Abstract
Recent evidence suggests that the ability of ceramides to induce apoptosis is due to a direct action on mitochondria. Mitochondria are known to contain enzymes responsible for ceramide synthesis and hydrolysis and mitochondrial ceramide levels have been shown to be elevated prior to the mitochondrial phase of apoptosis. Ceramides have been reported to induce the release of intermembrane space proteins from mitochondria, which has been linked to their ability to form large channels in membranes. The aim of this study was to determine if the membrane concentration of ceramide required for the formation of protein permeable channels is within the range that is present in mitochondria during the induction phase of apoptosis. Only a very small percentage of the ceramide actually inserts into the mitochondrial membranes. The permeability of the mitochondrial outer membrane correlates directly with the level of ceramide in the membrane. Importantly, the concentration of ceramide at which significant channel formation occurs is consistent with the level of mitochondrial ceramide that occurs during the induction phase of apoptosis (4 pmol ceramide/nanomole phospholipid). Similar results were obtained with short- and long-chain ceramide. Ceramide channel formation is specific to mitochondrial membranes in that no channel formation occurs in the plasma membranes of erythrocytes even at concentrations 20 times higher than those required for channel formation in mitochondrial outer membranes. Thus, ceramide channels are good candidates for the pathway by which proapoptotic proteins are released from mitochondria during the induction phase of apoptosis.
Keywords: Mitochondria, Apoptosis, Pore, Sphingolipid, Ceramide, Channel
1. Introduction
Mitochondria are known to play a major regulatory role in apoptosis (see for example, Crompton, 1999; Susin et al., 1998). Early in apoptosis, there is an increase in the permeability of the mitochondrial outer membrane, that leads to the release of intermembrane space proteins, including cytochrome c, procaspases, apoptosis inducing factor (AIF), heat shock proteins, Smac/Diablo, and endonuclease G (reviewed in Saelens et al., 2004). The release of intermem-brane space proteins into the cytoplasm is crucial for the activation of specific caspases and DNases that are responsible for the execution of apoptosis.
Ceramide is a sphingosine-based lipid that is known to be involved in the regulation of apoptosis. Its generation is a common cellular response of a variety of cell types following exposure to apoptosis-inducing agents (for review see Siskind, 2005). Elevations in cellular ceramide in response to proapoptotic stimuli occur prior to the mitochondrial phase of apoptosis (for example, Hannun, 1996; Dbaibo et al., 1997; Bose et al., 1995; Witty et al., 1996; Thomas et al., 1999; Raisova et al., 2000; Rodriguez-Lafrasse et al., 2001; Kroesen et al., 2001). Recent evidence indicates that ceramides act to induce apoptosis through a direct affect on mitochondria (Birbes et al., 2001, 2005). Mitochondria contain enzymes capable of generating ceramide (ceramide synthase and reverse ceramidase, El Bawab et al., 2000; Shimeno et al., 1998; Bionda et al., 2004) and several apoptotic stimuli have been shown to induce apoptosis via an increase in mitochondrial ceramide levels (Birbes et al., 2005; Garcia-Ruiz et al., 1997; Matsko et al., 2001; Dai et al., 2004).
Ceramides have been reported to have numerous effects on mitochondria, including enhanced generation of reactive oxygen species, alteration of calcium homeostasis of mitochondria and endoplasmic reticulum, ATP depletion, collapse in the inner mitochondrial membrane potential; inhibition and/or activation of the activities of various components of the mitochondrial electron transport chain, and release of inter-membrane space proteins (Arora et al., 1997; Di Paola et al., 2000, 2004; France-Lanord et al., 1997; Garcia-Ruiz et al., 1997; Ghafourifar et al., 1999; Gudz et al., 1997; Muriel et al., 2000; Pinton et al., 2001; Quillet-Mary et al., 1997; Siskind et al., 2002; Zamzami et al., 1995). Short-chain cell permeable ceramide analogues, such as N-acetyl-d-erythro-sphingosine (C2-ceramide) and N-hexanoyl-d-erythro-sphingosine (C6-ceramide) have been shown to induce cytochrome c release and apoptosis when added to whole cell cultures and isolated mitochondria (Zamzami et al., 1995; Susin et al., 1997a,b; Zhang et al., 1997; Di Paola et al., 2000, 2004; Arora et al., 1997; Ghafourifar et al., 1999); this cytochrome c release was preventable by pre-incubation with or overexpression of the anti-death protein Bcl-2 or transfection of cells with Bcl-xL (Ghafourifar et al., 1999; Zhang et al., 1996; Geley et al., 1997; Gottschalk et al., 1994; Wiesner et al., 1997). Long-chain naturally occurring ceramides have also been shown to induce the release of cytochrome c from isolated mitochondria (Di Paola et al., 2000, 2004). In addition to cytochrome c, long-and short-chain ceramides have been shown to induce the release of AIF, AK-2, and adenylate kinase from isolated mitochondria (Di Paola et al., 2004; Siskind et al., 2002). Until recently, it was not clear how ceramide increased the permeability of the mitochondrial outer membrane to inter-membrane space proteins. Recent evidence indicates that it is due to the ability of ceramide to form large protein permeable channels in planar phospholipid membranes, mitochondrial membranes and liposomes (Siskind, 2005; Siskind and Colombini, 2000; Siskind et al., 2002, 2003; Montes et al., 2002; Pajewski et al., 2005).
Ceramides form oligomeric barrel-stave channels with estimated diameters larger than 10 nm in planar phospholipid membranes (Siskind et al., 2003). In mitochondrial outer membranes, ceramide channels allow the release of proteins up to 60 kDa in size (Siskind et al., 2002). Even though this cut-off was measured under denaturing conditions and thus is most likely an underestimate, it is still in line with the size of proapoptotic proteins released from mitochondria during apoptosis (cytochrome c 12 kDa (Dickerson et al., 1971); endonuclease G 28 kDa (Schafer et al., 2004); AIF 57 kDa (Mate et al., 2002); Smac/DIABLO 42 kDa (Chai et al., 2001)). Work with ceramide channels in planar phospholipid membranes and isolated mitochondria indicate that these channels are good candidates for the pathway by which proapoptotic proteins are released into the cytosol during apoptosis. Ceramide channels would be even stronger candidates if it was known whether or not the in vitro effects of ceramide on isolated mitochondria occurred at physiologically relevant levels of ceramide in the membrane. It is not currently known what percentage of the ceramide added to solution actually inserts into membranes and whether the ceramide that inserts into the membrane is metabolized. Here, we show that only a small percentage of the ceramide that is added to isolated mitochondria actually inserts into the membrane and that very little if any is actually metabolized during the experimental period. The results of this study show that the permeability of the mitochondrial outer membrane directly correlates with the level of ceramide in the membrane. As little as 4 pmol ceramide per nanomole mitochondrial phospholipid is required to form ceramide channels large enough to allow cytochrome c to permeate. Thus, the concentration of ceramide at which channel formation occurs is consistent with the level of mitochondrial ceramide that occurs during the induction phase of apoptosis.
2. Materials and methods
2.1. Reagents
The following reagents were purchased from Avanti Polar Lipids (Alabaster, AL): C2-ceramide, C2-dihydroceramide, C16-ceramide. Antimycin A, 2,4-dinitrophenol (DNP), horse heart cytochrome c, fatty acid-depleted BSA, and sodium ascorbate were purchased from Sigma. The following reagents were purchased from American Radiolabeled Chemical, Inc. (St Louis, MO): [4,5-³H] N-acetyl-d-erythro-dihydro-sphingosine, [acetyl-1-14C] N-acetyl-d-erythro-sphingosine, and [palmitoyl-1-14C] N-palmitoyl-d-erythro-sphingosine.
2.2. Preparation of mitochondria
Rat liver mitochondria were isolated by differential centrifugation of tissue homogenate as described previously (Parsons et al., 1966) as modified (Siskind et al., 2002). Mitochondrial intactness was determined by the rate of oxidation of exogenously-added cytochrome c as compared to the rate measured with mitochondria with hypotonically lysed outer membranes as previously described (Douce et al., 1987) as modified (Siskind et al., 2002).
2.3. Percent insertion of ceramide into mitochondrial membranes
Mitochondria (0.47 mg/mL mitochondrial protein) were incubated with fatty acid-depleted BSA for 5 min prior to the addition of sphingolipids (where indicated) at a molar ratio of 0.5 BSA to sphingolipids. Mitochondria were incubated with the indicated concentrations of [14C] C2-ceramide, [14C] C16-ceramide, or [³H] C2-dihydroceramide for the indicated time periods. Half the mitochondria were then pelleted (at 12,000×g for 5 min) and the other half left unspun. Five-hundred microlitres of supernatant was then subjected to scintillation counting. Percent insertion of radiolabeled sphingolipids was determined from the difference between total radioactivity in the solution and that after removal of mitochondria by centrifugation.
2.4. Assessment of hydrolysis of ceramides by mitochondria
A 100 µL sample of the mitochondrial supernatant from the above centrifuged samples exposed to [14C] C2-ceramide was removed for assessment of ceramide hydrolysis. Separation of ceramide hydrolysis products (sphingosine and [14C] acetyl) into their corresponding organic and aqueous phases was performed according to Bligh and Dyer (1959). Briefly, 0.5 mL H2O was added to the 100 µL centrifuged supernatant sample followed by the addition of 0.8 mL CH3OH and 1.6 mL CHCl3. The samples were vortexed and put onto ice for 60 min. The aqueous phase was subjected to scintillation counting. Hydrolysis of [14C]-C16-ceramide was assessed by separation of released hydrolysis products ([14C] palmitate and sphingo-sine) from both the above mitochondrial pellet and supernatant samples according to Yavin and Gatt (1969).
2.5. Permeability of the mitochondrial outer membrane
Mitochondria (0.47 mg/mL mitochondrial protein) were incubated with fatty acid-depleted BSA for 5 min prior to the addition of sphingolipids at a molar ratio of BSA to sphingolipids of 0.5. Mitochondria were then incubated with the indicated concentrations of ceramide for the indicated time periods. Exogenously-added reduced cytochrome c was then added and the rate of oxidation was assessed as described previously (Siskind et al., 2002) by spectrophotometrically monitoring the rate of absorbance decrease at 550 nm. The permeability of the mitochondrial outer membrane was expressed as a percentage of the rate of CN-sensitive oxidation of exogenously added cytochrome c by mitochondria with hypotonically lysed outer membranes.
2.6. Erythrocyte experiments
Erythrocytes were isolated as previously described (Siskind et al., 2005). Briefly, erythrocytes were obtained from decapitated male Sprague-Dawley rats in a solution of 150 mM NaCl, 4 mM EGTA, 5 mM HEPES pH 7.4 to prevent clotting. They were used within 3 days. Erythrocytes were washed in successive centrifugation steps followed by resuspension in the above buffer. 0.5 mL erythrocytes (4 mg protein/mL stock) were incubated with fatty acid-depleted BSA at a molar ratio of BSA to ceramide of 0.5 for 5 min. Varying concentrations of C2- or C16-ceramide were added for a 15 min incubation period. Following the incubation, the erythrocytes were either evaluated for channel formation or percent ceramide insertion. Channel formation in erythrocyte plasma membranes was assessed by monitoring erythrocyte lysis as described in Siskind et al. (2005). Briefly, following incubation with ceramides, the cells were sedimented (5 min at 12,000×g). Four-hundred microlitres of supernatant was added to 400 µL of Drabkin’s reagent (Sigma Technical Bulletin No. 525) and the absorbance measured at 540 nm after 5 min. The percent lysis of the erythrocytes was determined from the maximum possible absorbance at 100% lysis obtained after the addition of Triton X-100 (0.5% (w/v) final). Insertion of [14C] C2- or C16-ceramide into the erythrocytes was evaluated by pelleting half the erythrocyte samples (at 12,000×g for 5 min) and leaving the other half unspun. Four-hundred microlitres of supernatant was then subjected to scintillation counting. Percent insertion of radiolabeled ceramides was determined from the difference between total radioactivity in the solution and that after removal of mitochondria by centrifugation.
3. Results and discussion
3.1. Only a small percentage of the ceramide added to solution inserts into the mitochondrial membranes
Numerous studies have examined the effect of ceramides on isolated mitochondria (for example, Di Paola et al., 2000, 2004; Siskind et al., 2002, 2005). Because ceramide exerts its effects on the mitochondrial membrane, it is its concentration in the membrane that is relevant and not its absolute concentration added to solution. Therefore, we first wanted to determine the amount of ceramide added to isolated mitochondrial suspensions that actually inserts into the mitochondrial membranes. Before this could be directly measured, several obstacles had to be overcome. Both long- and short-chain ceramides, as well as dihydroceramide, when added to an aqueous solution form aggregates that sediment in a centrifuge at the same speed as mitochondria (data not shown). In addition, ceramides adhere to the surfaces of glass, plastic, and Teflon (data not shown). These problems were overcome by the addition of a small amount of fatty-acid depleted BSA to the solution prior to the addition of the ceramide. The molar ratio of BSA to ceramide that was able to prevent both aggregation in solution and adherence to surfaces while allowing for insertion of ceramide into the mitochondrial membranes was 0.5. Higher levels of BSA prevented the insertion of all ceramides tested.
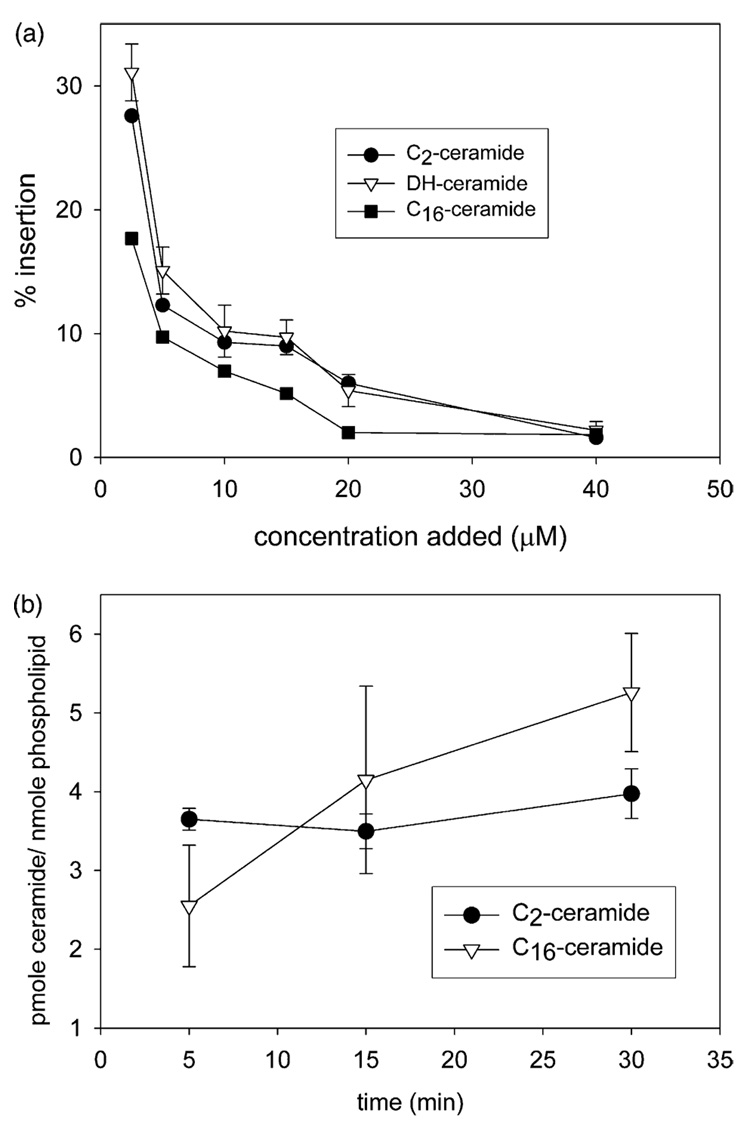
Fig. 1 shows the percent insertion of C2-ceramide, C2-dihydroceramide, and C16-ceramide as a function of concentration added to solution (Fig. 1a) and time (Fig. 1b). The percent insertion of all three ceramides into mitochondria decreases as the concentration of ceramide added to solution increases. For example, at 5 min of incubation when 2.5 µM ceramide is added to solution, 28±2, 31±2, and 18±2% of the C2-ceramide, C2-dihydroceramide, and C16-ceramide, respectively, inserts into the mitochondrial membranes (Fig. 1a). However, when 20 µM ceramide is added to solution, only 6.0±1.5, 5.4±1.3, and 2.0±0.9% of the C2-ceramide, C2-dihydroceramide, and C16-ceramide, inserts respectively (Fig. 1a). The percent insertion decreases to less than 3% for all three ceramides at 40 µM for 5 min incubation time (Fig. 1a).
Fig. 1.
Ceramide insertion into mitochondria as a function of absolute concentration added to solution and time. (a) Percent insertion of ceramides as a function of concentration added to solution. Isolated mitochondria were incubated with the indicated concentrations of [14C] C2- or C16-ceramide or [³H] C2-dihydroceramide for 5 min and the percent insertion into the mitochondrial membranes was measured. (b) Ceramide insertion as a function of incubation time. Isolated mitochondria were incubated with 20 µM [14C] C2- or C16-ceramide for the indicated incubation times and the percent insertion was measured. Results are a representative experiment of three independent experiments. Error bars are the standard deviations.
Notice that the insertion of C2-ceramide and C2-dihydroceramide is essentially identical. Thus, the inability of dihydroceramide to induce apoptosis is not due a lack of its insertion into the membrane. Dihydroceramide differs from ceramide only by the reduction of one double bond. Ceramide-channel formation requires the presence of the 4–5 trans double bond as dihydroceramide does not form channels even at concentrations up to 25 times higher than that required for ceramide channel formation (Siskind and Colombini, 2000). Thus, the channel forming ability of ceramides correlates with its apoptotic activity. Molecular dynamic simulations indicate that the presence of the double bond stabilizes ceramide channels via adjacent double bond-double bond interactions (Anishkin et al., 2006). An alternative hypothesis that could explain the importance of the double bond for channel formation is that the trans double bond in ceramide augments intramolecular hydration/hydrogen bonding in the polar region (Li et al., 2002; Brockman et al., 2004).
The long-chain naturally occurring C16-ceramide consistently has a significantly lower percent insertion than either of the short-chain ceramide analogues at 5 min incubation. However, while C2-ceramide insertion does not change with time, C16-ceramide insertion increases with incubation time and is greater than the short-chain ceramide at the longer incubation times of 15 and 30 min (Fig. 1b, the C16-ceramide 5 and 30 min points are statistically different, P < 0.05, by the Student’s t-test). No hydrolysis of C2-ceramide was detected at any time period. Less than 5% of the C16-ceramide that inserted into the mitochondria was metabolized, which was determined to be statistically insignificant (data not shown).
The permeability of the mitochondrial outer membrane directly correlates with the level of C2- or C16-ceramide in the membrane:
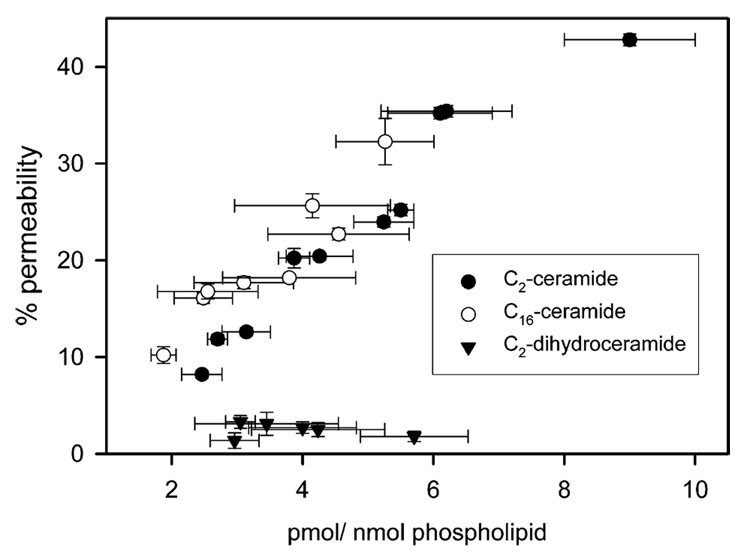
The permeability of the mitochondrial outer membrane can be determined by measuring the bidirectional flux of cytochrome c as monitored by the CN-sensitive oxidation of externally-added reduced cytochrome c. We previously employed this method to show that C2- and C16-ceramide form channels in the mitochondrial outer membrane that are large enough to allow for the bidirectional flux of cytochrome c (Siskind et al., 2002). However, only the concentration of ceramide added to solution was known. We therefore determined what level of ceramide in the mitochondrial membranes was required to form channels large enough for cytochrome c to permeate. As shown in Fig. 2, the permeability of the mitochondrial outer membrane directly correlates with the level of C2- or C16-ceramide in the membrane. Regardless of the concentration of dihydroceramide, there was no increase in the permeability of the mitochondrial outer membrane (Fig. 2). However, as little as 4–6 pmol C2- or C16-ceramide per nanomole mitochondrial phospholipids is required for a significant permeability increase in the mitochondrial outer membrane. This level of ceramide is on the order of the level of mitochondrial ceramide increase found during the induction phase of apoptosis (before or at the time of cytochrome c release; Birbes et al., 2005; Garcia-Ruiz et al., 1997; Rodriguez-Lafrasse et al., 2002). Mitochondrial ceramide levels increased by about 6.5 pmol ceramide/nanomole phospholipid in MCF7 cells treated with TNFα (Birbes et al., 2005). Garcia-Ruiz et al. (1997) reported a similar increase in isolated mitochondria from TNF treated hepatocytes (an increase of about 4.5 pmol ceramide/nanomole phospholipid). γ-radiation of Jurkat cells induced the formation of 4 pmol mitochondrial ceramide/nanomole phospholipid (Rodriguez-Lafrasse et al., 2002). Thus, the level of mitochondrial ceramide required for the formation of protein permeable channels correlates closely with level of mitochondrial ceramide formed during the induction phase of apoptosis.
Fig. 2.
The permeability of the mitochondrial outer membrane correlates with the level of ceramide in the membrane. Isolated mitochondria were incubated with [14C] C2- or C16-ceramide or [³H] C2-dihydroceramide and the concentration of ceramide in the membrane (as determined from the percent insertion) as well as the corresponding permeability of the mitochondrial outer membrane measured via the initial linear rate of oxidation of externally added reduced cytochrome c. Data are means±SD of triplicates from three separate experiments.
It is important to point out that while there have been several reports of ceramide-induce cytochrome c release from isolated mitochondria (Di Paola et al., 2000, 2004; Arora et al., 1997; Ghafourifar et al., 1999), there have also been reports to the contrary (Yuan et al., 2003; Novgorodov et al., 2005; Kristal and Brown, 1999). These contradictory reports in the literature can be explained by the method used to add the ceramide to the mitochondria and/or the incubation conditions. Rapid mixing while simultaneously adding ceramide to solutions is essential to the efficient incorporation of ceramide into mitochondria. In addition, a large molar excess of fatty acid-free BSA is able to prevent the incorporation of ceramides into mitochondria; this could explain the negative results obtained for either C2- or C16-ceramide as reported by Yuan et al. (2003). When only cytochrome c release is examined as opposed to measuring the permeability of the mitochondrial outer membrane to exogenously-added cytochrome c, the ionic strength of the solution plays an important role. Under low ionic strength conditions, cytochrome c may not detach from the surface of the mitochondrial inner membrane unless large amplitude swelling occurs; this may explain the negative results obtained for C6-ceramide in Novgorodov et al. (2005) and for C2-ceramide in Kristal and Brown (1999).
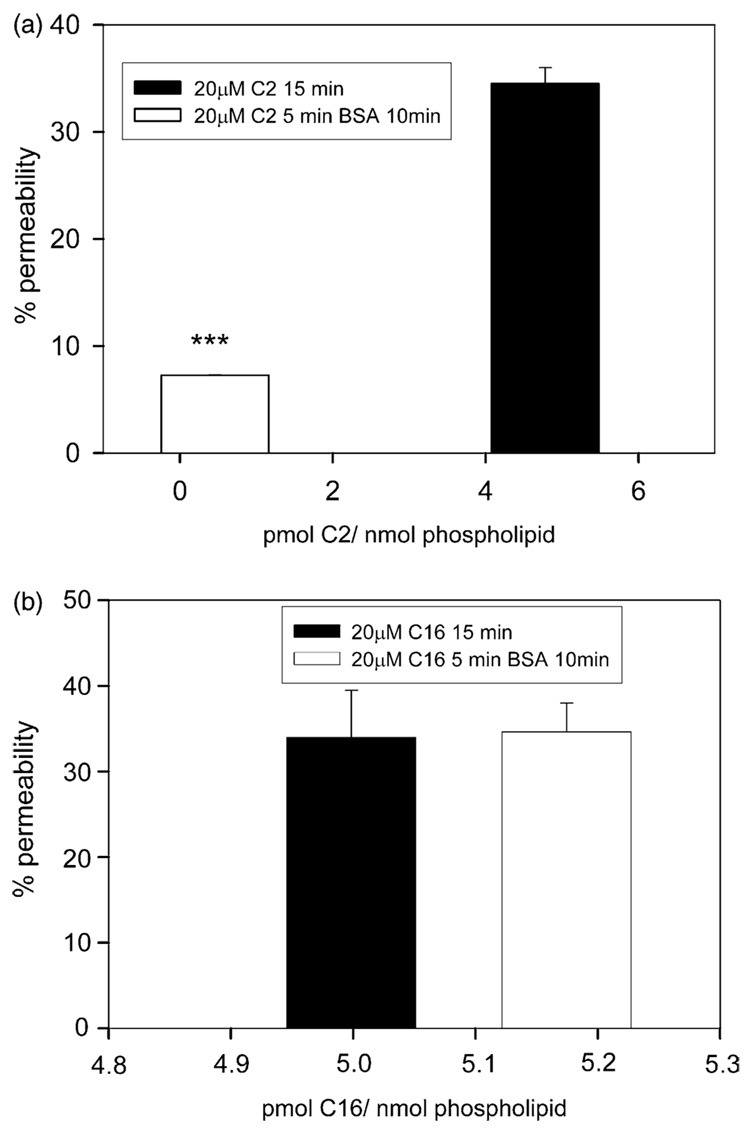
Mitochondrial depletion of C2-ceramide restores the permeability barrier of the mitochondrial outer membrane.
We previously reported that a fivefold molar excess of fatty-acid depleted BSA induced C2-ceramide channel disassembly in planar phospholipids membranes (Siskind et al., 2002). In addition, this same excess of BSA reversed the C2-ceramide-induced permeability increase in the mitochondrial outer membrane (Siskind et al., 2002). We reasoned that ceramide channels were in dynamic equilibrium with ceramide monomers and that the ability of BSA to disassemble C2-ceramide channels was due to its ability to bind ceramide monomers in solution and induce channel disassembly by mass action. Fig. 3a, shows that the ability of BSA to restore the permeability barrier of the mitochondrial outer membrane is indeed due its ability to deplete C2-ceramide from the mitochondrial membrane. A 15 min incubation of mitochondria with 20 µM C2-ceramide results in a membrane concentration of 4.8±1.3 pmol ceramide/nanomole phospholipid and a permeability increase of 34.5 ± 1.5% (Fig. 3a). A 5 min pre-incubation of isolated mitochondria with 20 µM C2-ceramide followed by a 10 min incubation with excess of BSA (5:1, BSA: C2-ceramide mole ratio) resulted in a membrane concentration of 0.46±0.32 pmol C2-ceramide/nanomole phospholipids and a permeability increase of only 7.8±0.03% (Fig. 3a). Thus, the ability of BSA to restore the permeability barrier of the mitochondrial outer membrane is indeed due to the depletion of ceramide from the membrane.
Fig. 3.
Depletion of C2-ceramide from the mitochondrial membrane restores the permeability barrier of the mitochondrial outer membrane. (a) Isolated mitochondria were incubated with 20 µM [14C] C2-ceramide for 15 min (black bar) or for 5 min followed by a 10 min incubation with a five molar excess of fatty acid-depleted BSA (white bar). The level of statistical significance from control (20 µM [14C] C2-ceramide for 15 min) are: ***, P≤0.001. (b) The same experiments as in (a) except using [14C] C16-ceramide. Results are a representative experiment from three independent experiments. Error bars are the standard deviations.
We previously reported that an excess of BSA was not able to reverse or prevent the permeability increase induced by C16-ceramide (Siskind et al., 2002). Fig. 3b, shows that this is due to an inability of excess BSA to deplete C16-ceramide from the membrane; the level of C16-ceramide in the membrane as well as the corresponding permeability increase was essentially identical in the presence or absence of BSA. The inability of BSA to deplete C16-ceramide from the membrane is most likely due to a greater affinity of C16-ceramide for membranes as opposed to BSA. C16-ceramide has about double the hydrophobic portion of that of C2-ceramide and thus has about double the hydrophobic component of the interaction energy between ceramide and the membrane. This could make the C16-ceramide-membrane interactions stronger than the C16-ceramide-BSA interactions. Alternatively, the much lower concentration of free C16-ceramide in solution may reduce the rate of exchange of C16-ceramide resulting in a process that is too slow to detect under our experimental conditions.
3.2. The ability of ceramide to form channels in membranes is membrane type dependent
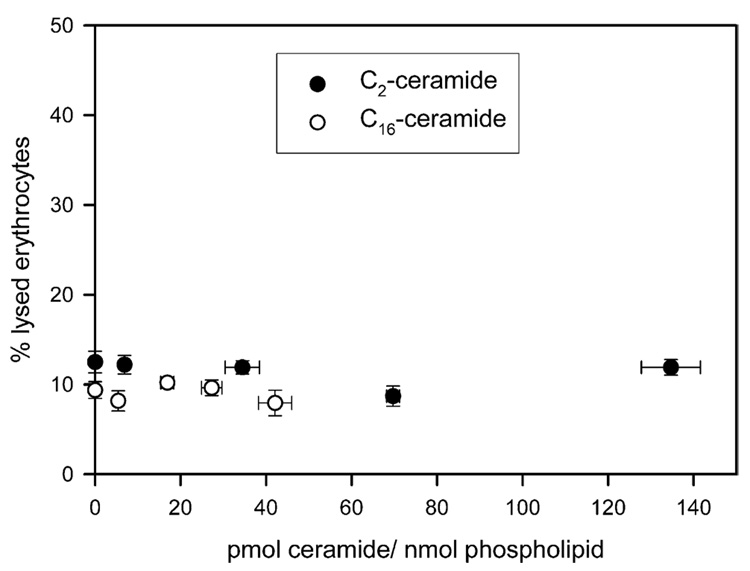
Recent studies indicate that only ceramide generation in mitochondria, as opposed to other organelles, leads to the release of cytochrome c and apoptosis (Birbes et al., 2001). Ceramides induce apoptosis when they are added exogenously to whole cells (for example, Obeid et al., 1993; Jarvis et al., 1994; Quintans et al., 1994; Cifone et al., 1994). If ceramide could form protein permeable channels indiscriminately in any type of membrane at levels as low as 4–6 pmol ceramide per nanomole phospholipids, then when added to whole cells it would induce necrosis and not apoptosis. We therefore wanted to determine if ceramide could form channels in plasma membranes using erythrocytes.
Erythrocytes contain osmotically-active solutes, especially hemoglobin and its counterions that must be balanced by extracellular solutes to prevent bulk water movement and cell lysis. The insertion of a channel into the erythrocyte membrane that allows extracellular solutes to enter results in an osmotic influx of water, increasing the cell volume and intracellular pressure until the cell membrane tears and hemoglobin is released. Thus, ceramide channels present in the plasma membranes of the erythrocytes only need to be large enough to allow the passage of small ions (Na+ and Cl−) for erythrocyte lysis will occur. As shown in Fig. 4, regardless of the level of C2- or C16-ceramide in the erythrocyte membrane, no ceramide channel is observed. Ceramides do not form channels in the plasma membranes of erythrocytes even at membrane concentrations 20 times higher than that required for channel formation in mitochondrial membranes. Thus ceramide can insert into the membrane but does not form a channel.
Fig. 4.
Ceramides do not form channels in the plasma membranes of erythrocytes. Erythrocytes (4 mg/mL) were incubated with varying concentrations of [14C] C2- or C16-ceramide. Following, a 15 min incubation, the concentration of ceramide in the membrane and the percent lysis of the erythrocytes were measured. The percent lysis of the erythrocytes is the amount of hemoglobin released expressed as a percentage of the total hemoglobin released after addition of Triton X-100. The data are means±SD of three separate experiments. Note that the y-axis has been enlarged twofold so as to better view the data.
The membrane specificity of ceramide channel formation could be due to several factors. The difference in the lipid composition of the plasma membrane and the mitochondrial outer membrane could have a dramatic effect on ceramide channel function. Ceramide is thought to be a major participant in reorganizing microscopic membrane rafts into signaling platforms in response to stress. Consistent with this notion, evidence indicates that ceramide generation in plasma membranes occurs within rafts (for review see Gulbins and Kolesnick, 2003). It is possible that ceramide forms alternative structures in rafts because of the local concentration of particular lipids and membrane proteins. Future studies will investigate the lipid specificity of ceramide channel formation further.
4. Conclusion
The results of this study strengthen the hypothesis that ceramide channels are indeed good candidates for the pathway with which proapoptotic proteins are released from the mitochondrial intermembrane space into the cytoplasm during the induction phase of apoptosis. Mitochondria contain the enzymes necessary for ceramide synthesis and hydrolysis and mitochondrial ceramide levels have been shown to increase prior to the release of proapoptotic proteins. The level of mitochondrial ceramide generated during the induction phase of apoptosis closely matches the membrane concentration required for the formation of protein permeable ceramide channels. Thus, the permeability of the mitochondrial outer membrane can be tightly regulated by controlling the size of the ceramide channels via the level of ceramide in the membrane.
Acknowledgements
This work was supported by the National Institutes of Health grant NS42025.
Abbreviations used
- AIF
apoptosis-inducing factor
- C2-ceramide
N-acetyl-d-erythro-sphingosine
- C6-ceramide
N-hexanoyl-d-erythro-sphingosine
- C16-ceramide
N-hexadecyl-d-erythro-sphingosine
- DNP
2,4-dinitrophenol
- BSA
bovine serum albumin
References
- Anishkin A, Sukharev S, Colombini M. Searching for the molecular arrangement of transmembrane ceramide channels. Biophys. J. 2006;90:2414–2426. doi: 10.1529/biophysj.105.071977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora AS, Jones BJ, Patel TC, Bronk SF, Gores GJ. Ceramide induces hepatocyte cell death through disruption of mitochondrial function in the rat. Hepatology. 1997;25:958–963. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem. J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. Fed. Am. Soc. Exp. Biol. J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem. J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- Brockman HL, Momsen MM, Brown RE, He L, Chun J, Byun HS, Bittman R. The 4,5-double bond of ceramide regulates its dipole potential, elastic properties, and packing behavior. Biophys. J. 2004;87:1722–1731. doi: 10.1529/biophysj.104.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Cifone MG, De Maria R, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J. Exp. Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochemic. J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Liu J, Chen J, Durrant D, McIntyre TM, Lee RM. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene. 2004;23:3650–3658. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- Dbaibo GS, Perry DK, Gamard CJ, Platt R, Poirier GG, Obeid LM, Hannun YA. Cytokine response modifier A (CrmA) inhibits ceramide formation in response to tumor necrosis factor (TNF)-alpha: CrmA and Bcl-2 target distinct components in the apoptotic pathway. J. Exp. Med. 1997;185:481–490. doi: 10.1084/jem.185.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson RE, Takano T, Eisenberg D, Kallai OB, Samson L, Cooper A, Margoliash E. Ferricytochrome c.I. General features of the horse and bonito proteins at 2.8 A resolution. J. Biol. Chem. 1971;246:1511. [PubMed] [Google Scholar]
- Di Paola M, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6620–6628. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Zaccagnino P, Montedoro G, Cocco T, Lorusso M. Ceramide induces release of pro-apoptotic proteins from mitochondria by either a Ca2+ -dependent or a Ca2+ -independent mechanism. J. Bioener. Biomem. 2004;36:165–170. doi: 10.1023/b:jobb.0000023619.97392.0c. [DOI] [PubMed] [Google Scholar]
- Douce R, Bourguignon J, Brouquisse R, Neuburger M. Isolation of plant mitochondria: general principles and criteria of integrity. Methods Enzymol. 1987;148:403–412. [Google Scholar]
- El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. J. Biol. Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson’s disease. J. Neurochem. 1997;69:1612–1621. doi: 10.1046/j.1471-4159.1997.69041612.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- Geley S, Harmann BL, Kofler R. Ceramides induce a form of apoptosis in human acute lymphoblastic leukemia cells that is inhibited by Bcl-2, but not by CrmA. Fed. Eur. Biochem. Soc. Lett. 1997;400:15–18. doi: 10.1016/s0014-5793(96)01284-7. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J. Biol. Chem. 1999;274:6080–6084. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- Gottschalk A, Boise L, Thompson C, Quintáns J. Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc. Natl Acad. Sci. USA. 1994;91:7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Kolesnick RN, Fornari FA, Traylor RS, Gewirtz DA, Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc. Natl Acad. Sci. USA. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal BS, Brown AM. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J. Biol. Chem. 1999;274:23169–23175. doi: 10.1074/jbc.274.33.23169. [DOI] [PubMed] [Google Scholar]
- Kroesen BJ, Pettus B, Luberto C, Busman M, Sietsma H, Leij LD, Hannun YA. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. J. Biol. Chem. 2001;276:13606–13614. doi: 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- Li L, Tang X, Taylor KG, DuPre DB, Yappert MC. Conformational characterization of ceramides by nuclear magnetic resonance spectroscopy. Biophys. J. 2002;82:2067–2080. doi: 10.1016/S0006-3495(02)75554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mate MJ, Ortiz-Lombardia M, Boitel B, Haouz A, Tello D, Susin SA, Penninger J, Kroemer G, Alzari PM. The crystal structure of the mouse apoptosis-inducing factor AIF. Nat. Struct. Biol. 2002;9:442–446. doi: 10.1038/nsb793. [DOI] [PubMed] [Google Scholar]
- Matsko CM, Hunter OC, Rabinowich H, Lotze MT, Amoscato AA. Mitochondrial lipid alterations during Fas- and radiation-induced apoptosis. Biochem. Biophys. Res. Commun. 2001;287:1112–1120. doi: 10.1006/bbrc.2001.5696. [DOI] [PubMed] [Google Scholar]
- Montes LR, Ruiz-Arguello MB, Goñi FM, Alonso A. Membrane restructuring via ceramide results in enhanced solute efflux. J. Biol. Chem. 2002;277:11788–11794. doi: 10.1074/jbc.M111568200. [DOI] [PubMed] [Google Scholar]
- Muriel M-P, Lamberg N, Darios F, Michel PP, Hirsch EC, Agid Y, Ruberg M. Mitochondrial free calcium levels (Rhod-2 fluorescence) and ultrastructural alterations in neuronally differentiated PC12 cells during ceramide-dependent cell death. J. Comp. Neurol. 2000;426:297–315. doi: 10.1002/1096-9861(20001016)426:2<297::aid-cne10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Novgorodov SA, Szulc ZM, Luberto C, Jones JA, Bielawski J, Bielawska A, Hannun YA, Obeid LM. Positively charged ceramide is a potent inducer of mitochondrial permeabilization. J. Biol. Chem. 2005;280:16096–16105. doi: 10.1074/jbc.M411707200. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Pajewski R, Djedovic N, Harder E, Ferdani R, Schlesinger PH, Gokel GW. Pore formation in and enlargement of phospholipid liposomes by synthetic models of ceramides and sphingomyelin. Bioorg. Med. Chem. 2005;13:29–37. doi: 10.1016/j.bmc.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Parsons DF, Williams GR, Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann. NY Acad. Sci. 1966;137:643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. Eur. Mol. Biol. Org. J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillet-Mary A, Jaffrézou J, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J. Biol. Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- Quintans J, Kilkus J, McShan CL, Gottschalk AR, Dawson G. Ceramide mediates the apoptotic response of WEHI 231 cells to anti-immunoglobulin, corticosteroids and irradiation. Biochem. Biophys. Res. Commun. 1994;202:710–714. doi: 10.1006/bbrc.1994.1988. [DOI] [PubMed] [Google Scholar]
- Raisova M, Bektas M, Wieder T, Daniel P, Eberle P, Orfanos CE, Geilen CC. Resistance to CD95/Fas-induced and ceramide-mediated apoptosis of human melanoma cells is caused by a defective mitochondrial cytochrome c release. Fed. Eur. Biochem. Soc. Lett. 2000;473:27–32. doi: 10.1016/s0014-5793(00)01491-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Alphonse G, Broquet P, Aloy M, Louisot P, Rousson R. Temporal relationships between ceramide production, caspase activation and mitochondrial dysfunction in cell lines with varying sensitivity to anti-Fas-induced apoptosis. Biochem. J. 2001;357:407–416. doi: 10.1042/0264-6021:3570407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Alphonse G, Aloy MT, Ardail D, Gerard JP, Louisot P, Rousson R. Increasing endogenous ceramide using inhibitors of sphingolipid metabolism maximizes ionizing radiation-induced mitochondrial injury and apoptotic cell killing. Int. J. Cancer. 2002;101:589–598. doi: 10.1002/ijc.10652. [DOI] [PubMed] [Google Scholar]
- Saelens X, Festjens N, Walle LV, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- Schafer P, Scholz SR, Gimadutdinow O, Cymerman IA, Bujnicki JM, Ruiz-Carrillo A, Pingoud A, Meiss G. Structural and functional characterization of mitochondrial EndoG, a sugar non-specific nuclease which plays an important role during apoptosis. J. Mol. Biol. 2004;338:217–228. doi: 10.1016/j.jmb.2004.02.069. [DOI] [PubMed] [Google Scholar]
- Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T. Partial purification and characterization of sphingosine N-acyltrans-ferase (ceramide synthase) from bovine liver mitochondrion-rich fraction. Lipids. 1998;33:601–605. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- Siskind LJ. Mitochondrial ceramide and the induction of apoptosis. J. Bioenerg. Biomem. 2005;37:143–153. doi: 10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Davoody A, Lewin N, Marshall S, Colombini M. Enlargement and contracture of C2-ceramide channels. Biophys. J. 2003;85:1560–1575. doi: 10.1016/S0006-3495(03)74588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Fluss S, Bui M, Colombini M. Sphingosine forms channels in membranes that differ greatly from those formed by ceramide. J. Bioenerg. Biomem. 2005;37:227–236. doi: 10.1007/s10863-005-6632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Castedo M, Daugas E, Wang EG, Geley S, Fassy F, Reed JC, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J. Exp. Med. 1997a;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Larochette N, Dallaporta B, Marzo I, Brenner C, Hirsch T, Petit PX, Geuskens M, Kroemer G. A cytofluorometric assay of nuclear apoptosis induced in a cell-free system: application to ceramide-induced apoptosis. Exp. Cell Res. 1997b;236:397–403. doi: 10.1006/excr.1997.3733. [DOI] [PubMed] [Google Scholar]
- Susin SA, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim. Biophys. Acta-Bioenerg. 1998;1366:151–165. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]
- Thomas RL, Jr, Matsko CM, Lotze MT, Amoscato AA. Mass spectrometric identification of increased C16 ceramide levels during apoptosis. J. Biol. Chem. 1999;274:30580–30588. doi: 10.1074/jbc.274.43.30580. [DOI] [PubMed] [Google Scholar]
- Wiesner DA, Kilkus JP, Gottschalk AR, Quintáns J, Dawson G. Anti-immunoglobulin-induced apoptosis in WEHI 231 cells involves the slow formation of ceramide from sphingomyelin and is blocked by bcl-XL. J. Biol. Chem. 1997;272:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- Witty JP, Bridgham JT, Johnson AL. Induction of apoptotic cell death in hen granulosa cells by ceramide. Endocrinology. 1996;137:5269–5277. doi: 10.1210/endo.137.12.8940345. [DOI] [PubMed] [Google Scholar]
- Yavin E, Gatt S. Enzymatic hydrolysis of sphingolipids. 8. Further purification and properties of rat brain ceramidase. Biochemistry. 1969;8:1692–1698. doi: 10.1021/bi00832a052. [DOI] [PubMed] [Google Scholar]
- Yuan H, Williams SD, Adachi S, Oltersdorf T, Gottlieb RA. Cytochrome c dissociation and release from mitochondria by truncated Bid and ceramide. Mitochondrion. 2003;2:237–244. doi: 10.1016/S1567-7249(02)00106-X. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc. Natl Acad. Sci. USA. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Liu B, Kang SW, Soe MS, Rhee SG, Obeid LM. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J. Biol. Chem. 1997;272:30615–30618. doi: 10.1074/jbc.272.49.30615. [DOI] [PubMed] [Google Scholar]