Abstract
PURPOSE
To describe the prevalence and risk factors of diabetic retinopathy in a multi-ethnic US population of whites, blacks, hispanics, and chinese.
DESIGN
Cross-sectional study of 778 individuals from ages 45 to 85 years with diabetes, participating in the Multi-Ethnic Study of Atherosclerosis (MESA).
METHODS
Retinal photographs were obtained with a 45° nonmydriatic digital fundus camera. Presence and severity of diabetic retinopathy were graded at a central reading center on the basis of a modification of the Airlie House classification system. All participants underwent a standardized interview, examination, and laboratory investigations.
RESULTS
In this population with diabetes, the prevalence of any retinopathy was 33.2% and macular edema 9.0%. The prevalence of any diabetic retinopathy and macular edema was significantly higher in blacks (36.7% and 11.1%) and hispanics (37.4% and 10.7%) than in whites (24.8% and 2.7%) and chinese (25.7% and 8.9%) (P = .01 and P = .007, comparing racial/ethnic differences for retinopathy and macular edema, respectively). Significant independent predictors of any retinopathy were longer duration of diabetes, higher fasting serum glucose, use of diabetic oral medication or insulin, and greater waist-hip ratio. Race was not an independent predictor of any retinopathy.
CONCLUSIONS
This study provides contemporary data on the prevalence of and risk factors for diabetic retinopathy among whites, blacks, hispanics, and chinese participating in the MESA.
Diabetic retinopathy, the most common specific complication of type 2 diabetes, is the leading cause of blindness among Americans age 20 to 64 years.1,2 The epidemiology of diabetic retinopathy has been previously described, largely in white populations.3–6 In the Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR), the prevalence of retinopathy in 1980 to 1982 among persons with type 2 diabetes was reported at 39% for those not receiving insulin treatment, and 70% for those receiving insulin treatment.3 A recent review of data pooled from eight studies suggests the prevalence of retinopathy may have declined in recent years.7 However, of these eight studies, only two collected data in the past decade, and none in the past 5 years. Thus, there is a need for new contemporary data.
Epidemiological studies, again largely conducted in whites, have identified several risk factors for diabetic retinopathy.8–17 The most consistent of these are longer duration of diabetes, hyperglycemia, and hypertension.3–5,8,9 Associations with hyperlipidemia,11–15 obesity,15,16 and other cardiovascular risk factors14,15 have been less consistently documented.
In contrast to whites, there are fewer population-based data on either the prevalence of or the risk factors for retinopathy in other racial/ethnic groups with diabetes in the United States.17–20 Because diabetes prevalence appears to vary between these racial/ethnic groups,21–23 it has been suggested that the frequency of retinopathy may also vary by race/ethnicity. The few studies conducted suggest that in comparison to studies in whites, African Americans (blacks) and hispanics have a higher prevalence of retinopathy.24–26 These differences have been attributed in part to racial/ethnic differences in diabetes duration, glycemic control, and blood pressure levels in blacks, but not in hispanics. There are no data on the epidemiology of diabetic retinopathy in Chinese Americans (chinese).
The purposes of our current study were to describe the prevalence of diabetic retinopathy among whites, blacks, hispanics, and chinese diagnosed with diabetes participating in the Multi-Ethnic Study of Atherosclerosis (MESA), to quantify risk factors of diabetic retinopathy, and to examine racial/ethnic differences.
METHODS
The mesa is a prospective cohort study of men and women 45 to 85 years of age initially free of clinical evidence of cardiovascular disease and living in six United States communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan, New York; and St Paul, Minnesota).27 The study objective of MESA was to identify risk factors for subclinical and clinical cardiovascular disease. Selection of study population has been reported in detail elsewhere.27 At the baseline examinations, there were 6814 participants: 1086 from Baltimore; 1164 from Chicago; 1077 from Forsyth County; 1319 from Los Angeles County; 1102 from New York; and 1066 from St Paul.
Of the 6814 participants, 959 had diabetes defined as fasting glucose ≥7.0 mmol/l (≥126 mg/dl) or use of insulin or oral hypoglycemic medication.28 Fundus photography was performed at the second examination immediately after the baseline examination, from August 2002 to January 2004. At this examination, 827 (86.2%) of 959 of the participants with diabetes underwent retinal photography. Of these, we excluded 49 without gradeable photographs for diabetic retinopathy severity in either eye, leaving 778 participants who were included in this analysis. The proportion of people ungradeable for diabetic retinopathy was not significantly different between whites (1.5%), blacks (2.4%), hispanics (1.3%), and chinese (0.7%) (P = .80).
Tenets of the Declaration of Helsinki were followed, and institutional review board approval was granted at each study site. Written informed consent was obtained from each participant.
Fundus photography was performed at each site following a standardized protocol. Participants were seated in a darkened room. Both eyes of each participant were photographed with a 45 degree 6.3-megapixel digital nonmydriatic camera (Canon, Lake Success, New York, USA). Two photographic fields were taken of each eye; the first centered on the optic disk (field 1), and the second centered on the fovea (field 2). Standard software was used for image acquisition and archiving (Eye QSL; Digital Healthcare Inc, Cambridge, England). Images were then sent from the field centers to the University of Wisconsin, Madison, for assessment of retinopathy and other retinal diseases. Photographs were evaluated in semiquantitative fashion by a grader with a custom written Microsoft Access database, EyeQ Lite (an image-processing database for storage, retrieval, and manipulation of digital images), and a dual-monitor computer display.
Retinopathy was considered to be present if any characteristic lesion as defined by the Early Treatment Diabetic Retinopathy Study29 severity scale was present: microaneurysms (MAs), hemorrhages, cotton wool spots (CWSs), intraretinal microvascular abnormalities (IRMAs), hard exudates (HEs), venous beading, and new vessels. For each eye, a retinopathy severity score was assigned as follows according to a scale modified from the Airlie House Classification system30: level 10, no retinopathy present; level 14, any combination of definite HE, CWS, IRMA, or venous loops in the absence of definite MA; level 15, hemorrhage present without any definite MA; level 20, MA only with no other diabetic lesions present; level 31, MA and one or more of the following: hemorrhage or MA < standard photograph 2A, HE, venous loops, questionable CWS, IRMA, or venous beading; level 41, MA and one or more of the following: CWS, IRMA < standard photograph 8A; level 51, MA and one or more of the following: venous beading, hemorrhage or MA ≥2A, IRMA ≥8A; level 60, fibrous proliferation with no other proliferative lesions; level 61–64, laser scatter photocoagulation scars with retinopathy levels 31–51; level 65, proliferative diabetic retinopathy less than high-risk characteristics, as defined in the Diabetic Retinopathy Study; level 70, proliferative diabetic retinopathy ≥ high-risk characteristics; and level 80, total vitreous hemorrhage.
Macular edema was defined by HE in the presence of MA and blot hemorrhage within one disk diameter from the foveal center, or presence of focal photocoagulation scars in the macular area. Clinically significant macular edema (CSME) was considered present when the macular edema involved or was within 500 μm of the foveal center, or if focal photocoagulation scars were present in the macular area.
We defined three primary outcomes for this study on the basis of the severity scores of the worse eye. Any diabetic retinopathy was defined as level 14 and above. This was further divided into minimal nonproliferative diabetic retinopathy (NPDR) (levels 14 to 20), early-moderate NPDR retinopathy (levels 31 to 41) and severe NPDR-proliferative retinopathy (levels 51 to 80). Macular edema was defined as present and absent, and further divided into those with and without CSME. Vision-threatening retinopathy was defined as the presence of severe NPDR-proliferative retinopathy or CSME. If an eye was ungradeable, the scores for the other eye were used to define these outcomes.
Participants underwent an extensive assessment of atherosclerotic disease and its risk factors during the course of the study. Data for this analysis were based on those collected at the baseline examination. Resting blood pressure was measured three times with participants in the seated position (Dinamap model Pro 100 automated oscillometric sphygmomanometer; Critikon, Tampa, Florida, USA).31 The average of the last measurements was used in analysis. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medications.31 Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. The waist-hip ratio was defined as the ratio of the waist circumference and the hip circumference, measured in centimeters.
Standardized questionnaires were used to obtain information about medical history, education level, marital status, health insurance status, occupation, annual household income, cigarette smoking and alcohol consumption, and use of hormone replacement therapy and antihypertensive and antidiabetic medications. Marital status was defined as married or other (single, widowed, divorced). Smoking was defined as current, former, or never. Duration of diabetes was estimated from the age of first use of diabetic medication. Diabetic medication use was defined to include oral hypoglycemic medications and/or insulin.
Blood samples were assayed for putative biochemical risk factors, including total and high-density lipoprotein (HDL) plasma cholesterol, plasma triglycerides, and serum glucose levels measured after a 12-hour fast. Analyses were performed at a central site at the Collaborative Studies Clinical Laboratory at Fairview–University Medical Center (Minneapolis, Minnesota, USA). Low-density lipoprotein (LDL) cholesterol was calculated with the Friedewald equation.
We compared characteristics of participants with diabetes by race and estimated the prevalence of diabetic retinopathy, macular edema, CSME, and vision-threatening diabetic retinopathy according to age group, gender, and race/ethnicity. Differences in means and proportions were tested by the analysis of variance or χ2 tests, respectively.
We constructed logistic regression models to determine the odds ratio (OR) and 95% confidence intervals (CIs) for the three primary outcomes (any diabetic retinopathy, CSME, and vision-threatening retinopathy) in association with putative risk factors (eg, presence vs absence of hypertension, or quartiles of blood pressure). This was initially performed for the total diabetic sample (n = 778) and then for the four racial/ethnic groups separately. All initial models were adjusted for age, gender, race, and center (and age and gender for race/ethnicity-specific models). Test of trend was determined by treating categorical risk factors (eg, quartiles of blood pressure) as continuous variables, and the χ2 statistic for the parameter estimate was computed. Significant predictors (P < .10) in the initial models were selected for inclusion in the multivariable logistic models. All analyses were performed by SPSS version 12.0.1 (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Participant characteristics comparing those with and without diabetes, as well as participants with diabetes in each of the four racial/ethnic groups, are listed in Table 1. There were significant differences between participants with and without diabetes; participants with diabetes were older and more likely to have less than 8 years of education, hypertension, and higher glucose, BMI, and waist-hip ratios than participants without diabetes. When we compared racial/ethnic groups among participants with diabetes, we found that blacks, hispanics, and chinese were more likely to have less than 8 years of education, blacks were more likely to have hypertension, and blacks and hispanics were more likely to have higher glucose levels, BMI, and waist-hip ratios.
TABLE 1.
Characteristics of Participants
| Characteristic | No Diabetes
(N = 5322) |
Total Diabetes Sample
(N = 778) |
White
(N = 153) |
Black
(N = 289) |
Hispanic
(N = 235) |
Chinese
(N = 101) |
|---|---|---|---|---|---|---|
| Gender, male, n (%) | 2497 (46.9) | 410 (52.7) | 95 (62.1) | 142 (49.1) | 118 (50.2) | 55 (54.5) |
| Education, <8 years, n (%) | 477 (9.0) | 129 (16.6)* | 2 (1.3) | 11 (3.8) | 94 (40.0) | 22 (21.8) |
| Hypertension, n (%) | 2136 (40.1) | 510 (65.6)* | 91 (59.5) | 229 (79.2) | 141 (60.0) | 49 (48.5) |
| Oral diabetic medication, n (%) | — | 437 (56.2) | 71 (46.4) | 166 (57.4) | 138 (58.7) | 62 (61.4) |
| Insulin, n (%) | — | 79 (10.2) | 16 (10.5) | 34 (11.8) | 26 (11.1) | 3 (3.0) |
| Current cigarette smoker, n (%) | 676 (12.8) | 92 (11.9) | 15 (9.9) | 44 (15.3) | 26 (11.1) | 7 (6.9) |
| Age, years, mean ± SD | 61.5 ± 102 | 64.0 ± 9.2* | 64.3 ± 9.5 | 63.8 ± 8.9 | 63.4 ± 9.5 | 65.9 ± 9.0 |
| Diabetes duration, years, mean ± SD | — | 8.4 ± 8.1 | 8.5 ± 8.0 | 8.5 ± 8.7 | 8.8 ± 7.8 | 6.5 ± 6.1 |
| Serum glucose, mg/dl, mean ± SD | 95.9 ± 9.8 | 157.1 ± 52.9* | 149.0 ± 50.5 | 155.7 ± 51.4 | 166.0 ± 55.8 | 152.4 ± 51.6 |
| Systolic blood pressure, mm Hg, mean ± SD | 125.0 ± 20.9 | 132.5 ± 21.1 | 129.7 ± 20.6 | 135.9 ± 21.3 | 131.6 ± 21.8 | 129.0 ± 18.7 |
| Body mass index, kg/m2, mean ± SD | 28.0 ± 5.3 | 30.7 ± 5.9* | 31.3 ± 5.7 | 31.6 ± 5.4 | 31.6 ± 6.0 | 25.1 ± 3.2 |
| Waist-hip ratio, mean ± SD | 0.92 ± 0.08 | 0.97 ± 0.06* | 0.97 ± 0.07 | 0.96 ± 0.06 | 0.98 ± 0.06 | 0.96 ± 0.06 |
| Total cholesterol, mg/dl, mean ± SD | 194.8 ± 34.7 | 189.7 ± 39.9* | 189.2 ± 44.4 | 185.9 ± 37.6 | 194.0 ± 41.8 | 191.6 ± 33.2 |
N = number at risk.
P < .05 based on χ2 or ANOVA, comparing differences between no diabetes and total diabetes sample.
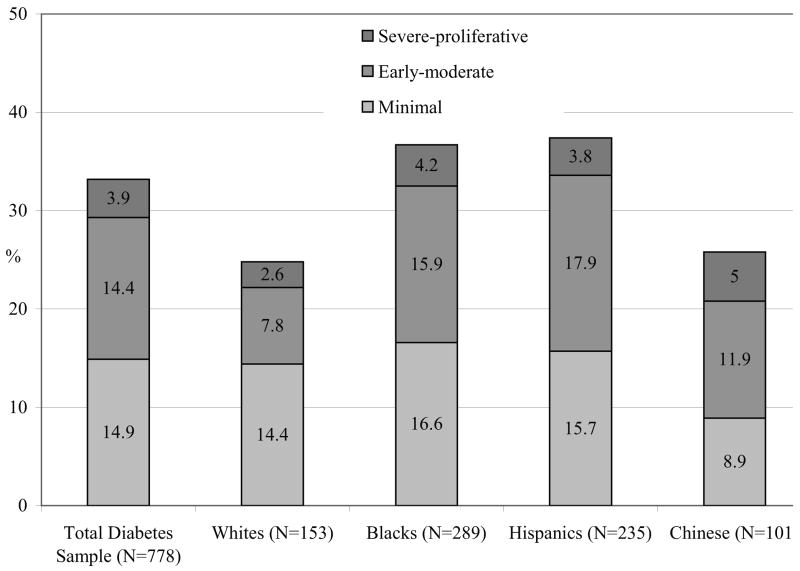
The prevalence of any retinopathy, macular edema, CSME, and vision-threatening retinopathy in the total diabetic sample and in each of the four racial/ethnic groups is listed in Table 2. In the total diabetic sample, the overall prevalence of any retinopathy was 33.2%, any macular edema 9.0%, CSME 5.6%, and vision-threatening retinopathy 7.9%. Minimal NPDR was seen in 14.9% of the total diabetic sample, early-moderate NPDR in 14.4%, and severe NPDR–proliferative retinopathy in 3.9%. The prevalence of any diabetic retinopathy and macular edema was significantly higher in blacks (36.7% and 11.1%) and hispanics (37.4% and 10.7%) than in whites (24.8% and 2.7%) and chinese (25.7% and 8.9%) (P = .01 and P = .007, comparing racial/ethnic differences for retinopathy and macular edema, respectively, Table 2). The prevalence of vision-threatening retinopathy was also higher in blacks and hispanics than in whites and chinese, but this difference was not statistically significant (P = .28). There was no noticeable or significant trend in prevalence of retinopathy or vision-threatening retinopathy with age or gender in any of the four racial/ethnic groups (data not shown).
TABLE 2.
Prevalence and Severity of Diabetic Retinopathy and Macular Edema
| Characteristic | Total Diabetes
Sample (N = 778), n (%) |
White
(N = 153), n (%) |
Black
(N = 289), n (%) |
Hispanic
(N = 235), n (%) |
Chinese
(N = 101), n (%) |
P Value* |
|---|---|---|---|---|---|---|
| No retinopathy | 520 (66.8) | 115 (75.2) | 183 (63.3) | 147 (62.6) | 75 (74.3) | |
| Retinopathy | 258 (33.2) | 38 (24.8) | 106 (36.7) | 88 (37.4) | 26 (25.7) | .01 |
| Minimal | 116 (14.9) | 22 (14.4) | 48 (16.6) | 37 (15.7) | 9 (8.9) | |
| Early-moderate | 112 (14.4) | 12 (7.8) | 46 (15.9) | 42 (17.9) | 12 (11.9) | |
| Severe-proliferative | 30 (3.9) | 4 (2.6) | 12 (4.2) | 9 (3.8) | 5 (5.0) | |
| No macular edema | 694 (91.0) | 146 (97.3) | 248 (88.9) | 208 (89.3) | 92 (91.1) | |
| Macular edema present | 69 (9.0) | 4 (2.7) | 31 (11.1) | 25 (10.7) | 9 (8.9) | .007 |
| CSME | 43 (5.6) | 3 (2.0) | 21 (7.5) | 16 (6.9) | 3 (3.0) | |
| Vision-threatening retinopathy | 60 (7.9) | 7 (4.5) | 26 (9.3) | 20 (8.7) | 7 (6.9) | .28 |
N = number at risk; n = number with retinopathy endpoint; % = prevalence.
P value based on χ2 test, comparing racial/ethnic differences for any retinopathy, macular edema, and vision-threatening retinopathy.
Table 3 shows risk factor associations of any diabetic retinopathy and vision-threatening retinopathy in the total diabetic sample. After adjusting for age, gender, and center, the prevalence of any diabetic retinopathy was higher in blacks (OR 1.84, 95% CI 1.18 to 2.87) and hispanics (OR 1.70, 95% CI 1.07 to 2.72) as compared with whites. After adjustment for age, gender, race, and MESA center, significant predictors for both outcomes (any retinopathy and vision-threatening retinopathy) were longer duration of diabetes, use of diabetic medication (known and treated diabetes vs unknown and untreated diabetes) and higher systolic blood pressure. Additional predictors for any retinopathy included higher serum glucose levels and greater waist-hip ratio; additional predictors for vision-threatening retinopathy included a history of hypertension. Associations for CSME were largely similar to results presented for any retinopathy and vision-threatening retinopathy (data not shown).
TABLE 3.
Risk Factors for Diabetic Retinopathy and Vision-Threatening Retinopathy, Total Diabetes Sample (N = 778)
| Any Retinopathy
|
Vision-Threatening Retinopathy
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Risk Factor | Number at Risk | % | OR (95% CI)* | P Value | Number at Risk | % | OR (95% CI)* | P Value |
| Gender | ||||||||
| Male | 410 | 34.4 | 1.00 | .42 | 403 | 6.9 | 1.00 | .35 |
| Female | 368 | 31.8 | 0.89 (0.66–1.20) | 361 | 8.9 | 1.29 (0.76–2.18) | ||
| Race | ||||||||
| White | 153 | 24.8 | 1.00 | .63 | 153 | 4.5 | 1.00 | .34 |
| Black | 289 | 36.7 | 1.84 (1.18–2.87) | 280 | 9.3 | 2.10 (0.88–4.99) | ||
| Hispanic | 235 | 37.4 | 1.70 (1.07–2.72) | 229 | 8.7 | 2.06 (0.83–5.13) | ||
| Chinese | 101 | 25.7 | 0.93 (0.50–1.74) | 101 | 6.9 | 1.56 (0.49–5.03) | ||
| Diabetes medication | ||||||||
| No | 262 | 17.9 | 1.00 | <.001 | 262 | 1.5 | 1.00 | <.001 |
| Yes | 516 | 40.9 | 3.36 (2.33–4.85) | 499 | 11.2 | 8.15 (2.92–22.73) | ||
| Diabetes duration, years | ||||||||
| Less than 3 years | 486 | 21.0 | 1.00 | <.001 | 486 | 2.7 | 1.00 | <.001 |
| 3–10 years | 158 | 41.1 | 2.63 (1.78–3.88) | 153 | 5.3 | 2.02 (0.82–4.99) | ||
| 10 years or longer | 134 | 67.9 | 9.09 (5.87–14.08) | 125 | 31.2 | 16.72 (8.45–33.07) | ||
| Serum glucose, mg/dl | ||||||||
| 1st Quartile, <127 | 194 | 29.9 | 1.00 | <.001 | 192 | 9.9 | 1.00 | .16 |
| 2nd Quartile, 127–141 | 197 | 21.8 | 0.65 (0.41–1.04) | 198 | 3.5 | 0.36 (0.15–0.87) | ||
| 3rd Quartile, 141–173 | 191 | 30.9 | 1.04 (0.68–1.61) | 191 | 6.3 | 0.64 (0.30–1.36) | ||
| 4th Quartile, ≥173 | 196 | 50.0 | 2.30 (1.50–3.53) | 183 | 12.0 | 1.53 (0.77–3.01) | ||
| Hypertension status | ||||||||
| Absent | 268 | 31.3 | 1.00 | .18 | 259 | 3.9 | 1.00 | .009 |
| Present | 510 | 34.1 | 1.25 (0.90–1.75) | 505 | 9.9 | 2.62 (1.28–5.36) | ||
| Systolic BP, mm Hg | ||||||||
| 1st Quartile, <117 | 200 | 32.0 | 1.00 | .01 | 195 | 4.1 | 1.00 | <.001 |
| 2nd Quartile, 117–131 | 195 | 29.2 | 0.93 (0.61–1.44) | 191 | 4.7 | 1.14 (0.43–3.03) | ||
| 3rd Quartile, 131–145 | 197 | 31.0 | 1.05 (0.68–1.62) | 192 | 8.3 | 2.02 (0.83–4.90) | ||
| 4th Quartile, ≥145 | 185 | 41.1 | 1.74 (1.13–2.71) | 185 | 14.6 | 3.73 (1.60–8.70) | ||
| Diastolic BP, mm Hg | ||||||||
| 1st Quartile, <65 | 200 | 37.0 | 1.00 | .13 | 193 | 8.3 | 1.00 | .82 |
| 2nd Quartile, 65–72 | 199 | 29.6 | 0.66 (0.43, 1.02) | 199 | 8.5 | 1.14 (0.55–2.36) | ||
| 3rd Quartile, 72–79 | 183 | 36.1 | 0.84 (0.54–1.31) | 176 | 8.0 | 1.15 (0.52–2.54) | ||
| 4th Quartile, ≥79 | 195 | 30.3 | 0.64 (0.41–1.01) | 195 | 6.7 | 0.91 (0.41–2.03) | ||
| Body mass index, kg/m2 | ||||||||
| 1st Quartile, <26.4 | 194 | 36.6 | 1.00 | .61 | 190 | 9.5 | 1.00 | .66 |
| 2nd Quartile, 26.4–29.8 | 191 | 30.4 | 0.74 (0.48–1.15) | 191 | 5.8 | 0.63 (0.28–1.39) | ||
| 3rd Quartile, 29.8–33.8 | 193 | 31.6 | 0.79 (0.51–1.22) | 191 | 6.8 | 0.76 (0.35–1.66) | ||
| 4th Quartile, >33.8 | 200 | 34.0 | 0.87 (0.55–1.36) | 192 | 9.4 | 1.15 (0.54–2.47) | ||
| Waist-hip ratio, unit | ||||||||
| 1st Quartile, <0.94 | 193 | 21.8 | 1.00 | <.001 | 192 | 6.3 | 1.00 | .06 |
| 2nd Quartile, 0.94–0.97 | 203 | 36.0 | 2.10 (1.33–3.31) | 198 | 6.1 | 1.05 (0.45–2.42) | ||
| 3rd Quartile, 0.97–1.01 | 206 | 37.4 | 2.31 (1.46–3.65) | 203 | 9.4 | 1.72 (0.79–3.73) | ||
| 4th Quartile, >1.01 | 176 | 37.5 | 2.31 (1.43–3.73) | 171 | 9.9 | 1.88 (0.84–4.19) | ||
| Total cholesterol, mg/dl | ||||||||
| 1st Quartile, <164 | 200 | 32.0 | 1.00 | .37 | 200 | 9.0 | 1.00 | .82 |
| 2nd Quartile, 164–187 | 191 | 29.8 | 0.89 (0.58–1.37) | 187 | 7.0 | 0.75 (0.36–1.58) | ||
| 3rd Quartile, 187–212 | 197 | 37.1 | 1.27 (0.84–1.93) | 190 | 6.3 | 0.66 (0.31–1.43) | ||
| 4th Quartile, ≥212 | 190 | 33.7 | 1.09 (0.71–1.68) | 187 | 9.1 | 0.96 (0.47–1.95) | ||
| Marital status | ||||||||
| Married | 460 | 30.9 | 1.00 | .03 | 450 | 6.2 | 1.00 | .11 |
| Others | 318 | 36.5 | 1.42 (1.04–1.95) | 314 | 10.2 | 1.60 (0.91–2.80) | ||
| Cigarette smoking | ||||||||
| Past/Never | 291 | 31.6 | 1.00 | .51 | 285 | 7.4 | 1.00 | .80 |
| Current | 484 | 34.1 | 1.12 (0.81–1.54) | 476 | 8.2 | 1.08 (0.61–1.92) | ||
| Alcohol consumption | ||||||||
| Past/Never | 191 | 36.1 | 1.00 | .18 | 193 | 8.8 | 1.00 | .89 |
| Current | 587 | 32.2 | 0.77 (0.52–1.13) | 571 | 7.5 | 1.05 (0.54–2.05) | ||
= prevalence. Each risk factor is in separate models.
Adjusted for age, gender, race, and center (except for gender and race).
Final multivariable models for any retinopathy and vision-threatening retinopathy for the total diabetic sample are listed Table 4. Significant independent predictors of any retinopathy were longer duration of diabetes, increased fasting glucose, greater waist-hip ratio, and use of diabetic medications. Significant independent predictors of vision-threatening retinopathy in the total diabetic sample were longer duration of diabetes, increased fasting glucose, greater waist-hip ratio, and increased systolic blood pressure. The pattern of associations was largely similar among the different racial/ethnic groups (data not shown). Table 4 also shows that the racial differences in the prevalence of any diabetic retinopathy and vision-threatening retinopathy were no longer significant when adjustment was made for other risk predictors.
TABLE 4.
Independent Predictors of Diabetic Retinopathy and Vision-Threatening Retinopathy, Total Diabetes Sample (N = 778)
| Any Retinopathy
|
Vision-Threatening Retinopathy
|
|||
|---|---|---|---|---|
| Risk Factor | OR (95% CI)* | P Value | OR (95% CI)* | P Value |
| Age | 0.97 (0.95–0.99) | .01 | 0.99 (0.95–1.02) | .46 |
| Gender, male vs female | 1.11 (0.77–1.61) | .58 | 0.68 (0.36–1.28) | .23 |
| Race | ||||
| White | 1.00 | .27 | 1.00 | .13 |
| Black | 1.57 (0.94–2.63) | 1.84 (0.67–5.05) | ||
| Hispanic | 1.44 (0.85–2.44) | 1.75 (0.63–4.85) | ||
| Chinese | 1.53 (0.80–2.94) | 3.24 (0.93–11.34) | ||
| Duration of diabetes, per 10 years | 2.98 (2.23–3.98) | <.001 | 3.72 (2.59–5.34) | <.001 |
| Serum glucose, per 10 mg/dl | 1.10 (1.07–1.14) | <.001 | 1.07 (1.01–1.13) | .02 |
| Waist hip ratio, per 0.1 unit | 1.58 (1.17–2.13) | .003 | 1.77 (1.05–2.98) | .03 |
| Systolic BP, per 10 mmHg | 1.08 (0.99–1.17) | .09 | 1.18 (1.03–1.35) | .02 |
| Use of diabetes medication, yes vs no | 1.61 (1.03–2.51) | .04 | 2.05 (0.66–6.34) | .21 |
Of any retinopathy and vision-threatening retinopathy, adjusted for all variables listed.
Other risk factors examined in this study that were not significantly associated with diabetic retinopathy, CSME, or vision-threatening retinopathy include HDL cholesterol, LDL cholesterol, triglycerides, self-reported kidney disease, use of oral contraceptives, use of antihypertensive medication, use of hormone replacement therapy, occupation, income, marital status, and health insurance status (data not shown).
DISCUSSION
Our study provides new data on the prevalence of and risk factors for diabetic retinopathy in four racial/ethnic groups participating in the MESA study, a prospective study of cardiovascular disease in six United States communities (Figure). We found an overall prevalence of retinopathy of 33.2%, CSME of 5.6%, and vision-threatening retinopathy (severe retinopathy or CSME) of 7.9% among participants with diabetes. The rates of diabetic retinopathy in whites in the MESA were lower than that reported in the WESDR,3 which was conducted approximately 20 years ago. In WESDR, among adult whites with diabetes 40 years and older, the prevalence of any retinopathy was 50%, and vision-threatening retinopathy was 10%.7 More contemporary studies suggest a lower prevalence of diabetic retinopathy.4–6 For example, in a recent metaanalysis of seven population-based studies that included whites, blacks, and hispanics, the prevalence of retinopathy was 35.8% and vision-threatening retinopathy 7.3%,7 similar to the current MESA analysis (33.2% and 7.9%, respectively), which also included Chinese Americans.
FIGURE.
Prevalence of diabetic retinopathy in the Multi-Ethnic Study of Atherosclerosis subdivided into minimal, mild-moderate, and severe-proliferative grades, in the total diabetes sample and in different racial/ethnic groups.
There are a number of possible explanations for the apparent decline in prevalence of diabetic retinopathy over time. First, it is possible that improvements in the clinical management of diabetes (eg, better glycemic and blood pressure control) may have led to a gradual decline in retinopathy frequency.32,33 Brown and colleagues32 observed that people with type 2 diabetes who were under care in the Kaiser Permanente Northwest health maintenance organization in 1997 to 1998 had lower rates of retinopathy as compared with patients with type 2 diabetes in the WESDR, which may reflect lower mean glycosylated hemoglobin levels (7.9% vs 10.4%) and lower mean systolic blood pressure levels (139 vs 147 mmHg) in their study compared with the WESDR cohort. Second, more recent studies may have included participants who had diabetes diagnosed earlier in the course of the disease, through screening programs or other processes.34 Third, the changing definition of diabetes from fasting blood glucose of 140 mg/dl or higher to 126 mg/dl or higher may have led to inclusion of subjects with diabetes in more contemporary studies, such as the MESA, with less severe diabetes. Finally, the lower prevalence of retinopathy in the MESA may be related to the use of 45 degree nonstereoscopic, nonmydriatic photographs to grade retinopathy.35 In other studies,3–5 such as in the WESDR,3 retinopathy was detected by grading of seven stereoscopic fundus photographs. In a pilot study of 72 eyes of 36 subjects with diabetes, retinopathy lesions were detected more frequently from seven stereoscopic film photographs than from nonstereoscopic, nonmydriatic digital photographs (Moss SE, ARVO Meeting, 2004, abstract). Thus, the lack of stereoscopic effect and the lower magnification of photographs taken with the nonmydriatic camera may explain the lower rates of retinopathy and macular edema in the MESA as compared with the WESDR.
There are limited epidemiological studies of diabetic retinopathy on non-whites,17–19 and few have examined racial/ethnic differences in prevalence of diabetic retinopathy,24,25,36 despite substantial data that demonstrate variation in diabetes prevalence by racial/ethnic group.21–23 Three population-based studies, the Atherosclerosis Risk in Communities (ARIC) study,24 the National Health and Nutrition Examination Survey (NHANES) III,25 and the Cardiovascular Health Study,36 showed that retinopathy was more prevalent in blacks with type 2 diabetes than in whites. In the ARIC study, the higher prevalence of retinopathy in blacks (27.7%) as compared with whites (16.7%) largely disappeared after controlling for differences in glycemic control, duration of diabetes, and blood pressure, which suggests that these factors may explain differences in retinopathy prevalence between whites and blacks in the ARIC study cohort.24 Similarly, hispanics have been suggested to have higher prevalence of diabetic retinopathy, although factors that may explain differences between hispanics and whites are less clear.19,20,26 In one study, despite adjustment for glycemic levels and other risk factors, diabetic retinopathy was still twice as common in hispanics living in San Antonio, Texas, USA as compared with whites in Madison, Wisconsin, USA.19 Similarly, in the NHANES III, despite controlling for duration of diabetes and levels of glycemia and blood pressure, hispanics had higher prevalence and more severe retinopathy than whites in that study.25
In the current study, we showed that blacks and hispanics had a significantly higher prevalence of diabetic retinopathy than whites and chinese. These differences were attenuated when analyses accounted for diabetes duration, glycemic levels, and increased waist-hip ratio, suggesting that these risk factors may in part explain the higher rates of retinopathy in blacks and hispanics. It is possible that other factors not examined here, such as racial/ethnic variation in the time of diabetes diagnosis (eg, blacks and hispanics with diabetes may be diagnosed later than whites and chinese), may also explain these differences.
To our knowledge, the MESA provides the first data on the prevalence of diabetic retinopathy in Chinese Americans in the United States. The prevalence among the chinese sample (25.7%) was similar to whites (24.8%) in the MESA and is consistent with the findings of a clinic-based study of 2131 patients with type 2 diabetes attending a Beijing Hospital in China (27.3%).37 We note that vision-threatening retinopathy was higher in chinese women (12.5%) than men (1.9%), and was seen across all age groups. This observation, which is based on very small numbers of this endpoint in MESA, may deserve further attention and additional studies.
In the MESA, the prevalence of diabetic retinopathy did not vary substantially by age, a finding consistent with other studies.7 This contrasts with the WESDR, which showed the prevalence of retinopathy declined in older people,3 perhaps reflecting increased diabetes severity and a higher risk of cardiovascular diseases and other diabetes complications in older people. In addition, in that study, younger persons with type 2 diabetes were more likely to require insulin and have poorer glycemic control. With improved survival of patients with diabetes, it is possible that this pattern may not be as distinctive in more contemporary populations such as in the MESA.
With regard to risk factors for diabetic retinopathy, longer duration of disease, increased fasting glucose, greater waist-hip ratio, and use of diabetic medications or insulin were significant independent predictors of any retinopathy, whereas longer duration of diabetes, increased fasting glucose, greater waist-hip ratio, and increased systolic blood pressure were significant independent predictor of vision-threatening retinopathy. The role of diabetes duration, hyperglycemia, and hypertension in retinopathy development is well established and has been confirmed in the WESDR3 and in clinical trials.38–40
We did not find associations of plasma lipids and either retinopathy or macular edema. Data from the Early Treatment Diabetic Retinopathy Study showed that higher levels of serum lipids (LDL cholesterol, very LDL cholesterol, and triglycerides) were associated with increased risk of HE in the macula and vision loss in persons with diabetes.12 In the WESDR, however, associations between serum lipids and HE were found only in persons with type 1 but not type 2 diabetes.13 Ongoing randomized controlled clinical trials will provide further insights into the role of plasma lipids in incidence and progression of retinopathy in persons with diabetes.
Diabetic retinopathy was also associated with larger waist-hip ratio in the MESA cohort, independent of diabetes duration and glycemia levels. The association of obesity and diabetic retinopathy has not been consistently demonstrated in all studies.15,16 In the WESDR, for example, higher BMI was related to retinopathy only in the type 2 participants with diabetes who did not use insulin.16 In addition, there was a U-shaped relationship between both lower and higher BMI and retinopathy, which may reflect a “severe” phase of diabetes in underweight type 2 subjects, or these underweight patients may be a subset of late-onset type 1 diabetes.16 However, we could not confirm a U-shaped pattern in the MESA (data not shown).
The strengths of this study include the high frequency of gradeable fundus photographs and the use of objective means of detecting retinopathy by standardized grading protocols. Limitations of this study should also be noted. First, as discussed above, subtle differences in prevalence and risk factors of diabetic retinopathy in the MESA compared with other studies may be related to differences in retinal photography (eg, 45 degree nonstereoscopic digital photographs were taken without pharmacological dilation in MESA, as compared with 30 degree stereoscopic film-based photographs taken with pharmacological mydriasis in other studies3–5). Second, the cross-sectional nature of the study limits our ability to judge the temporal sequence of some of the associations reported. Third, although the MESA cohort is community based, the sampling scheme was not designed to be statistically representative of the population, and therefore estimates of the prevalence of diabetic retinopathy are not directly generalizable to the United States. Finally, the participation rate was approximately 30% of those contacted, which was low enough that bias could have been introduced, although similar recruitment methods were used in all ethnic groups, and the participation among those screened (for whom race/ethnicity was collected) was 70% of whites, 61% of blacks, 59% of hispanics, and 48% of chinese. Exclusion of persons with symptomatic cardiovascular disease could also have operated differentially across ethnic groups as a result of differences in access to care and diagnosis of disease.
In summary, the current MESA provides new data on the frequency of risk factors for diabetic retinopathy in four racial/ethnic populations in six United States communities. In this sample, we showed that approximately one in three persons with diabetes have some retinopathy, and one in 12 have signs of vision-threatening retinopathy (severe nonproliferative, proliferative retinopathy, or CSME). We confirm the strong independent associations of diabetic retinopathy with classic risk factors, including duration of diabetes, hyperglycemia, and hypertension.
REPORTING VISUAL ACUITIES
The AJO encourages authors to report the visual acuity in the manuscript using the same nomenclature that was used in gathering the data provided they were recorded in one of the methods listed here. This table of equivalent visual acuities is provided to the readers as an aid to interpret visual acuity findings in familiar units.
Table of Equivalent Visual Acuity Measurements
| Snellen Visual Acuities
|
||||
|---|---|---|---|---|
| 4 Meters | 6 Meters | 20 Feet | Decimal Fraction | LogMar |
| 4/40 | 6/60 | 20/200 | 0.10 | +1.0 |
| 4/32 | 6/48 | 20/160 | 0.125 | +0.9 |
| 4/25 | 6/38 | 20/125 | 0.16 | +0.8 |
| 4/20 | 6/30 | 20/100 | 0.20 | +0.7 |
| 4/16 | 6/24 | 20/80 | 0.25 | +0.6 |
| 4/12.6 | 6/20 | 20/63 | 0.32 | +0.5 |
| 4/10 | 6/15 | 20/50 | 0.40 | +0.4 |
| 4/8 | 6/12 | 20/40 | 0.50 | +0.3 |
| 4/6.3 | 6/10 | 20/32 | 0.63 | +0.2 |
| 4/5 | 6/7.5 | 20/25 | 0.80 | +0.1 |
| 4/4 | 6/6 | 20/20 | 1.00 | 0.0 |
| 4/3.2 | 6/5 | 20/16 | 1.25 | −0.1 |
| 4/2.5 | 6/3.75 | 20/12.5 | 1.60 | −0.3 |
| 4/2 | 6/3 | 20/10 | 2.00 | −0.3 |
From Ferris FL III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol 1982;94:91–96.
Acknowledgments
Supported in part by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. A full list of participating MESA investigators and institutions is available at http://www.mesa-nhlbi.org. Additional support was provided by National Institutes of Health grants HL69979-03 (R.K. and T.Y.W.). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article in manuscript.
Biography
Biosketch
Tien Yin Wong, MD, PhD, is an Associate Professor in Ophthalmology at the Centre for Eye Research Australia, University of Melbourne, and a Retinal consultant at the Royal Victorian Eye and Ear Hospital. He has a PhD in Epidemiology from the Johns Hopkins University. His clinical and research interest is in retinal vascular diseases, including diabetic and hypertensive retinopathy. He is on the Editorial Board of the British Journal of Ophthalmology and IOVS.

Footnotes
See accompanying Editorial on page 539.
References
- 1.Klein R, Klein BE. Vision disorders in diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, editors. Diabetes in America. 2. Bethesda, MD: National Institute of Health; 1995. pp. 293–338. NIH Publication 95-1468. [Google Scholar]
- 2.Fong DS, Aiello L, Gardner TW, et al. for the American Diabetes Association. Diabetic retinopathy. Diabetes Care. 2003;26:S99–S102. doi: 10.2337/diacare.26.2007.s99. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Linton KL. The Beaver Dam Eye Study: retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology. 1992;99:58–62. doi: 10.1016/s0161-6420(92)32011-1. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell P, Smith W, Wang JJ, Attebo K. Prevalence of diabetic retinopathy in an older community: the Blue Mountains Eye Study. Ophthalmology. 1998;105:406–411. doi: 10.1016/S0161-6420(98)93019-6. [DOI] [PubMed] [Google Scholar]
- 6.McKay R, McCarty CA, Taylor HR. Diabetic retinopathy in Victoria, Australia: the Visual Impairment Project. Br J Ophthalmol. 2000;84:865–870. doi: 10.1136/bjo.84.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempen JH, O’Colmain BJ, Leske MC, et al. for the Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Moss SE, et al. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988;260:2864–2871. [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy. Arch Intern Med. 1994;154:2169–2178. [PubMed] [Google Scholar]
- 10.UK Prospective Diabetes Study Group (UKPDS) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes. Br Med J. 1998;317:703–715. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferris FL, 3rd, Chew EY, Hoogwerf BJ Early Treatment Diabetic Retinopathy Study Research Group. Serum lipids and diabetic retinopathy. Diabetes Care. 1996;19:1291–1293. doi: 10.2337/diacare.19.11.1291. [DOI] [PubMed] [Google Scholar]
- 12.Chew EY, Klein ML, Ferris FL, 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 13.Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, XII: relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–1265. doi: 10.1016/s0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- 14.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 15.van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003;121:245–251. doi: 10.1001/archopht.121.2.245. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Moss SE. Is obesity related to microvascular and macrovascular complications in diabetes? The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med. 1997;157:650–656. [PubMed] [Google Scholar]
- 17.West SK, Klein R, Rodriguez J, et al. Diabetes and diabetic retinopathy in a Mexican American population: Proyecto VER. Diabetes Care. 2001;24:1204–1209. doi: 10.2337/diacare.24.7.1204. [DOI] [PubMed] [Google Scholar]
- 18.Varma R, Torres M, Pena F, Klein R, Azen SP for the Los Angeles Latino Eye Study Group. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1298–1306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM, Fong D, Stern MP, et al. Diabetic retinopathy in Mexican Americans and non-hispanic whites. Diabetes. 1988;37:878–884. doi: 10.2337/diab.37.7.878. [DOI] [PubMed] [Google Scholar]
- 20.Hamman RF, Mayer EJ, Moo-Young GA, Hildebrandt W, Marshall JA, Baxter J. Prevalence and risk factors of diabetic retinopathy in non-hispanic whites and hispanics with NIDDM: San Luis Valley Diabetes Study. Diabetes. 1989;38:1231–1237. doi: 10.2337/diab.38.10.1231. [DOI] [PubMed] [Google Scholar]
- 21.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien TR, Flanders WD, Decoufle P, Boyle CA, DeStefano F, Teutsch S. Are racial differences in the prevalence of diabetes in adults explained by differences in obesity? JAMA. 1989;262:1485–1488. [PubMed] [Google Scholar]
- 23.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403–408. doi: 10.2337/diacare.22.3.403. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Sharrett AR, Klein BE, et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the Atherosclerosis Risk in Communities Study. Ophthalmology. 2002;109:1225–1234. doi: 10.1016/s0161-6420(02)01074-6. [DOI] [PubMed] [Google Scholar]
- 25.Harris MI, Klein R, Cowie CC, et al. Is the risk of diabetic retinopathy greater in non-hispanic blacks and Mexican Americans than in non-hispanic whites with type 2 diabetes? A US population study. Diabetes Care. 1998;21:1230–1235. doi: 10.2337/diacare.21.8.1230. [DOI] [PubMed] [Google Scholar]
- 26.Haffner SM, Hazuda HP, Stern MP, et al. Effects of socioeconomic status on hyperglycemia and retinopathy levels in Mexican Americans with NIDDM. Diabetes Care. 1989;12:128–134. doi: 10.2337/diacare.12.2.128. [DOI] [PubMed] [Google Scholar]
- 27.Bild DE, Bluemke DA, Burke GL, et al. The Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 28.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 29.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 30.Diabetic Retinopathy Study Group. Diabetic retinopathy study. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21:210–226. [PubMed] [Google Scholar]
- 31.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertension. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Brown JB, Pedula KL, Summers KH. Diabetic retinopathy: contemporary prevalence in a well-controlled population. Diabetes Care. 2003;26:2637–2642. doi: 10.2337/diacare.26.9.2637. [DOI] [PubMed] [Google Scholar]
- 33.Ozmen B, Boyvada S. The relationship between self-monitoring of blood glucose control and glycosylated haemoglobin in patients with type 2 diabetes with and without diabetic retinopathy. J Diabetes Complications. 2003;17:128–134. doi: 10.1016/s1056-8727(02)00200-3. [DOI] [PubMed] [Google Scholar]
- 34.Klein R. Has the frequency of proliferative diabetic retinopathy declined in the US? Diabetes Care. 2003;26:2691–2692. doi: 10.2337/diacare.26.9.2691. [DOI] [PubMed] [Google Scholar]
- 35.Liu DP, Molyneaux L, Chua E, et al. Retinopathy in a Chinese population with type 2 diabetes: factors affecting the presence of this complication at diagnosis of diabetes. Diabetes Res Clin Pract. 2002;56:125–131. doi: 10.1016/s0168-8227(01)00349-7. [DOI] [PubMed] [Google Scholar]
- 36.Klein R, Klein BEK, Neider MW, Hubbard LD, Meuer SM, Brothers RJ. Diabetic retinopathy as detected using ophthalmoscopy, a nonmydriatic camera and a standard fundus camera. Ophthalmology. 1985;92:485–491. doi: 10.1016/s0161-6420(85)34003-4. [DOI] [PubMed] [Google Scholar]
- 37.Klein R, Marino EK, Kuller LH, et al. The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:84–90. doi: 10.1136/bjo.86.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 39.UK Prospective Diabetes Study Group (UKPDS) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. UKPDS 33. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 40.UK Prospective Diabetes Study Group (UKPDS) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes. UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]