Abstract
Studies using brain imaging methods have shown that neuronal activity in the orbitofrontal cortex, a brain area thought to promote the ability to control behavior according to likely outcomes or consequences, is altered in drug addicts. These human imaging findings have led to the hypothesis that core features of addiction like compulsive drug use and drug relapse are mediated in part by drug-induced changes in orbitofrontal function. Here, we discuss results from laboratory studies using rats and monkeys on the effect of drug exposure on orbitofrontal-mediated learning tasks and on neuronal structure and activity in orbitofrontal cortex. We also discuss results from studies on the role of the orbitofrontal cortex in drug self-administration and relapse. Our main conclusion is that while there is clear evidence that drug exposure impairs orbitofrontal-dependent learning tasks and alters neuronal activity in orbitofrontal cortex, the precise role these changes play in compulsive drug use and relapse has not yet been established.
Keywords: drug cues, orbitofrontal cortex, reinstatement, relapse, reversal learning, stress
Introduction
Drug addiction is characterized by compulsive drug-seeking and high frequency of relapse to drug use 1-3. For decades, basic research on drug addiction has been largely devoted to understanding the mechanisms underlying the acute rewarding effects of drugs 4. This research indicates that the mesolimbic dopamine system and its efferent and afferent connections is the neural substrate for the rewarding effects of drugs of abuse 4-7. In recent years, however, it has become clear that the acute rewarding effects of drugs cannot account for several major features of addiction, including relapse to drug use following prolonged abstinence 8-10 and the transition from controlled drug intake to excessive and compulsive drug use 11-14.
Based on several lines of evidence, it has been hypothesized that compulsive drug-seeking and drug relapse is mediated in part by drug-induced changes in the orbitofrontal cortex (OFC) 14-18. Hypermetabolic activity in OFC has been implicated in the etiology of obsessive compulsive disorders (OCD) 19-22, and there is evidence that the incidence of OCD in drug abusers is higher than the rate in the general population 23-25. Imaging studies in cocaine 26; 27, methamphetamine 28; 29 and heroin 15 users reveal altered metabolism in the OFC and increased neuronal activation in response to drug-associated cues 15; 30. Although it is difficult to know whether metabolic changes reflect enhanced or disrupted neural function, altered neuronal signaling in both OCD patients and drug addicts likely reflects abnormal integration of input from afferent areas. Consistent with this speculation, drug addicts, like patients with OFC damage 31, fail to respond appropriately in several variants of the ‘gambling’ task 32-34. This poor performance is accompanied by abnormal activation of OFC 35. The results from these clinical studies indicate that OFC function is impaired in drug addict, but importantly these data cannot distinguish whether changes in OFC function are induced by drug exposure or represent a pre-existing condition that predispose individuals to drug addiction. This issue can be addressed in studies using animal models.
In this review, we first discuss the putative function of the OFC in guiding behavior. We then discuss evidence from laboratory studies on the effect of drug exposure on OFC-mediated behaviors and on neuronal structure and activity in OFC. We then discuss the limited literature on the role of the OFC in drug self-administration and drug relapse in animal models. We conclude that while there is clear evidence that drug exposure causes long-lasting changes in neuronal structure and activity in OFC and impairs OFC-dependent behaviors, the precise role these changes play in compulsive drug use and relapse has not yet been established. Table 1 provides a glossary of terms used in our review (italic letters in the text).
Role of OFC in guiding behavior
Broadly speaking, behavior can be mediated by a desire to obtain a particular outcome, which involves active representation of the value of that outcome, or by habits, which dictate a particular response in a particular circumstance regardless of the value or desirability (or undesirability) of the outcome. Ample evidence now demonstrates that a circuit including the OFC is particularly critical for promoting behavior based on active representation of the value of the expected outcome 36. This function is evident in the ability of animals to rapidly adjust responses when predicted outcomes change 37-39. In rats and monkeys, this ability is often assessed in reversal learning tasks in which a cue predictive of reward becomes predictive of non-reward (or punishment) and a cue predictive of non-reward (or punishment) becomes predictive of reward. Imaging studies implicate OFC in reversal learning in humans 40-42, and rats and primates with damage to the OFC are impaired at learning reversals even when learning for the original materials is intact 38; 43-51. This deficit is illustrated in rats in Figure 1A. OFC lesions may disrupt a similar function in ‘gambling’ tasks in which intact subjects learn to change their responding for a cue that initially predicts a high value, but later comes to predict a high risk of losses 31. Although it is currently a controversial topic in cognitive neuroscience, there is evidence that the role of the OFC in the gambling task is largely accounted for by the requirement for reversal learning that is inherent in the design of most gambling tasks 51.
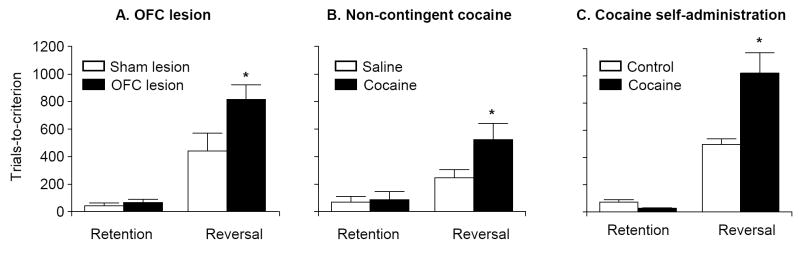
Figure 1. Cocaine exposure induces OFC-dependent reversal learning deficits that are of similar magnitude to learning deficits induced by OFC lesions.
(A) Effect of OFC lesions on reversal learning. Sham and OFC-lesioned rats were tested on serial reversals of a post-operatively acquired 2-odor go, no-go discrimination. One odor predicted sucrose availability, while a second odor predicted quinine. Rats had to learn to respond for sucrose but withhold responding for quinine; criterion was 90% correct responding in a block of 20 trials. OFC lesions had no effect on retention but impaired reversal learning. (B) Effect of repeated non-contingent cocaine exposure (30 mg/kg/day X 14 days) on reversal learning. Rats were injected with cocaine or saline and were then tested on the same odor discrimination reversal task used in (A) after approximately 1 month of withdrawal from the drug. Cocaine exposure had no effect on retention but impaired reversal learning. (C) Effect of contingent cocaine self-administration (0.75 mg/kg/infusion, 4 h/day X 14 days) on reversal learning. Rats were trained to self-administer cocaine and then tested on the same odor discrimination reversal task used in (A) after approximately 3 months of withdrawal from the drug. Cocaine self-administration had no effect on retention but impaired reversal learning. * Different from the respective controls, p < 0.05. Data in (A), (B), and (C) were adapted from references 49, 64 and 80, respectively.
The involvement of OFC in representing the value of predicted outcomes can be isolated in reinforcer devaluation tasks, in which the value of the outcome is directly manipulated via pairing with illness or selective satiation 52. In these settings, normal animals will respond less for predictive cues after devaluation of the predicted outcome. Rats and non-human primates with damage to the OFC fail to show this effect of outcome devaluation 37; 38; 53. These studies reveal a specific deficit in the ability of OFC-lesioned animals to utilize a representation of the outcome’s current value to guide their behavior, particularly in response to conditioned cues. As a result, behavior evoked by the cues becomes less based on the value of the expected outcome and, by default, more habit-like. Though these studies have been done in laboratory animals, imaging studies have shown that cue-evoked BOLD responses in OFC are highly sensitive to devaluation of the foods they predict 54. Below, we discuss evidence that repeated drug exposure induces alterations in neuronal and molecular markers of function in OFC; these changes likely mediate the observed impairments in OFC-mediated behaviors in drug-experienced laboratory animals. Such changes might also lead, in part, to the habit-like response patterns evident in the behavior of addicts and drug-experienced animals.
Effect of drug exposure on OFC
It remains an open question what brain areas and changes mediate the inability of addicts to control their behavior. One way to address this question is to examine whether normal behaviors, which depend on particular brain regions or circuits, are affected by drug exposure, and to relate changes in normal learning with drug-seeking behavior in a relevant animal model. If the loss of control over drug seeking reflects drug-induced changes in particular brain circuits, then the impact of these changes should be evident in behaviors that depend on those circuits. In this regard, drug exposure has been shown to affect several learned behaviors mediated by prefrontal regions, amygdala, and striatum in rats 55-58. Drug exposure also alters how neurons process learned information in these brain areas 59; 60. Among these studies, there is now evidence that cocaine exposure disrupts outcome-guided behavior that depends on the OFC. For example, rats previously exposed to cocaine for 14 days (30 mg/kg/day, i.p.) failed to modify conditioned responding after reinforcer devaluation approximately 1 month after withdrawal 57. Cocaine-experienced rats also respond impulsively when reward size and time to reward is manipulated in choice tasks several months after withdrawal 61; 62. These deficits are similar to those caused by OFC lesions 37; 63.
Reversal learning is also impaired after cocaine exposure. This was first shown by Jentsch and Taylor 64 in monkeys given chronic intermittent exposure to cocaine for 14 days (2 or 4 mg/kg/day, i.p.). These monkeys were slower to acquire reversals of object discriminations when tested 9 and 30 days after withdrawal from cocaine. Similarly, we have found that rats previously exposed to cocaine (30 mg/kg/day i.p. for 14 days) exhibit impaired reversal performance approximately 1 month after withdrawal from the drug 65. As illustrated in Figure 1B, this deficit in reversal learning is of similar magnitude to that of rats with OFC lesions 50; 65; 66.
This reversal learning deficit is associated with a failure of OFC neurons to signal the expected outcomes appropriately 59. Neurons were recorded from the OFC in a task similar to the one used above to demonstrate reversal-learning impairments; each day the rats learned a novel go, no-go odor discrimination, in which they responded to odor cues to obtain sucrose and to avoid quinine. The OFC neurons, recorded in rats exposed to cocaine over a month earlier, fired normally to the sucrose and quinine outcomes, but failed to develop cue-selective responses after learning. In other words, neurons in the cocaine-treated rats did not signal the outcomes during odor sampling, when that information could be used to guide the response. The loss of this signal was particularly apparent during sampling of the cue that predicted the aversive quinine outcome and was associated with abnormal changes in response latencies on these aversive trials. Furthermore, upon reversal of the cue-outcome associations, the OFC neurons in cocaine-treated rats with enduring reversal impairments failed to reverse their cue-selectivity. These results are consistent with the hypothesis that cocaine-induced neuroadaptations disrupt the normal outcome signaling function of the OFC, thereby altering the ability of the animal to engage adaptive decision-making processes that depend on this function 14; 67. These results also suggest that abnormal OFC function observed in addicts likely reflects drug-induced changes rather than or in addition to pre-existing OFC dysfunction.
Of course, there are substantial perils in using the results of lesion studies to infer what areas are affected by drug exposure. The effects of drug exposure are clearly not equivalent to a lesion, and distal effects in other structures could well mimic the effects of lesions. Yet work in laboratory animals demonstrates that psychostimulant exposure does cause changes in markers of function in the OFC. For example, rats trained to self-administer amphetamine exhibit long-lasting decreases in OFC dendritic density 68. In addition, amphetamine-experienced rats exhibit less plasticity in their dendritic fields in the OFC after instrumental training when compared to controls 68. Notably, these results stand in contrast to findings in most other brain areas that have been studied, including other parts of prefrontal cortex, where psychostimulant exposure typically increases dendritic spine density, likely reflecting increased neuronal plasticity 69-71. These results specify the OFC as an area that exhibits a lasting decline in plasticity – or the ability to encode new information – as a result of exposure to psychostimulants. Consistent with this, cocaine addicts show decreased gray matter concentration in the OFC 72.
There are several issues to consider regarding the relevance of the results of the behavioral studies reviewed above to the human condition. One issue is that in all of the studies reviewed above, drugs were given non-contingently, using exposure regimens that lead to enduring psychomotor sensitization 73; 74. Several studies have shown important differences in the effects of contingent and non-contingent drug exposure on brain function and behavior 75-78. In addition, there is little evidence that psychomotor sensitization is manifested in either chronic cocaine addicts or in monkeys with extensive history of cocaine self-administration 79. Thus, it is important to establish that deficits in OFC-dependent functions observed following non-contingent cocaine exposure regimens are also observed in drug addiction models that incorporate contingent drug use (i.e., drug self-administration). Accordingly, we have recently reported that rats trained to self-administer cocaine for 14 d for 3 h/d (0.75 mg/kg/infusion) demonstrated a profound reversal learning deficit up to three months after withdrawal from the drug 80. As illustrated in Figure 1C, this reversal deficit was similar in magnitude to that observed after non-contingent cocaine exposure 65 or after OFC lesions 50.
Another issue to consider is that in all of these studies, OFC deficits were demonstrated in laboratory animals that were abstinent for some period of time. As a result, the time course and duration of the effect of drug-exposure on OFC function is largely unknown. One exception is a study by Kantak and colleagues 81 in which they tested the effect of ongoing cocaine exposure on an OFC-dependent odor-guided win-shift task 82. These authors reported that behavior in this task was impaired by contingent but not non-contingent cocaine in rats that were tested immediately after ongoing cocaine self-administration sessions. This result shows that cocaine exposure can have an immediate effect on OFC-dependent functions. Interestingly, the failure of non-contingent cocaine exposure on OFC-mediated behaviors in this study compared to the reports reviewed above suggests that the impact of drug-exposure on OFC function may increase after withdrawal from the drug.
In conclusion, cocaine exposure (either contingent or non-contingent) leads to long-lasting deficits in OFC-dependent behaviors that are similar in magnitude to those observed after OFC lesions. Non-contingent cocaine exposure also leads to structural changes in OFC neurons, likely reflecting decreased plasticity in these neurons, as well as abnormal neuronal encoding in the OFC. Next, we describe results from studies that have examined the role of OFC in drug reward and relapse, as measured in the drug self-administration 83 and reinstatement 84 models.
Role of OFC in drug self-administration and relapse
The data reviewed above indicate that OFC function is altered by repeated drug exposure. A question derived from these data is what role the OFC plays in mediating drug-taking behavior in animal models. Surprisingly few papers have assessed this question directly. In an early study, Phillips et al. 85 reported that four rhesus monkeys reliably self-administered amphetamine (10-6 M) into the OFC. Surprisingly, the same monkeys did not self-administer amphetamine into the nucleus accumbens, an area known to be involved in the rewarding effects of amphetamine in rats 86. Hutcheson and Everitt 87 and Fuchs et al. 88 reported that neurotoxic OFC lesions did not impair the acquisition of cocaine self-administration under a fixed-ratio-1 reinforcement schedule in rats. Hutcheson and Everitt 87 also reported that OFC lesions had no effect on the dose-response curve for self-administered cocaine (0.01 to 1.5 mg/kg). Although it is difficult to compare the rat and monkey studies because of differences in drug used and routes of administration, and potential species differences in OFC anatomy 89, the results of the rat studies suggest that the OFC is not critical for the rewarding effects of self-administered intravenous cocaine. This observation is similar to results in normal learning studies, which show that OFC lesions typically have no effect on learning to respond for non-drug rewards in a variety of settings 37; 50; 90.
By contrast, Hutcheson and Everitt 87 found that the OFC was required for the conditioned reinforcing effects of cocaine-associated cues, as measured in a second-order schedule of reinforcement procedure 91; 92. They reported that neurotoxic OFC lesions impaired the ability of the cocaine Pavlovian cues to maintain instrumental responding. Similarly, Fuchs et al. 88 reported that reversible inactivation of the lateral (but not medial) OFC with a mixture of a GABAa+GABAb agonists (muscimol+baclofen) impaired the conditioned reinforcing effects of cocaine cues, as measured in a discrete cue-induced reinstatement procedure. Additional potential evidence for OFC’s role in cue-induced cocaine seeking is that exposure to cues previously paired with cocaine self-administration increases the expression of the immediately early gene Zif268 (a marker of neuronal activation) in this region 93. Together these data indicate that the OFC plays an important role in mediating the specific ability of drug-associated cues to motivate drug-seeking behavior. Such a role may reflect the OFC’s previously described role in the acquisition and use of cue-outcome associations 37; 38; 53. Indeed, OFC lesions impair responding for conditioned reinforcement in non-drug settings 94-96 and have also been recently reported to affect Pavlovian-to-instrumental transfer 90, indicating that the OFC supports the ability of Pavlovian cues to guide instrumental responding.
Interestingly, Fuchs et al. 88 reported a different pattern of results when they made lesions of the lateral or medial OFC prior to training. They found that these pre-training lesions had no effect on cue-induced reinstatement of cocaine seeking. Because these lesions were made prior to self-administration training, the OFC was not available to participate in acquisition of the cue-cocaine associations. As a result, the lesioned rats may have learned to rely more on other brain areas that are involved in cue-induced cocaine seeking 97.
Finally, the OFC also appears to be important for stress-induced reinstatement of drug-seeking. Previous studies using a reinstatement procedure 10; 98 have shown that exposure to intermittent footshock stress reinstates drug seeking after training for drug self-administration and subsequent extinction of the drug-reinforced responding 99; 100. Recently, Capriles et al. 101 compared the role of the OFC in stress-induced reinstatement and reinstatement induced by cocaine priming injections. They found that reversible inactivation of the OFC with tetrodotoxin decreased footshock stress- but not cocaine-induced reinstatement of cocaine seeking. They also reported that injections of the D1-like receptor antagonist SCH 23390 but not the D2-like receptor antagonist raclopride into the OFC blocked stress-induced reinstatement.
In conclusion, the limited literature reviewed above suggest that the OFC likely does not mediate the acute rewarding effects of self-administered cocaine, but is involved in the ability of cocaine cues and stressors to promote drug seeking. In addition, D1-like dopamine receptors in the OFC are involved in stress-induced relapse to cocaine seeking.
Conclusions and future directions
The results of studies using self-administration and reinstatement procedures suggest a complex role of the OFC in drug reward and relapse. We would draw several tentative conclusions from these pre-clinical studies. First, the OFC does not appear to play an important role in the acute rewarding effect of cocaine or in relapse induced by acute exposure to the drug. This result is consistent with data showing that the OFC is rarely necessary for animals to learn to respond for reward, presumably due to the operation of multiple, parallel learning systems 37; 50; 90.
Second, the OFC does appear to play an important role in the ability of drug-associated cues to provoke cocaine seeking. These findings are in agreement with results from imaging studies demonstrating strong activation of the OFC by drug-associated cues 15. Lesions or reversible inactivation of the OFC may decrease cue-induced drug seeking, because of a failure to normally activate information regarding the expected value of the drug 36. One question for future research is the time-course of drug-induced changes in OFC and whether the OFC is involved in the time-dependent increases in cue-induced cocaine seeking after withdrawal 102-104, a phenomenon termed incubation of craving.
Third, the OFC also appears to be important for stress-induced reinstatement of cocaine seeking. It has been reported that the effect of footshock stress on reinstatement of cocaine seeking is dependent on the presence of a discrete tone-light cue 105. Thus, the role of the OFC in mediating stress-induced reinstatement may be secondary to the effect of the stress manipulations on cue-controlled responding.
It is important to emphasize that our conclusions regarding the role of the OFC in drug self-administration and relapse are somewhat speculative given the very limited data. One issue to consider is that the contribution of the OFC to drug-seeking behaviors may reflect changes in the OFC caused by previous exposure to the drug. Because of this consideration, interpreting the effects of lesions or other pharmacological manipulations of the OFC on drug-seeking induced by cues or stress in rats with a history of drug self-administration must be done with caution.
A second and perhaps more fundamental issue to consider is that the current animal models of drug self-administration and relapse may not be suitable for assessing what role the OFC plays in human drug addiction. In addition to its general role in mediating outcome-guided behaviors, the OFC seems to be particularly important for recognizing and responding to changes in expected outcomes 38; 43; 50. This is particularly evident when outcomes change from good to bad or when they become delayed or probabilistic 37; 50; 63; 106-108. Here we have reviewed evidence that this particular function of the OFC is disrupted by exposure to addictive drugs, leading to maladaptive and impulsive decision-making 57; 58; 61; 62; 64; 65; 80. Given that drug-seeking behavior in humans is likely the consequence of the balance between the momentary desire for the drug and the evaluation of the typically probabilistic and often delayed consequences of drug seeking 109-111, effects of drugs on the ability of the OFC to correctly signal delayed or probabilistic outcomes might underlie the inability of addicts to forgo the short-term and immediate gratification of drug use. Yet such effects would not be evident in most current models of drug use and relapse, which typically do not model the addict’s conflict between immediate and delayed outcomes.
Although earlier studies did incorporate punishment procedures for assessing the drug reinforcement 112; 113, only recently have several addiction researchers returned to these models. These researchers have reported that some rats with an extensive history of exposure to drugs will continue to engage in drug-taking behavior when confronted with punishment or adverse consequences that normally would suppress drug- or food-taking responding 114-116. Punishment- or conflict-based procedures were also recently introduced to assess drug-priming- and cue-induced relapse to drug seeking 117. These procedures may be better suited to isolate the role of the OFC in drug addiction, because they more closely model the known roles of the OFC in behavior as well as behavior of the human drug addict. Thus, assessing the role of the OFC in punishment or conflict models is an important area of future research. In this regard, based on the findings on the reversal learning deficits after cocaine exposure, we predict that cocaine-induced alterations in OFC functioning will be associated with a diminished ability to suppress responding in the presence of adverse consequences.
Supplementary Material
Acknowledgments
The writing of this review was supported by R01-DA015718 (GS) and the Intramural Research Program of the National Institute on Drug Abuse (YS).
Footnotes
Financial disclosures: Drs. Schoenbaum and Shaham have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leshner AI. Drug abuse and addiction treatment research. The next generation. Arch Gen Psychiatry. 1997;54:691–694. doi: 10.1001/archpsyc.1997.01830200015002. [DOI] [PubMed] [Google Scholar]
- 2.Mendelson JH, Mello NK. Management of cocaine abuse and dependence. N Engl J Med. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- 4.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA. Catecholamine theories of reward: A critical review. Brain Res. 1978;152:215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- 6.Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- 7.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 10.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 12.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolffgramm J, Galli G, Thimm F, Heyne A. Animal models of addiction: models for therapeutic strategies? J Neural Transm. 2000;107:649–668. doi: 10.1007/s007020070067. [DOI] [PubMed] [Google Scholar]
- 14.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 16.Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cerebral Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 18.Porrino LJ, Lyons D. Orbital and medial prefrontal cortex and psychostimulant abuse: studies in animal models. Cerebral Cortex. 2000;10:326–333. doi: 10.1093/cercor/10.3.326. [DOI] [PubMed] [Google Scholar]
- 19.Micallef J, Blin O. Neurobiology and clinical pharmacology of obsessive-compulsive disorder. Clin Neuropharmacol. 2001;24:191–207. doi: 10.1097/00002826-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry. 1998;(Suppl):26–37. [PubMed] [Google Scholar]
- 21.Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, et al. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology. 1999;21:683–693. doi: 10.1016/S0893-133X(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 22.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 23.Friedman I, Dar R, Shilony E. Compulsivity and obsessionality in opioid addiction. J Nerv Ment Dis. 2000;188:155–162. doi: 10.1097/00005053-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Crum RM, Anthony JC. Cocaine use and other suspected risk factors for obsessive-compulsive disorder: a prospective study with data from the Epidemiologic Catchment Area surveys. Drug Alcohol Depend. 1993;31:281–295. doi: 10.1016/0376-8716(93)90010-n. [DOI] [PubMed] [Google Scholar]
- 25.Fals-Stewart W, Angarano K. Obsessive-compulsive disorder among patients entering substance abuse treatment. Prevalence and accuracy of diagnosis. J Nerv Ment Dis. 1994;182:715–719. doi: 10.1097/00005053-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 27.Stapleton JM, Morgan MJ, Phillips RL, Wong DF, Yung BC, Shaya EK, et al. Cerebral glucose utilization in polysubstance abuse. Neuropsychopharmacology. 1995;13:21–31. doi: 10.1016/0893-133X(94)00132-J. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 29.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Archives in General Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 30.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 33.Bechara A, Dolan S, Denburg N, Hindes A, Andersen SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 34.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 35.Bolla KI, Eldreth DA, London ED, Keihl KA, Mouratidis M, Contoreggi C, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenbaum G, Roesch MR. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. Journal of Neuroscience. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izquierdo AD, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baxter MG, Parker A, Lindner CCC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbitofrontal cortex. Journal of Neuroscience. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hampton AN, Bossaerts P, O’Doherty JP. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision-making in humans. Journal of Neuroscience. 2006;26:8360–8367. doi: 10.1523/JNEUROSCI.1010-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris JS, Dolan RJ. Dissociable amygdala and orbitofrontal responses during reversal fear conditioning. Neuroimage. 2004;22:372–380. doi: 10.1016/j.neuroimage.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown VJ, McAlonan K. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioral Brain Research. 2003;146:97–130. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Ragozzino KE. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology of Learning and Memory. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: Decision-making and reversal learning. Brain and Cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 47.Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 48.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 49.Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 50.Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning and Memory. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- 52.Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- 53.Pickens CL, Setlow B, Saddoris MP, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 55.Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. Journal of Neuroscience. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implication for drug addiction. Neurobiology of Learning and Memory. 2006;86:305–310. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cerebral Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- 58.Nelson A, Killcross S. Amphetamine exposure enhances habit formation. Journal of Neuroscience. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stalnaker TA, Roesch MR, Franz TM, Burke KA, Schoenbaum G. Abnormal associative encoding in orbitofrontal neurons in cocaine-experienced rats during decision-making. European Journal of Neuroscience. 2006;24:2643–2653. doi: 10.1111/j.1460-9568.2006.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. Journal of Neuroscience. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. Journal of Neuroscience. 2007;27:245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behavioral Neuroscience. doi: 10.1037/0735-7044.121.3.543. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 64.Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 65.Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. European Journal of Neuroscience. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- 66.Schoenbaum G, Nugent S, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 67.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 68.Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cerebral Cortex. 2004;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 69.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by experience with amphetamine. Journal of Neuroscience. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 71.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. European Journal of Neuroscience. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 72.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 73.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 74.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 75.Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- 76.Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- 77.Kiyatkin EA, Brown PL. Fluctuations in neural activity during cocaine self-administration: clues provided by brain thermorecording. Neuroscience. 2003;116:525–538. doi: 10.1016/s0306-4522(02)00711-x. [DOI] [PubMed] [Google Scholar]
- 78.Kalivas PW, Hu XT. Exciting inhibition in psychostimulant addiction. Trends in Neurosciences. 2006;29:610–616. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology. 2007;191:705–717. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- 80.Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learning and Memory. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology. 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- 82.DiPietro N, Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Complementary tasks to measure working memory in distinct prefrontal cortex subregions in rats. Behavioral Neuroscience. 2004;118:1042–1051. doi: 10.1037/0735-7044.118.5.1042. [DOI] [PubMed] [Google Scholar]
- 83.Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- 84.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 85.Phillips AG, Mora F, Rolls ET. Intracerebral self-administration of amphetamine by rhesus monkeys. Neurosci Lett. 1981;24:81–86. doi: 10.1016/0304-3940(81)90363-3. [DOI] [PubMed] [Google Scholar]
- 86.Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 87.Hutcheson DM, Everitt BJ. The effects of selective orbitofrontal cortex lesions on the acquisition and performance of cue-controlled cocaine seeking in rats. Ann N Y Acad Sci. 2003;1003:410–411. doi: 10.1196/annals.1300.038. [DOI] [PubMed] [Google Scholar]
- 88.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 90.Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental learning. Journal of Neuroscience. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology. 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- 92.Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- 93.Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. European Journal of Neuroscience. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- 94.Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. Journal of Neuroscience. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burke KA, Miller DN, Franz TM, Schoenbaum G. Orbitofrontal lesions abolish conditioned reinforcement mediated by a representation of the expected outcome. Annals of the New York Academy of Science . 2007 in press. [Google Scholar]
- 96.Cousens GA, Otto T. Neural substrates of olfactory discrimination learning with auditory secondary reinforcement. I. Contributions of the basolateral amygdaloid complex and orbitofrontal cortex. Integrative Physiological and Behavioral Science. 2003;38:272–294. doi: 10.1007/BF02688858. [DOI] [PubMed] [Google Scholar]
- 97.See RE. Neural substrates of conditioned-cue relapse to drug-seeking behavior. Pharmacology, Biochemistry, and Behavior. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 98.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 99.Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking under opioid maintenance: the effects of opioid withdrawal, heroin priming and stress. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 101.Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 102.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 104.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shelton KL, Beardsley PM. Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in rats. Int J Comp Psychol. 2005;18:154–166. [Google Scholar]
- 106.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nature Neuroscience. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 107.Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. Journal of Neuroscience. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roesch MR, Taylor AR, Schoenbaum G. Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron. 2006;51:509–520. doi: 10.1016/j.neuron.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 110.Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Epstein DE, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith SG, Davis WM. Punishment of amphetamine and morphine self-administration behavior. Psychol Rec. 1974;24:477–480. [Google Scholar]
- 113.Johanson CE. The effects of electric shock on responding maintained by cocaine injections in a choice procedure in the rhesus monkey. Psychopharmacology. 1977;53:277–282. doi: 10.1007/BF00492364. [DOI] [PubMed] [Google Scholar]
- 114.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 115.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 116.Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- 117.Panlilio LV, Thorndike EB, Schindler CW. Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology. 2003;168:229–235. doi: 10.1007/s00213-002-1193-0. [DOI] [PubMed] [Google Scholar]
- 118.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug -related cues and cocaine craving. Psychopharnacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 119.Katzir A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. doi: 10.1007/s00213-007-0827-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Brien CP, Childress AR, Mclellan TA, Ehrman R. Classical conditioning in drug dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 121.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 122.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 123.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 124.De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- 125.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychostimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 126.Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- 127.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.