Abstract
In most cell types, a key event in apoptosis is the release of proapoptotic intermembrane space proteins from mitochondria to the cytoplasm. In general, it is the release of these intermembrane space proteins that is responsible for the activation of caspases and DNases that are responsible for the execution of apoptosis. The mechanism for the increased permeability of the mitochondrial outer membrane during the induction phase of apoptosis is currently unknown and highly debated. This review will focus on one such proposed mechanism, namely, the formation of ceramide channels in the mitochondrial outer membrane. Ceramides are known to play a major regulatory role in apoptosis by inducing the release of proapoptotic proteins from the mitochondria. As mitochondria are known to contain the enzymes responsible for the synthesis and hydrolysis of ceramide, there exists a mechanism for regulating the level of ceramide in mitochondria. In addition, mitochondrial ceramide levels have been shown to be elevated prior to the induction phase of apoptosis. Ceramide has been shown to form large protein permeable channels in planar phospholipid and mitochondrial outer membranes. Thus, ceramide channels are good candidates for the pathway with which proapoptotic proteins are released from mitochondria during the induction phase of apoptosis.
Keywords: Ceramide, mitochondria, ceramide synthase, Bcl-2, apoptosis, sphingosine, cytochrome c, channel
INTRODUCTION
Early in apoptosis, there is often an increase in the permeability of the mitochondrial outer membrane that leads to the release of intermembrane space proteins, including cytochrome c, procaspases, apoptosis-inducing factor (AIF), heat shock proteins, Smac/Diablo, and endonuclease G (for review see Saelens et al., 2004). The release of intermembrane space proteins into the cytoplasm is crucial for the activation of specific caspases and DNases that are responsible for the execution of apoptosis. The mechanism for the increased permeability of the mitochondrial outer membrane during the induction phase of apoptosis is currently unknown and highly debated. It is thought to occur by either the permeability transition or the creation of a pore in the mitochondrial outer membrane (for review see Henry-Mowatt et al., 2004). Several pathways have been proposed for this pore. This review will focus on one such proposed pathway, namely, ceramide channels.
Ceramide is a membrane sphingolipid that is comprised of an N-acylated (14–26 carbons) sphingosine (18 carbons); carbons 1–5 of the sphingosine backbone constitute the biologically important part of the molecule and consist of hydroxyl groups at C1 and C3, a trans double bond across C4 and C5, and an amide group that serves as the fatty acyl linkage at C2 (Fig. 1(a) and Pettus et al., 2002). Ceramide is known to regulate several cellular processes, including differentiation, growth suppression, cell senescence, and apoptosis. In fact, the role of ceramide specifically in apoptosis has attracted much attention in recent years. Recent evidence suggests that ceramide-induced apoptosis is due at least in part to its ability to form protein-permeable channels in mitochondrial membranes. Since mitochondria contain the enzymes responsible for ceramide synthesis and hydrolysis, and mitochondrial ceramide levels increase prior to the mitochondrial stage of apoptosis, there exists a mechanism for controlling the permeability of the mitochondrial outer membrane via regulation of mitochondrial ceramide levels.
Fig. 1.

Ceramides form channels in phospholipid membranes. (a) General structure of a ceramide molecule, which consists of a sphingoid base backbone and a N-linked fatty acyl chain that can vary in length from a n = 1 to more than 23. (b) Example ceramide conductance increments observed following the addition of 5 µM C22-ceramide to the aqueous phase on one side of a solvent free planar phospholipid membrane formed from monolayers composed of 0.5% (w/v). DiPyPC, 0.5% (w/v) asolectin, 0.2% (w/v) cholesterol. The applied voltage was clamped at 10 mV (trans side was ground). The aqueous solution bathing both sides of the membrane was composed of 1 M KCl, 1 mM MgCl2, 5 mM PIPES (pH 6.8).
GENERATION OF CERAMIDE
Ceramide can be generated in cells by sphingomyelin hydrolysis or de novo synthesis. Sphingomyelinases (SMases) catalyze the hydrolysis of sphingomyelin to form ceramide and phosphocholine and are classified by their pH optima and/or subcellular location (for reviews see Marchesini and Hannun, 2004; Samet and Barenholz, 1999; Stoffel, 1999). Acid SMase is localized mainly in the lysosomes and has optimal enzymatic activity at ∼ pH 4.5–5 (see Stoffel, 1999 for review). However, plasma membrane (in caveolae, Liu and Anderson, 1995) and secreted forms (Tabas, 1999) of acidic SMase have also been identified. There are neutral SMases that are further classified as Mg2+/Mn2+ dependent or independent. Neutral SMases have optimal activity at a neutral pH and are mainly located in the plasma membrane, cytosol, endoplasmic recticulum or nuclear membranes (Chaterjee, 1999; Liu and Hannun, 1997; Samat and Barenholz, 1999; Birbes et al., 2002). An alkaline SMase exists, that is activated by bile salts and is thought to exist only in intestinal cells (Nilsson and Duan, 1999).
The de novo synthesis of ceramide occurs in the endoplasmic recticulum (ER) (Mandon et al., 1992) and in mitochondria (Bionda et al., 2004; Shimeno et al., 1998) and begins with the condensation of serine and palmitoyl-CoA to form 3-ketosphinganine as catalyzed by serine palmitoyl transferase. 3-Ketosphinganine is then reduced to form sphinganine, which is acylated to generate dihydroceramide. The acylation of sphinganine to form dihydroceramide is catalyzed by ceramide synthase. A trans double bond is then introduced at the 4–5 position to generate ceramide. The enzyme ceramide synthase can utilize both sphingosine and sphinganine as substrates, and thus, ceramide can be directly generated from sphingosine and a fatty acyl-CoA. Ceramide synthase has been localized to the endoplasmic recticulum (Michel and van Echten-Deckert, 1997) and mitochondrial outer and inner membranes (Bionda et al., 2004).
Ceramide serves as the precursor to other sphingolipids. Ceramide can be glycosylated to form complex glycosphingolipids in the Golgi apparatus or can be used to form sphingomyelin as catalyzed by sphingomyelin synthase. Sphingomyelin synthase catalyzes the transfer of a phosphocholine headgroup from phosphatidylcholine to ceramide to generate sphingomyelin and diacylglycerol. In addition, ceramidases catalyze the breakdown of ceramide via the removal of the N-linked fatty acyl chain to form sphingosine. Three different types of mammalian ceramidases have been identified. Acid ceramidases are lysosomal (Gatt, 1963), whereas neutral/alkaline ceramidases have been localized to mitochondria (Bionda et al., 2004; El Bawab et al., 2000), nuclear membranes (Shiraishi et al., 2003), and the endoplasmic recticulum (Mao et al., 2001). A purely alkaline ceramidase has been localized to the Golgi apparatus and endoplasmic reticulum (Mao et al., 2001; Birbes et al., 2002). The neutral/alkaline ceramidase has also been shown to catalyze the reverse reaction to generate ceramide from sphingosine and fatty acids (Gatt, 1966; Tani et al., 2000; Mao et al., 2000; El Bawab et al., 2001). Mitochondria have been shown to be capable of generating ceramide via the action of reverse ceramidase (Bionda et al., 2004; El Bawab et al., 2000; Siskind et al., 2005).
Sphingosine generated via ceramidase catalyzed hydrolysis of ceramide, other than being used to reform ceramide, can also be used to generate sphingosine-1-phosphase. Phosphorylation of sphingosine is catalyzed by sphingosine kinase, which is known to exist in the cytosol and endoplasmic recticulum (Ghosh et al., 1994; Olivera et al., 1999). Sphingosine-1-phosphate has been shown to be protective/anti-apoptotic. In fact, it has been proposed that it is the ratio of sphingosine-1-phosphate to ceramide/sphingosine that determines the fate of a cell (Cuvillier et al., 1996; Olivera and Spiegel, 1993).
A CONSERVED ROLE FOR CERAMIDE IN APOPTOSIS
There are a number of observations that support a proapoptotic role for ceramide in apoptosis. First, ceramide generation is a common cellular response of a variety of cell types following exposure to apoptosis-inducing agents. These include TNFα (Obeid et al., 1993; Modur et al., 1996; García-Ruiz et al., 1997; Geilen et al., 1997), interleukin-1 (Masamune et al., 1996), CD95/Fas/APO-1 (Cifone et al., 1994; Cremesti et al., 2001; Brenner et al., 1998; Gulbins et al., 1995; Paris et al., 2001; Tepper et al., 1995), γ-interferon (Birbes et al., 2002), NO (Takeda et al., 1999), ionizing radiation (Vit and Rosselli, 2003; Alphonse et al., 2002), serum withdrawal (Caricchio et al., 2002), heat (Jenkins, 2003), etoposide (Sawada et al., 2000a,b), staurosporine (Wiesner and Dawson, 1996), daunorubicin (Come et al., 1999), and the corticosteroid dexamethasone (Cifone et al., 1999). Second, the effective doses of these agents required to induce ceramide generation closely matches the dose required to induce apoptosis (Kolesnick and Kröonke, 1998). Third, elevations in cellular ceramide in response to these agents occurs prior to the execution phase of apoptosis (for example, Birbes et al., 2002; Hannun, 1996; Dbaibo et al., 1997). Fourth, exogenous addition of cell-permeable ceramide analogues induces apoptosis in a variety of cell lines (for example, Obeid et al., 1993; Jarvis et al., 1994; Quintans et al., 1994; Cifone et al., 1994). Fifth, ceramide-induced apoptosis is very specific, as the naturally occurring ceramide precursor dihydroceramide (lacks the 4–5 trans double bond present in ceramide) does not induce apoptosis (Obeid et al., 1993; Bielawska et al., 1993). Sixth, apoptosis can be inhibited upon blockage of ceramide generation and cells that are incapable of generating ceramide are often incapable of undergoing apoptosis (Selzner et al., 2001; Chmura et al., 1997a,b; Bruno et al., 1998; Sautin et al., 2000; Alphonse et al., 2002; Riboni et al., 2002).
Apoptosis can often be induced in cancer cells by elevation of endogenous ceramide levels via the addition of inhibitors of ceramide metabolism (for example, Chmura et al., 1997a; Raisova et al., 2002; Rodriguez-Lafrasse et al., 2002; Alphonse et al., 2004; Bielawska et al., 1996; Selzner et al., 2001) or by exogenously added cell-permeable ceramide analogues (for example, Sautin et al., 2000; Lozano et al., 2001; Selzner et al., 2001; Kimura et al., 2003). Ceramidase inhibitors have been shown to elevate ceramide levels and induce apoptosis in HL-60 leukemia cells (Bielawska et al., 1996) human colon cancer cells (Selzner et al., 2001), HaCaT keratinocytes, and human melanoma cells (Raisova et al., 2002). Defective ceramide generation has been correlated with resistance to radiation-induced apoptosis (Chmura et al., 1997a,b; Bruno et al., 1998; Sautin et al., 2000; Alphonse et al., 2002). In fact, Alphonse et al., 2002 demonstrated that SQ20B adenocarcinoma cells are resistant to both anti-Fas and γ-irradiation because of an inability to generate ceramide. In a further study, they showed that the apoptotic death pathway is present, but not functional, in the absence of a certain threshold of ceramide generation (Alphonse et al., 2004). Thus, ceramide clearly plays an important role in apoptosis induction. However, the mechanism by which ceramide induces apoptosis is still highly debated. It is thought that ceramide-induced apoptosis involves a direct action of ceramide on mitochondria.
MITOCHONDRIAL CERAMIDE GENERATION AND APOPTOSIS
Mitochondria have been shown to contain ceramide with a three-fold higher concentration in the outer membranes than the inner membranes (Ardail et al., 2001). In fact, mitochondria isolated from healthy rat livers have higher levels of dihydroceramide (lacks the 4–5 trans double bond present in ceramide, not apoptotic) than ceramide (Ardail et al., 2001). This is not surprising given the fact that higher levels of ceramide in mitochondria would not be expected in healthy cells. Thus, conversion of dihydroceramide to ceramide would be one way to rapidly generate ceramide in mitochondria and induce apoptosis.
Increases in cellular ceramide levels during apoptosis have been shown to occur prior to the mitochondrial phase of apoptosis (Witty et al., 1996; Raisova et al., 2000; Bose et al., 1995; Charles et al., 2001; Rodriguez-Lafrasse et al., 2001; Kroesen et al., 2001; Thomas et al., 1999). As stated above, mitochondria contain the enzymes responsible for ceramide synthesis and hydrolysis, namely, ceramide synthase and ceramidase (El Bawab et al., 2000; Shimeno et al., 1998; Bionda et al., 2004). In fact, Bionda et al., 2004 reconfirmed the presence of ceramide synthase and ceramidase in ultrapure mitochondria free from contaminating endoplasmic recticulum membranes (mitochondrial associated membranes; MAM), and microsomes. They were able to show that both mitochondrial outer and inner membranes are capable of generating ceramide. CD95-, TNFα-radiation, and UV-induced apoptosis have all been shown to occur via an increase in mitochondrial ceramide levels (Birbes et al., 2005; Vance, 1990; Garcia-Ruiz et al., 1997; Matsko et al., 2001; Dai et al., 2004). Garcia-Ruiz et al., 1997 showed that mitochondria isolated from TNF treated cells had a two- to three-fold increase in ceramide levels than mitochondria from untreated cells. A similar increase in mitochondrial ceramide levels was also shown by Birbes et al., 2005 in TNF treated MCF7 breast cancer cells. Dai et al., 2004 showed that UV irradiation of HeLa cells results in increased mitochondrial ceramide levels that preceded cytochrome c release and apoptosis. Inhibitors of sphingolipid metabolism that prevented ceramide synthesis after UV irradiation also prevented apoptosis (Dai et al., 2004). Birbes et al., 2001, targeted the bacterial sphingomyelinase protein (bSMase) to different intracellular locations in MCF7 breast cancer cells in order to generate ceramide solely in these locations. Only when the bSMase was targeted to mitochondria and ceramide generated in mitochondria was there cytochrome c release and apoptosis. A mutant inactive bSMase targeted to mitochondria resulted in no ceramide generation and no apoptosis. Targeting the bSMase to the plasma membrane, cytoplasm, endoplasmic reticulum, the Gogli, and the nucleus did not result in apoptosis despite the generation of ceramide in these compartments. Thus, ceramide-induced apoptosis occurs at the level of mitochondria.
Ceramides have been reported to have numerous affects on mitochondria, including enhanced generation of reactive oxygen species (Zamzami et al., 1995; Di Paolaet al., 2000; Quillet-Mary et al., 1997; France-Lanord et al., 1997; Garcia-Ruiz et al., 1997), alteration of calcium homeostasis of mitochondria and endoplasmic reticulum (Zamzami et al., 1995; Quillet-Mary et al., 1997; Garcia-Ruiz et al., 1997; Pinton et al., 2001; Muriel et al., 2000), ATP depletion (Arora et al., 1997), collapse in the inner mitochondrial membrane potential (Zamzami et al., 1995; Arora et al., 1997; Di Paola et al., 2000; Ghafourifar et al., 1999), inhibition and/or activation of the activities of various components of the mitochondrial electron transport chain (Di Paola et al., 2000; Gudz et al., 1997), and release of intermembrane space proteins (Ghafourifar et al., 1999; Di Paola et al., 2000, 2004; Siskind et al., 2002). Short-chain cell permeable ceramide analogues, such as N-acetyl-D-erythro-sphingosine (C2-ceramide) and N-hexanoyl-D-erythro-sphingosine (C6-ceramide) have been shown to induce cytochrome c release when added to whole cell cultures (for example, Zamzami et al., 1995; Castedo et al., 1996; Susin et al., 1997a,b; DeMaria et al., 1997; Zhang et al., 1997; Amarante-Mendes et al., 1998) and isolated mitochondria (Arora et al., 1997; Di Paola et al., 2000, 2004; Ghafourifar et al., 1999); this cytochrome c release was preventable by preincubation with or overexpression of the anti-death protein Bcl-2 (Ghafourifar et al., 1999; Zhang et al., 1996; Geley et al., 1997), or transfection of cells with Bcl-xL (Gottschalk et al., 1994; Wiesner et al., 1997). Long chain naturally occurring ceramides have also been shown to induce the release of both cytochrome c (Di Paola et al., 2000, 2004) from isolated mitochondria. In addition to cytochrome c, long- and short-chain ceramides have been shown to induce the release of apoptosis-inducing factor (AIF) (Di Paola et al., 2004), AK-2 (Di Paola et al., 2004), and adenylate kinase (Siskind et al., 2002) from isolated mitochondria. Until recently, it was not clear how ceramide increased the permeability of the mitochondrial outer membrane to intermembrane space proteins. Recent evidence indicates that it is due to a direct action of ceramide on the mitochondrial outer membrane. In fact, ceramides have been shown to form large protein permeable channels in planar phospholipid and mitochondrial outer membranes (Siskind and Colombini, 2000; Siskind et al., 2002, 2003).
CERAMIDE CHANNEL FORMATION
Both short- and long-chain naturally occurring ceramides form large channels in planar phospholipid membranes. The addition of ceramide to the aqueous phase on either one or both sides of a planar phospholipid membrane results in pore formation as indicated by discrete stepwise current increases (Siskind and Colombini, 2000; Siskind et al., 2002, 2003, 2005; Fig. 1(b)). These discrete increments in conductance are, by definition, channels, reflecting the formation of continuous water-filled pathways through the membrane. Ceramide channel formation requires the presence of the 4–5 trans double bond as dihydroceramide does not form channels even at concentrations up to 25 times higher than that required for ceramide channel formation (Siskind and Colombini, 2000). Recall that dihydroceramide is the metabolic precursor to ceramide. It does not induce cytochrome c release or apoptosis. Thus, the channel forming ability of ceramides correlates with its apoptotic activity.
Similar ceramide channels form in the mitochondrial outer membrane. Ceramide does not induce a cytochrome c secretion or release mechanism, but simply raises the permeability of the mitochondrial outer membrane, via ceramide channel formation, to include all small proteins. Evidence for ceramide channel formation in mitochondrial outer membranes comes from work with isolated mitochondria. Ceramide channels allow not only the release of cytochrome c (Arora et al., 1997; Di Paola et al., 2000, 2004; Ghafourifar et al., 1999; Siskind et al., 2005), but also its bi-directional flux across the mitochondrial outer membrane (Fig. 2(a); Siskind et al., 2002). Entry to and exit from the intermembrane space through ceramide channels was measured by monitoring the oxidation of exogenously-added reduced cytochrome c to isolated mitochondria. Because of the exceedingly small volume of the mitochondrial intermembrane space, cytochrome c would need to cross the outer membrane twice for appreciable oxidation to take place and be detected spectrophotometrically. Mitochondria with intact outer membranes or mitochondria exposed to dihydroceramide were unable to oxidize exogenously-added cytochrome c. However, short- and long-chain ceramides caused a rapid increase in the rate of cytochrome c oxidation in a concentration and time-dependent manner (Siskind et al., 2002). Thus, the rate of oxidation of exogenously-added cytochrome c is a good measure of the permeability of the mitochondrial outer membrane. Importantly, ceramides form protein permeable channels in mitochondrial outer membranes at physiologically relevant concentrations. Until recently, it was not known whether the levels of ceramide required to induce cytochrome c release and/or the bidirectional flux of cytochrome c was physiologically relevant. However, we have found that less than 4% of the ceramide added to isolated mitochondria actually inserts into the membrane (L. Siskind unpublished observations). Thus, the insertion of only 4–8 pmol of ceramide per nanomole mitochondrial phospholipids is required for the formation of protein-permeable ceramide channels in the mitochondrial outer membrane. This level of ceramide is on the order of the level of mitochondrial ceramide increase found during the induction phase of apoptosis (before or at the time of cytochrome c release; Birbes et al., 2005).
Fig. 2.

Ceramides form protein permeable channels in mitochondrial outer membranes of isolated mitochondria. (a) Ceramide addition to isolated rat liver mitochondrial suspensions allows the oxidation of exogenously added reduced cytochrome c. Mitochondria were incubated with the following treatments: mitochondria controls (M; untreated mitochondria, vehicle controls); 20 µM C2-dihydroceramide for 10 min (DH); 20 µM C2-ceramide for 10 min (C2); 20 µM C16-ceramide for 10 min (C16); mitochondria with lysed outer membranes were incubated with 20 µM C2-dihydroceramide for 10 min (L-DH); lysed mitochondrial controls (L; untreated lysed mitochondria, lysed mitochondria incubated with C2- and C16-ceramide for 10 min, lysed mitochondria vehicle controls). Reduced cytochrome c was then added and its oxidation monitored by measuring the absorbance decrease at 550 nm. (b) The permeability increase induced by C2-ceramide can be reversed with bovine serum albumin (BSA). Isolated mitochondria were incubated with 20 µM C2-ceramide and were indicated 83 µM BSA for the indicated time periods. Reduced cytochrome c was then added and the absorbance was then monitored at 550 nm. (c) C2- and C16-ceramide increase the permeability of the mitochondrial outer membrane to intermembrane space proteins with a cut-off of 60 kDa. Mitochondria were incubated with C2- or C16-ceramides or C2-dihydroceramide at a level of 18 nmol ceramide added/mg mitochondrial protein for 10 min. Released proteins were run on a 15% acrylamide SDS-PAGE and the gel stained with GelCode Blue stain (Pierce). Densitometry from the gel was performed and the individual background for each lane on the gel and the untreated mitochondrial control subtracted out. The results were expressed as a percent of the total protein (mitochondria with lysed outer membranes) versus the RF value.
Ceramide channels in mitochondrial outer membranes and planar phospholipid membranes are eliminated upon removal of ceramide. Thus, removal of short-chain ceramide from mitochondria using bovine serum albumin (BSA) results in the restoration of the permeability barrier of the mitochondrial outer membrane to cytochrome c (Fig. 2(b); Siskind et al., 2002). This was confirmed using 14C-ceramide (unpublished observations). Ceramide channels in planar phospholipid membranes can also be reversed/disassembled by removal with BSA (Siskind et al., 2002). The fact that the integrity of planar membranes and the outer membranes of isolated mitochondria suspensions can be restored upon removal of ceramide argues against an indirect action by ceramide.
Ceramide channels in mitochondrial outer membranes are not specific to cytochrome c. In fact, ceramide channels allow the release of low molecular weight proteins from mitochondria, but not high molecular weight ones. There is a sharp molecular weight cut-off of 60,000 for the size of intermembrane space proteins that are released through the ceramide channels (Fig. 2(c); Siskind et al., 2002). Even though this cut-off was measured under denaturing conditions and thus is most likely an underestimate, it is still in line with the size of proapoptotic proteins released from mitochondria during apoptosis (cytochrome c 12 kDa, (Dickerson et al., 1971); endonuclease G 28 kDa, (Schafer et al., 2004); AIF 57 kDa, (Mate et al., 2002); Smac/DIABLO 42 kDa, (Chai et al., 2001)).
The finding that ceramide forms large protein permeable channels in membranes is somewhat surprising given the fact that ceramide is a lipid. However, ceramides differ from many other lipids in that they have several hydrogen-bond donating and accepting groups, namely the two hydroxyl groups, the amide nitrogen, and the carbonyl oxygen (Fig. 1(a)). We developed a structural model to explain ceramide channel formation that is based on their ability to form intermolecular hydrogen bonds (Pascher et al., 1976; Moore et al., 1997) and extensive characterization of the channels from electrophysiological recordings. We propose the formation of columns of ceramides on the membrane surface, held together by intermolecular hydrogen bonds between amide nitrogens and carbonyl groups located on opposite surfaces of the ceramide molecule (Fig. 3(a) for the proposed structure of a ceramide column). These columns could swing into the membrane, forming an annulus stabilized by hydrogen bonding of the hydroxyl groups lining the channel (Fig. 3(b)). This hydrogen-bonded network would be similar to the structure of ice, and thus, should form a good interface between the water in the channel and the nonpolar portion of the ceramide molecule (Figs. 3(b) and (c) for a top and cut-away longitudinal view, respectively). Each transmembrane column would consist of 6–7 individual ceramide molecules (sufficient to span the membrane). This column has a dipole moment due to the alignment of the amide linkages, and thus, we propose that adjacent columns be oriented in opposite directions so that the dipoles attract (Fig. 3(d)). The number of columns in the annulus determines the size of the channel; the insertion or removal of columns would result in channel enlargement or contracture, respectively. We propose that the curvature of the headgroups of the surrounding phospholipids at the outer edge of the channel is such that they minimize the exposure of the hydrophobic regions of the channel edge (Fig. 3(c)).
Fig. 3.
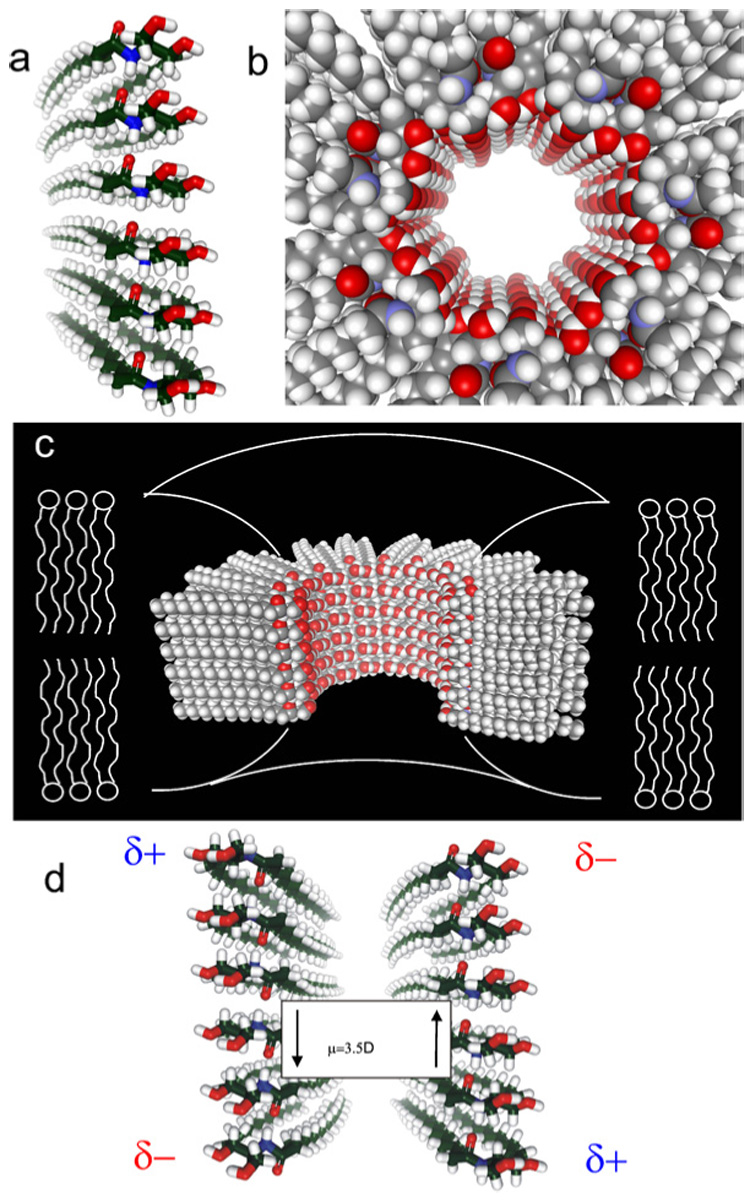
Structural model for ceramide channels. (a) A column of ceramide residues held together by intermolecular hydrogen bonds between amide nitrogens and carbonyl groups. This column would span the hydrophobic portion of the membrane and in association with other columns would form channels of varying sizes. (b) Top view of a ceramide channel consisting of 12 columns of ceramide molecules. Adjacent columns are oriented in an antiparallel fashion so that amide dipoles attract. The columns are held together via intermolecular hydrogen bonds between hydroxyl groups proposed to line the channel lumen. (c) A longitudinal cut-away of a ceramide channel, consisting of 14 columns of ceramide molecules, where 4 columns have been removed to show the interior of the channel. The curvature of the phospholipids of the membrane at the channel interface would minimize the exposure of the hydrophobic regions of the outer surface of the channel to the aqueous solution. (d) Two adjacent ceramide columns oriented in an antiparallel fashion so that opposite dipoles attract. This antiparallel orientation adds to the stability of the channel.
The above structural model is supported by data obtained from electrophyiological recordings of ceramide channels in planar phospholipid membranes. Ceramide channel conductance increments and decrements represent the enlargement and contracture of ceramide channels rather than the formation and dissolution of individual channels (Siskind et al., 2003). The primary evidence for channel enlargement stems from observations of multiple conductance increments followed by one large decrement to baseline (Siskind et al., 2003). Channel enlargement is supported by selectivity measurements; increases in channel size are usually accompanied by a decrease in ion selectivity, whereas channels conducting in parallel have the same selectivity. Initial ceramide conductances favor cations, but this selectivity drops dramatically with increasing total membrane conductance until the ceramide channel is no longer selective (Siskind et al., 2003, 2005), indicating channel enlargement. La+3 induces rapid ceramide channel disassembly in a manner indicative of large conducting structures (Siskind et al., 2003). The addition of La+3 to the ceramide channels is followed by a brief delay, in which the total membrane conductance remains constant, and then a sudden decrease in conductance to baseline (Siskind et al., 2003). This is distinctly different from the exponential conductance decrease that is expected for the inhibition of a population of channels.
Ceramide channels have a propensity to contract by a defined size. Larger ceramide conductance decrements tend to occur as multiples of 4 nS (Siskind et al., 2003), which indicates that the channels disassemble by means of a fundamental structural unit. This structural unit cannot be a single ceramide molecule. Rather, a ceramide column or pairs of ceramide columns (see Fig. 3(b) for the structure of a ceramide column) as in a barrel-stave model may represent the larger structural unit. The conductance of a channel (G) can be calculated from the radius and length of the channel as follows (Hille, 1992):
| (1) |
where r is the radius of the channel, κsp the specific conductance (equal to 112 for 1.0M KCl; Robinson and Stokes, 1965) and L the length of the channel (estimated to be ∼5 nm). The length of the channel is increased by 0.5(πr) to take into account the axis resistance. For very large channels, the channel length is negligible as compared to its radius. Thus, the conductance of the channel is proportional not to the cross-sectional area (πr²), but to the radius or circumference of the channel. According to the above structural model for ceramide channels, the circumference is equal to the number of ceramide columns in the channel multiplied by the width of a ceramide column. Hence, the change in the conductance is proportional to a change in the number of columns comprising the ceramide channel and equation (1) becomes:
| (2) |
at 1 M KCl and G in nS (see Siskind et al., 2003 for an in-depth description and underlying mathematical details). Because the number of columns lost from the channel must always be an integer, the change in conductance would equal to 4 nS if the column width were 1.12 nm. This is compatible with the dimension of two ceramide columns, indicating that the fundamental unit of disassembly is a two-column unit. Thus, two columns may line up in opposite directions resulting in a favorable dipole–dipole interaction (Fig. 3(d)).
The larger conductance decrements occurring as multiples of 4 nS is only consistent with ceramide channels that are cylindrical structures with preferred diameters rather than a continuum of sizes or shapes (Siskind et al., 2003). The electrophysiological data provides evidence that ceramide channels are not flaccid structures, but rather rigid cylinders whose structure is determined by the hydrogen-bonded network forming the inner lining of the channel. Other models that propose that ceramides act by simply disrupting the membrane or forming rafts that result in defects between these and the surrounding phospholipids are all in sharp disagreement with the observed behavior of ceramide channels. Taken together, the data indicates that ceramides form barrel-stave channels whose size can change by the loss or insertion of multiples of columns.
FUTURE DIRECTIONS
Future studies need to be aimed at determining the exact submitochondrial location of ceramide generation during the induction phase of apoptosis. It is known that mitochondrial ceramide levels increase prior to the release of propapoptotic intermembrane space proteins and that mitochondrial outer and inner membranes are capable of generating ceramide. However, it is not known in which mitochondrial membrane the ceramide is generated during the induction phase of apoptosis. Generation of ceramide in the mitochondrial outer membrane would lead to the formation of pathways that would allow the release of intermembrane space contents. Alternatively, ceramide generation in the mitochondrial inner membrane would lead to the collapse in the mitochondrial inner membrane potential and swelling similar to a mitochondrial permeability transition. So the submitochondrial location of the ceramide channels will determine whether mitochondrial mediated apoptosis occurs via the formation of a pore in the mitochondrial outer membrane and release of inter-membrane space proteins without a collapse in the mitochondrial inner membrane potential or alternatively, the permeability transition.
In addition, the role that the Bcl-2 family members play in ceramide-induced apoptosis, needs to be established. As stated above, ceramide-induced cytochrome c release is inhibited by preincubation with or overexpression of the anti-death protein Bcl-2 (Ghafourifar et al., 1999; Zhang et al., 1996; Geley et al., 1997), or transfection of cells with Bcl-xL (Gottschalk et al., 1994; Wiesner et al., 1997). However, it is still debated whether this is through a direct interaction with ceramide (for example, inhibition of ceramide channels) or an indirect action (for example, altering the activity of one or more enzymes responsible for ceramide synthesis). Overexpression of Bcl-xL in the B-lymphocyte cell line WEHI 231 protected against ceramide-induced apoptosis, but not ceramide formation (Wiesner et al., 1997). There have also been several reports that overexpression of Bcl-2 blocked ceramide-induced apoptosis without inhibiting ceramide generation (Zhang et al., 1996; Allouche et al., 1997; Birbes et al., 2001), indicating that ceramide acts upstream of the anti-apoptotic Bcl-2 proteins. For example, Ghafourifar et al., 1999 showed that ceramide addition to isolated mitochondria induces cytochrome c release and this cytochrome c release was completely prevented by preincubation with Bcl-2. Alternatively, other studies have shown that Bcl-2 inhibits ceramide-induced apoptosis by preventing ceramide accumulation (Yoshimura et al., 1998; Tepper et al., 1999; Sawada et al., 2000b; Kawatani et al., 2003).The conflicting reports on the mode of Bcl-2 and/or Bcl-xL inhibition of ceramide-induced apoptosis may be due to different pathways for the generation of ceramide. Some inducers of apoptosis that act via ceramide generation, do so via activation of the de novo synthesis pathway, whereas others do so via activation of sphingomyelin hydrolysis. Until recently, it was thought that mitochondrial ceramide generation only occurred via the de novo synthesis pathway. However, recent studies indicate that a mitochondrial pool of sphingomyelin and ceramide exist and are involved in apoptosis (Birbes et al., 2001, 2005). Thus, the manner in which Bcl-2 and/or Bcl-xL inhibit ceramide-induced apoptosis, whether down- or up-stream of ceramide generation, may depend on whether mitochondrial ceramide generation occurs via de novo synthesis or sphingomyelin hydrolysis. Future work needs to determine the mechanism of Bcl-2 and/or Bcl-xL protection from ceramide-induced apoptosis.
Evidence indicates that pro-apoptotic Bcl-2 family members act in concert with ceramide. For example, Pastorino et al., 1999 showed that exogenously-added ceramide potentiates the ability of Bax to initiate apoptosis. It has been reported that for some cell types and tissues, Bax translocation from the cytosol to mitochondria occurs following ceramide production (Kim et al., 2001). Birbes et al., 2005 showed that treatment of MCF7 cells with TNF resulted in increased mitochondrial ceramide levels that were associated with Bax translocation to mitochondria. Kashkar et al., 2005 reported that addition of C16-ceramide to isolated mitochondria stimulated Bax translocation to mitochondria and subsequent cytochrome c/Smac release. They found that the ceramide-induced Bax conformational change occurred in isolated mitochondria fractions and not in mitochondrial protein lysates or cytosolic fractions (Kashkar et al., 2005). While ceramide can clearly induce cytochrome c release and apoptosis under Bax deficient conditions, the presence of Bax often enhances ceramide-induced apoptosis (von Haefen et al., 2002). Clearly Bax is not required for ceramide channel formation (Siskind and Colombini, 2000; Siskind et al., 2002, 2003) as ceramide channels are observed in planar phospholipid membranes free of proteins and ceramide channels allow the release of intermembrane space proteins less than 60 kDa in size from isolated rat liver mitochondria (reported to lack Bax, Polster et al., 2001). However, it is possible that Bax and ceramide may form hybrid channels. Alternatively, Bax could promote the enlargement or stabilization of ceramide channels (or vice versa) or ceramide could promote the insertion of Bax into mitochondrial membranes. Further investigation is required in order to better understand how ceramide channels are regulated in vivo.
CONCLUSIONS
Ceramide forms large and stable barrel-stave channels in membranes. These channels are large enough to allow mitochondrial proapoptotic intermembrane space proteins to cross the outer membrane and thus are good candidates for the pathway by which proapoptotic proteins are released from mitochondria during the induction phase of apoptosis. Mitochondrial ceramide levels have been shown to be elevated during apoptosis, specifically prior to the mitochondrial phase of apoptosis (Witty et al., 1996; Raisova et al., 2000; Bose et al., 1995; Charles et al., 2001; Rodriguez-Lafrasse et al., 2001; Kroesen et al., 2001; Thomas et al., 1999). Recent studies indicate that only ceramide generation in mitochondria, as opposed to other organelles, leads to the release of cytochrome c and apoptosis (Birbes et al., 2001).Mitochondrial membranes contain the enzymes responsible for ceramide synthesis and hydrolysis, namely, ceramide synthase and ceramidase. In fact, Bionda et al., 2004 recently showed that mitochondrial outer membranes contain ceramide synthase activity. Thus, the permeability of the mitochondrial outer membrane can be directly regulated by controlling the level of ceramide in the membrane and thus the size of the ceramide channels.
Interestingly, it was recently found that the ceramide metabolite sphingosine forms channels that are too small to allow proteins to permeate, but large enough to allow metabolites to pass (Siskind et al., 2005). Sphingosine channels have actually been proposed to exert a protective role if sphingosine is generated in the mitochondrial outer membrane as the channels would allow for the exchange of metabolites across the mitochondrial outer membrane (in the case when VDAC is closed), but would not allow the release of intermembrane space proteins (Siskind et al., 2005).Conversion to ceramide would lead to the formation of much larger channels that would then allow the release of intermembrane space proteins. Thus, the conversion between ceramide and sphingosine could regulate the permeability of the mitochondrial outer membrane and thus the life and death of the cell. Alternatively, as stated previously, dihydroceramide does not form channels in membranes. As healthy mitochondria are known to contain a higher percentage of dihydroceramide in their outer membranes than ceramide (Ardail et al., 2001), interconversion between dihydroceramide and ceramide would also determine the fate of the cell.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grant NS42025.
Key to abbreviations
- AIF
apoptosis inducing factor
- BSA
bovine serum albumin
- C16-ceramide
N-hexadecyl-D-erythro-sphingosine
- DiPyPC
diphytanoylphosphatidylcholine
- nS
nanoSiemens
- PIPES
piperazine-N,N′-bis[2-ethanesulfonic acid]
- PT
permeability transition
- TLC
thin layer chromatography
REFERENCES
- Allouche M, Bettaieb A, Vindis C, Rousse A, Grignon C, Laurent G. Oncogene. 1997;14:1837–1845. doi: 10.1038/sj.onc.1201023. [DOI] [PubMed] [Google Scholar]
- Alphonse G, Aloy MT, Broquet P, Gerard JP, Louisot P, Rousson R, Rodriguez-Lafrasse C. Int. J. Radiat. Biol. 2002;78:821–835. doi: 10.1080/09553000210153943. [DOI] [PubMed] [Google Scholar]
- Alphonse G, Bionda C, Aloy M-T, Ardail D, Rousson R, Rodriguez-Lafrasse C. Oncogene. 2004;23:2703–2715. doi: 10.1038/sj.onc.1207357. [DOI] [PubMed] [Google Scholar]
- Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, Green DR, Bhalla K. Blood. 1998;91:1700–1705. [PubMed] [Google Scholar]
- Ardail D, Popa I, Alcantara K, Pons A, Zanetta JP, Louisot P, Thomas L, Portoukalian J. FEBS Lett. 2001;488:160–164. doi: 10.1016/s0014-5793(00)02332-2. [DOI] [PubMed] [Google Scholar]
- Arora AS, Jones BJ, Patel TC, Bronk SF, Gores GJ. Hepatology. 1997;25:958–963. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- Bielawska A, Crane HM, Liotta D, Obeid LM, Hannun YA. J. Biol. Chem. 1993;268:26226–26232. [PubMed] [Google Scholar]
- Bielawska A, Greenberg MS, Perry D, Jayadev S, Shayman JA, McKay C, Hannun YA. J. Biol. Chem. 1996;271:12646–12654. doi: 10.1074/jbc.271.21.12646. [DOI] [PubMed] [Google Scholar]
- Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Biochem. J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Hannun YA, Obeid LM. FASEB J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Obeid LM, Hannun YA. Adv. Enzyme Regul. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. Biochem. J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- Brenner B, Ferlinz K, Grassmé H, Weller M, Koppenhoefer U, Dichgans J, Sandhoff K, Lang F, Gulbins E. Cell Death Differ. 1998;5:29–37. doi: 10.1038/sj.cdd.4400307. [DOI] [PubMed] [Google Scholar]
- Bruno AP, Laurent G, Averbeck D, Demur C, Bonnet J, Bettaieb A, Levade T, Jaffrezou JP. Cell Death Differ. 1998;5:172–182. doi: 10.1038/sj.cdd.4400330. [DOI] [PubMed] [Google Scholar]
- Caricchio R, D’Adamio L, Cohen PL. Cell Death Differ. 2002;9:574–580. doi: 10.1038/sj.cdd.4400996. [DOI] [PubMed] [Google Scholar]
- Castedo M, Hirsch T, Susin SA, Zamzami N, Marchetti P, Macho A, Kroemer G. J. Immunol. 1996;157:512–521. [PubMed] [Google Scholar]
- Charles AG, Han TY, Liu YY, Hansen N, Giuliano AE, Cabot MC. Cancer Chemother. Pharmacol. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- Cifone MG, De Maria R, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R. J. Exp. Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifone MG, Migliorati G, Parroni R, Marchetti C, Millimaggi D, Santoni A, Riccardi C. Blood. 1999;93:2282–2296. [PubMed] [Google Scholar]
- Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y, Dataa P. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Chaterjee S. Chem. Phys. Lipids. 1999;102:79–96. doi: 10.1016/s0009-3084(99)00077-8. [DOI] [PubMed] [Google Scholar]
- Chmura SJ, Mauceri HJ, Advani S, Heimann R, Beckett MA, Nodzenski E, Quintans J, Kufe DW, Weichselbaum RR. Cancer Res. 1997a;57:4340–4347. [PubMed] [Google Scholar]
- Chmura SJ, Nodzenski E, Beckett MA, Kufe DW, Quintans J, Weichselbaum RR. Cancer Res. 1997b;57:1270–1275. [PubMed] [Google Scholar]
- Come MG, Bettaieb A, Skladanowski A, Larsen AK, Laurent G. Int. J. Cancer. 1999;81:580–587. doi: 10.1002/(sici)1097-0215(19990517)81:4<580::aid-ijc13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. J. Biol. Chem. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Dai Q, Liu J, Chen J, Durrant D, McIntyre TM, Lee RM. Oncogene. 2004;23:3650–3658. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- Dbaibo GS, Perry DK, Gamard CJ, Platt R, Poirier GG, Obeid LM, Hannun YA. J. Exp. Med. 1997;185:481–490. doi: 10.1084/jem.185.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria R, Lenti L, Malisan F, d’Agostino F, Tomasini B, Zeuner A, Rippo MR, Testi R. Science. 1997;277:1652–1655. doi: 10.1126/science.277.5332.1652. [DOI] [PubMed] [Google Scholar]
- Dickerson RE, Takano T, Eisenberg D, Kallai OB, Samson L, Cooper A, Margoliash E. J. Biol. Chem. 1971;246:1511. [PubMed] [Google Scholar]
- Di Paola M, Cocco T, Lorusso M. Biochemistry. 2000;39:6620–6628. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Zaccagnino P, Montedoro G, Cocco T, Lorusso M. J. Bioenerg. Biomembr. 2004;36:165–170. doi: 10.1023/b:jobb.0000023619.97392.0c. [DOI] [PubMed] [Google Scholar]
- El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. J. Biol. Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- El Bawab S, Birbes H, Roddy P, Szulc ZM, Bielawska A, Hannun YA. J. Biol. Chem. 2001;276:16758–16776. doi: 10.1074/jbc.M009331200. [DOI] [PubMed] [Google Scholar]
- France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. J. Neurochem. 1997;69:1612–1621. doi: 10.1046/j.1471-4159.1997.69041612.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. J. Biol. Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- Gatt S. J. Biol. Chem. 1963;238:3131–3133. [PubMed] [Google Scholar]
- Gatt S. J. Biol. Chem. 1966;241:3724–3730. [PubMed] [Google Scholar]
- Geilen CC, Bektas M, Wieder T, Kodelja V, Goerdt S, Orfanos CE. J. Biol. Chem. 1997;272:8997–9001. doi: 10.1074/jbc.272.14.8997. [DOI] [PubMed] [Google Scholar]
- Geley S, Harmann BL, Kofler R. FEBS Lett. 1997;400:15–18. doi: 10.1016/s0014-5793(96)01284-7. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. J. Biol. Chem. 1999;274:6080–6084. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- Ghosh TK, Bian J, Gill DL. J. Biol. Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- Gottschalk A, Boise L, Thompson C, Quintáns J. Proc. Natl. Acad. Sci. USA. 1994;91:7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E, Bissonnette R, Mahboubi A, Martin S, Nishioka W, Brunner T, Baier G, Baier-Bitterlich G, Byrd C, Lang F, Kolesnick R, Altman A, Green D. Immunity. 1995;2:341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Gudz TI, Tserng K-Y, Hoppel CL. J. Biol. Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- Hannun YA. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Henry-Mowatt J, Dive C, Martinou J-C, James D. Oncogene. 2004;23:2850–2860. doi: 10.1038/sj.onc.1207534. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2nd edn. Sunderland, MA: Sinauer Association; 1992. [Google Scholar]
- Jarvis WD, Kolesnick RN, Fornari FA, Traylor RS, Gewirtz DA, Grant S. Proc. Natl. Acad. Sci USA. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM. Cell Mol. Life Sci. 2003;60:701–710. doi: 10.1007/s00018-003-2239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkar H, Wiegmann K, Yazdanpanah B, Haubert D, Krönke M. J. Biol. Chem. 2005;280:20804–20813. doi: 10.1074/jbc.M410869200. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Uchi M, Simizu S, Osada H, Imoto M. Exp. Cell Res. 2003;286:57–66. doi: 10.1016/s0014-4827(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Mun JY, Chun YJ, Choi KH, Kim MY. FEBS Lett. 2001;505:264–268. doi: 10.1016/s0014-5793(01)02836-8. [DOI] [PubMed] [Google Scholar]
- Kimura K, Markowski M, Edsall LC, Spiegel S, Gelmann EP. Cell Death Differ. 2003;10:240–248. doi: 10.1038/sj.cdd.4401145. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN, Kronke M. Annu. Rev. Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Kroesen B-J, Pettus B, Luberto C, Busman M, Sietsma H, Leij LD, Hannun YA. J. Biol. Chem. 2001;276:13606–13614. doi: 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- Liu B, Hannun YA. J. Biol. Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- Liu P, Anderson RG. J. Biol. Chem. 1995;270:27179–27185. doi: 10.1074/jbc.270.45.27179. [DOI] [PubMed] [Google Scholar]
- Lozano J, Menendez S, Morales A, Ehleiter D, Liao WC, Wagman R, Haimovitz-Friedman A, Fuks Z, Kolesnick R. J. Biol. Chem. 2001;276:442–448. doi: 10.1074/jbc.M006353200. [DOI] [PubMed] [Google Scholar]
- Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K. J. Biol. Chem. 1992;267:11144–11148. [PubMed] [Google Scholar]
- Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SM, Obeid LM. J. Biol. Chem. 2001;276:26577–26588. doi: 10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- Mao C, Xu R, Bielawska A, Obeid LM. J. Biol. Chem. 2002;275:6876–6884. doi: 10.1074/jbc.275.10.6876. [DOI] [PubMed] [Google Scholar]
- Marchesini N, Hannun YA. Biochem. Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- Masamune A, Igarashi Y, Hakomori S. J. Biol. Chem. 1996;271:9368–9375. doi: 10.1074/jbc.271.16.9368. [DOI] [PubMed] [Google Scholar]
- Mate MJ, Ortiz-Lombardia M, Boitel B, Haouz A, Tello D, Susin SA, Penninger J, Kroemer G, Alzari PM. Nat. Struct. Biol. 2002;9:442–446. doi: 10.1038/nsb793. [DOI] [PubMed] [Google Scholar]
- Matsko CM, Hunter OC, Rabinowich H, Lotze MT, Amoscato AA. Biochem. Biophys. Res. Commun. 2001;287:1112–1120. doi: 10.1006/bbrc.2001.5696. [DOI] [PubMed] [Google Scholar]
- Michel C, van Echten-Deckert G. FEBS Lett. 1997;416:153–155. doi: 10.1016/s0014-5793(97)01187-3. [DOI] [PubMed] [Google Scholar]
- Modur V, Zimmermann GA, Prescott SM, McIntyre TM. J. Biol. Chem. 1996;271:13094–13102. doi: 10.1074/jbc.271.22.13094. [DOI] [PubMed] [Google Scholar]
- Muriel M-P, Lamberg N, Darios F, Michel PP, Hirsch EC, Agid Y, Ruberg M. J. Comp. Neurol. 2000;426:297–315. doi: 10.1002/1096-9861(20001016)426:2<297::aid-cne10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Duan RD. Chem. Phys. Lipids. 1999;102:97–105. doi: 10.1016/s0009-3084(99)00078-x. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Nature. 1993;356:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. J. Cell. Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris F, Grassmé H, Cremesti A, Zager J, Fong Y, Haimovitz-Friedman A, Fuks Z, Gulbins E, Kolesnick R. J. Biol. Chem. 2001;276:8297–8305. doi: 10.1074/jbc.M008732200. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Tafani M, Rothman RJ, Marcineviciute A, Hoek JB, Farber JL. J. Biol. Chem. 1999;274:31734–31739. doi: 10.1074/jbc.274.44.31734. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA. Biochim. Biophys. Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster BM, Kinnally KW, Fiskum G. J. Biol. Chem. 2001;276:37887–37894. doi: 10.1074/jbc.M104552200. [DOI] [PubMed] [Google Scholar]
- Quillet-Mary A, Jaffrézou J, Mansat V, Bordier C, Naval J, Laurent G. J. Biol. Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- Quintans J, Kilkus J, McShan CL, Gottschalk AR, Dawson G. Biochem. Biophys. Res. Commun. 1994;202:710–714. doi: 10.1006/bbrc.1994.1988. [DOI] [PubMed] [Google Scholar]
- Raisova M, Bektas M, Wieder T, Daniel P, Eberle P, Orfanos CE, Geilen CC. FEBS Lett. 2000;473:27–32. doi: 10.1016/s0014-5793(00)01491-5. [DOI] [PubMed] [Google Scholar]
- Raisova M, Goltz G, Bektas M, Bielawska A, Riebeling C, Hossini AM, Eberle J, Hannun YA, Orfanos CE, Geilen CC. FEBS Lett. 2002;516:47–52. doi: 10.1016/s0014-5793(02)02472-9. [DOI] [PubMed] [Google Scholar]
- Riboni L, Campanella R, Bassi R, Villani R, Gaini SM, Martinelli-Boneschi F, Viani P, Tettamanti G. Glia. 2002;39:105–113. doi: 10.1002/glia.10087. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Alphonse G, Broquet P, Aloy M, Louisot P, Rousson R. Biochem. J. 2001;357:407–416. doi: 10.1042/0264-6021:3570407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Alphonse G, Aloy M-T, Ardail D, Gérard J-P, Louisot P, Rousson R. Int. J. Cancer. 2002;101:589–598. doi: 10.1002/ijc.10652. [DOI] [PubMed] [Google Scholar]
- Saelens X, Festjens N, Walle LV, van Gurp M, van Loo G, Vandenabeele P. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- Samet D, Barenholz Y. Chem. Phys. Lipids. 1999;102:65–77. doi: 10.1016/s0009-3084(99)00076-6. [DOI] [PubMed] [Google Scholar]
- Sawada M, Nakashima S, Banno Y, Yamakawa H, Hayashi K, Takenaka K, Nishimura Y, Sakai N, Nozawa Y. Cell Death Differ. 2000a;7:761–772. doi: 10.1038/sj.cdd.4400711. [DOI] [PubMed] [Google Scholar]
- Sawada M, Nakashima S, Banno Y, Yamakawa H, Takenaka K, Shinoda J, Nishimura Y, Sakai N, Nozawa Y. Oncogene. 2000b;19:3508–3520. doi: 10.1038/sj.onc.1203699. [DOI] [PubMed] [Google Scholar]
- Sautin Y, Takamura N, Shklyaev S, Nagayama Y, Ohtsusu A, Namba H, Yamashita S. Thyroid. 2000;10:733–740. doi: 10.1089/thy.2000.10.733. [DOI] [PubMed] [Google Scholar]
- Schafer P, Scholz SR, Gimadutdinow O, Cymerman IA, Bujnicki JM, Ruiz-Carrillo A, Pingoud A, Meiss G. J. Mol. Biol. 2004;338:217–228. doi: 10.1016/j.jmb.2004.02.069. [DOI] [PubMed] [Google Scholar]
- Selzner M, Bielawska A, Morse MA, Rudiger HA, Sindram D, Hannun YA, Clavien PA. Cancer Res. 2001;61:1233–1240. [PubMed] [Google Scholar]
- Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T. Lipids. 1998;33:601–605. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- Shiraishi T, Imai S, Uda Y. Biol. Pharm. Bull. 2003;26:775–779. doi: 10.1248/bpb.26.775. [DOI] [PubMed] [Google Scholar]
- Siskind LJ, Colombini M. J. Biol. Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M. J. Biol. Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Davoody A, Lewin N, Marshall S, Colombini M. Biophys. J. 2003;85:1560–1575. doi: 10.1016/S0006-3495(03)74588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Fluss S, Bui M, Colombini M. J. Bioener. Biomembr. 2005 doi: 10.1007/s10863-005-6632-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W. Chem. Phys. Lipids. 1999;102:107–121. doi: 10.1016/s0009-3084(99)00079-1. [DOI] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Castedo M, Daugas E, Wang EG, Geley S, Fassy F, Reed JC, Kroemer G. J. Exp. Med. 1997a;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Larochette N, Dallaporta B, Marzo I, Brenner C, Hirsch T, Petit PX, Geuskens M, Kroemer G. Exp. Cell Res. 1997b;236:397–403. doi: 10.1006/excr.1997.3733. [DOI] [PubMed] [Google Scholar]
- Tabas I. Chem. Phys. Lipids. 1999;102:123–130. doi: 10.1016/s0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Tashima M, Takahashi A, Uchiyama T, Okazaki T. J. Biol. Chem. 1999;274:10654–10660. doi: 10.1074/jbc.274.15.10654. [DOI] [PubMed] [Google Scholar]
- Tani M, Okino N, Mori K, Tanigawa T, Izu H, Ito M. J. Biol. Chem. 2000;275:11229–11234. doi: 10.1074/jbc.275.15.11229. [DOI] [PubMed] [Google Scholar]
- Tepper CG, Jayadev S, Liu B, Bielawska A, Wolff R, Yonehara S, Hannun YA, Seldin MF. Proc. Natl. Acad. Sci. USA. 1995;92:8443–8447. doi: 10.1073/pnas.92.18.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper AD, de Vries E, van Bitterswijk WJ, Borst J. J. Clin. Invest. 1999;103:971–978. doi: 10.1172/JCI5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RL, Jr, Matsko CM, Lotze MT, Amoscato AA. J. Biol. Chem. 1999;274:30580–30588. doi: 10.1074/jbc.274.43.30580. [DOI] [PubMed] [Google Scholar]
- Vance JE. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Vit JP, Rosselli F. Oncogene. 2003;22:8645–8652. doi: 10.1038/sj.onc.1207087. [DOI] [PubMed] [Google Scholar]
- Von Haefen C, Wieder T, Gillissen B, Stärck L, Graupner V, Dörken B, Daniel PT. Oncogene. 2002;21:4009–4019. doi: 10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- Wiesner DA, Dawson G. J Neurochem. 1996;66:1418–1425. doi: 10.1046/j.1471-4159.1996.66041418.x. [DOI] [PubMed] [Google Scholar]
- Wiesner DA, Kilkus JP, Gottschalk AR, Quintáns J, Dawson G. J. Biol. Chem. 1997;272:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- Witty JP, Bridgham JT, Johnson AL. Endocrinology. 1996;137:5269–5277. doi: 10.1210/endo.137.12.8940345. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Banno Y, Nakashima S, Takenaka K, Sakai H, Nishimura Y, Sakai N, Shimizu S, Eguchi Y, Tsujimoto Y, Nozawa Y. J. Biol. Chem. 1998;273:6921–6927. doi: 10.1074/jbc.273.12.6921. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marchette P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. J. Exp. Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Liu B, Kang SW, Soe MS, Rhee SG, Obeid LM. J. Biol. Chem. 1997;272:30615–30618. doi: 10.1074/jbc.272.49.30615. [DOI] [PubMed] [Google Scholar]
- Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Proc. Natl. Acad. Sci. USA. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]


