Abstract
Ceramides are known to play a major regulatory role in apoptosis by inducing cytochrome c release from mitochondria. We have previously reported that C2- and C16-ceramide, but not dihydroceramide, form large channels in planar membranes (Siskind, L. J., and Colombini, M. (2001) J. Biol. Chem. 275, 38640-38644). Here we show that ceramides do not trigger a cytochrome c secretion or release mechanism, but simply raise the permeability of the mitochondrial outer membrane, via ceramide channel formation, to include small proteins. Exogenously added reduced cytochrome c was able to freely permeate the mitochondrial outer membrane with entry to and exit from the intermembrane space facilitated by ceramides in a dose- and time-dependent manner. The permeability pathways were eliminated upon removal of C2-ceramide by bovine serum albumin, thus ruling out a detergent-like effect of C2-ceramide on membranes. Ceramide channels were not specific to cytochrome c, as ceramides induced release of adenylate kinase, but not fumerase from isolated mitochondria, showing some specificity of these channels for the outer mitochondrial membrane. SDS-PAGE results show that ceramides allow release of intermembrane space proteins with a molecular weight cut-off of about 60,000. These results indicate that the ceramide-induced membrane permeability increases in isolated mitochondria are via ceramide channel formation and not a release mechanism, as the channels that allow cytochrome c to freely permeate are reversible, and are not specific to cytochrome c.
Ceramide is a sphingosine-based lipid involved in the regulation of several cellular processes, including differentiation, growth suppression, cell senescence, and apoptosis (1 –5). Ceramide can be generated in cells via de novo synthesis or sphin-gomyelin hydrolysis. Several inducers of cellular stress leading to apoptosis have been shown to cause a net increase in cellular ceramide levels (2–4). Increases in cellular ceramide levels often precede the mitochondrial phase of apoptosis (6–13). Mitochondria are believed to be the targets in ceramide-mediated apoptosis and mitochondria are known to play a major regulatory role in cell death via apoptosis (14–18). Thus, ceramides may act on mitochondria to induce apoptosis.
In most cell types, a key event in apoptosis is the release of proteins from the intermembrane space of mitochondria to the cytoplasm, including apoptosis-inducing factor, cytochrome c, procaspases, and heat shock proteins (14–17, 19). In general, it is the release of these intermembrane space proteins that activates caspases and DNase that are responsible for the execution of apoptosis. Short-chain cell permeable ceramide analogues such as N-acetyl-d-erythro-sphingosine (C2-ceramide)1 and N-hexanoyl-d-erythro-sphingosine (C6-ceramide) have been shown to induce cytochrome c release when added to whole cell cultures (20–26) and isolated mitochondria (27–29); this cytochrome c release was preventable by preincubation with or overexpression of the anti-death protein, Bcl-2 (29–31), or transfection of cells with Bcl-xL (32, 33). In addition, Di Paola et al. (28) reported the release of cytochrome c from isolated mitochondrial suspensions by solubilized, long-chain, naturally occurring N-hexadecyl-d-erythro-sphingosine (C16-ceramide). We hypothesize that ceramide channels forming in the outer mitochondrial membrane are responsible for the ceramide-induced cytochromec release. We have reported that both short- and long-chain ceramides form large channels in planar membranes (34) and that some of these are predicted to be large enough to allow cytochrome c to permeate. Others have reported ceramide-induced increases in the permeability of liposomes (35–37). For example, Montes et al. (37) showed that long-chain ceramides, both externally added or enzymatically produced, can induce release of vesicle contents. They showed that sphingomyelinase treatment of large unilamellar vesicles containing sphingomyelin gives rise to release of fluorescein-derivatized dextrans of molecular weight of about 20,000, i.e. larger than cytochrome c (37). Dihydroceramide, despite the fact that it differs from ceramide only by the reduction of one double bond, does not induce apoptosis nor form channels (34). The ceramide channels are composed of many ceramide monomers and thus their formation is exceedingly sensitive to the free ceramide concentration. Altering the activity of local enzymes that synthesize and catabolize ceramide would cause channels to assemble or disassemble thus regulating the permeability of the outer membrane to small proteins.
Ceramides have been reported to have other effects on mitochondria including enhanced generation of reactive oxygen species (20, 28, 38–40), alteration of calcium homeostasis of mitochondria and endoplasmic recticulum (20, 38, 40–42), ATP depletion (27), collapse of the inner mitochondrial membrane potential (ΔΨ) (20, 27–29), and inhibition and/or activation of the activities of various components of the mitochondrial electron transport chain (28, 43). It is therefore unclear whether ceramide acts directly or indirectly on cytochrome c release.
Here we show that treatment of rat liver mitochondria with either C2- or C16-ceramide causes the outer membrane to be freely permeable to cytochrome c, not just cytochrome c release, and allows the release of proteins of up to about 60 kDa from the intermembrane space. The permeability increase induced by C2-ceramide is largely reversed by treatment with fatty acid-depleted bovine serum albumin (BSA). These results bolster the hypothesis that ceramide-induced cytochrome c release from mitochondria is via the formation of ceramide channels in dynamic equilibrium with ceramide in other structural states.
EXPERIMENTAL PROCEDURES
Reagents
The following reagents were purchased from Avanti Polar Lipids (Alabaster, AL): C2-ceramide, C2-dihydroceramide, C16-ceramide, C18-dihydroceramide, and asolectin (polar extract of soybean phospholipids). Antimycin A, 2,4-dinitrophenol (DNP), fatty acid-depleted BSA, horse heart cytochrome c, and sodium ascorbate were purchased from Sigma.
Electrophysiological Recordings
Planar membranes were formed by the monolayer method (44), as modified (45), across a 100-µm diameter hole in a Saran partition using 1% (w/v) asolectin (soybean phospholipids), 0.2% (w/v) cholesterol in hexane solution. The aqueous solution contained 1.0 m KCl, 1 mm MgCl2, and 5 mm HEPES or 5 mm PIPES (pH 7.0). The voltage was clamped (trans-side was ground) and the current was recorded. C2-ceramide was stirred into the water phase from a Me2SO solution, whereas C16-ceramide was first dissolved in ethanol at 37 °C prior to addition. In both cases, the final concentration of the vehicle was no more than 0.5%. Fatty acid-depleted BSA was prepared as a stock solution (0.5 mm) in the same aqueous solution bathing the membrane with 0.5% a zide (for storage purposes).
Preparation of Mitochondria
Rat liver mitochondria were isolated by differential centrifugation of tissue homogenate as previously described (46). Briefly, livers from male Sprague-Dawley rats (fasted overnight with water ad labitum) were quickly excised, cut, washed repeatedly in cold isolation medium (70 mm sucrose, 210 mm mannitol, 0.1 mm EGTA, 1.0 mm Tris-Cl, pH 7.4), and minced. Homogenization and differential centrifugation were performed with isolation medium supplemented with 0.5% (w/v) BSA. BSA was removed by a final 9000 × g centrifugation and subsequently mitochondria were suspended in BSA-free medium.
Preparation of Reduced Cytochrome c
1 mm horse heart cytochrome c was reduced by an excess of ascorbate (20 mm), 0.2 mTris-Cl (pH 7.5). The reduced cytochrome c was separated from the ascorbate using a Sephadex G-10 gel filtration column. The concentration of reduced cytochrome c was determined spectrophotometrically (ϵ550 nm(Red.-Ox.) = 18.5 mm−1 cm−1).
Detection of Cytochrome c Permeation through the Outer Membrane
Mitochondria were diluted 20–40-fold in isolation buffer to achieve an appropriate rate of cytochrome c oxidation after hypotonic shock and kept on ice. 20 µl of diluted mitochondria were added to 750 µl of isolation buffer supplemented with 0.5 mm DNP and 5 µm antimycin A (final concentration of 0.3–1.2 mg of mitochondrial protein/ml) and allowed to reach room temperature. Ceramide was added in such a way that the vehicle was no more than 1% of the total volume. Me2SO was the vehicle for C2-ceramide and C2-dihydroceramide. C16-ceramide and C18-ceramide were dissolved in 100% ethanol at 37 °C as described in Ref. 28 and added to mitochondria at room temperature. After the indicated incubation period, 20 µl of reduced cytochrome c (25–35 µm final concentration in the cuvette) was added and the initial rate of oxidation was assayed spectrophotometrically as a decrease in absorbance at 550 nm (ΔϵRed.-Ox. = 18.5 mm−1 cm−1) or the difference in absorbance at 550 and 536 nm (ϵ550 nm = 27.7 mm−1 cm−1; ϵ536 nm = 7.7 mm−1 cm−1). All initial rates were expressed as nanomoles of cytochrome c oxidized per s/mg of mitochondrial protein. KCN was used to inhibit cytochrome c oxidase and hence stop the reaction. Equal volumes of vehicle and/or dihydroceramide were used as controls.
Assessing the Ability of Ceramides to Release Adenylate Kinase and Fumarase
The intermembrane space enzyme adenylate kinase (47) and the matrix enzyme fumarase (48) were assayed by standard methods. To increase the concentration of any released enzyme in the medium (for detection purposes) following exposure to ceramide, it was necessary to use higher mitochondrial concentrations than those used to measure cytochrome c permeation through the outer membrane. To achieve comparable conditions in both types of experiments, the ceramide to mitochondria ratio was kept constant rather than the total concentration of added ceramide. This can be justified by recalling that ceramide exerts its effect on the membranes and that the critical concentration should be the concentration of ceramide in the membrane; a constant ratio of ceramide to mitochondrial protein should result in a constant level of ceramide in the mitochondrial outer membrane. The literature supports this notion. Muriel et al. (42) found that the concentration of C2-ceramide that induced the death of 50% of the PC12 cells after 24 h depended on the cell density used. Similarly, Simon and Gear (35) found that C2-ceramide inhibition of platelet aggregation and its ability to induce 6-carboxyfluorescein release from vesicles was dependent on the ratio of ceramide to total lipid, as opposed to the absolute ceramide concentration. Thus, we scaled the amount of ceramide to the amount of mitochondria used (i.e. moles of ceramide to milligrams of mitochondrial protein).
The mitochondrial preparation was diluted 5-fold in isolation buffer without antimycin A or DNP. A 250-µl aliquot (5 –10 mg of protein/ml) was incubated for 10 min with C2- or C16-ceramide at either a high or low molar ratio, 5 and 20 µm equivalent ratios of ceramide to milligrams of mitochondrial protein as in the accompanying cytochrome c oxidation experiments. The mitochondria were then pelleted at 12,000 rpm for 5 min and 200 µl of supernatant was then added to 700 µl of either adenylate kinase reaction mixture (50 mm Tris-HCl, pH 7.5, 5 mm MgSO4, 10 mm glucose, 5 mm ADP, 0.2 mm NADP, 10 units of hexokinase, and 10 units of glucose-6-phosphate dehydrogenase) or fumarase reaction mixture (50 mm sodium phosphate and 50 mm l-malate, pH 7.3). Adenylate kinase was detected as an increase in absorbance at 340 nm. Fumarase was detected as an increase in absorbance at 250 nm. Untreated mitochondria and mitochondria with lysed outer (adenylate kinase) or lysed outer and inner (fumarase) membranes served as negative and positive controls, respectively. In the case of the adenylate kinase assay, the outer mitochondrial membrane was lysed hypotonically. In the fumarase assay, the outer and inner mitochondrial membranes were lysed via sonication under severe hypo-osmotic conditions in the presence of 1 mm EDTA as described in Ref. 49. Equal volumes of vehicle and dihydroceramide were used as controls.
Assessment of Ceramide-induced Protein Release
Five milliliters of mitochondria that was diluted 5-fold (5–10 mg/ml) with isolation buffer (without antimycin A or DNP) was allowed to reach room temperature and then incubated for 10 min with C2- or C16-ceramide at 20 µm equivalent ratios of moles of ceramide to mitochondrial protein. Untreated mitochondria, dihydroceramide, and vehicles served as controls. The mitochondria were then spun at 35,000 rpm (50Ti rotor) for 30 min at 4 °C. The supernatant was removed and treated with 10% trichloroacetic acid overnight at 4 °C to pellet the proteins. The pellet was washed repeatedly with 1:1 ethanol:ether (v/v) to remove the trichloroacetic acid. The pellets were redissolved by adding Tris-OH, β-mercaptoethanol, and SDS in amounts equivalent to those used in the SDS-PAGE sample buffer and heated to near boiling for 10 min. After returning to room temperature, bromphenol blue was added and then HCl was added just until the color changed. 8 m urea was added to help stabilize the solution and provide for the added density (instead of glycerol). Proteins from the hypotonically lysed mitochondria were diluted 4-, 8-, and 16-fold. Samples were separated on a 15% acrylamide SDS-PAGE supplemented with 4 m urea and the bands stained with GelCode Blue stain (Pierce).
Determination of Protein Concentration
Mitochondrial protein was measured using the BCA method (Pierce). Bovine serum albumin was the standard.
RESULTS
Ceramide Increases the Permeability of the Mitochondrial Outer Membrane to Cytochrome c
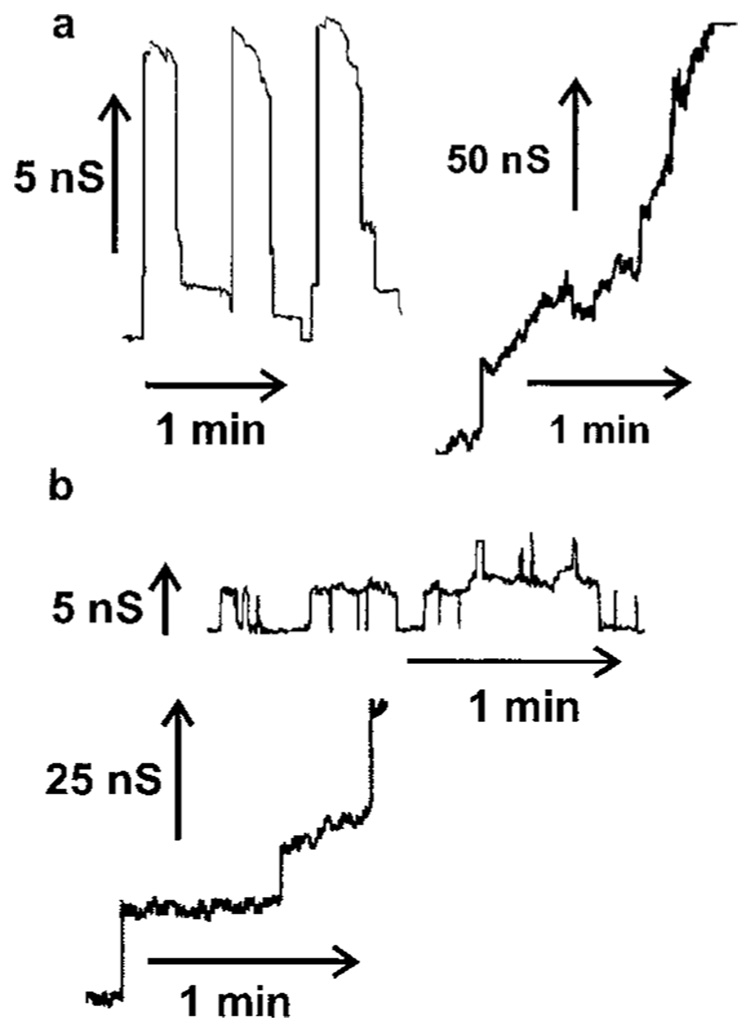
It has already been reported that addition of either C2- or C16-ceramide to isolated mitochondria results in cytochrome c release (27–29). However, from the literature it is not clear how this release might come about. We previously demonstrated that ceramides form large channels in phospholipid membranes (34), whereas the biologically inactive C2- and C18-dihydroceramides do not (34). The addition of either C16- or C2-ceramide to the aqueous phase on either one or both sides of a planar phospholipid membrane results in pore formation as indicated by discrete stepwise current increases (Fig. 1). Discontinuous changes in current are diagnostic of channel formation.
FIG. 1. Sample traces of C2- and C16-ceramide channels in planar membranes.
Continuous current recordings induced by the addition of ceramide to the aqueous solution as described under “Experimental Procedures.” The applied voltage was clamped at 10 mV. a, 1 µm C16-ceramide was added only to one side (the cis side) of the membrane.b, 5 µm C2-ceramide was added to both sides of the membrane.
Ceramide channels have a wide distribution of discrete conductance increases (34) (Fig. 1), ranging from 1 nanoSiemens to more than 200 nanosiemens. Small channels are seen early on when the total membrane conductance is low. With time, larger discrete conductance increases are evident reflecting the formation of larger structures. Eventually the conductance grows with fluctuations of current overlapping into current noise. Depicted in Fig. 1 are representative examples of some of these small and large channels observed with C2- and C16-ceramide. According to the bulk properties of water, pores with conductance ranging from 1 to 200 nanosiemens would have estimated diameters ranging from 0.8 to 11 nm, respectively. We hypothesize that similar channels form in the outer mitochondrial membrane, channels large enough to allow cytochrome c to exit. In this hypothesis, ceramide does not trigger a cytochrome c secretion or release mechanism, but simply raises the permeability of the outer mitochondrial membrane, via ceramide channel formation, to include small proteins. The hypothesis predicts the following: 1) cytochrome c should freely permeate and thus both entry to and exit from the intermembrane space should be facilitated; 2) the permeability pathways should be eliminated by removal of ceramide; 3) the release should not be specific to cytochrome c.
To test the first prediction, we did not measure cytochrome c release (already reported in Ref. 20–Ref. 29), but rather the ability of cytochrome c to permeate through the outer membrane. We determined the rate at which cytochrome oxidase of the mitochondrial inner membrane would oxidize exogenously added reduced cytochrome c. Because of the exceedingly small volume of the mitochondrial intermembrane space, cytochrome c would need to cross the outer membrane twice for appreciable oxidation to take place and be detected spectrophotometrically.
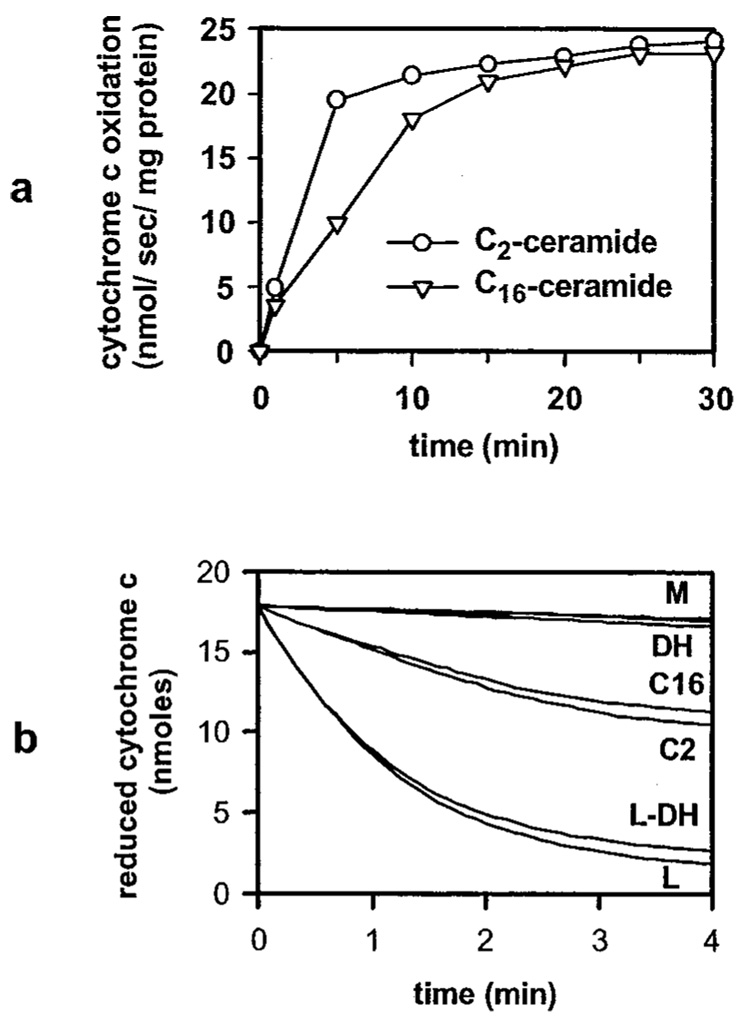
Both C2- and C16-ceramide caused a rapid increase in the rate of cytochrome c oxidation (Fig. 2a). The ceramide-induced permeability increase of the mitochondrial outer membrane occurred in a dose-dependent manner in the concentration range 0.5 to 40 µm. Dihydroceramide and vehicle controls were essentially identical to untreated mitochondria controls (Fig. 2b). To ensure that the decrease in absorbance at 550 nm in mitochondria incubated with ceramide reflected the oxidation of exogenously added reduced cytochrome c and not mitochondrial volume changes (swelling), mitochondria were exposed to C2- and C16-ceramide without adding exogenously reduced cytochrome c and the absorbance was recorded at 540 and 550 nm. We detected no changes in absorbance at 540 or 550 nm (data not shown). Therefore, the decrease in absorbance at 550 nm in mitochondria incubated with ceramide reflects the oxidation of exogenously added reduced cytochrome c and not volume changes. This does not rule out the possibility that ceramides can cause mitochondrial volume changes as previously reported (50). However, it does exclude the possibility that absorbance changes associated with volume changes were causing part of the recorded change in absorbance.
FIG. 2. C2- and C16-ceramide increase the permeability of the mitochondrial outer membrane to cytochrome c in a dose- and time-dependent manner.
Mitochondria were incubated for the indicated time periods and with the indicated concentrations of C2- (circles) or C16-ceramide (triangles) as described under “Experimental Procedures.” Reduced cytochrome c was then added and the absorbance was monitored at 550 nm. The initial rates plotted were in nanomoles of cytochrome c oxidized per s/mg of mitochondrial protein. Results are representative experiment of at least 3 performed on separate mitochondrial preparations. a mitochondria incubated for the indicated time periods with 20 µm ceramides. b, mitochondria incubated with the following treatments: mitochondrial controls (M; untreated mitochondria, vehicle controls, 83 µm BSA for 15 min, with overlapping lines); 20 µm C2-dihydroceramide for 10 min (DH); 20 µm C2-ceramide for 10 min (C2); 20 µm C16-ceramide for 10 min (C16); lysed mitochondria were incubated for 10 min with 20 µm C2-dihydroceramide (L-DH); lysed mitochondrial controls (L; untreated lysed mitochondria, lysed mitochondria incubated for 10 min with 20 µm C2-ceramide, C16-ceramide, or vehicles or 15 min with 83 µm BSA, with overlapping lines).
The measured rates of cytochrome c oxidation were not influenced by direct effects of ceramide on mitochondrial enzymes. Di Paola et al. (28) reported that C2- and C16-ceramide had stimulatory and inhibitory effects on cytochrome c oxidase, respectively, measured as ascorbate/TMPD oxidase activity, in isolated rat heart mitochondria or isolated respiratory complexes from bovine heart. However, we found that when mitochondria with lysed outer membranes were incubated with C2- or C16-ceramide, the rate of cytochrome c oxidation was identical to the control (Fig. 2b). Similarly, Gudz et al. (43) found no effect of C2-ceramide on cytochrome c oxidase activity in isolated rat heart mitochondria, using the ascorbate/TMPD oxidase system in the same range of C2-ceramide concentrations as utilized here.
In addition, ceramides have been reported to affect complex III (43), other components of the electron transport system (28), and the inner mitochondrial membrane potential (ΔΨ) (20, 27–29). Although important, these effects were not relevant as the medium was supplemented with both the complex III inhibitor antimycin A and the uncoupler DNP. Antimycin A prevents re-reduction of cytochrome c and DNP eliminates the protonmotive force that could slow down cytochrome oxidase activity. Contrary to the findings of Ghafourifar et al. (29) that ceramide-induced cytochrome c release was blocked in the absence of ΔΨ (achieved when they blocked respiration and uncoupled mitochondria), we detect cytochrome c permeation through the outer mitochondrial membrane in both uncoupled (Fig. 2, a and b) and coupled (data not shown) mitochondria incubated with C2- and C16-ceramide.
The Permeability Increase Induced by C2-ceramide Can Be Reversed
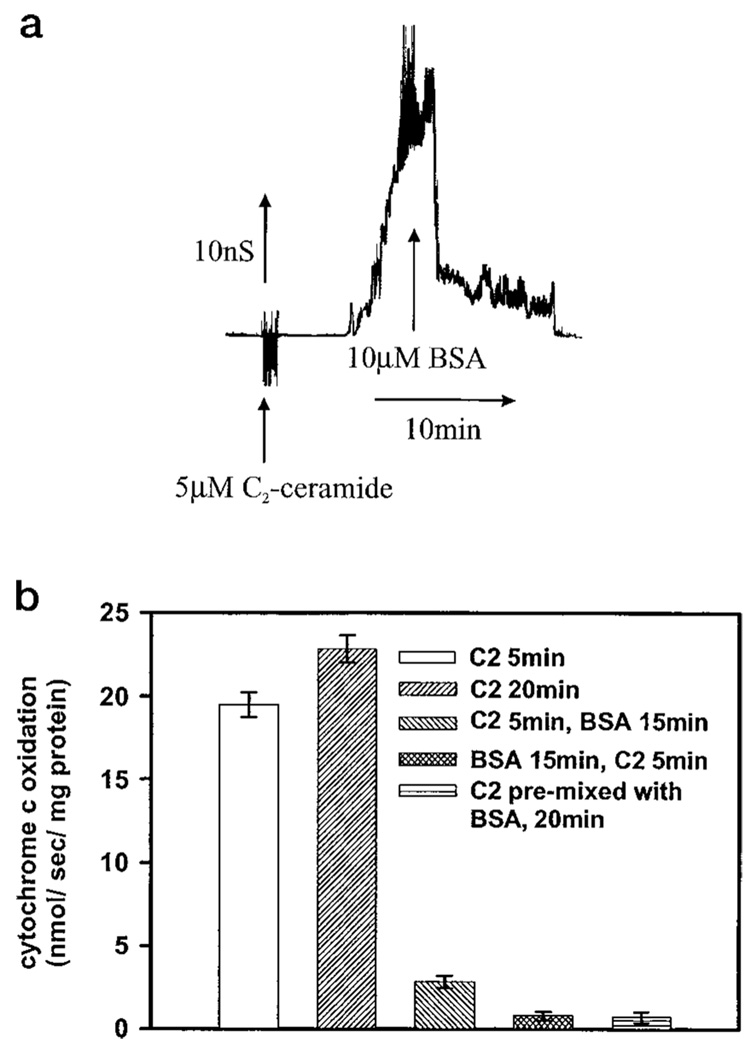
If the C2-ceramide-induced increase in permeability of the mitochondrial outer membrane to cytochrome c is because of ceramide channels in dynamic equilibrium with monomers then the normal permeability should be restored when ceramide monomers are removed from solution, resulting in channel disassembly (prediction number 2). Experiments with planar membranes showed that fatty acid-depleted BSA could be used to remove added ceramide. In the representative experiment in Fig. 3a, 10 µm fatty acid-depleted BSA (a 2:1 mol ratio of BSA to C2-ceramide) was added to the aqueous phase on both sides of a phospholipid membrane containing C2-ceramide channels. The membrane conductance decreased in a stepwise manner until the total membrane conductance returned to baseline. The time required for a complete disassembly of ceramide channels by BSA varied greatly between experiments. Although the conductance decreased soon after BSA addition, it took between 5 min and 2 h before all the channels disassembled. This may indicate that the structures formed have a varying degree of structural stability. In any case, BSA can be used as a tool to disassemble C2-ceramide channels.
FIG. 3. The permeability increase induced by C2-ceramide can be reversed with BSA.
a, BSA was added (10 µm final) to the aqueous phase on both sides of a planar phospholipid membrane containing C2-ceramide channels (5 µm C2-ceramide on both sides). The applied voltage was clamped at 10 mV. b, mitochondria were incubated with 20 µm C2-ceramide for the indicated time periods and where indicated with 83 µm BSA for the indicated time periods as described under “Experimental Procedures.” Reduced cytochrome c was then added and the absorbance was monitored at 550 nm. The initial rates were plotted as nanomoles of cytochrome c oxidized per s/mg of mitochondrial protein. Error bars represent standard deviations. Results are a representative experiment of at least 3 performed on separate mitochondrial preparations.
This approach was applied to intact mitochondria. A 5-min preincubation of isolated mitochondria with 20 µm C2-ceramide was followed by a 15-min incubation with excess BSA (83 µm; 5:1, BSA:C2-ceramide mole ratio). This resulted in a marked drop in the cytochrome c oxidation rate indicating an almost complete restoration of outer membrane permeability characteristics (Fig. 3b). If the BSA was simply preventing any further effects of the short-chain ceramide on the outer membrane permeability instead of actually reversing the ceramide channels then the rate of oxidation following the 5-min 20 µm C2-ceramide and then the 15-min 83 µm BSA incubation would only be lower than a 20-min incubation with 20 µm C2-ceramide. Instead, the rate of oxidation was substantially lower than the rate seen with a 5-min exposure of the mitochondria to 20 µm C2-ceramide and was almost as low as the untreated mitochondria. Incubation of the mitochondria with BSA prior to C2-ceramide addition or mixing the BSA and C2-ceramide prior to their addition also had very low rates of oxidation, showing that BSA can both reverse and inhibit C2-ceramide channel formation (Fig. 3b).
Complete reversal was not achieved, in contrast with the observations made in the planar membrane experiments, and this was likely because of the inability to incubate the mitochondria at room temperature for a sufficient amount of time to allow for 100% reversal without their degradation. The time required for complete reversal of the C2-ceramide channels in the planar membrane experiments was highly variable and for the most part required a longer time period than 15 min. Mitochondria with lysed outer membranes exposed to BSA had essentially identical rates of oxidation of the reduced cytochrome c as lysed mitochondria not exposed to BSA, ruling out an effect of the BSA on cytochrome oxidase activity. An inert molecule impermeable to the outer membrane, polyvinyl pyrrolidine (40 kDa), was used as a control for osmotic changes and the possibility that a reduction in the volume of the intermembrane space might somehow affect the rate of cytochrome c oxidation. Addition of 83 µm polyvinyl pyrrolidine had no effect.
Only C2-ceramide was used for the BSA reversal experiments because of the inability of BSA to reverse or prevent the permeability induced by C16-ceramide, which could be because of a greater affinity of C16-ceramide for membranes as opposed to BSA. C16-ceramide has two long fatty acyl chains like phospholipids, whereas C2-ceramide has only one as in the free fatty acids that bind to BSA. By doubling the non-polar portion of ceramide one would expect that the hydrophobic component of the interaction energy between ceramide and membranes would also double. This change could not only make ceramide-membrane interactions stronger than ceramide-BSA interactions, but also greatly reduce the free ceramide concentration in solution, favoring the dissociation of ceramide from BSA. In addition, BSA is known to bind associated forms of organic ligands poorly relative to unassociated forms (monomers) (51)
C2- and C16-ceramides Allow the Release of Low Molecular Weight Proteins from the Intermembrane Space
It has been proposed that cytochrome c release is the prime mitochondrial target of ceramide (29). However, if C2- and C16-ceramide induced cytochrome c release was because of ceramide channel formation in the outer mitochondrial membrane then these channels should not be specific to the release of cytochrome c, but should also allow the release of other proteins from the mitochondrial intermembrane space (prediction number 3). We therefore assayed for adenylate kinase (26 kDa) (52) release from the intermembrane space of isolated mitochondria by C2- and C16-ceramide addition. To obtain a sufficient amount of adenylate kinase activity, a higher amount of mitochondria was used and therefore 5 and 20 µm equivalent ceramide to mitochondria ratios (labeled low and high) were used to achieve comparable conditions as in the cytochrome c permeability assays. Mitochondria incubated for 10 min with C2- and C16- ceramide, but not their dihydroceramide counterparts, released adenylate kinase from the intermembrane space; an increased amount of adenylate kinase was released at the higher ceramide dose (Table I). Vehicles and dihydroceramides were essentially identical to the untreated mitochondrial control. Therefore, the ceramide channels allow the passage of adenylate kinase as well as cytochrome c.
Table I. Effects of ceramides on the release of the intermembrane space enzyme adenylate kinase and the matrix enzyme fumerase.
Enzymatic activity (absorbance change/min ± S.D.) released from mitochondria (2.4 (adenylate kinase) or 1.8 (fumerase) mg of protein) after they were untreated (M) or treated with C2-ceramide (C2), C16-ceramide (C16), or C18-dihydroceramide (DH) or hypotonically shocked to damage either the outer membrane (L, adenylate kinase) or both membranes (L, fumarase). Treatments utilized low or high levels of ceramide (4 or 16 for adenylate kinase and 5 or 20 for fumarase in nanomoles/mg of protein). The results are representative of three different experiments using separate mitochondrial preparations.
| Treatment | Adenylate kinase |
Fumerase |
||
|---|---|---|---|---|
| Specific activity | % of L | Specific activity | % of L | |
| L | 23.72 ± 1.25 | 100 | 33.54 ± 0.58 | 100 |
| M | 1.3 ± 0.07 | 6 | 0.74 ± 0.02 | 2.2 |
| Low C2 | 3.9 ± 0.22 | 17 | 0.85 ± 0.09 | 2.5 |
| High C2 | 9.8 ± 0.77 | 42 | 0.88 ± 0.11 | 2.6 |
| Low C16 | 9.5 ± 0.65 | 40 | 0.76 ± 0.05 | 2.2 |
| High C16 | 13.7 ± 0.82 | 58 | 0.77 ± 0.04 | 2.3 |
| High DH | 1.3 ± 0.09 | 6 | 0.81 ± 0.05 | 2.4 |
To assess whether the inner membrane was also being permeabilized to proteins, we tested for the release of fumarase (50 kDa) (53), a soluble matrix enzyme, in mitochondrial supernatants. This is a typical test for damage/disruption of the mitochondrial inner membrane (53). No increase in fumarase activity was detected in supernatants from mitochondria incubated with C2- or C16-ceramide at either low or high ceramide to mitochondrial protein ratios (Table I).
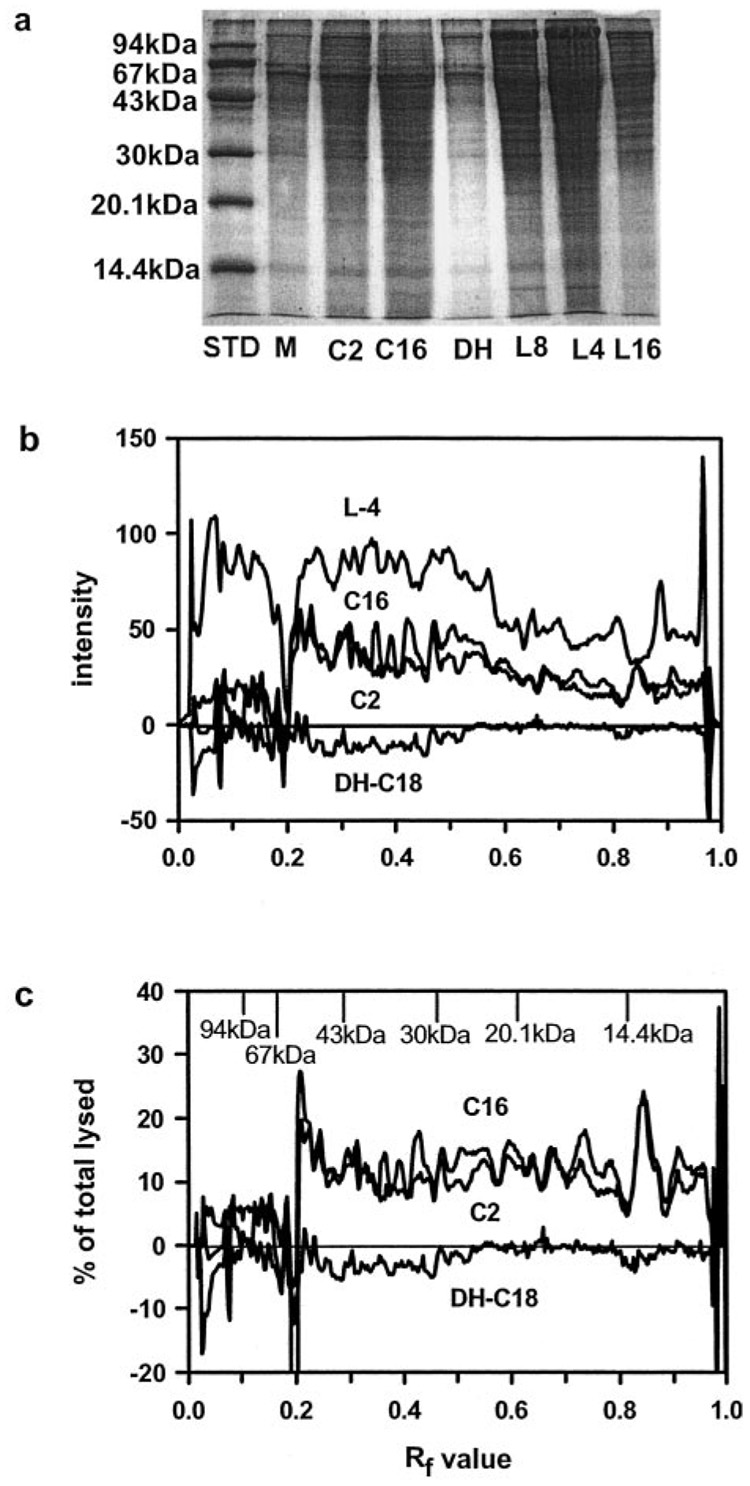
If ceramide channels were forming in the mitochondrial outer membrane that allow the release of relatively small soluble proteins from the intermembrane space, then other proteins of similar molecular weight should also be released. SDS-PAGE results show that C2- and C16-ceramides favor the release of low molecular weight proteins from mitochondria over high molecular weight ones (Fig. 4, a–c). The bands from the representative gel in Fig. 4a were quantified using densitometry and the individual gel background for each lane in the gel was subtracted out. To more accurately reflect the differences between the ceramides, dihydroceramide, and positive control (mitochondria with hypo-osmotically lysed outer membranes), the data from the untreated mitochondrial control (the background) was also subtracted out. Fig. 4b quantitatively represents the differences in the proteins released by the ceramides and those that were present in the intermembrane space. C18-dihydroceramide was essentially identical to the untreated mitochondria, as its intensity was basically zero when the data from the mitochondrial control was subtracted out (Fig. 4b). Mitochondria with hypo-osmotically lysed outer membranes show proteins both above and below the RF value of 0.2 (Mr of 60,000). However, mitochondria incubated with C2- and C16-ceramides show proteins only above the RF value of 0.2 (Fig. 4b). Therefore, there was a sharp molecular weight cut-off of the proteins released by C2- and C16-ceramides (Fig. 4b).
FIG. 4. C2-C16-ceramide increase the permeability of the mitochondrial outer membrane to intermembrane space proteins with a cut-off of about 60 kDa.
Mitochondria were incubated with or without ceramides as described under “Experimental Procedures” and the released proteins were run on a 15% acrylamide SDS-PAGE. The ceramide level was 18 nmol of ceramide/mg of mitochondrial protein. Results are a representative experiment of 3 performed on separate mitochondrial preparations. The following abbreviations are used: STD, standard proteins; M, mitochondrial control; C2, C2-ceramide; C16, C16-ceramide; DH or DH-C18, C18-dihydroceramide; L8, lysed mitochondria diluted 8-fold; L4, lysed mitochondria diluted 4-fold; L16, lysed mitochondria diluted 16-fold. a, SDS-PAGE was stained with GelCode Blue stain (Pierce). b, densitometry was performed on the gel from a with individual backgrounds for each lane and the mitochondrial control was subtracted out. Results were expressed as intensity of staining versus the RF value. c, results were expressed as the % of lysed (undiluted) versus the RF value.
Upon initial examination of Fig. 4b, it appears that more of the high molecular weight proteins (less than 60,000, but greater than about 20,000) were released by the ceramides than the lower molecular weight ones (less than about 20,000). However, when the data was expressed as the percent of total proteins in the intermembrane space (released by osmotic shock), on average the same percentage of proteins less than the cut-off of 60,000 was released by the ceramides (Fig. 4c). It appears that at a ceramide dose of 18 (nanomoles of ceramide/mg of mitochondrial protein) of either C2- or C16-ceramide increases the permeability of the outer membranes of about 10–15% of mitochondria to proteins of molecular weight ≤ 60,000 (Table II).
Table II. Effects of ceramides on the release of intermembrane space proteins.
Individual bands from polyacrylamide gels such as the one illustrated in Fig. 4 were quantitated by densitometry. The intensity was corrected for gel background and for the intensity of the corresponding band in the untreated mitochondrial control. The results were expressed as a percentage of the intensity of the corresponding band for protein released after selective damage of the outer membrane by hypotonic shock. Errors represent the S.E. of the mean for three separate mitochondrial preparations.
| Band | Release as % of intermembrane space content |
|
|---|---|---|
| C16-ceramide | C2-ceramide | |
| kDa | ||
| 55.7 ± 0.5 | 15.3 ± 1.5 | 12.7 ± 1.8 |
| 44.4 ± 0.9 | 15.1 ± 2.3 | 14.7 ± 2.7 |
| 36.3 ± 1.5 | 13.0 ± 2.7 | 12.6 ± 2.2 |
| 22.6 ± 0.4 | 14.1 ± 1.5 | 12.8 ± 1.7 |
| 18.7 ± 0.2 | 16.1 ± 1.2 | 14.9 ± 1.3 |
| 12.6 ± 0.3 | 12.4 ± 1.8 | 12.3 ± 2.0 |
| 10.5 ± 0.3 | 16.7 ± 2.1 | 15.9 ± 2.6 |
DISCUSSION
The results of this paper extend our previous observations that C2- and C16-ceramide form large stable channels in planar membranes to isolated mitochondria. Here, several predictions/criteria for channel formation were utilized to test our hypothesis that ceramide channels, similar to the ones that we observe in planar phospholipid membranes, form in the mitochondrial outer membrane, raising its permeability to intermembrane space proteins. The results of this paper show that by fulfilling all of the criteria for channel formation, the ceramide-induced increases in the permeability of isolated rat liver mitochondrial outer membranes were in fact because of ceramide channels.
First, as expected for a channel, the ceramides do not simply allow the release of cytochrome c or trigger a release mechanism, but allow its bidirectional flux across the outer mitochondrial membrane (Fig. 2). Our results strongly argue for specific pathways, channels of a size capable of allowing the passage of proteins. They are inconsistent with a specific pathway for cytochrome c. Ghafourifar et al. (29) showed an increase in the release of mitochondrial proteins apart from cytochrome c upon treatment of isolated rat liver mitochondria with C2- and C6-ceramide. However, it should be noted that in their experiments they only found release of cytochrome c and other mitochondrial proteins by ceramide when cytochrome c was mainly oxidized, achieved after blocking complex III (29). In contrast, we found release of adenylate kinase (Table I) and other proteins of similar size from mitochondria (Fig. 4, a and b) when complex III was not blocked. Similarly, Di Paola et al. (28) reported C2- and C16-ceramide-induced cytochrome c release from isolated mitochondria without blocking complex III. In addition, we found that exogenously added reduced cytochrome c was able to cross the outer mitochondrial membrane of mitochondria incubated with either C2- or C16-ceramide; hence, we found no correlation between the redox state of cytochrome c and its ability to transverse the outer mitochondrial membrane via ceramide channels.
Second, as would be expected for a channel, the permeability pathways in the mitochondrial outer membranes were eliminated by removal of ceramide. The permeability barrier of the mitochondrial outer membranes was almost completely restored when BSA removed C2-ceramide and this mirrors exactly the results obtained with C2-ceramide channels reconstituted into planar membranes. The simplest conclusion is that C2-ceramide forms the same channels in both mitochondrial and phospholipid membranes and these channels are in dynamic equilibrium with monomers in solution. As BSA binds monomers reducing the monomer concentration, the channels disassemble. The fact that the integrity of planar membranes and the outer membranes of isolated mitochondrial suspensions can be restored upon removal of ceramide monomers argues against an indirect action by ceramide in our system. Ceramides might act indirectly by triggering the permeability transition or catalyzing the insertion and channel formation by Bax or by triggering lipid reorganization in a detergent-like manner. Whereas not specifically excluding any of these possibilities for the in vivo situation, clearly our results in isolated mitochondria can be easily explained by ceramide channel formation without the need for ancillary factors. In fact, Polster et al. (54) found no Bax associated with mitochondria isolated from rat liver. In addition, in the planar membrane experiments no proteins were used except for BSA.
Third, the ceramide-induced increase in the permeability of the outer mitochondrial membranes was not specific to cytochrome c. The ceramide channels allow the release of other intermembrane space proteins and, as expected for a channel, there was a distinct molecular weight cut-off for the proteins released at about 60,000. This cut-off is in line with the sizes of proteins that were released from the mitochondrial intermembrane space during apoptosis (55–57). The observed cut-off is a lower limit because the proteins have been reduced to individual polypeptides by treatment with SDS under reducing conditions. In addition, no effects were observed when the biologically inactive C18-dihydroceramide was used and this was in harmony with the inability of this molecule to form channels in phospholipid membranes (34).
It has been suggested that C2-ceramide can cause unspecific increases in membrane permeability via perturbation of the membrane structure (1, 28, 35, 58). In addition, there has been recent controversy over the type of ceramide used in apoptosis research. The majority of experiments with cells and isolated mitochondria have used short-chain cell-permeable ceramides (such as C2- or C6-ceramide) because of their ease in handling. However, these short-chain ceramides are not the major forms found in cells and their use has been criticized because they were thought to cause an unspecific increase in membrane permeability (28, 58). For example, Di Paola et al. (28) showed that only C2-ceramide, but not C16-ceramide, was able to dissipate the inner mitochondrial membrane potential and the potential in complex III-reconstituted liposomal vesicles. However, we argue that the differences between short- and long-chain ceramides lies in their ability to insert and exchange between membranes. The virtually non-existent second acyl chain leads to a much higher water solubility and thus short-chain ceramides would be expected to be able to partition promiscuously into different populations of membranes, whereas long-chain ceramides would be expected to exert their effects only locally. Indeed, Simon et al. (59) found that the rate of exchange of C16-ceramide between populations of lipid vesicles was on the order of days.
Short-chain ceramides have also been proposed to have detergent-like effects on membranes. Amphipathic molecules are labeled as detergents based on a variety of properties such as the ability to dissolve relatively insoluble molecules into an aqueous environment, ability to disrupt membranes by solubilizing components and favoring the formation of micelles, possessing a relatively high critical micellar concentration, and the like. Often, molecules that act as channels or carriers display detergent-like behavior by destabilizing membranes. However, the fact that ceramides do not destabilize solvent-free planar phospholipid membranes and form channels in said membranes demonstrates that they are not significantly detergent-like. We argue that C2-ceramide does not cause cytochrome c release by simply rupturing or dissolving the outer membrane in a detergent-like manner because its effects are reversible, i.e. the low permeability of the membrane can be restored. In addition, whereas some might argue that a detergent-like effect may also be reversible with BSA, disruption of the outer membrane with digitonin was not reversed by washing away the digitonin. In addition, detergents would not have a molecular weight cut-off for the proteins released from the mitochondrial intermembrane space as was the case for ceramides. Finally, erythrocytes are sensitive to detergent-induced lysis, but we found no such effect with C2-ceramide added at a ratio of ceramide to surface membrane lipids that was 4-fold higher than those used in the experiments reported here (data not shown). Most importantly, it was the combined results of testing all three predictions of channel activity that allows us to conclude that ceramide-induced increases in the permeability of outer membranes isolated from rat liver mitochondria was through ceramide channel formation.
To apply our results obtained with isolated mitochondria to the in vivo situation, one must first consider the levels of ceramide used and whether or not they were physiologically relevant. At a ratio of 18 nmol of ceramide/mg of mitochondrial proteins, if all of the added ceramide inserted into the mitochondrial outer membrane then the ceramide would constitute about 14 mol % of the outer membrane phospholipids. The ceramide content of isolated healthy rat liver mitochondria has been measured at 1.7 nmol/mg of protein (60). If all of the ceramide was inserted into the mitochondria then there would be about a 10-fold increase in ceramide above the level of ceramide in healthy rat liver mitochondria. Apoptotic agents can cause up to 20-fold increases in cellular ceramide levels (1) and these represent average increases for the entire cell. Local increases in ceramide may very well be of a much higher magnitude. (Ongoing research in our laboratory indicates that only about 10 % of the C2-ceramide added actually gets into the mitochondria.)
Another consideration was the way ceramide levels could be raised locally. Enzymes capable of both de novo synthesis (ceramide synthase) and hydrolysis (ceramidase) have been found in mitochondria (61, 62). (The de novo synthetic pathway was distinct from ceramide generation in the plasma membrane through the breakdown of sphingomyelin.) Altering the activity of one or both of these mitochondrial enzymes would change the local steady-state level of ceramide thus increasing or decreasing the propensity for channel formation. Garcia-Ruiz et al. (40) reported that mitochondria isolated from cells treated with tumor necrosis factor showed a 2–3-fold increase in mitochondrial ceramide concentrations as compared with control cells. Their failure to increase mitochondrial ceramide levels by adding sphingomyelinases (40) indicates that the increase was most likely through de novo synthesis. Ionizing radiation has been shown to induce both a prolonged ceramide generation exclusively within mitochondrial membranes and an increase in mitochondrial ceramide synthase activity.2 Other reports show that many chemotherapy drugs and radiation that induce ceramide generation do so via the de novo synthesis route (9, 64–70). For example, Charles et al. (10) found that exposure of human breast cancer cells to Taxol resulted in an enhanced formation of ceramide that was inhibited by both fumonisin B1 (a ceramide synthase inhibitor) and l-cycloserine (a serine palmitoyltransferase inhibitor). Additionally, Perry et al. (8) reported ceramide accumulation following etoposide treatment of Molt-4 human leukemia cells as a result of activation of the de novo synthesis enzyme serine palmitoyltransferase. Kroesen et al. (12) reported early generation of de novo derived C16- ceramide in response to B-cell receptor cross-linking that was linked to a loss of mitochondrial function and subsequent activation of the apoptotic program. They showed that fumonisin B1 completely prevented not only ceramide production, but also a drop in ΔΨ, mitochondrial swelling and disruption of mitochondrial membranes, poly(ADP-ribose) polymerase cleavage, and DNA fragmentation (12). Kawatani et al. (63) reported a decrease in cytochrome c release upon treatment of cells with fumonisin B1. These studies implicate a role for de novo generated ceramide in cytochrome c release and subsequent activation of downstream effectors that lead to the execution of apoptosis.
In conclusion, ceramide channels have the right biophysical properties to be responsible for the release of pro-apoptotic factors from mitochondria. When added to isolated mitochondria they increase the permeability of the outer membrane in a way that is consistent with the formation of dynamic channels. The appropriate enzymes capable of regulating ceramide levels are located in mitochondria. There is evidence that the activity of synthetic enzymes is elevated early in apoptosis. Taken together, these findings make a compelling case that ceramide channels are good candidates for the pathway in the outer membrane that is responsible for the release of pro-apoptotic factors leading to irreversible apoptosis.
Acknowledgement
We are indebted to Amirparviz Davoody for assistance in preparing mitochondria and adenylate kinase assays.
Footnotes
The abbreviations used are: C2-ceramide, N-acetyl-d-erythro-sphingosine; C6-ceramide, N-hexanoyl-d-erythro-sphingosine; C2-dihydroceramide, N-acetyl-d-erythro-sphinganine; C16-ceramide, N-hexadecyl-d-erythro-sphingosine; C18-dihydroceramide, N-octadecyl-d-erythro-sphinganine; ΔΨ, inner mitochondrial membrane potential; BSA, bovine serum albumin; DNP, 2,4-dinitrophenol; PIPES, 1,4-pipera-zinediethanesulfonic acid.
H. Lee, Z. Fuks, A. Rimmer, D. Ehleiter, J. Reed, W. Liao, and R. Kolesnick, manuscript in preparation.
REFERENCES
- 1.Radin NS. Eur. J. Biochem. 2001;268:193–204. doi: 10.1046/j.1432-1033.2001.01845.x. [DOI] [PubMed] [Google Scholar]
- 2.Kolesnick RN, Krönke M. Annu. Rev. Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 3.Ariga T, Jarvis WD, Yu RK. J. Lipid Res. 1998;39:1–16. [PubMed] [Google Scholar]
- 4.Hannun YA. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 5.Kolesnick RN, Goñi FM, Alonoso A. J. Cell. Physiol. 2000;184:285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Witty JP, Bridgham JT, Johnson AL. Endocrinology. 1996;137:5269–5277. doi: 10.1210/endo.137.12.8940345. [DOI] [PubMed] [Google Scholar]
- 7.Raisova M, Bektas M, Wieder T, Daniel P, Eberle P, Orfanos CE, Geilen CC. FEBS Lett. 2000;473:27–32. doi: 10.1016/s0014-5793(00)01491-5. [DOI] [PubMed] [Google Scholar]
- 8.Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. J. Biol. Chem. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- 9.Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 10.Charles AG, Han TY, Liu YY, Hansen N, Giuliano AE, Cabot MC. Cancer Chemother. Pharmacol. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Lafrasse C, Alphonse G, Broquet P, Aloy M, Louisot P, Rousson R. Biochem. J. 2001;357:407–416. doi: 10.1042/0264-6021:3570407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroesen B-J, Pettus B, Luberto C, Busman M, Sietsma H, Leij LD, Hannun YA. J. Biol. Chem. 2001;276:13606–13614. doi: 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RL, Jr, Matsko CM, Lotze MT, Amoscato AA. J. Biol. Chem. 1999;274:30580–30588. doi: 10.1074/jbc.274.43.30580. [DOI] [PubMed] [Google Scholar]
- 14.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Eur. J. Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 15.Crompton M. Biochem. J. 1999;342:233–249. [PMC free article] [PubMed] [Google Scholar]
- 16.Kroemer G, Dallaporta B, Resche-Rigon M. Annu. Rev. Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 17.Susin SA, Zamzami N, Kroemer G. Biochim. Biophys. Acta. 1998;1366:151–165. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 18.Green DR, Reed JC. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 19.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, Israels S, Ballester M, Virmani R, Saxna S, Kharbanda S. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. J. Exp. Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castedo M, Hirsch T, Susin SA, Zamzami N, Marchetti P, Macho A, Kroemer G. J. Immunol. 1996;157:512–521. [PubMed] [Google Scholar]
- 22.Susin SA, Zamzami N, Castedo M, Daugas E, Wang EG, Geley S, Fassy F, Reed JC, Kroemer G. J. Exp. Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susin SA, Zamzami N, Larochette N, Dallaporta B, Marzo I, Brenner C, Hirsch T, Petit PX, Geuskens M, Kroemer G. Exp. Cell Res. 1977;236:397–403. doi: 10.1006/excr.1997.3733. [DOI] [PubMed] [Google Scholar]
- 24.DeMaria R, Lenti L, Malisan F, d’Agostino F, Tomasini B, Zeuner A, Rippo MR, Testi R. Science. 1997;277:1652–1655. doi: 10.1126/science.277.5332.1652. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P, Liu B, Kang SW, Seo MS, Rhee SG, Obeid LM. J. Biol. Chem. 1997;272:30615–30618. doi: 10.1074/jbc.272.49.30615. [DOI] [PubMed] [Google Scholar]
- 26.Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, Green DR, Bhalla K. Blood. 1998;91:1700–1705. [PubMed] [Google Scholar]
- 27.Arora AS, Jones BJ, Patel TC, Bronk SF, Gores GJ. Hepatology. 1997;25:958–963. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- 28.Di Paola M, Cocco T, Lorusso M. Biochemistry. 2000;39:6620–6628. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 29.Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. J. Biol. Chem. 1999;274:6080–6084. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geley S, Hartmann BL, Kofler R. FEBS Lett. 1997;400:15–18. doi: 10.1016/s0014-5793(96)01284-7. [DOI] [PubMed] [Google Scholar]
- 32.Gottschalk A, Boise L, Thompson C, Quintáns J. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiesner DA, Kilkus JP, Gottschalk AR, Quintáns J, Dawson G. J. Biol. Chem. 1997;272:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- 34.Siskind LJ, Colombini M. J. Biol. Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon CG, Jr, Gear ARL. Biochemistry. 1998;37:2059–2069. doi: 10.1021/bi9710636. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Argüello MB, Basañez G, Goñi FM, Alonoso A. J. Biol. Chem. 1996;271:26616–26621. doi: 10.1074/jbc.271.43.26616. [DOI] [PubMed] [Google Scholar]
- 37.Montes LR, Ruiz-Argüello MB, Goñi FM, Alonoso A. J. Biol. Chem. 2002;277:11788–11794. doi: 10.1074/jbc.M111568200. [DOI] [PubMed] [Google Scholar]
- 38.Quillet-Mary A, Jaffrézou J, Mansat V, Bordier C, Naval J, Laurent G. J. Biol. Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 39.France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. J. Neurochem. 1997;69:1612–1621. doi: 10.1046/j.1471-4159.1997.69041612.x. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. J. Biol. Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 41.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muriel M-P, Lamberg N, Darios F, Michel PP, Hirsch EC, Agid Y, Ruberg M. J. Comp. Neurol. 2000;426:297–315. doi: 10.1002/1096-9861(20001016)426:2<297::aid-cne10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 43.Gudz TI, Tserng K-Y, Hoppel CL. J. Biol. Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 44.Montal M, Mueller P. Proc. Natl. Acad. Sci. U. S. A. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colombini M. Methods Enzymol. 1987;148:465–475. doi: 10.1016/0076-6879(87)48045-2. [DOI] [PubMed] [Google Scholar]
- 46.Parsons DF, Williams GR, Chance B. Ann. N. Y. Acad. Sci. 1966;137:643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- 47.Sotocassa GL, Kuylenstierna B, Ernster L, Bergstrand A. Methods Enzymol. 1967;10:448–463. [Google Scholar]
- 48.Hill RL, Bradshaw RA. Methods Enzymol. 1969;13:91–99. [Google Scholar]
- 49.Holden MJ, Colombini M. Biochim. Biophys. Acta. 1993;1144:393–402. doi: 10.1016/0005-2728(93)90126-z. [DOI] [PubMed] [Google Scholar]
- 50.de Gann M-P, Belaud-Rotureau MA, Voisin P, Leducq N, Belloc F, Canioni P, Diolez P. Cytometry. 1998;33:333–339. [PubMed] [Google Scholar]
- 51.Spector AA, John K, Fletcher JE. J. Lipid Res. 1969;10:56–67. [PubMed] [Google Scholar]
- 52.Tanabe T, Yamada M, Noma T, Kajii T, Nakazawa A. J. Biochem. (Tokyo) 1993;113:200–207. doi: 10.1093/oxfordjournals.jbchem.a124026. [DOI] [PubMed] [Google Scholar]
- 53.Tuboi S, Suzuki T, Sato M, Yoshid T. Adv. Enzyme Regul. 1990;30:289–304. doi: 10.1016/0065-2571(90)90023-u. [DOI] [PubMed] [Google Scholar]
- 54.Polster BM, Kinnally KW, Fiskum G. J. Biol. Chem. 2001;276:37887–37894. doi: 10.1074/jbc.M104552200. [DOI] [PubMed] [Google Scholar]
- 55.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 56.Du C, Fang M, Li Y, Li L, Wang X. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 57.Li LY, Luo X, Wang X. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman K, Dixit VM. Trends Biochem. Sci. 1999;24:226–227. doi: 10.1016/s0968-0004(99)01410-3. [DOI] [PubMed] [Google Scholar]
- 59.Simon CG, Jr, Holloway PW, Gear ARL. Biochemistry. 1999;38:14676–14682. doi: 10.1021/bi991537w. [DOI] [PubMed] [Google Scholar]
- 60.Ardail D, Popa I, Alcantara K, Pons A, Zanetta JP, Louisot P, Thomas L, Portoukalian J. FEBS Lett. 2001;488:160–164. doi: 10.1016/s0014-5793(00)02332-2. [DOI] [PubMed] [Google Scholar]
- 61.Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T. Lipids. 1998;33:601–605. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- 62.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. J. Biol. Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 63.Kawatani M, Simizu S, Osada H, Takada M, Arber N, Imoto M. Exp. Cell Res. 2000;259:389–397. doi: 10.1006/excr.2000.4986. [DOI] [PubMed] [Google Scholar]
- 64.Pauman MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. J. Biol. Chem. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki A, Iwasaki M, Kato M, Wagai N. Exp. Cell Res. 1997;233:41–47. doi: 10.1006/excr.1997.3498. [DOI] [PubMed] [Google Scholar]
- 66.Shimabukuro M, Zhou Y-T, Levi M, Unger RH. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garzotto M, White-Jones M, Jiang Y, Ehleiter D, Liao W-C, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Cancer Res. 1998;58:2260–2264. [PubMed] [Google Scholar]
- 68.Xu J, Yeh C-H, Chen S, He L, Sensi SL, Canzoniero LMT, Choi DW, Hsu CY. J. Biol. Chem. 1998;273:16521–16526. doi: 10.1074/jbc.273.26.16521. [DOI] [PubMed] [Google Scholar]
- 69.Cabot MC, Han T-Y, Giuliano AE. FEBS Lett. 1998;431:185–188. doi: 10.1016/s0014-5793(98)00744-3. [DOI] [PubMed] [Google Scholar]
- 70.Garzotto M, Haimovitz-Friedman A, Liao W-C, White-Jones M, Huryk R, Heston WDW, Cardon-Cardo C, Kolesnick R, Fuks Z. Cancer Res. 1999;59:5194–5201. [PubMed] [Google Scholar]