusion.
Cytotoxic assays.
NK cell cytotoxicity and VV-specific CTL activity in single-cell suspensions from BAL, lung, spleen, and MLN was assayed with a standard 51Cr-release assay. NK-mediated lysis was tested on YAC-1 cells, while P815 cells (H-2d, mastocytoma) were used as targets for VV-specific CTL lysis. Prior to labeling with Na251CrO4 (150 μCi per 3 × 106 cells), P815 cells were mock infected or infected with VV WR at 10 PFU per cell for 2 h at 37°C. Uninfected YAC-1 cells were labeled as described above. Serial dilutions of effector cells were incubated in triplicate cultures with either noninfected or VV-infected target cells in 100 μl of RF10 in 96-well V-bottomed plates at 37°C in 5% CO2. After 4 h (YAC-1) or 6 h (P815) cells were collected by centrifugation, and 50 μl of supernatant was transferred to a Lumaplate-96 (Packard Instrument Company, Inc.) and counted. The percentage of specific 51Cr release was calculated as specific lysis = [(experimental release − spontaneous release)]/(total detergent release − spontaneous release)] × 100. The spontaneous release values were always <10% of total lysis.
In some experiments, asialo-GM1+ or CD8+ cells were depleted from lung cell suspensions by incubation at 37°C with an anti-CD8 monoclonal antibody (MAb) (clone 3.115 [47]) or with anti-asialo-GM1 (Wako Pure Chemicals) in the presence of human complement. Analysis by flow cytometry revealed selective depletion of the desired cell populations. Depleted cells were added to cytotoxicity assays without adjustment for the depletion in cell number.
In vitro infection of lung macrophages.
To obtain lung macrophages, a BAL procedure was carried out on naive BALB/c mice as described above. Approximately 0.5 × 105 to 1 × 105 cells were consistently obtained, and more than 95% of these cells were macrophages. These cells were resuspended in DMEM10 and cultured in 96-well plates at 0.1 × 106 cells per well. After 4 h, nonadherent cells were removed by three vigorous washes and the adherent macrophages were cultured with VV at 10 PFU per cell for 2 h. Cells were washed three times and cultured for 24 h before medium was removed, centrifuged at 3,000 rpm for 10 min, and the clarified supernatants frozen at −20°C.
ELISA for cytokines.
Cytokines were measured in BAL and macrophage culture supernatants by ELISA. ELISA kits were from Pharmingen (IFN-γ, IL-10, and IL-4) or R&D Systems (IL-18).
Flow cytometric analysis of cell surface and intracellular antigens.
Single-cell supensions of BAL, lung, spleen, or MLN cells were blocked with 10% normal rat serum and 0.5 μg of Fc block (Pharmingen) in fluorescence-activated cell sorter (FACS) buffer (PBS containing 0.1% bovine serum albumin and 0.1% sodium azide) on ice for 20 min. Cells were stained with appropriate combinations of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or quantum red (QR)-labeled anti-CD3, anti-CD8, anti-CD4, or anti-pan NK (DX5) and the relevant isotype antibody controls (all from Pharmingen, San Diego, Calif.). The distribution of cell surface markers was determined on a FACScan flow cytometer with CellQUEST software (Becton Dickinson, Mountain View, Calif.). A lymphocyte gate was used to select at least 20,000 events.
To detect intracellular cytokines, 106 lung cells per ml was stimulated with 50 ng of phorbol myristate acetate (PMA) per ml (Sigma) and 500 ng of ionomycin per ml (Calbiochem) in the presence of 10 μg of brefeldin A per ml (Sigma) for 5 h at 37°C. Cells were washed with FACS buffer and stained with QR-conjugated CD4 and FITC-conjugated CD8 for 30 min on ice and then fixed for 30 min at room temperature with 2% paraformaldehyde in PBS. Samples were permeabilized with 0.5% saponin in FACS buffer for 10 min. PE-conjugated anti-mouse IFN-γ (clone XMG1.2; Pharmingen) was added for a further 30 min at room temperature and the cells were washed once with 0.5% saponin in FACS buffer and twice in FACS buffer alone. Cells were analyzed on a Becton Dickinson flow cytometer collecting data on at least 20,000 lymphocytes.
Nitrite determination.
Measurement of nitrite in BAL fluids provides an indirect indication of the amount of nitric oxide (NO) production in the lung. Nitrite was measured colorimetrically at 540 nm by mixing 100 μl of BAL fluid with freshly prepared Greiss' reagent as described elsewhere (16). Each sample was assayed in duplicate, and sodium nitrite was used to generate standard curves.
RESULTS
Virulence of VV lacking C12L (vΔC12L).
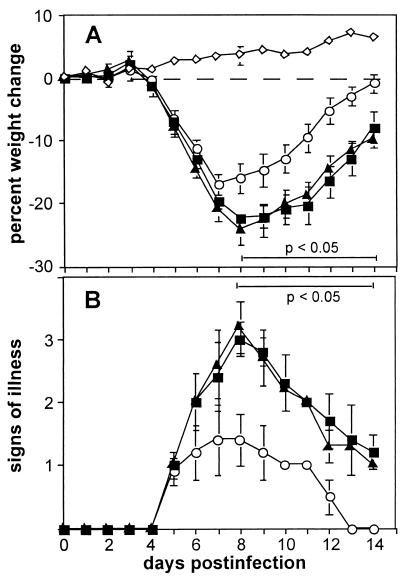
VV strain vΔC12L was attenuated in a murine model of infection (54). To investigate the basis for this phenotype, groups of mice were infected with wild-type (vC12L), deletion mutant (vΔC12L), or revertant (vC12L-rev) virus and monitored for weight loss (Fig. 1A), signs of illness (Fig. 1B), and infectious virus titers in lungs, spleen, and brain on days 3, 7, and 10 p.i. (Fig. 2). Levels of weight loss were similar between all infected groups on days 1 to 7; however, thereafter, mice infected with vΔC12L recovered more rapidly than controls. Signs of illness were also milder in animals infected with vΔC12L. Virus titers in lung homogenates were similar between all groups at day 3 p.i.; however, mice infected with vC12L or vC12L-rev virus retained significantly higher titers of virus at days 7 and 10 (Fig. 2).
FIG. 1.
Virulence of recombinant VVs in the murine intranasal model. Groups of five BALB/c mice were mock-infected (⋄) or infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). (A) Mice were weighed daily, and the results are expressed as the mean percent weight loss of each group ± standard error compared with the weight immediately prior to infection. Data are from five mice, except for the group infected with vC12L-rev, in which one mouse was sacrificed at day 9 and another at day 10. P values were determined by using Student's t test and indicate mean percent weight changes of mice infected with vΔC12L that were significantly different from those of mice infected with vC12L or vC12L-rev. (B) Animals were monitored daily for signs of illness, which was scored from 1 to 4 as described previously (2). Data from each day are expressed as the mean ± standard error from five mice, except for the group infected with C12L-rev, in which 1 mouse was sacrificed at day 9 and another at day 10. P values were determined by using Student's t test and indicate mean signs of illness of mice infected with vΔC12L that were significantly different from those of mice infected with vC12L or vC12L-rev.
FIG. 2.
Replication and spread of recombinant VVs in vivo. Mice were infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). On the indicated day p.i., five animals infected with each virus were sacrificed, and the titer of infectious virus in the lungs, brains, and spleens was determined by plaque assay on BS-C-1 cells. Virus titers are expressed as PFU per organ. The broken line indicates the minimum detection limit of the plaque assay.
In the intranasal model, inoculum doses of VV WR of ≥104 PFU are accompanied by extensive respiratory infection and virus dissemination (59, 60). To examine the ability of the three viruses to spread in vivo, homogenates of brain and spleen were assayed for infectious virus. At day 3 p.i., infectious virus was recovered from the brains of some animals and from the spleens of all animals infected with the recombinant VVs (Fig. 2). No differences in virus titer were observed between groups at this time. At day 7, less virus was detected in brain and spleen samples from vΔC12L-infected mice, and by day 10, virus had been cleared from the brains and spleens of all vC12L- or vC12L-rev-infected mice, but only three of five mice infected with either vC12L or vC12L-rev. In a second experiment, virus was recovered from the brains and spleens of none of five vΔC12L-infected mice, and two of five and three of five mice infected with vC12L or vC12L-rev, respectively, 10 days after infection. These findings indicate that the vΔC12L virus is capable of in vivo spread; however, it is cleared more rapidly than vC12L and vC12L-rev.
Effect of VV infection on numbers of cells in the BAL and lung.
To examine the cellular infiltrate, the number of cells from BAL fluids was determined. By day 3 p.i., cell numbers in lung and BAL preparations from virus-infected animals had increased compared to those in mock-infected controls, and this increase was more pronounced on days 7 and 10 p.i. At days 3, 7, and 10 p.i., the number of lung or BAL cells recovered from vΔC12L-infected animals was not significantly different from those recovered from mice infected with control viruses (data not shown), indicating that deletion of C12L has little effect on the overall number of cells recruited to the lung.
Enhanced NK cell cytotoxicity in mice infected with vΔC12L.
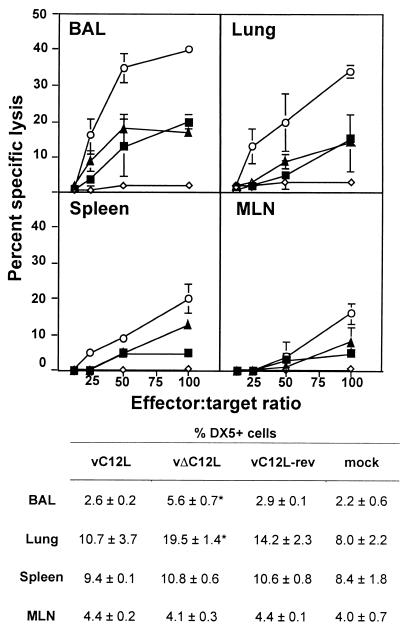
Because IL-18 induces NK cell cytotoxicity (25, 58) and the C12L protein inhibited murine IL-18 in vitro, we examined the role of C12L in NK cell activation in vivo. Cytotoxic activity was determined by lysis of YAC-1 tumor cells in a 51Cr-release assay. Effector cells were recovered from BAL, lung, spleen, and MLN at days 3 and 7; however, results from day 7 are not shown, because little NK cytotoxic activity was detected in cells from any of these sites at this time (<10% lysis at an effector-to-target [E:T] ratio of 100:1). Day 3 effector cells recovered from BAL, lung, spleen, and MLNs of vΔC12L-infected mice showed greater cytotoxicity toward YAC-1 targets than cells from vC12L- and vC12L-rev-infected mice (Fig. 3). To confirm that cytotoxicity was due to NK cells, lung cells taken 3 days p.i. with vΔC12L were depleted of CD8+ cells or asialo-GM1+ cells in the presence of human complement (Materials and Methods). Treatment with complement alone or complement plus anti-CD8 MAb had little effect on cytotoxic activity, but treatment with complement plus anti-asialo-GM1 removed virtually all activity (undepleted = 35%, complement alone = 33%, complement plus anti-CD8 = 26%, and complement plus anti-asialo-GM1+ = 1.5% lysis, at an E:T ratio of 100:1).
FIG. 3.
NK cytotoxicity in BAL, lung, spleen, and MLN after VV infection. Groups of six mice were mock infected (⋄) or infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). At day 3, NK cytotoxicity was determined in cell suspensions prepared from the BAL, lung, spleen, and MLN of mice. Specific lysis of YAC-1 cells was assessed by 51Cr-release assay. Data are expressed as the mean ± standard error from two groups of mice (n = 3 per group). Cells were also stained for expression of the pan-NK cell marker DX5 and examined by flow cytometry with a minimum of 20,000 cells analyzed in a lymphocyte gate. Results are expressed as the percentage of DX5+ cells in the total viable cell population. P values were determined by Student's t test and indicate the mean percentage of DX5+ cells from mice infected with vΔC12L that were significantly different from those from mice infected with vC12L or vC12L-rev. *, P < 0.05.
Effector cell populations were also stained for expression of the NK cell marker DX5 to ascertain if the enhanced NK cytotoxic activity was due to greater recruitment of NK cells or increased activation of those cells present. The percentage of DX5+ cells detected in BAL and lung was significantly greater (approximately double) from vΔC12L-infected mice than that from controls, but the percentages were similar in spleen and MLN preparations from all VV-infected groups (Fig. 3). The elevation in DX5+ cells recovered from BAL of vΔC12L-infected mice is not sufficient to account for all of the enhanced NK cytotoxic activity observed: for example, compare the ability of BAL cells from vΔC12L-infected mice to lyse targets at an E:T ratio of 50:1 with those from vC12L and vC12L-rev-infected mice at an E:T ratio of 100:1. These findings suggest that the activation state of NK cells is also enhanced in the lung.
Enhanced CTL activity in mice infected with vΔC12L.
To assess the effect of VV C12L on VV-specific CTL activity, the cytotoxic activity of effector cells from BAL, lung, spleen, and MLN was examined against uninfected or WR-infected P815 cells. No VV-specific CTL activity was observed with effector cells recovered from any site at day 3 p.i. (data not shown). At day 7, VV-specific CTL activity was higher in BAL and lung cells from vΔC12L-infected mice than that in vC12L- and vC12L-rev-infected controls (Fig. 4). A more modest enhancement was also observed in spleen and MLN. All effector populations showed low cytotoxic activity against uninfected P815 cells (<10% at an E:T ratio of 100:1) (data not shown). Treatment of day 7 lung cells from vΔC12L-infected mice with complement plus anti-CD8 MAb abrogated virtually all CTL activity (undepleted = 45%, complement alone = 39%, complement plus anti-CD8 = 2% lysis, at an E:T ratio of 100:1), confirming that cytotoxic activity against VV-infected P815 cells was mediated by CD8+ lung cells.
FIG. 4.
CTL activity in BAL, lung, spleen, and MLN after VV infection. Groups of six mice were mock infected (⋄) or infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). At day 7, CTL activity was determined in cell suspensions prepared from BAL, lung, spleen, and MLN of mice. Specific lysis of WR-infected P815 cells was assessed by 51Cr-release assay. Data are expressed as the mean ± standard error from two groups of mice (n = 3 per group). Lysis of noninfected P815s by day 7 effector cell populations was always <10% at an E:T ratio of 100:1 and is not shown. Cells were also stained for expression of CD8 and examined by flow cytometry with a minimum of 20,000 cells analyzed in a lymphocyte gate. Results are expressed as the percentage of CD8+ cells in the total viable cell population. P values were determined by Student's t test and indicate the mean percentage of CD8+ cells from mice infected with vΔC12L that were significantly different from those from mice infected with vC12L or vC12L-rev. *, P < 0.05.
The proportion of CD8+ T lymphocytes present in lung and BAL fluids from vΔC12L-infected mice on day 7 was approximately twofold greater than that from vC12L and vC12L-rev controls (Fig. 4); however, again, this difference does not fully explain the enhanced cytotoxic activity from these sites (compare the lysis at an E:T ratio of 50:1 for vΔC12L to 100:1 for vC12L or vC12L-rev cells). Evidently, more CTLs are present in the lungs of vΔC12L-infected mice, and these CTLs are in a higher state of activation than cells from control-infected animals. Similar proportions of CD8+ lymphocytes were detected in all spleen and MLN samples tested.
VV C12L inhibits IFN-γ induction in vivo.
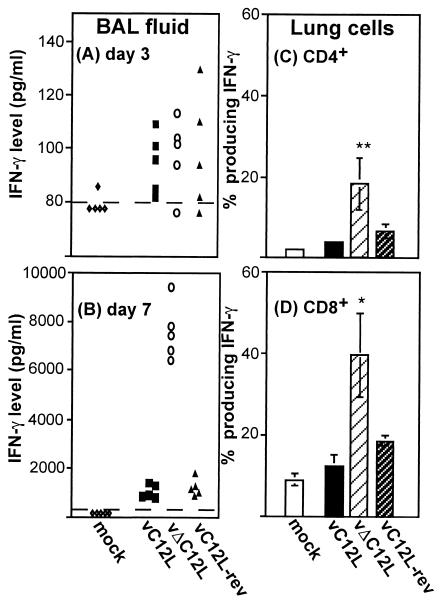
IL-18 induces IFN-γ production by murine T cells and NK cells (39), and IFN-γ possesses antiviral activity (43). We therefore examined levels of IFN-γ in the lungs of VV-infected mice. First, an ELISA was used to determine levels of IFN-γ in BAL on days 3 and 7 p.i. Low levels of IFN-γ were detected in BAL from vC12L-, vΔC12L-, and vC12L-rev-infected mice at day 3 (Fig. 5A). By day 7, there was a 10-fold increase in BAL IFN-γ levels from vC12L (972 ± 253 pg/ml) and vC12L-rev (1,128 ± 380 pg/ml)-infected mice (Fig. 5B). Most striking, however, is the enhancement of IFN-γ observed in BAL from vΔC12L-infected animals (7,602 ± 1,786 pg/ml), indicating an important role for IL-18 in augmenting IFN-γ production in response to VV infection at this time.
FIG. 5.
Production of IFN-γ in the lungs of VV-infected mice. Groups of four to six BALB/c mice were mock infected (⋄) or infected with 104 PFU of vC12L (▪), vΔC12L (○), or vC12L-rev (▴). At days 3 and 7, mice were sacrificed, BALs were performed, and single-cell suspensions were prepared from lung tissue. (A and B) Levels of IFN-γ in BAL fluids of VV-infected mice. BAL samples were centrifuged, and the levels of IFN-γ present in the supernatant were determined by ELISA at days 3 and 7. Values for individual mice are shown. The broken line represents the minimum detection level of the assay. (C and D) Intracellular production of IFN-γ by lung lymphocytes from mice 7 days pi. Lung cells were stimulated with PMA and iomnomycin for 4 h, and brefeldin A was added to retain cytokines in the cytoplasm. Cells were stained with FITC-labeled anti-CD8, QR-labeled anti-CD4, and, after permeabilization with saponin, PE-labeled anti-IFN-γ before analysis by three-color flow cytometry. Shown is the percentage of CD4+ (C) or CD8+ (D) T lymphocytes producing IFN-γ. Values are averaged from two groups (n = 3 per group). P values were determined by Student's t test and indicate the mean percentage of CD8+ cells from mice infected with vΔC12L that were significantly different from those from mice infected with vC12L or vC12L-rev. *, P < 0.05; **, P < 0.02.
To determine which cells in the lung were producing IFN-γ, we performed intracellular staining of lung lymphocytes for IFN-γ. At day 3, we examined IFN-γ production by DX5+ NK cells in the lung. Consistent with the low levels of IFN-γ in day 3 BAL fluids, IFN-γ was detected in less than 5% of DX5+ or DX5− lymphocytes at this time (data not shown). At day 7, more CD4+ (Fig. 5C) and CD8+ (Fig. 5D) lung lymphocytes from VV-infected animals stained for IFN-γ than did those from mock-infected controls. The proportion of both CD4+ and CD8+ lymphocytes that stained for IFN-γ was significantly greater from vΔC12L-infected mice than that from lung cells from animals infected with control viruses. IL-4 and IL-10 were not detected (<1% of total lymphocytes) in any lung cells by intracellular staining at day 3 or 7 (data not shown).
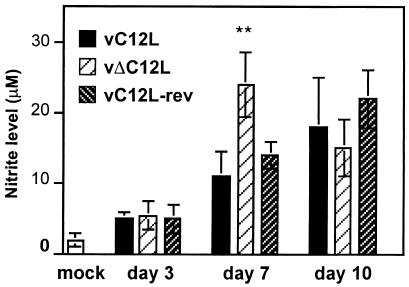
Pulmonary nitrite levels are elevated during infection with vΔC12L virus.
NO is produced by a wide range of mammalian cells and has been demonstrated to inhibit VV replication in vitro (28) and in vivo (45). The inducible nitric oxide synthase (iNOS) enzyme produces high levels of NO and is up-regulated by cytokines such as IFN-γ and tumor necrosis factor alpha (TNF-α). Given the marked elevation in IFN-γ levels in the lungs of vΔC12L-infected mice (Fig. 5), we investigated levels of nitrite in cell-free BAL fluid as a measure of NO activity. BAL nitrite levels peaked earlier (day 7) and at higher levels in vΔC12L-infected mice than in vC12L- or vC12L-rev-infected animals (Fig. 6). By day 10 p.i., BAL nitrite levels declined in vΔC12L-infected mice but remained high in C12L- or vC12L-rev-infected mice.
FIG. 6.
Nitrite levels in the cell-free BAL of mock-infected mice or mice infected with 104 PFU of vC12L, vΔC12L, or vC12L-rev. The data for each time point represent the mean nitrite level ± standard error of four to five individual mice. Columns marked with an asterisk indicate nitrite levels in BAL fluid from vΔC12L-infected mice that were statistically different from those from both vC12L- and vC12L-rev-infected animals. **, P < 0.02.
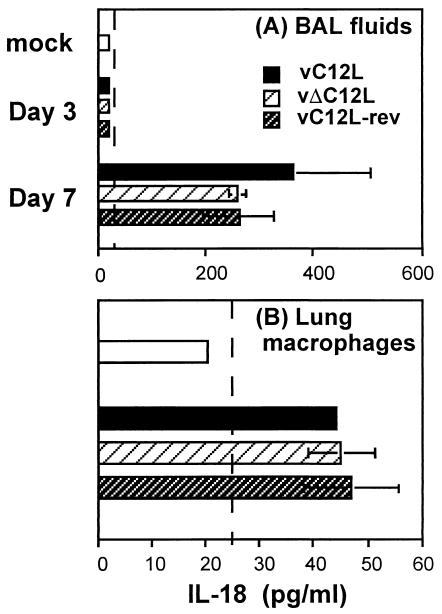
IL-18 is produced in response to VV infection in vivo and in vitro.
Many orthopoxviruses encode IL-18bps, yet the ability of these viruses to induce IL-18 production has not been examined. We used an ELISA to measure IL-18 levels in BAL from mice infected with 104 PFU of vC12L, vΔC12L, or vC12L-rev. Levels of IL-18 were below detection (<25 pg/ml) in BAL from mock-infected mice or from mice 3 days p.i. with VV, but could be detected at similar levels in BAL fluids 7 days p.i. with either vC12L, vΔC12L, or vC12L-rev (Fig. 7A). This showed that IL-18 is induced in response to VV infection in vivo and that expression of vIL-18bp does not affect levels of IL-18 in the lung at day 7 p.i. In a second experiment, recombinant vIL-18 bp containing a C-terminal six-histidine tag (C12L-His) was produced from recombinant baculoviruses and purified by metal chelate affinity chromatography. Addition of 0.1 μg of purified C12L-His per ml to BAL fluid taken from mice 7 days after infection with vΔC12L had no effect on the levels of IL-18 detected (data not shown), indicating that the ELISA could detect IL-18 even when bound by the vIL-18bp.
FIG. 7.
IL-18 production in response to VV infection in vivo and in vitro. (A) IL-18 levels in BAL. Mice were infected with 104 PFU of vC12L, vΔC12L, or vC12L-rev and were sacrificed 3 or 7 days later. BALs from four to five mice were pooled, and the level of total IL-18 protein was determined by ELISA. Data are expressed as the mean ± standard error from two independent experiments. (B) IL-18 release by lung macrophages. Lung macrophages were isolated from naive BALB/c mice and cultured in the presence of 10 PFU of vC12L, vΔC12L, or C12L-rev per cell. Supernatants were measured for IL-18 by ELISA. Results are expressed as the mean ± standard error of duplicate wells.
IL-18 is expressed in many different cell types, but macrophage-like cells are a major source of IL-18 production (15). Therefore, we investigated whether lung macrophages could release IL-18 in response to VV infection. We found significant amounts of IL-18 were released by lung macrophages 24 h p.i. with vC12L, vΔC12L, or vC12L-rev (Fig. 7B).
DISCUSSION
The VV WR gene C12L encodes a protein that inhibits murine IL-18 in vitro and promotes virulence in a murine intranasal model. A deletion mutant lacking C12L induced reduced viral titers in lungs, brain, and spleen; heightened cytotoxic NK and CTL activities in the lungs, spleen, and MLNs; and enhanced production of IFN-γ and nitrite in the lungs. IL-18 was also produced in response to VV infection both in vivo and in vitro. Together, these findings demonstrate a role for the vIL-18bp in modulating aspects of both the innate and specific host response to VV infection.
IL-18 treatment augments the antiviral response in mice after intravenous challenge with VV and enhances NK and CTL activity (56). Our findings highlight the role of IL-18 in the development of a protective immune response against VV. In addition to enhancing cell-mediated immunity, IL-18 possesses proinflammatory properties that are relevant for viral infections. NO, cytokines (TNF-α, IL-1β, and IL-6), and chemokines (IL-8, MCP-1, MIP-1α, and MIP-1β) (18, 23, 42) are induced in response to IL-18 and can exert direct antiviral activity (as shown, for example, by VV-infected cells' sensitivity to killing by both NO [29] and TNF-α [33]) or modulate the recruitment of inflammatory cells. In mouse lungs, IL-18 is expressed by pulmonary epithelium and mononuclear cells (12). Here we demonstrate for the first time that IL-18 is produced in response to VV infection; IL-18 was detected in BAL from VV-infected mice and in supernatants from naive lung macrophages infected with VV in vitro.
The intranasal model has allowed us to delineate the influence of vIL-18bp on innate and specific antiviral effector mechanisms. At day 3 p.i., cytotoxic activity against NK-sensitive YAC-1 cells was abrogated by in vitro treatment with anti-asialo-GM1, but not anti-CD8, antibodies. At day 7, NK cell cytotoxic activity was undetectable, and CD8+ cells were shown to mediate lysis of VV-infected, but not uninfected, P815 targets. Early in infection, loss of C12L promoted an enhanced NK cytotoxic response, but had little effect on IFN-γ production or viral load. However, by day 7, VV-specific CTL activity and production of IFN-γ were heightened from mice infected with vΔC12L, and this correlated with a reduction of virus titers in lungs, brain, and spleen. Although we have demonstrated a role for IL-18 in augmenting the innate response to VV infection, it is the ability to promote a more vigorous Th1-like response (characterized by heightened CTL activity and IFN-γ production) that is critical in mediating the accelerated clearance of vΔC12L.
Depletion of NK cells from mice lowered resistance to VV following intraperitoneal infection (10, 11). Our results suggest that in the intranasal model, while it is likely that NK cells and other innate effectors act to limit early viral replication, this effect is not enhanced significantly in the presence of IL-18. In contrast, IL-18 was an important inducer of NK cytotoxicity and IFN-γ production up to 3 days p.i. of mice with an EV mutant lacking vIL-18bp (8). A modest reduction in EV titer was reported in the liver at day 3, suggesting that the importance of the vIL-18bp in this model resides in the attenuation of NK effector function. For VV infection of the lung, we confirm a role for vIL-18bp in restricting the early NK cell response, but more importantly, we demonstrate that vIL-18bp suppresses the differentiation and activation of T lymphocytes to secrete IFN-γ and mediate CTL activity. It would be interesting to examine later Th-1 responses in the EV model and the role they play in mediating viral clearance.
The importance of IFN-γ in host defense against VV is well established. Mice infected with VV can clear infection in the absence of CD8+ T lymphocytes (53); however, experimental mice deficient in production of IFN-γ (14) or IFN-γ receptors (IFN-γR) (24, 38) or treated with a neutralizing antibody to IFN-γ (27, 46) are highly susceptible to infection despite the generation of virus-specific immune responses. The antiviral activity of IFN-γ is mediated through activation of NK cells, lymphocytes, and macrophages, and the induction of several proteins and enzymes. The enhanced levels of nitrite in BAL fluids from vΔC12L-infected mice 7 days after infection (Fig. 6) would be consistent with IFN-γ-upregulating enzymes such as iNOS in the lung, leading to the production of reactive nitrogen species that inhibit VV replication (28, 45). Chemokines such as MuMig (murine monokine induced by IFN-γ) and Crg (cytokine-responsive gene 2) are also induced by IFN-γ and play a role in IFN-mediated defense against VV infection in vivo (34). IFN-γ levels are 10- to 20-fold higher in BAL from vΔC12L-infected mice than in that from vC12L and vC12L-rev controls (Fig. 5B). Our data also demonstrate that IL-18 favors the differentiation and development of CD4+ and CD8+ T cells primed to produce IFN-γ in the lung (Fig. 5C and D). Together, these data demonstrate that vIL-18bp antagonizes the IFN-γ response to VV in vivo.
VV, cowpox virus, and camelpox virus encode an IFN-γR that binds and inhibits the biological activity of human, bovine, and rat IFN-γ, but fails to neutralize mouse IFN-γ (3, 4, 37, 55). Deletion of VV IFN-γR did not alter the virulence of VV strain WR in a murine intranasal model (55). In the absence of an effective viral receptor for murine IFN-γ, blockade of IL-18-induced IFN-γ production by the C12L protein would be an important mechanism to undermine the IFN-γ response of the host. In contrast, the EV IFN-γR neutralizes murine IFN-γ (37, 50), suggesting a more direct role in counteracting mouse IFN-γ in vivo.
In addition to secreted proteins that bind and inactivate host-derived cytokines, poxviruses encode proteins to interfere with intracellular antiviral pathways. To combat IFNs, VV expresses soluble receptors for IFN-α/β and IFN-γ, as well as intracellular proteins that block the IFN-induced inhibition of protein synthesis (6, 13). Similarly, IL-1β activity is neutralized by a secreted IL-1βR (2, 52) and an intracellular protein, termed B13R, which inhibits caspase 1 and blocks conversion of pro-IL-1β into mature IL-1β (30, 44). Pro-IL-18 is also cleaved into mature IL-18 by caspase 1 (21), suggesting that B13R may also inhibit IL-18 cleavage within virus-infected cells.
The data presented here illustrate how the response to infection can be influenced by the IL-18bp and suggest that deletion of this gene from VV strains, such as MVA, which expresses this protein (51, 54), might increase the safety and immunogenicity of these vaccines. These may be applicable to development of new vaccines for smallpox, other infectious diseases, and cancer. Consistent with this proposal, the Th1 and antigen-specific CTL responses of DNA vaccines were enhanced by coadministration of IL-18 (7, 17).
In summary, our data demonstrate an important role for IL-18 in the host response to pulmonary infection with VV, through the regulation of NK cell and T-cell cytotoxicity and the production of IFN-γ.
Acknowledgments
We thank Fiona Culley and Jonathan Dodd for assistance and helpful discussion.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, F. A. Osorio, C. Balinsky, G. F. Kutish, and D. L. Rock. 2002. The genome of swinepox virus. J. Virol. 76:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcamí, A., and G. L. Smith. 1992. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell 71:153-167. [DOI] [PubMed] [Google Scholar]
- 3.Alcamí, A., and G. L. Smith. 2002. The vaccinia virus soluble interferon-gamma receptor is a homodimer. J. Gen. Virol. 83:545-549. [DOI] [PubMed] [Google Scholar]
- 4.Alcamí, A., and G. L. Smith. 1995. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J. Virol. 69:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcamí, A., J. A. Symons, P. D. Collins, T. J. Williams, and G. L. Smith. 1998. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 160:624-633. [PubMed] [Google Scholar]
- 6.Beattie, E., J. Tartaglia, and E. Paoletti. 1991. Vaccinia-virus encoded eIF-2α homolog abrogates the antiviral effect of interferon. Virology 183:419-422. [DOI] [PubMed] [Google Scholar]
- 7.Billaut-Mulot, O., T. Idziorek, M. Loyens, A. Capron, and G. M. Bahr. 2001. Modulation of cellular and humoral immune responses to a multiepitopic HIV-1 DNA vaccine by interleukin-18 DNA immunization/viral protein boost. Vaccine 19:2803-2811. [DOI] [PubMed] [Google Scholar]
- 8.Born, T. L., L. A. Morrison, D. J. Esteban, T. VandenBos, L. G. Thebeau, N. Chen, M. K. Spriggs, J. E. Sims, and R. M. Buller. 2000. A poxvirus protein that binds to and inactivates IL-18, and inhibits NK cell response. J. Immunol. 164:3246-3254. [DOI] [PubMed] [Google Scholar]
- 9.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukowski, J. F., K. W. McIntyre, H. Yang, and R. M. Welsh. 1987. Natural killer cells are not required for interferon-mediated prophylaxis against vaccinia or murine cytomegalovirus infections. J. Gen. Virol. 68:2219-2222. [DOI] [PubMed] [Google Scholar]
- 11.Bukowski, J. F., B. A. Woda, S. Habu, K. Okumura, and R. M. Welsh. 1983. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J. Immunol. 131:1531-1538. [PubMed] [Google Scholar]
- 12.Cameron, L. A., R. A. Taha, A. Tsicopoulos, M. Kurimoto, R. Olivenstein, B. Wallaert, E. M. Minshall, and Q. A. Hamid. 1999. Airway epithelium expresses interleukin-18. Eur. Respir. J. 14:553-559. [DOI] [PubMed] [Google Scholar]
- 13.Chang, H.-W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello, C. A. 1999. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 103:11-24. [DOI] [PubMed] [Google Scholar]
- 16.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 17.Dupre, L., L. Kremer, I. Wolowczuk, G. Riveau, A. Capron, and C. Locht. 2001. Immunostimulatory effect of IL-18-encoding plasmid in DNA vaccination against murine Schistosoma mansoni infection. Vaccine 19:1373-1380. [DOI] [PubMed] [Google Scholar]
- 18.Fehniger, T. A., M. H. Shah, M. J. Turner, J. B. VanDeusen, S. P. Whitman, M. A. Cooper, K. Suzuki, M. Wechser, F. Goodsaid, and M. A. Caligiuri. 1999. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J. Immunol. 162:4511-4520. [PubMed] [Google Scholar]
- 19.Fujioka, N., R. Akazawa, K. Ohashi, M. Fujii, M. Ikeda, and M. Kurimoto. 1999. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J. Virol. 73:2401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghayur, T., S. Banerjee, M. Hugunin, D. Butler, L. Herzog, A. Carter, L. Quintal, L. Sekut, R. Talanian, M. Paskind, W. Wong, R. Kamen, D. Tracey, and H. Allen. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619-623. [DOI] [PubMed] [Google Scholar]
- 21.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, M. Kurimoto, T. Tanimoto, R. A. Flavell, V. Sato, M. W. Harding, D. J. Livingston, and M. S. Su. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 22.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J. Virol. 75:6705-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshino, T., R. H. Wiltrout, and H. A. Young. 1999. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J. Immunol. 162:5070-5077. [PubMed] [Google Scholar]
- 24.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 25.Hyodo, Y., K. Matsui, N. Hayashi, H. Tsutsui, S. Kashiwamura, H. Yamauchi, K. Hiroishi, K. Takeda, Y. Tagawa, Y. Iwakura, N. Kayagaki, M. Kurimoto, H. Okamura, T. Hada, H. Yagita, S. Akira, K. Nakanishi, and K. Higashino. 1999. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J. Immunol. 162:1662-1668. [PubMed] [Google Scholar]
- 26.Kanda, T., T. Tanaka, K. Sekiguchi, Y. Seta, M. Kurimoto, J. E. Wilson McManus, R. Nagai, D. Yang, B. M. McManus, and I. Kobayashi. 2000. Effect of interleukin-18 on viral myocarditis: enhancement of interferon-gamma and natural killer cell activity. J. Mol. Cell. Cardiol. 32:2163-2171. [DOI] [PubMed] [Google Scholar]
- 27.Karupiah, G., R. V. Blanden, and I. A. Ramshaw. 1990. Interferon-γ is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J. Exp. Med. 172:1495-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karupiah, G., and N. Harris. 1995. Inhibition of viral replication by nitric oxide and its reversal by ferrous sulfate and tricarboxylic acid cycle metabolites. J. Exp. Med. 181:2171-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karupiah, G., Q.-W. Xie, R. M. L. Buller, C. Nathan, C. Duarte, and J. D. MacMicking. 1993. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science 261:1445-1448. [DOI] [PubMed] [Google Scholar]
- 30.Kettle, S., A. Alcami, A. Khanna, R. Ehret, C. Jassoy, and G. L. Smith. 1997. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J. Gen. Virol. 78:677-685. [DOI] [PubMed] [Google Scholar]
- 31.Lee, H. J., K. Essani, and G. L. Smith. 2001. The genome sequence of Yaba-like disease virus, a yatapoxvirus. Virology 281:170-192. [DOI] [PubMed] [Google Scholar]
- 32.Lee, S. J., Y. S. Cho, M. C. Cho, J. H. Shim, K. A. Lee, K. K. Ko, Y. K. Choe, S. N. Park, T. Hoshino, S. Kim, C. A. Dinarello, and D. Y. Yoon. 2001. Both E6 and E7 oncoproteins of human papillomavirus 16 inhibit IL-18-induced IFN-gamma production in human peripheral blood mononuclear and NK cells. J. Immunol. 167:497-504. [DOI] [PubMed] [Google Scholar]
- 33.Li, M., and A. A. Beg. 2000. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J. Virol. 74:7470-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahalingam, S., J. M. Farber, and G. Karupiah. 1999. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity in vivo. J. Virol. 73:1479-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massung, R. F., J. J. Esposito, L.-I. Liu, J. Qi, T. R. Utterback, J. C. Knight, L. Aubin, T. E. Yuran, J. M. Parsons, V. N. Loparev, N. A. Selivanov, K. F. Cavallaro, A. R. Kerlavage, B. W. J. Mahy, and A. J. Venter. 1993. Potential virulence determinants in terminal regions of variola smallpox virus genome. Nature 366:748-751. [DOI] [PubMed] [Google Scholar]
- 36.Mori, I., M. J. Hossain, K. Takeda, H. Okamura, Y. Imai, S. Kohsaka, and Y. Kimura. 2001. Impaired microglial activation in the brain of IL-18-gene-disrupted mice after neurovirulent influenza A virus infection. Virology 287:163-170. [DOI] [PubMed] [Google Scholar]
- 37.Mossman, K., C. Upton, R. M. Buller, and G. McFadden. 1995. Species specificity of ectromelia virus and vaccinia virus interferon-gamma binding proteins. Virology 208:762-769. [DOI] [PubMed] [Google Scholar]
- 38.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 40.Novick, D., S. H. Kim, G. Fantuzzi, L. L. Reznikov, C. A. Dinarello, and M. Rubinstein. 1999. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 10:127-136. [DOI] [PubMed] [Google Scholar]
- 41.Pien, G. C., A. R. Satoskar, K. Takeda, S. Akira, and C. A. Biron. 2000. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J. Immunol. 165:4787-4791. [DOI] [PubMed] [Google Scholar]
- 42.Puren, A. J., G. Fantuzzi, Y. Gu, M. S. Su, and C. A. Dinarello. 1998. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J. Clin. Investig. 101:711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramshaw, I. A., A. J. Ramsay, G. Karupiaph, M. S. Rolph, S. Mahalingam, and J. C. Ruby. 1997. Cytokines and immunity to virus infections. Immunol. Rev. 159:119-135. [DOI] [PubMed] [Google Scholar]
- 44.Ray, C. A., R. A. Black, S. R. Kronheim, T. A. Greenstreet, P. R. Sleath, G. S. Salvensen, and D. J. Pickup. 1992. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell 69:597-604. [DOI] [PubMed] [Google Scholar]
- 45.Rolph, M. S., W. B. Cowden, C. J. Medveczky, and I. A. Ramshaw. 1996. A recombinant vaccinia virus encoding inducible nitric oxide synthase is attenuated in vivo. J. Virol. 70:7678-7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruby, J., and I. Ramshaw. 1991. The antiviral activity of immune CD8+ T cells is dependent on interferon-gamma. Lymphokine Cytokine Res. 10:353-358. [PubMed] [Google Scholar]
- 47.Sarmiento, M., A. L. Glasebrook, and F. W. Fitch. 1980. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J. Immunol. 125:2665-2672. [PubMed] [Google Scholar]
- 48.Shchelkunov, S. N., A. V. Totmenin, I. V. Babkin, P. F. Safronov, O. I. Ryazankina, N. A. Petrov, V. V. Gutorov, E. A. Uvarova, M. V. Mikheev, J. R. Sisler, J. J. Esposito, P. B. Jahrling, B. Moss, and L. S. Sandakhchiev. 2001. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 509:66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sims, J. E. 2002. IL-1 and IL-18 receptors, and their extended family. Curr. Opin. Immunol. 14:117-122. [DOI] [PubMed] [Google Scholar]
- 50.Smith, V. P., and A. Alcamí. 2002. Inhibition of interferons by ectromelia virus. J. Virol. 76:1124-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith, V. P., N. A. Bryant, and A. Alcami. 2000. Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18-binding proteins. J. Gen. Virol. 81:1223-1230. [DOI] [PubMed] [Google Scholar]
- 52.Spriggs, M. K., D. E. Hruby, C. R. Maliszewski, D. J. Pickup, J. E. Sims, R. M. Buller, and J. VanSlyke. 1992. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell 71:145-152. [DOI] [PubMed] [Google Scholar]
- 53.Spriggs, M. K., B. H. Koller, T. Sato, P. J. Morrissey, W. C. Fanslow, O. Smithies, R. F. Voice, M. B. Widmer, and C. R. Maliszewski. 1992. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc. Natl. Acad. Sci. USA 89:6070-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Symons, J. A., E. Adams, D. C. Tscharke, P. C. Reading, H. Waldmann, and G. L. Smith. 2002. The vaccinia virus C12L protein inhibits mouse IL-18 and promotes virulence in a mouse intranasal model. J. Gen. Virol. 83:2833-2844. [DOI] [PubMed] [Google Scholar]
- 55.Symons, J. A., D. C. Tscharke, N. Price, and G. L. Smith. 2002. A study of the vaccinia virus interferon-gamma receptor and its contribution to virus virulence. J. Gen. Virol. 83:1953-1964. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka-Kataoka, M., T. Kunikata, S. Takayama, K. Iwaki, K. Ohashi, M. Ikeda, and M. Kurimoto. 1999. In vivo antiviral effect of interleukin 18 in a mouse model of vaccinia virus infection. Cytokine 11:593-599. [DOI] [PubMed] [Google Scholar]
- 57.Tovey, M. G., J. F. Meritet, J. Guymarho, and C. Maury. 1999. Mucosal cytokine therapy: marked antiviral and antitumor activity. J. Interferon Cytokine Res. 19:911-921. [DOI] [PubMed] [Google Scholar]
- 58.Tsutsui, H., K. Nakanishi, K. Matsui, K. Higashino, H. Okamura, Y. Miyazawa, and K. Kaneda. 1996. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J. Immunol. 157:3967-3973. [PubMed] [Google Scholar]
- 59.Turner, G. S. 1967. Respiratory infection of mice with vaccinia virus. J. Gen. Virol. 1:399-402. [DOI] [PubMed] [Google Scholar]
- 60.Williamson, J. D., R. W. Reith, L. J. Jeffrey, J. R. Arrand, and M. Mackett. 1990. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J. Gen. Virol. 71:2761-2767. [DOI] [PubMed] [Google Scholar]
- 61.Xiang, Y., and B. Moss. 1999. IL-18 binding and inhibition of interferon gamma induction by human poxvirus-encoded proteins. Proc. Natl. Acad. Sci. USA 96:11537-11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xing, Z., A. Zganiacz, J. Wang, M. Divangahi, and F. Nawaz. 2000. IL-12-independent Th1-type immune responses to respiratory viral infection: requirement of IL-18 for IFN-gamma release in the lung but not for the differentiation of viral-reactive Th1-type lymphocytes. J. Immunol. 164:2575-2584. [DOI] [PubMed] [Google Scholar]