Abstract
Background
The tetracycline (Tet) responsive system is a valuable tool that is routinely used in a wide variety of mammalian cells for regulatable expression of gene products. However, technical difficulties such as harsh selection conditions and extensive screening processes to identify suitably responsive clones limit the generation of stable cell lines. Hence, application of this system in mammalian cells with relatively slow growth rates and / or the capacity to undergo terminal differentiation such as primary mouse keratinocytes is particularly challenging.
Objective
To our knowledge, no Tet-responsive stable cell lines have been generated from mouse keratinocytes, presumably due to their sensitivity to selection conditions. Our goal was to utilize a modified and robust Tet-expression system to generate a stable primary mouse keratinocyte cell line. These cells could be then utilized for conditional expression of potentially toxic proteins in an inducible fashion.
Methods
We utilized a eukaryotic promoter instead of a viral promoter to express a modified reverse tetracycline transactivator in mouse keratinocytes and optimized the selection process for generating stable cell lines.
Results
Here, we report the generation of a stable mouse keratinocyte cell line for Tet-regulated gene expression with minimal leakiness and high degree of Tet responsivity. This mouse keratinocyte cell line was further engineered for generation of a double stable cell line, which expresses the transcription factor AP-2α in an inducible manner. Importantly, the selected cells retain their inherent keratinocyte morphology, respond to differentiation signals and exhibit a persistent and highly tunable Tet inducibility upon continuous culturing.
Conclusion
We have generated a tetracycline inducible gene expression model system in mouse epidermal keratinocytes. Such inducible cell lines will serve as valuable in vitro models for future gain-of-function and loss-of-function studies.
Keywords: Keratinocytes, Tet-inducibility, Stable transfection, Differentiation
INTRODUCTION
The study of in vivo gene functions in specific tissues has been made feasible by advances in transgenic and knockout mouse technology. Although such mouse models have revealed a great deal about the biological role of genes in their native milieu, studies with these models are expensive, time consuming and often quite limited in their flexibility. In contrast to animal models, cell lines can be easily established and manipulated; therefore, a wide range of studies can be performed with much more ease and efficiency in cultured cells. As the predominant cell type present in the stratified squamous epithelia of mammalian skin epidermis, keratinocytes have been extremely valuable in broadening our understanding of many facets of skin biology. Studies on keratinocytes have been greatly facilitated by the fact that these cells can be isolated from mammalian skin and can be easily grown in culture, where they retain much of their in vivo properties [1]. Keratinocytes in culture exhibit polarity, grow as epithelial sheets and can be induced to undergo terminal squamous differentiation [2, 3, 4]. Therefore, these cells have been widely utilized to study various biological processes of epithelial cells such as cell cycle progression, differentiation, migration, and wound healing [4, 5].
Despite application of similar methods and conditions for establishment, culture, and passaging, studies with mouse epidermal keratinocytes have lagged behind those of human keratinocyte cell lines. Difficulties in growing mouse keratinocytes have been attributed in part to their species-specific properties, unique nutrient requirements and special growth conditions such as low (0.05 mM) extracellular calcium [6]. Indeed, an increase in extracellular Ca2+ concentration initiates a differentiation program in these cells, which is characterized by a loss of their proliferative capacity and alteration in their gene expression profile [7, 8]. Recent studies have led to formulation on several approaches that allow the selection, growth and passage of mouse keratinocytes in a variety of medium, including those that are serum-free [9]. To promote survival and propagation, mouse keratinocytes can also be cultured on fibroblast feeder layers. Although establishing primary cultures of mouse keratinocytes remains challenging, the propensity for these cells to get easily immortalized (unlike human keratinocytes) offers an unlimited supply of cells for downstream applications [10].
In view of the growing number of studies that involve knock out and transgenic mice, the importance of parallel and confirmatory gain-of-function and loss-of-function experiments using mouse keratinocytes cannot be stressed enough. In a typical scenario, these experiments would involve transient transfections for one-time usage or generation of stable cell lines as a permanent source of research material. However, mouse keratinocytes have proven to be recalcitrant to both these methods using conventional techniques. In addition, expression of genes encoding for very potent or potentially toxic proteins in keratinocytes has also proven to be a difficult task. This is often due to the accumulation of overexpressed deleterious gene products and / or altered levels of downstream targets affecting the survival of transfected cells. Inducible gene expression systems, such as the tetracycline regulated gene expression system have overcome this drawback by virtue of their ability to tightly control the expression of heterologous genes in mammalian cells [11, 12]. Indeed, a number of stable cell lines including Tet based systems have been generated from human keratinocytes, especially HaCaT cells, but similar mouse keratinocyte cell lines do not exist [13, 14].
The tetracycline regulated gene expression system has undergone several modifications [15, 16] and has been widely utilized for more than a decade since its inception. There are two variants of the Tet based system, the Tet-On version in which the transgene expression is induced in the presence of the tetracycline analog, doxycycline (Dox) and the Tet-Off version where the expression of the transgene is turned off by Dox. Typically, the Tet-on system has several advantages over its Tet-off equivalent especially in cell culture model systems. This includes a high sensitivity to the inducer Dox, quicker response and the generation of a graded, dose dependent expression of the gene product [17]. In addition, the Tet-on system also offers the advantage of not exposing the cells to doxycycline for prolonged periods of time during the process of selection and under non-inducing conditions.
The Tet-on system includes three functional components. The first component is the activator plasmid, encoding for a chimeric reverse tetracycline regulated transactivator (rtTA), a fusion of the E.coli reverse tetracycline repressor protein and the herpes simplex virus (HSV) VP16 transactivation domain. The expression of rtTA is usually controlled by strong viral promoters such as the cytomegalovirus (CMV) promoter or mammalian promoters that are active in specific cells or tissues. The activity of rtTA is regulated by the inducing agent Dox (second component). Only in the presence of Dox, can rtTA bind to the tetracycline response element (TRE) and activate transcription. Finally, the third component of this system is the Tet responder, which consists of the gene of interest under control of the TRE. Expression from the Tet responder is turned on only when the inducer, Dox is added to the system. Although this tripartite system allows for controlled gene expression, its widespread use has been limited by its relatively high basal activity or leaky expression. To overcome this drawback, improved versions of rtTA have been developed, such as rtTA2S-M2 [18, 19] which possesses a lower background activity, higher Dox sensitivity and inducibility in a wide range of mammalian cell lines and diverse organ systems in transgenic animals [20].
Establishing a tetracycline inducible cell line is a time consuming two-step process. The first step involves the generation of a ‘Tet-on’ cell line which stably expresses the activator plasmid (rtTA). The second step involves stable transfection of the response plasmid which encodes for the desired gene under TRE control in the Tet-on cells. The procedure entails two rounds of transfection, clonal selection and extensive screening of the isolated clones to identify those that lack basal expression of the desired gene product in the absence of Dox but are rapidly and strongly induced upon addition of Dox. One major drawback of this system is the fact that upon continuous culture, some cell lines spontaneously lose their inducibility, especially after successive rounds of selection [21]. Such a phenomenon has usually been attributed to the loss of expression of rtTA and to the inherent nature of the cell line used. Indeed, these events are particularly common in cell lines that proliferate slowly and possess the capacity to undergo differentiation.
As demonstrated in this report, we have successfully generated multiple mouse keratinocyte cell lines that constitutively express enhanced green fluorescent protein (EGFP) and the tetracycline transactivator, rtTA. We further show that one of these cell lines exhibits virtually no basal expression of Tet responsive genes, but is highly activated upon Dox administration and more importantly, retains normal keratinocyte behavior and function. Finally, we have established Tet-on mouse keratinocytes that can express AP-2α in a tightly regulated fashion. AP-2α is a transcription factor that plays an important role in keratinocyte growth and differentiation [22]. It has been difficult till now to stably express AP-2α in cultured cells because of its deleterious effect on cell survival [23]. Thus, our studies provide proof of principle for the notion that potent or potentially toxic proteins can be inducibly expressed in mouse keratinocytes. Thus, development of the Tet-on keratinocyte system is a significant advance in epithelial biology.
MATERIALS AND METHODS
Plasmids
The rtTA2S-M2 construct was originally generated by Urlinger et al., by random and directed mutagenesis of TetR and subsequent selection of the mutants for higher specificity and inducibility at lower concentrations of Dox [11, 18]. The modified reverse tetracycline transactivator (pN1pβactin-rtTA2S-M2-IRES-EGFP) plasmid was a generous gift from A. Welman (Paterson Institute for Cancer Research, Manchester, UK) [28]. pTRE2H-HA-mAP-2α was constructed by inserting the mouse AP-2α cDNA (mRNA: NM_011547.2; genomic DNA: NC_000079.5) with a 5′ HA tag into the BamHI and XhoI sites of the pTRE2H plasmid (BD Biosciences, Clontech). pTRE-Tt-Luc consists of the luciferase reporter gene under the control of the modified TRE (BD Biosciences, Clontech). pTet-Op-LacZ encodes for the β-galactosidase reporter gene under control of the Tet operator (a kind gift from L. Chodosh). pCMV-LacZ includes the β-galactosidase reporter gene under the control of CMV promoter.
Cell lines
Primary mouse keratinocytes were isolated as previously described with slight modifications [6]. The dorsal skin from newborn mice (strain C57/Bl6) was dissected out and incubated in Dispase II (Roche Diagnostics) with the epidermal side facing up overnight at 4°C. The epidermis was mechanically separated from the underlying dermis, placed in TrypLE Express (GIBCO / Invitrogen Corporation) and disaggregated with a pair of NO. 15 Brad Parker knives to separate the keratinocytes. The TrypLE Express was subsequently neutralized with a serum rich low Ca2+ medium calcium free DMEM/F12 [3:1], containing 15% Chelex (Biorad Laboratories) treated Fetal Bovine Serum). The suspension was filtered through a 100μm cell strainer (BD Biosciences) and the epidermal keratinocytes were obtained by centrifugation at 500 rpm for 5 minutes. The keratinocytes were then plated out and maintained in low Ca2+ media prepared with the Tet system approved Fetal Bovine Serum (BD Biosciences, Clontech). Keratinocyte differentiation was induced by the addition of calcium chloride to the culture medium to a final concentration of 1.2mM for various periods of time (early differentiation- 3 hours; intermediate differentiation- 6 hours and late differentiation- 12 hours).
Transfection of mouse keratinocytes and selection of stable clones
Separate killing curve assays were performed for G-418 (GIBCO / Invitrogen) and hygromycin (Invitrogen) to determine the optimal concentration of each antibiotic to be used for selection. Keratinocytes were grown in a series of dilutions of the individual antibiotic and the concentration that caused 100% cell death at 7 days was chosen as the optimum selection concentration.
Mouse keratinocytes were transfected with pN1pβactin-rtTA2S-M2-IRES-EGFP 1.0μg/well in 35 mm dishes using Fugene6 transfection agent (Roche Diagnostics), as per manufacturer’s instructions. Selection with 60μg/ml of G-418 was begun 36 hours post-transfection and maintained hence forth. After 2 days of G-418 selection, the cells were trypsinized and transferred to 150 mm dishes. This facilitates dispersal of cells, so that the stable transfectants will be sufficiently separated from each other. Two weeks later, 24 individual colonies were transferred to a 24 well plate for further propagation and clonal expansion, after which they were screened for EGFP expression. For continuous propagation, the TMK-17 clone was cultured in the presence of G-418. For the second round of transfection, cells from the TMK17 clone were transfected with 1.0μg/well of pTRE2H-HA-mAP-2α and selection was performed with 7.5μg/ml of hygromycin and 60μg/ml of G-418. The cells were treated with 0.5μg/ml of doxycycline (BD Biosciences, Clontech) for 48 hours where indicated before being harvested for luciferase assay, measurement of β-galactosidase activity, immunofluorescence or immunoblot analysis.
EGFP detection
The cells were plated on coverslips and after 48 hours of culture, fixed in 10% neutral buffered formalin, rinsed in 1× PBS and mounted with 50% glycerol in 1× PBS. The cells were photographed using Zeiss Axioplan microscope fitted with a Q-imaging Retiga EXi color camera and analyzed with OpenLab imaging software.
Luciferase reporter assay
The luciferase reporter assays were performed as described [24]. Briefly, cells were plated at a density of 125,000 cells/well in 6 well plates and were transfected using Fugene6 reagent (Roche Diagnostics) at a confluence of 40-60% with pTRE-Tt-Luc (0.5 μg) and pCMV-LacZ (0.25 μg) for luciferase reporter assays. For β-galactosidase reporter assays, the cells were transfected as above with pTet-Op-LacZ (0.5 μg) in duplicate. Four hours post transfection, 0.5μg/ml of doxycycline was added to one of the duplicate wells. Cells were harvested 48 hours post transfection in Reporter lysis buffer (Promega) and luciferase assays were performed using the Luciferase assay system (Promega). β-galactosidase values were measured using the Galacton plus kit (Applied Biosystems). The Luciferase and β-galactosidase values were measured using a Lumat LB 9501 Luminometer (Berthold). For luciferase reporter assays, luciferase values were normalized to β-galactosidase levels to accommodate for transfection efficiency. Reporter assays were performed in duplicates of at least three independent experiments.
Western blots
Whole cell extracts were prepared using Laemmli sample buffer (Biorad Laboratories) from TMK clones transiently transfected with pTRE2H-HA-mAP-2α or untransfected TMK-AP2 clones in the presence or absence of Dox. Equal amounts of lysates were loaded on a 10% SDS-polyacrylamide gel and transferred on to Immun-Blot PVDF membrane (Biorad Laboratories). After blocking in 5% non-fat dry milk in 1×TBST (Tris- buffered Saline Tween-20), the membrane was incubated with a 1:5000 dilution of rat anti-HA antibody (Roche Diagnostics) overnight at 4°C. After washing in 1×TBST, the membrane was incubated in horseradish peroxidase (HRPO) conjugated goat anti-Rat secondary antibody (KPL) at 1:20,000 dilution for 45 minutes at room temperature. Specific bands were detected by chemiluminescence (KPL).
Indirect immunofluorescence
Cells were cultured to 80% confluence on coverslips in the presence or absence of Dox for 48 hours and fixed with 4% paraformaldehyde in 1× PBS on ice for 20 minutes, followed by three washes in 1× PBS at room temperature. Cells were blocked in 5% BSA in 1× PBS for 1 hour at RT, then incubated with the primary rat anti-HA antibody (1:500 dilution) and mouse anti-AP2α antibody (1:100 dilution) (3B5, a gift from Trevor Williams, University of Colorado Health Sciences Center, Denver) in 5% BSA for 1 hour at RT. After three 1× PBS washes, the cells were incubated in 1:500 dilution of FITC conjugated goat anti-mouse antibody and Alexa-568 conjugated goat anti-rat antibody. Antibodies against keratinocyte markers – keratin14, keratin10, involucrin and filaggrin (a generous gift from Julia Segre, National Human Genome Research Institute, Bethesda) and the FITC conjugated anti-Rabbit secondary antibodies were used at a dilution of 1:500. The cells were counterstained with DAPI (4’, 6-diamidino-2-phenylindole) to mark the nuclei and coverslips were mounted with 50% glycerol in 1× PBS. The cells were viewed and photographed with an Axiophot Zeiss microscope, equipped with a Hamamatsu ORCA-ER camera. The contrast and brightness of the images were adjusted using the Adobe Photoshop and ImageJ software.
RESULTS
Use of a eukaryotic promoter driven Reverse Tetracycline Transactivator to generate Tet-on mouse keratinocyte (TMK) clones
Most of the currently available Tet-on cell lines utilize rtTA driven by a strong viral promoter [25]. Recently, eukaryotic housekeeping gene promoters have been favored over viral promoters to drive the expression of rtTA in mammalian cell lines. For example, Human EF-1α [26, 27] and chicken β-actin [28] promoters have been employed effectively in rtTA expression plasmids in human colon cancer cell lines. These promoters are typically cell type and cell cycle independent and are less subject to epigenetic modifications. Hence, they can maintain their activity at relatively comparable levels under various physiological conditions. To generate a stable mouse keratinocyte cell line, we utilized the plasmid pN1pβactin-rtTA2S-M2-IRES-EGFP where the modified version of reverse tetracycline transactivator rtTA2S-M2 is under control of the chicken β-actin promoter [29]. This construct contains a Neomycin resistance cassette; hence the transfected cells can be selected using G-418. Cells transfected with this plasmid will also express EGFP; this feature aids in the easy identification, sorting and selection of transfected clones.
A primary epidermal keratinocyte cell line was established from newborn mouse skin by enzymatic separation of epidermis and mechanical disaggregation, and was propagated for multiple passages to ensure homogeneity and robustness among the cells. The keratinocytes were first examined for their sensitivity to the selection antibiotics G-418 and hygromycin, by antibiotic killing curve assay. Interestingly, these cells were extremely sensitive to routinely used concentrations of the antibiotics. For instance, the optimal concentrations required for selection in HaCaT keratinocytes were 550μg/ml of G-418 and 75μg/ml of hygromycin (our unpublished data). On the other hand, the mouse keratinocytes could tolerate only 60μg/ml and 7.5μg/ml of G-418 and hygromycin respectively. This difference in antibiotic sensitivity could be a reflection of the inter-species difference or the fact that HaCaT cells have been in culture for an extensive period and are immortalized [30].
Mouse keratinocytes were transfected with pN1pβactin-rtTA2S-M2-IRES-EGFP and then selected with G-418. The use of very low antibiotic concentrations accommodates for the sensitivity and slow growing nature of mouse keratinocytes, thereby allowing for efficient selection of transfected cells. After 14 days of culture under selection, 24 individual Tet-on mouse keratinocyte (TMK) clones were chosen for further propagation. At this stage, the cells were examined for EGFP expression and as expected, all the clones were positive. Though the clones varied in the degree of EGFP expression, within a single clone there was a uniform intensity of EGFP fluorescence suggesting homogeneity among clonal populations.
Tet-responsiveness of TMK clones
Three representative TMK clones were chosen for further analysis. These clones, TMK1, TMK16 and TMK17 were selected based on the criterion that they expressed uniform and high levels of EGFP throughout the cell population (Fig. 1A). In addition, they displayed normal morphological characteristics and growth behavior similar to the parent (untransfected) keratinocyte cell line. The Dox inducibility of these clones was tested by transiently transfecting the cells with two different reporter genes under the control of the tetracycline response element (TRE). The clones were transiently transfected with pTet-Op-LacZ and the β-galactosidase activity was measured under inducing and non-inducing conditions (Fig. 1B). The β-galactosidase levels of TMK1 and TMK16 did not vary significantly in the presence or absence of Dox, suggesting that these clones were not very Dox responsive. On the other hand, clone TMK17 was promising in that it displayed very low basal levels of β-galactosidase activity, which was increased approximately 61 fold upon addition of Dox. The higher background and weak induction for TMK1 and TMK16 was surprising but could be attributed to the nature of the pTet-Op-LacZ reporter construct. The β-galactosidase in this plasmid is driven by the Tet operator sequence (TetO), which has been shown to be leaky in its expression and to produce higher background.
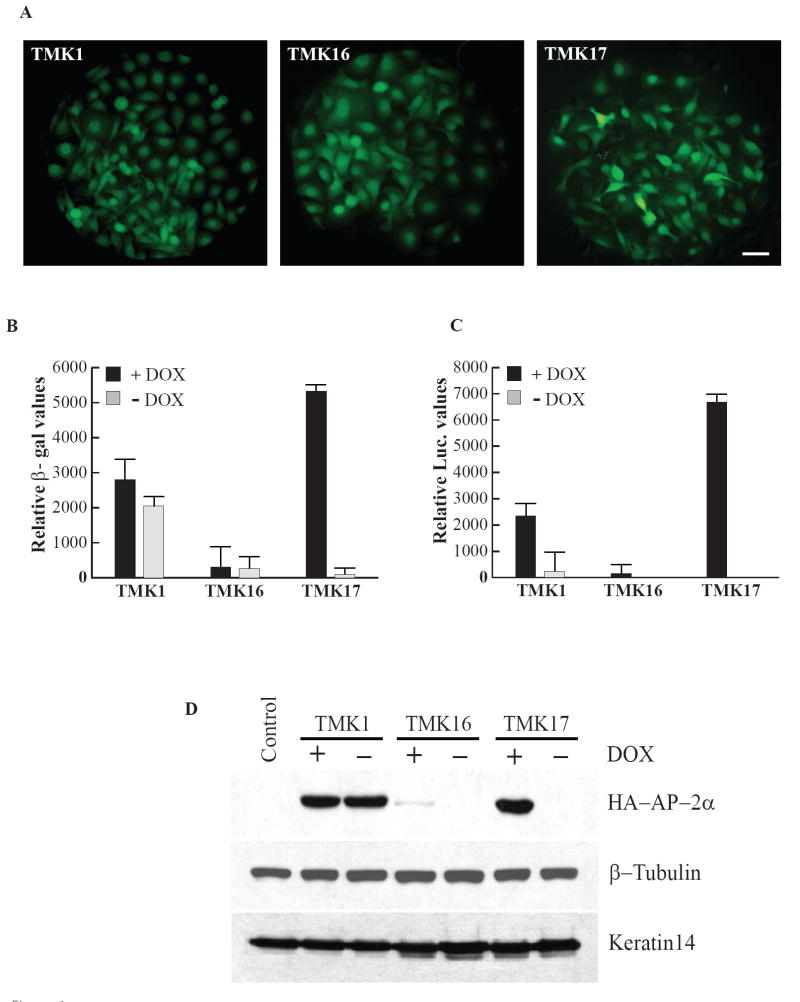
Figure 1. Generation and characterization of TMK, Tet-on mouse keratinocyte clones.
A. EGFP fluorescence of the three representative TMK clones, Scale bar - 20μm. B. β-galactosidase reporter assay: Comparison of Dox responsivity of the TMK clones by transient transfection with pTet-Op-LacZ construct. C. Luciferase reporter assay: Dox responsive inducibility of the TMK clones determined by transient transfection with pTRE-Tt-Luc reporter plasmid. D. Immunoblot for HA-mAP-2α expressed under the control of TRE by transient transfection of pTRE-HA-mAP-2α in the TMK clones. Loading controls: β-Tubulin and K14.
Therefore, we examined the Dox inducibility of these clones with a different reporter construct pTRE-Tt-Luc, in which the luciferase reporter gene is under the control of the ‘TRE-Tight’ promoter. TRE-Tight (TRE-Tt) is an improved version of TRE, consisting of a modified Tet responsive element fused to a minimal CMV promoter; its activity has been shown to be far less leaky when compared to that of the Tet operator. While TMK16 demonstrated very low luciferase values in the presence of Dox and almost no activity in its absence (28 fold difference), TMK1 was quite leaky in its expression in spite of its overall higher response to Dox (Fig. 1C), resulting in only a 10 fold difference. On the other hand, TMK17 displayed a strong response to Dox with minimal basal level of expression in its absence (approximately 685 fold over Dox-). The data comparing the reporter activity of the three TMK clones clearly indicate that they are divergent in their sensitivity to Dox and that Dox responsiveness does not always correlate with the intensity of EGFP fluorescence; for example, though the three Tet-on mouse keratinocyte clones express similar levels of EGFP (Fig. 1A), they differed noticeably in their Dox responsivity (Fig. 1B and 1C). This difference in responsiveness could be an integration site specific effect: in case of clone TMK1, the plasmid could have integrated close to an extremely active site of transcription, while in clone TMK16, the genomic integration could have occurred within a region of heterochromatin.
Transient induction of AP-2α
In addition to reporter assays, which offer an indirect readout of Dox inducibility of the clones, we also examined them by directly monitoring the levels of Tet responsive protein expression. For this purpose, the clones were transiently transfected with pTRE2H-HA-mAP-2α. This plasmid allows the expression of epitope tagged transcription factor, AP-2α under the control of the Tet-responsive promoter. The Dox responsive expression of HA-mAP2α was examined by western blotting with anti-HA antibodies. Clone TMK1 showed strong expression of HA-mAP-2α irrespective of the presence or absence of Doxycycline (Fig. 1D). Clone TMK16 showed a very weak band only in the presence of Dox, suggesting that it might be a weak responder. TMK17 on the other hand, displayed a very strong band corresponding to HA-mAP2α only in the presence of Dox, with no leakiness in its activity under non-inducing conditions. These data on the expression of AP-2α protein tallies well with the reporter assays and taken together suggest that clone TMK17 is the first reported stable mouse keratinocyte cell line with outstanding conditional gene expression properties. Based on these results, we chose to use clone TMK17 for further studies.
Response to keratinocyte differentiation signals of the parental TMK17 cell line
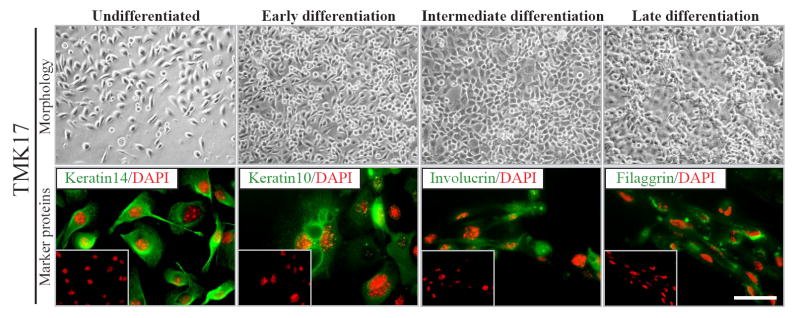
Owing to their extremely sensitive nature, the physiologic properties and therefore the behavior of mouse keratinocytes can be easily altered upon antibiotic selection. To rule out the possibility that the processes of transfection and selection could have altered the normal physiology of TMK17, we examined the keratinocytes for their capacity to differentiate. TMK17 cells were induced to differentiate by the addition of Ca2+. The ability to undergo a process of terminal differentiation and expression of specific marker proteins were examined at various stages (Fig. 2). Upon addition of Ca2+, the cells of TMK17 clone underwent changes in morphology that culminated in the formation of an epithelial sheet, the terminally differentiated state of keratinocytes in culture within 24 hours. Also, they expressed stage specific structural proteins as they went through the multi-step process of differentiation. In the undifferentiated state, the cells expressed keratin14. Upon addition of Ca2+, they sequentially switched on the expression of keratin10, involucrin and filaggrin at early, intermediate and late stages of differentiation respectively. Thus, as judged by immunoflorescence, the differentiation profile and marker expression of clone TMK17 were found to be normal. The EGFP expression of TMK17 was examined upon induction of differentiation at various time points and was found to be constant, suggesting continuous activity of the β-actin promoter in all states of keratinocyte differentiation (data not shown). Clone TMK17 was maintained in culture for multiple passages and its activity in terms of EGFP expression and Dox responsiveness was monitored and found to be unaltered over time, even when cultured in the absence of G-418 for several weeks (data not shown).
Figure 2. Analysis of physiological properties of TMK17 clone.
Examination of differentiation profile of TMK17 clone. Bright Field (top) images of differentiation pattern of TMK17; fluorescent (bottom) images of specific markers of keratinocyte differentiation. Scale bar - 20μm.
Generation and characterization of TMK-AP2 stable clones
Though a very tight expression of potentially harmful target genes has been demonstrated by the Tet system [31], generation of double transfectants after the second round of stable transfection has been a particularly difficult task in most cell lines. Generally, these failures have been contributed to leaky expression of potentially harmful gene product from the second plasmid before its stable integration into the genome, which could compromise the survival of the transfected cells even at very low levels.
AP-2α is transcription factor that has been implicated in cellular processes such as proliferation, cell cycle progression, differentiation and apoptosis [32]. In keratinocytes, AP-2α functions in developmental events associated with growth and differentiation and plays a key role in keratinocyte specific gene expression [22, 33]. The presence of supra-physiological levels of AP-2α has been shown to block cell cycle progression [34] and induce cellular apoptosis [23, 35]; thus evading attempts at stable transfection. Consequently overexpression studies of AP-2α in cell culture model systems have been limited to transient transfections. Therefore, we chose to utilize the TMK17 clone to generate a stable mouse keratinocyte cell line that expresses AP-2α in an inducible fashion.
A second round of transfection was performed in clone TMK17 with pTRE2H-HA-mAP-2α. Double stable transfectants were selected with hygromycin and G-418. Expression of HA-mAP-2α was examined by performing western blots with anti-HA antibodies on the clones grown in the presence and absence of Dox. Of the 20 clones screened, one clone TMK-AP-2 clone #9 (hereafter TMK-AP2 clone) showed a tight Dox responsive expression of HA-mAP-2α (Fig. 3A). Immunofluorescence on these cells showed a tight Dox dependent expression of HA-mAP-2α. The cells demonstrated negligible background, if any, in non-inducing conditions, i.e. in the absence of Dox. On the other hand, upon addition of Dox, the cells displayed a robust induction and homogeneous expression of the overexpressed protein. Being a transcription factor, AP-2α is localized predominantly in the keratinocyte nuclei. Similar to endogenous AP-2α, the exogenous protein was also localized in the nucleus (Fig. 3B). The induction of HA-mAP-2α expression can be detected as early as 6 hours after the addition of Dox to the culture media and reaches a peak around 24 hours. Upon withdrawal of Dox, the decline in HA-mAP-2α levels was discernible in just 6 hours, suggesting that the TMK-AP2 clone was highly Dox responsive and its withdrawal turns the system off immediately (data not shown). This is in contrast to several animal models where Dox withdrawal does not produce an early effect, presumably due to the presence of considerable quantities of Dox in tissue stores.
Figure 3. Generation of TMK-AP2 clone and examination of its characteristics.
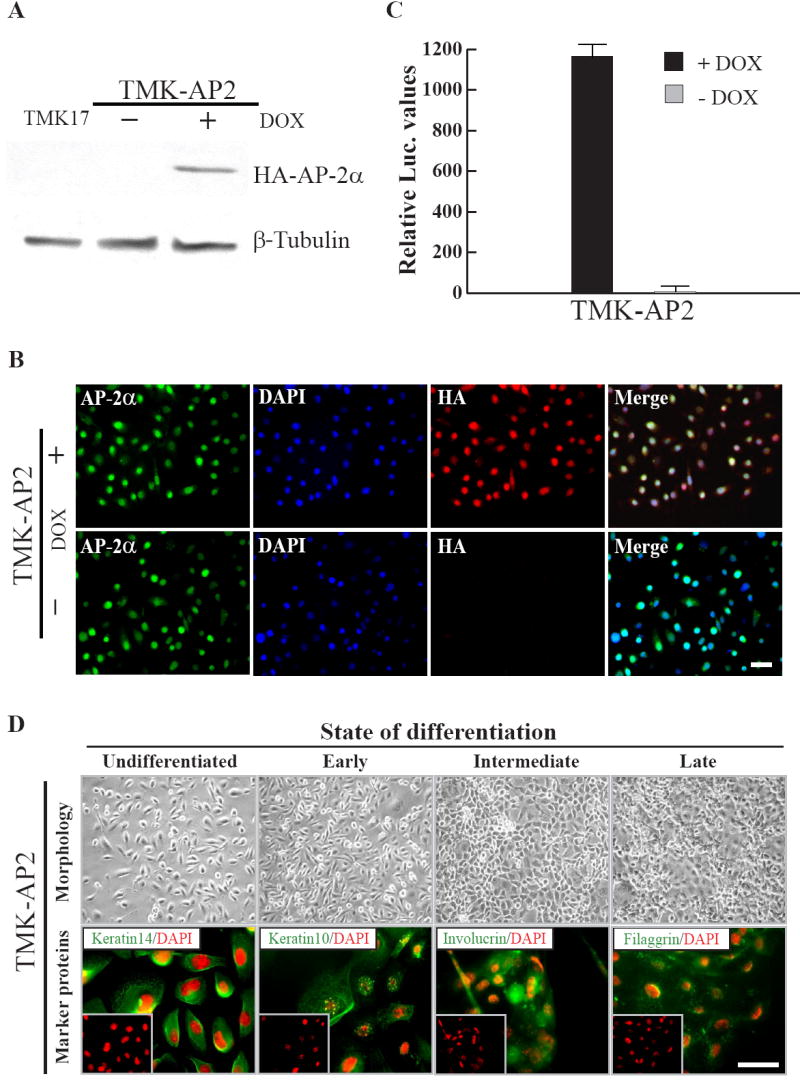
A. Immunoblot for HA-mAP-2α after stable transfection and selection of TMK17 clone with pTRE-HA-mAP-2α. TMK-AP2 clone shows Dox responsive expression of HA-mAP-2α. B. Immunofluorescence on TMK-AP2 clone in the presence and absence of Dox, Scale bar - 20μm. C. Examination of Dox inducibility in TMK-AP2 clone by transient transfection with pTRE-Tt-Luc reporter plasmid. D. Evaluation of in vivo properties of TMK-AP2 clone. Bright field (top) images of differentiating TMK-AP2 keratinocytes and immunofluorescence (bottom) for keratinocyte markers. Scale bar - 20μm.
Analysis of the TMK-AP2 stable clones
Transient transfection of TMK-AP2 clone with a Tet responsive luciferase reporter construct, suggested that the clone retained its Dox responsivity (233 fold approximately) even after undergoing two rounds of transfection and antibiotic selection and several passages (Fig. 3C). Differentiation studies on the TMK-AP2 double stable transfectant showed that these cells maintain their inherent keratinocyte characteristics such as squamous differentiation in response to increased extracellular calcium concentrations and expression of differentiation state specific marker proteins (Fig. 3D). This suggests that two rounds of transfection and selection did not affect the inherent properties of TMK-AP2 keratinocytes. Therefore, TMK-AP2 cells can express supra-physiological levels of AP-2α in an inducible fashion, without compromising the survival of the cell line. This conditional expression system will allow us to effectively explore the keratinocyte specific functions of AP-2α, specifically its role in keratinocyte proliferation, differentiation and programmed cell death. In conclusion, overexpression of AP-2α in cell culture model systems has now been extended to stable transfections.
DISCUSSION
Tetracycline regulated system is one of the most versatile gene expression tools available today. The system is tightly regulated, robust and is relatively non-toxic to cells in culture. In addition, the induction of target gene expression is rapid and reversible. These favorable features have led to an extensive use of this system in several cell culture, transgenic animal and even plant model systems [36]. However, the efficiency of this system has been shown to differ in different cell lines, especially in terms of Dox inducibility [21]. Therefore, the system must be carefully adapted in different cell lines. We have optimized the use of this system for conditional gene expression in mouse keratinocytes and generated an inducible mouse keratinocyte cell line for the overexpression of a potent gene.
Traditionally viral promoters such as the cytomegalovirus (CMV) promoter have been utilized to direct the expression of a gene of interest in mammalian cell lines [19]. One major caveat to the use of viral promoters for such applications is loss of gene expression upon long-term culture of cell lines [21]. To circumvent this problem, eukaryotic promoters have recently been put to use in the place of viral promoters. This has resulted in a long-term, sustained and strong target gene expression in mammalian cell lines [26, 27, 28]. These eukaryotic promoters include those that control housekeeping genes or are cell-type specific. To target specific expression in keratinocytes, promoters such as those of keratins (K5, K14 and K10) and involucrin [37, 38, 39, 40] have been effectively used in transgenic mice. However, the use of these promoters in cell culture system is tricky, since in most cases their activity in keratinocytes is differentiation state specific. For instance, the use of involucrin or keratin 10 promoters in cell culture necessitates induction of keratinocyte differentiation to initiate strong transgene expression. On the other hand, though keratin 5 and keratin 14 promoters possess the advantage of being extensively active in proliferating keratinocytes, their activity is diminished upon induction of differentiation (our unpublished data). Hence, the use of a promoter that is ubiquitously active and is not altered by a change in the state of differentiation or by cell cycle progression is desirable. For this purpose we utilized the plasmid pN1pβactin-rtTA2S-M2-IRES-EGFP where the modified version of reverse tetracycline transactivator rtTA2S-M2 is under control of the chicken β-actin promoter [29]. The advantage of the β-actin promoter was to provide prolonged and sustained transgene expression. Similarly the use of the improved mutant of the Dox-regulated reverse transcriptional transactivator (rtTA2S-M2) allowed for favorable kinetics of inducible gene expression as shown by previous studies [28]. Finally the use of EGFP under an IRES cassette enabled easy selection and subsequent monitoring of the keratinocytes.
The development of the inducible mouse keratinocyte cell line serves many important purposes and offers numerous advantages. As we have shown with the generation of AP-2α overexpressing clones, the TMK17 cell line can be utilized for construction of double-stable cell lines inducibly expressing a protein of interest. This is of particular importance when there is a need to express toxic proteins. As we demonstrate by generation of the double stable cell line, this approach obviates the need for viral expression systems, which is time consuming and presents safety issues. There have been many published and anecdotal evidence that traditional methods of establishing stable cell lines for proteins involved in cell cycle, differentiation and cell growth often lead to weakly expressing clones, presumably due to selection advantage. Furthermore, many stable cell lines stop expressing ectopic proteins over time due to shut down of viral promoters by epigenetic silencing. The generation of the Tet inducible cell line utilizing a mammalian promoter circumvents such problems and offers the option to modulate the levels of protein expressed in a graded fashion. The fact that the system is reversible and gene expression can be easily turned off as desired, affords added and useful experimental maneuverability.
The TMK17 cell line is very well suited for transient transfection studies, for example, to test whether a newly generated expression construct indeed expresses the protein of choice in a Tet inducible fashion (as we have demonstrated for AP-2α). The transient transfection assay will save time and facilitate the optimization of the conditions before embarking on a long-term experiment such as generation of transgenic animals. There has been a rapid growth and use of RNAi to study the effects of knockdown of genes of interest in cell culture [41, 42]. Our TMK17 cells will also serve as a useful resource to generate RNAi in an inducible fashion and thus can be utilized to inhibit the expression a wide range of genes that are involved in cell growth, proliferation and differentiation in a dynamic and reversible fashion.
Finally, these Tet-on cells have been grown in culture for a long time period and have been passaged extensively. Interestingly, these cells have proven to be remarkably hardy and have retained their growth and keratinocyte-specific differentiation properties and Tet-inducibility. The fact that the keratinocytes can be easily assessed by periodically checking the cells for EGFP expression during routine culture is an added benefit. Our study suggests that although it is possible to generate mouse keratinocytes that are stably transfected by conventional methods, it is important to emphasize the need for calibration and optimization of the antibiotic selection conditions. Not surprisingly, these conditions vary significantly from those of human cells, in accordance with the distinct physiological differences in mouse and human keratinocyte growth characteristics in culture. Since such species-specific differences might be biologically important, it is important that studies be performed in the appropriate cell type. Our generation of a Tet-inducible mouse keratinocyte cell line fulfills a void in the field and offers researchers a valuable system to study various cellular processes. Considering the general applicability of the system, it can be efficiently tailored to suit other sensitive and difficult-to-grow cell lines such as melanocytes, cardiomyocytes, neurons and insect cells as well.
Acknowledgments
We would like to thank Dr. Arkadiusz Welman, Paterson Institute for Cancer Research, Manchester, UK for kindly providing the pN1pβactin-rtTA2S-M2-IRES-EGFP construct. We are also very grateful to Dr. Julia Segre, NIH for the antibodies against keratinocyte markers. We would also like to thank Dr. Ramakumar Tummala, Roswell Park Cancer Institute for his help in establishing the mouse keratinocyte cell line. This work was supported by grant from the National Institutes of Health to S.S (AR049238).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Priyadharsini Nagarajan, Email: pn7@buffalo.edu.
Satrajit Sinha, Email: ssinha2@buffalo.edu.
References
- 1.Hakkinen L, Koivisto L, Larjava H. An improved method for culture of epidermal keratinocytes from newborn mouse skin. Methods Cell Sci. 2001;23:189–196. doi: 10.1023/a:1016385109922. [DOI] [PubMed] [Google Scholar]
- 2.Maas-Szabowski N, Fusenig NE, Stark HJ. Experimental models to analyze differentiation functions of cultured keratinocytes in vitro and in vivo. In: Turksen K, editor. Epidermal cells: Methods and protocols. Humana Press Totowa; New Jersey: 2005. pp. 47–60. [DOI] [PubMed] [Google Scholar]
- 3.Bruegel Sanchez VL, Zhou J, LaCivita D, Milstone LM. Long-term murine keratinocyte cultures become tetraploid, yet maintain the ability to stratify. The Journal of investigative dermatology. 2004;123:403–404. doi: 10.1111/j.0022-202X.2004.23218.x. [DOI] [PubMed] [Google Scholar]
- 4.Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- 5.Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Pirrone A, Hager B, Fleckman P. Primary mouse keratinocyte culture. In: Turksen K, editor. Epidermal cells: Methods and protocols. Humana Press Totowa; New Jersey: 2005. pp. 3–14. [DOI] [PubMed] [Google Scholar]
- 7.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 8.Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertolero F, Kaighn ME, Gonda MA, Saffiotti U. Mouse epidermal keratinocytes. Clonal proliferation and response to hormones and growth factors in serum-free medium. Experimental cell research. 1984;155:64–80. doi: 10.1016/0014-4827(84)90768-7. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- 11.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gossen M, Bonin AL, Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem Sci. 1993;18:471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- 13.Boukamp P, Stanbridge EJ, Foo DY, Cerutti PA, Fusenig NE. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990;50:2840–2847. [PubMed] [Google Scholar]
- 14.Goren I, Linke A, Muller E, Pfeilschifter J, Frank S. The suppressor of cytokine signaling-3 is upregulated in impaired skin repair: implications for keratinocyte proliferation. J Invest Dermatol. 2006;126:477–485. doi: 10.1038/sj.jid.5700063. [DOI] [PubMed] [Google Scholar]
- 15.Berens C, Hillen W. Gene regulation by tetracyclines. Constraints of resistance regulation in bacteria shape TetR for application in eukaryotes. Eur J Biochem. 2003;270:3109–3121. doi: 10.1046/j.1432-1033.2003.03694.x. [DOI] [PubMed] [Google Scholar]
- 16.Gossen M, Bujard H. Studying gene function in eukaryotes by conditional gene inactivation. Annu Rev Genet. 2002;36:153–173. doi: 10.1146/annurev.genet.36.041002.120114. [DOI] [PubMed] [Google Scholar]
- 17.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 18.Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu Z, Thottassery JV, Van Ginkel S, et al. Homogeneity and long-term stability of tetracycline-regulated gene expression with low basal activity by using the rtTA2S-M2 transactivator and insulator-flanked reporter vectors. Gene. 2004;327:61–73. doi: 10.1016/j.gene.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Biol. 2002;13:121–128. doi: 10.1016/s1084-9521(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 21.Ackland-Berglund CE, Leib DA. Efficacy of tetracycline-controlled gene expression is influenced by cell type. Biotechniques. 1995;18:196–200. [PubMed] [Google Scholar]
- 22.Wang X, Bolotin D, Chu DH, Polak L, Williams T, Fuchs E. AP-2alpha: a regulator of EGF receptor signaling and proliferation in skin epidermis. J Cell Biol. 2006;172:409–421. doi: 10.1083/jcb.200510002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller FU, Loser K, Kleideiter U, et al. Transcription factor AP-2alpha triggers apoptosis in cardiac myocytes. Cell death and differentiation. 2004;11:485–493. doi: 10.1038/sj.cdd.4401383. [DOI] [PubMed] [Google Scholar]
- 24.Romano RA, Birkaya B, Sinha S. Defining the regulatory elements in the proximal promoter of DeltaNp63 in keratinocytes: Potential roles for Sp1/Sp3, NF-Y, and p63. J Invest Dermatol. 2006;126:1469–1479. doi: 10.1038/sj.jid.5700297. [DOI] [PubMed] [Google Scholar]
- 25.Foecking MK, Hofstetter H. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene. 1986;45:101–105. doi: 10.1016/0378-1119(86)90137-x. [DOI] [PubMed] [Google Scholar]
- 26.Gopalkrishnan RV, Christiansen KA, Goldstein NI, DePinho RA, Fisher PB. Use of the human EF-1alpha promoter for expression can significantly increase success in establishing stable cell lines with consistent expression: a study using the tetracycline- inducible system in human cancer cells. Nucleic Acids Res. 1999;27:4775–4782. doi: 10.1093/nar/27.24.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teschendorf C, Warrington KH, Jr, Siemann DW, Muzyczka N. Comparison of the EF-1 alpha and the CMV promoter for engineering stable tumor cell lines using recombinant adeno-associated virus. Anticancer Res. 2002;22:3325–3330. [PubMed] [Google Scholar]
- 28.Welman A, Cawthorne C, Barraclough J, et al. Construction and characterization of multiple human colon cancer cell lines for inducibly regulated gene expression. J Cell Biochem. 2005;94:1148–1162. doi: 10.1002/jcb.20342. [DOI] [PubMed] [Google Scholar]
- 29.Fregien N, Davidson N. Activating elements in the promoter region of the chicken beta-actin gene. Gene. 1986;48:1–11. doi: 10.1016/0378-1119(86)90346-x. [DOI] [PubMed] [Google Scholar]
- 30.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee P, Morley G, Huang Q, et al. Conditional lineage ablation to model human diseases. Proc Natl Acad Sci U S A. 1998;95:11371–11376. doi: 10.1073/pnas.95.19.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilger-Eversheim K, Moser M, Schorle H, Buettner R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene. 2000;260:1–12. doi: 10.1016/s0378-1119(00)00454-6. [DOI] [PubMed] [Google Scholar]
- 33.Sinha S, Degenstein L, Copenhaver C, Fuchs E. Defining the regulatory factors required for epidermal gene expression. Mol Cell Biol. 2000;20:2543–2555. doi: 10.1128/mcb.20.7.2543-2555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wajapeyee N, Somasundaram K. Cell cycle arrest and apoptosis induction by activator protein 2alpha (AP-2alpha) and the role of p53 and p21WAF1/CIP1 in AP-2alpha-mediated growth inhibition. The Journal of biological chemistry. 2003;278:52093–52101. doi: 10.1074/jbc.M305624200. [DOI] [PubMed] [Google Scholar]
- 35.Wajapeyee N, Britto R, Ravishankar HM, Somasundaram K. Apoptosis induction by activator protein 2alpha involves transcriptional repression of Bcl-2. J Biol Chem. 2006;281:16207–16219. doi: 10.1074/jbc.M600539200. [DOI] [PubMed] [Google Scholar]
- 36.Weinmann P, Gossen M, Hillen W, Bujard H, Gatz C. A chimeric transactivator allows tetracycline-responsive gene expression in whole plants. Plant J. 1994;5:559–569. doi: 10.1046/j.1365-313x.1994.5040559.x. [DOI] [PubMed] [Google Scholar]
- 37.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 38.Xie W, Chow LT, Paterson AJ, Chin E, Kudlow JE. Conditional expression of the ErbB2 oncogene elicits reversible hyperplasia in stratified epithelia and up-regulation of TGFalpha expression in transgenic mice. Oncogene. 1999;18:3593–3607. doi: 10.1038/sj.onc.1202673. [DOI] [PubMed] [Google Scholar]
- 39.Bailleul B, Surani MA, White S, et al. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell. 1990;62:697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- 40.Jaubert J, Patel S, Cheng J, Segre JA. Tetracycline-regulated transactivators driven by the involucrin promoter to achieve epidermal conditional gene expression. J Invest Dermatol. 2004;123:313–318. doi: 10.1111/j.0022-202X.2004.23203.x. [DOI] [PubMed] [Google Scholar]
- 41.Cullen LM, Arndt GM. Genome-wide screening for gene function using RNAi in mammalian cells. Immunology and cell biology. 2005;83:217–223. doi: 10.1111/j.1440-1711.2005.01332.x. [DOI] [PubMed] [Google Scholar]
- 42.Scherr M, Eder M. Gene silencing by small regulatory RNAs in mammalian cells. Cell cycle. 2007;6:444–449. doi: 10.4161/cc.6.4.3807. [DOI] [PubMed] [Google Scholar]