Abstract
Patients with early onset seizure disorder tend to have less cognitive decline following surgical resection than patients with late onset seizure disorder. Differential opportunity for presurgical cerebral functional reorganization has been proposed to account for this “age of onset” effect. However, the relationships between age of seizure onset, functional organization, and neuropsychological outcome remain incompletely understood. To shed additional light on these issues, we investigated 66 patients with anterior temporal lobectomies (37 left; 29 right), all of whom completed comprehensive neuropsychological assessment prior to and following surgical resection. The sample was divided into two groups: 34 patients with early onset (EO) epilepsy and 32 patients with late onset (LO) epilepsy. We found the typical age of onset effect: post-surgically, EO patients demonstrated relatively better outcome in multiple neuropsychological domains, especially verbal memory, compared to LO patients. Based on presurgical Wada testing, 23 patients were classified as having traditional cerebral functional organization (TFO) and 35 were classified as having nontraditional cerebral functional organization (NTFO). The NTFO group had an earlier age of seizure onset than the TFO group, and NTFO was more frequent in EO patients (70%) than LO patients (50%). NTFO patients demonstrated relatively better post-surgical outcome in several neuropsychological domains, compared to TFO patients. Our findings are consistent with the notion that functional reorganization may be an explanatory factor for the better neuropsychological outcome of EO epilepsy patients following anterior temporal lobectomy.
INTRODUCTION
Epilepsy is a neurological condition characterized by repeated seizure activity. It affects 2 million people in the U.S. annually, and .5 to 1% of the world’s population (Hauser & Hesdorffer, 1990). Efficacy data indicate that 30–40% of people with epilepsy do not obtain reasonable control over seizures through use of antiepileptic medications (Engel et al., 2002). Anterior temporal lobectomy is the most common form of surgical intervention for epilepsy, and has been demonstrated to be superior to medications and other forms of surgery (i.e., focal cortical resection) in producing seizure control (Engel et al., 2002; Wiebe, Blume, Girvin & Eliasziw, 2001). Advances in diagnostic procedures over the last several decades have increased the sophistication of presurgical evaluations and have allowed for the identification of greater numbers of candidates for surgical intervention to treat medically refractory seizures.
Neuropsychological outcome following temporal lobectomy
Both medication and surgical treatments of epilepsy are accompanied by risk for cognitive side effects. The research literature indicates significant variability in neuropsychological outcome following anterior temporal lobectomy for intractable seizures. Factors such as practice effects and improved seizure control have been purported to account for observed improvements in cognitive functioning following surgery (Novelly et al., 1984; Ojemann & Dodrill, 1985). Deterioration in cognitive functions such as language and memory has been shown to be associated with a variety of factors, including left hemisphere resection (Gleissner, Helmstaedter, Schramm & Elger, 2002; Stroup et al. 2003), older age at the time of surgery (Gleissner et al., 2002), and male gender (Trenerry et al., 1993). Pre-operative cognitive performance and the neuropathological status of the resected tissue have been shown to interact with post-surgical outcome (Stroup et al., 2003). In particular, individuals with normal appearing hippocampi and strong preoperative memory performances are at increased risk for memory impairment following surgery (Hermann et al., 1994; Loring, 1997; Powell, Polkey & McMillan, 1985; Trenerry et al., 1993). Interestingly, extent of tissue resection, per se, is not a consistent predictor of cognitive outcome following anterior temporal lobectomy (Gleissner et al., 2002; Ojemann & Dodrill, 1985; Wolf et al., 1993).
Age of onset
Age of epilepsy onset has emerged as an important predictor of cognitive outcome following temporal lobectomy. Powell, Polkey, and McMillan (1985) found that subjects with later onset of epilepsy (which the authors defined as > 11 years) demonstrated poorer outcomes following temporal lobectomy than did subjects with earlier onset of epilepsy. Further, these authors demonstrated an interaction with lobectomy hemisphere, such that subjects undergoing left temporal lobectomy demonstrated greater deterioration on verbal measures, and subjects undergoing right temporal lobectomy deteriorated more on non-verbal measures. Saykin, Gur, Sussman, O’Connor, and Gur (1989) also found that late onset subjects (which the authors defined as > 5 years) demonstrated deterioration in verbal memory performances, compared with no deterioration in early onset subjects. In contrast to the Powell et al. findings, however, Saykin et al. found that early onset subjects deteriorated in visual memory performances following surgery, whereas late onset subjects did not.
Stafiniak and colleagues (Stafiniak et al., 1990) demonstrated that post-operative anomia was predicted by relatively late age at first risk for seizures (defined as > 5 years). Subjects with early risk for seizures were less likely to show anomia following temporal lobectomy than those with no early risk. Wolf et al. (1993) found older age at seizure onset to be associated with increased risk for post-surgical cognitive deficits on measures of verbal reasoning and memory in subjects undergoing left temporal lobectomy. Subjects undergoing left temporal lobectomy, with epilepsy onset after 10 years of age, were also found to be at greater risk for post-surgical decline in verbal memory performances in a study by Hermann, Seidenberg, Haltiner, and Wyler (1995). Similarly, Gleissner et al. (2002) demonstrated that verbal memory decline following left temporal lobectomy was associated with later age of onset.
No investigations to date have systematically examined cognitive outcome as a function of age of epilepsy onset, using a comprehensive neuropsychological battery in a chronic post-surgical population. Several authors have focused on outcome in the domain of memory (i.e., Gleissner et al., 2002; Hermann et al., 1995; Saykin et al., 1989), while others have expanded their investigation to the domains of memory and intellectual functioning (Powell et al., 1985; Wolf et al., 1993). In most studies reviewed previously, subjects were tested during the acute epoch, with retest times ranging from as short as 2 weeks post surgically (Stafaniak et al., 1990; Wolf et al., 1993) to 1 month post surgically (Powell et al., 1985; Saykin et al., 1989). In several studies, age of onset findings were incidental in nature, and the authors did not control for potentially confounding factors, such as seizure type and frequency, and number of anticonvulsant medications (Gleissner et al., 2002; Wolf et al., 1993).
Functional reorganization
The nature and severity of pre-operative cognitive impairments depend on age of onset and duration of epilepsy, among other factors. Early-onset seizure patients have been consistently shown to perform more poorly on a wide range of neuropsychological measures as adults, when compared with late-onset patients, and the observed deficits appear to be more generalized and pervasive than would be expected based on what is known about the extent of structural lesion in these patients (Dikmen, Matthews & Harley, 1975; Dikmen, Matthews & Harley, 1977; Hermann et al., 2002; Lespinet, Bresson, N’Kaoua, Rougier & Claverie 2002). O’Leary, Seidenberg, Berent, and Boll (1981) found this age of onset effect even in young children and when controlling for potentially confounding variables such as seizure frequency. Furthermore, there is growing evidence of pervasive changes in cerebral and white matter volumes in early-onset patients who have focal seizure disorders (Hermann et al., 2002). Thus, research in epilepsy populations suggests that even relatively focal injury, when accompanied by ongoing seizures, can significantly disrupt cerebral function and cognitive performance. (This effect could also contribute to the age of seizure onset effect reviewed previously, whereby earlier onset is associated with better outcome: early onset patients may have relatively less ground to lose, compared to late onset patients.)
The situation is complicated further by various views of what can be termed the “reorganization hypothesis.” Powell et al. (1985), for example, hypothesized that there is significant reorganization in early onset patients, whereby the undamaged temporal lobe assumes functions of the damaged temporal lobe, explaining why there is less change in functions like memory following surgical resection in early onset patients. This idea was echoed in a more recent study by Pataraia et al. (2004), who reported that early onset of seizures was strongly associated with atypical language lateralization (mainly, a displacement of receptive language functions to the nondominant hemisphere). These investigators have also reported intrahemispheric shifts in receptive language mediation following anterior temporal lobectomy, especially in patients with left hemisphere language dominance preoperatively (Pataraia et al., 2005). It should also be noted that both of the Pataraia et al. studies utilized MEG as a technique for language mapping (coregistered with structural MRI data), and there are important cautions about source localization with MEG data that should be kept in mind when interpreting those studies. In regard to language, Stafiniak et al. (1990) provided a somewhat different view of this idea, proposing that early onset patients have not had time for complete lateralization of language to occur, and thus reorganization is more likely in such individuals and the resultant effects of surgery on language are diminished. Several studies have demonstrated a relationship whereby smaller hippocampal volume (or greater hippocampal sclerosis) was associated with poorer preoperative memory performance, but relatively less postoperative memory decline (e.g., Hermann et al., 1994; Trenerry et al., 1993).
The current study
The goals of the current study were to examine the relationship between age of seizure onset and neuropsychological outcome following anterior temporal lobectomy, and to investigate whether there were systematic differences in cerebral functional organization in patients with early onset and late onset seizure disorder. Following the preponderance of evidence from the literature, we hypothesized that: (1) Age of seizure onset would have an influence on neuropsychological outcome following temporal lobectomy. Specifically, we predicted that (a) early onset would be associated with relatively better neuropsychological outcome, whereas (b) late onset would be associated with relatively poorer neuropsychological outcome. For the purpose of this study, we define better outcome as stable or improved performances on neuropsychological measures on post-testing. We also hypothesized that: (2) Age of seizure onset would influence cerebral functional organization (as assessed by intra-carotid amobarbital testing). Specifically, we predicted that early onset would be associated with a higher rate of “nontraditional” cerebral functional organization, compared to late onset. Finally, irrespective of whether Hypothesis 2 was upheld, it was of interest to explore the question of whether non-traditional functional organization would be associated with better neuropsychological outcome than traditional functional organization, following anterior temporal lobectomy; Hypothesis 3 was that we would observe this to be the case.
METHOD
Participants
Participants included 66 individuals with a history of intractable complex partial seizures who underwent anterior temporal lobectomy (37 left; 29 right) between 1984 and 2002. All participants had given informed consent to participate in a larger research study investigating the effects of focal lesions on cognitive and behavioral function, according to standard institutional and federal guidelines. Participants included 33 men and 33 women, with a mean age of 33.8 years (SD = 10.6), and an average of 12.8 (SD = 1.9) years of education. The modified Geshwind-Oldfield Handedness Questionnaire (Oldfield, 1971) was used to assess manual dominance, and the specific breakdown was as follows: 52 participants were fully right-handed (+100); 3 were primarily or partially right-handed (+95, +90, +20); 6 were fully left-handed (−100); 5 were primarily or partially left-handed (−90, −80, −70, −60, −30). 81% of the sample was right-eye dominant; 80% of the sample was right-foot dominant. All subjects but one were Caucasian. Demographic information about the participants is presented in Table 1.
Table 1.
Demographics as a function of age of onset classification
| Group | Age* Mean (SD) | Gender1 M:F | Education* Mean (SD) | Handedness1 R:L |
|---|---|---|---|---|
| Early onset (n = 34) | 32.4 (10.3) | 17:17 | 12.9 (2.0) | 26:8 |
| Late onset (n = 32) | 35.3 (10.9) | 16:16 | 12.8 (1.8) | 29:3 |
Note Independent samples t-test revealed no differences between groups;
Chi-square test revealed no differences between groups.
The operationalization of the age of onset factor was established using a cutoff of 9 years of age, so that participants with epilepsy onset prior to 9 years old were classified as “early onset” and participants with epilepsy onset after 9 years old were classified as “late onset.” This cutoff was used because it is within the range of cutoff ages used in prior studies investigating age of onset (i.e., 5–14 years old; Hermann et al., 1995; Powell et al., 1985; Saykin et al., 1989; Stafiniak et al., 1990; Wolf et al., 1993).1 More specifically, early onset (EO) epilepsy was defined as the onset of continuous seizures at or before age 9, and late onset (LO) epilepsy was defined as the onset of continuous seizures after age 9. Thus, we had two groups, EO and LO, which happened to have similar N’s (34 v. 32, respectively). The groups did not differ with respect to demographic characteristics (Table 1). Neither did the groups differ on epilepsy or surgical characteristics (e.g., age at surgery, pre- and post-surgical seizure frequency, pre- and post-surgical number of antiepileptic medications), except, as expected based on the classification criterion, age at onset (Table 2). As is common following surgical intervention for intractable seizures, all subjects remained on at least one antiepileptic medication at the time of post-surgical follow-up. Interestingly, there was a somewhat higher incidence of left-handedness (24%) in the EO group, compared to the LO group (9%) (the latter figure is closer to the population baserate of left-handedness, which is around 10%), although this difference was not statistically significant in a Chi square test.
Table 2.
Epilepsy and surgery characteristics as a function of age of onset classification
| Group | Follow-up time*(mths)Mean (SD) | Age at onset1 (yrs)Mean (SD) | Age at surgery* (yrs) Mean (SD) | Lobectomy hemisphere2 (L:R) |
|---|---|---|---|---|
| Early onset (n = 34) | 19.3 (22.2) | 3.6 (2.9) | 32.9 (10.7) | 18:16 |
| Late onset (n = 32) | 23.4 (27.1) | 18.1 (8.0) | 35.6 (11.0) | 19:13 |
| Pre surgery # seizuresy* (per day) | Post surgery # seizures* (per day) | Pre surgery # meds* mean (SD) | Post surgery # meds* mean (SD) | |
| Early onset (n = 34) | .92 (1.8) | .04 (.18) | 1.9 (.55) | 1.5 (.51) |
| Late onset (n = 32) | 1.3 (2.3) | .14 (.55) | 2.0 (.74) | 1.5 (.68) |
Independent samples t-test revealed no differences between groups;
t(64) = −10.0, p < .0001;
Chi-square test revealed no differences between groups.
Materials and procedure
Neuropsychological, demographic, and medical data were collected retrospectively from research files and medical records. All subjects completed comprehensive neuropsychological assessment prior to surgery, and then again during the chronic epoch (defined as at least three months after surgery) (the availability of both datasets was a criterion for entry into the study). Mean follow-up time was 21.3 months (SD = 24.6; range: 4.2 months – 146.1 months). More specifically, all but two (64/66; both in the EO group) of the subjects were tested post-surgically when they were at least 6 months out from the lobectomy operation. Twenty-eight subjects (17 EO, 11 LO) were tested between 6 and 12 months post-surgically, and the remaining 36 (15 EO, 21 LO) were tested more than 12 months post-surgically. As indicated in Table 2, there was no difference between the EO and LO groups in terms of time since surgery that the postoperative testing was conducted, and given that the vast majority of subjects were tested more than 6 months after surgery (97%), we are confident that the post-surgical assessment represents a valid indicator of chronic-epoch functioning.
The neuropsychological test battery comprised measures for which complete pre- and post-surgical data sets were available for the majority of subjects. In contrast to the more limited focus of many prior studies, the current test battery allowed for comprehensive assessment of cognitive functioning across multiple domains, including attention and speed of information processing, verbal and visual memory, language, visuospatial skills, psychomotor speed/dexterity, and executive functions. The specific neuropsychological measures are enumerated in Table 3. Tests were administered according to standard administration procedures by trained technicians, who were blind to the hypotheses and predictions of the current investigation (see Tranel, 1996, and Tranel, in press, for methods and references for tests). IQ testing was performed with the WAIS-R (some subjects received testing with the WAIS-III as well, but to ensure comparability of the subtest scores, we utilized WAIS-R data in the current study). For tests for which comparable parallel forms exist, alternate forms were used in the second (post-surgical) evaluation; specifically, this included the AVLT, BVRT, and COWAT. This procedure was designed to reduce potential practice effects. Of course, such effects could still play some role in the pre-to-post change scores, even when parallel alternate forms were used; however, we do not know of any evidence that practice effects would interact with the primary independent variables (age of seizure onset; functional organization), and hence, this should not bias the basic analyses reported below. (Practice effects could tend to mitigate adverse effects of surgery on neuropsychological functioning in the sample as a whole, although we doubt that such effects would tend to wield a very profound influence. At any rate, such effects should not confound our interpretation of the separate effects of age of seizure onset and functional organization, which are the primary focus of our study.)
Table 3.
Pre- and postsurgical neuropsychological scores for EO v. LO patients
| Test | EO pre mean (SD) | EO post mean (SD) | LO pre mean (SD) | LO post mean (SD) |
|---|---|---|---|---|
| WAIS-R Digit Span scaled scorea | 8.4 (2.8) n = 31 | 9.0 (2.8) n = 33 | 7.8 (2.7) n = 29 | 8.6 (2.6) n = 28 |
| WAIS-R Arithmetic scaled scorea | 8.9 (3.3) n = 26 | 9.5 (3.0) n = 30 | 9.0 (2.7) n = 18 | 8.8 (3.0) n = 26 |
| WAIS-R Similarities scaled scoreg | 8.2 (3.2) n = 23 | 8.0 (2.3) n = 26 | 8.7 (2.6) n = 23 | 8.7 (2.8) n = 22 |
| WAIS-R Block Design scaled scored | 9.0 (2.8) n = 33 | 10.0 (2.9) n = 33 | 9.8 (2.6) n = 31 | 10.2 (3.0) n = 30 |
| WAIS-R Digit Symbol scaled scorea | 8.1 (2.6) n = 30 | 9.3 (3.1) n = 33 | 8.4 (2.7) n = 26 | 9.8 (3.5) n = 28 |
| AVLT trial 5 raw scoreb | 10.8 (2.7) n = 32 | 11.5 (2.4) n = 33 | 11.1 (2.4) n = 31 | 9.7 (3.2) n = 31 |
| AVLT Delayed Recall raw scoreb | 8.0 (3.4) n = 32 | 9.0 (2.9) n = 33 | 8.5 (3.3) n = 31 | 7.3 (3.7) n = 31 |
| AVLT Recognition Correct raw scoreb | 13.8 (1.8) n = 30 | 13.9 (1.5) n = 32 | 14.1 (1.5) n = 29 | 12.8 (3.1) n = 31 |
| CFT Copy raw scored | 30.0 (5.5) n = 32 | 31.1 (4.2) n = 34 | 30.3 (4.5) n = 28 | 32.0 (3.1) n = 29 |
| CFT Recall raw scorec | 13.6 (4.4) n = 32 | 17.2 (6.8) n = 34 | 15.5 (6.0) n = 28 | 17.1 (6.8) n = 29 |
| BVRT Correct raw scorec | 6.9 (1.8) n = 31 | 7.0 (1.9) n = 33 | 6.4 (1.9) n = 26 | 6.0 (1.9) n = 31 |
| BVRT Errors raw scorec | 4.9 (3.2) n = 31 | 4.2 (3.6) n = 33 | 5.8 (3.8) n = 26 | 5.9 (4.0) n = 31 |
| RMT Words raw scoreb | 43.3 (5.2) n = 18 | 44.2 (4.9) n = 21 | 43.6 (5.6) n = 17 | 43.4 (5.5) n = 16 |
| RMT Faces raw scorec | 37.8 (3.3) n = 18 | 39.3 (4.9) n = 21 | 38.5 (4.3) n = 16 | 37.4 (4.9) n = 15 |
| BNT raw scoree | 44.6 (9.5) n = 18 | 47.2 (7.8) n = 27 | 43.2 (12.9) n = 18 | 42.7 (12.7) n = 25 |
| COWAT age corrected raw scoree | 33.9 (12.2) n = 34 | 35.4 (12.2) n = 32 | 31.4 (11.0) n = 30 | 33.4 (9.2) n = 29 |
| Benton Facial Discrimination Test age corrected raw scored | 43.8 (5.0) n = 28 | 43.9 (3.9) n = 27 | 44.0 (5.9) n = 23 | 43.2 (4.6) n = 20 |
| Grooved Pegboard test dominant hand raw score (sec)f | 97.4 (66.1) n = 23 | 82.9 (50.9) n = 23 | 77.0 (22.6) n = 19 | 74.8 (16.3) n = 23 |
| Grooved Pegboard test nondominant hand raw score (sec)f | 88.6 (36.9) n = 23 | 77.0 (16.5) n = 23 | 85.8 (21.0) n = 18 | 78.0 (14.0) n = 23 |
| Trailmaking Test A raw score (sec)a | 32.9 (21.5) n = 27 | 28.5 (15.3) n = 28 | 29.6 (14.4) n = 26 | 29.9 (12.6) n = 29 |
| Trailmaking Test B raw score (sec)g | 88.4 (84.4) n = 27 | 80.1 (56.7) n = 28 | 102.8 (75.6) n = 26 | 78.2 (37.6) n = 29 |
Note. AVLT = Auditory-Verbal Learning Test; CFT = Complex Figure Test; BVRT = Benton Visual Retention Test; RMT = Warrington Recognition Memory Test; BNT = Boston Naming Test; COWAT = Controlled Oral Word Association Test. The superscript letters denote which tests were grouped together to comprise various composite scores, as follows:
Attention/Speed of Information Processing Composite
Verbal Memory Composite
Visual Memory Composite
Visuospatial Composite
Language Composite
Motor Composite
Executive Functioning Composite.
For the purpose of conducting statistical analyses of the outcome data, neuropsychological composite scores were generated by averaging standardized difference scores for tests representing like cognitive domains. Standardized difference scores were obtained by converting raw pre- and post-surgical neuropsychological scores to standardized scores (i.e., z-scores) using the appropriate normative dataset for each measure. Then pre-surgical z-scores were subtracted from post-surgical z-scores, and the values of the differences were averaged across like tests. Using this procedure, seven composite scores were generated. The seven composite domains and the tests that comprise them are as follows: Attention/Speed of Information Processing (WAIS-R Digit Span total score, WAIS-R Arithmetic total score, WAIS-R Digit Symbol total score, Trailmaking Test Part A time); Verbal Memory (Auditory-Verbal Learning Test Trial 5 score, Auditory-Verbal Learning Test Delayed Recall score, Auditory-Verbal Learning Test Recognition Correct score, Recognition Memory Test-Words total score); Visual Memory (Complex Figure Test Recall score, Recognition Memory Test-Faces total score, Benton Visual Retention Test Correct score, Benton Visual Retention Test Errors score); Visuospatial (WAIS-R Block Design total score, Complex Figure Test Copy total score, Benton Facial Discrimination Test total score); Language (Boston Naming Test total score, Controlled Oral Word Association Test total score); Motor (Grooved Pegboard Test dominant hand time, Grooved Peg-board Test nondominant hand time); and Executive Functioning (WAIS-R Similarities total score, Trailmaking Test Part B time). Some of the neuropsychological tests contributed more than one score to the pertinent composite; e.g., the AVLT contributed both the Trial 5 score, the delayed recall score, and the recognition score to the Verbal Memory composite. We felt that the gain in reliability from using as many scores as possible was worth the tradeoff of potential influence from colinearity.
An overall neuropsychological change score (“global”) was also computed, using the average of the 7 domain scores. This score and the 7 composite domain scores served as dependent measures in the analyses. The overall score is obviously not independent from the 7 domain scores, and we took this into account in the statistical analyses and in our interpretation of the results (see below).
RESULTS
Effect of age of onset on neuropsychological outcome
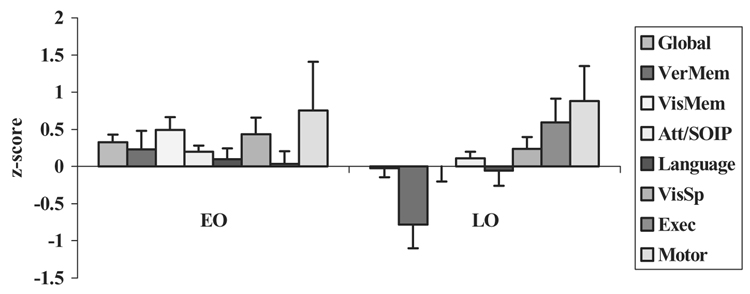
Pre- and post-surgical neuropsychological scores for the EO and LO groups are presented in Table 3. A series of one-way ANOVAs with group (EO versus LO) as the independent variable was conducted on the 8 neuropsychological measures (7 composite scores plus 1 global score). We used one-way ANOVAs rather than MANOVA, due to the fact that not all participants received all tests; this also explains small variations in the degrees of freedom for the various ANOVAs reported below. The data are plotted in Figure 1. There was a significant group effect for the Global measure, that is, overall neuropsychological outcome, F(1,65) = 5.1, p < .05, and for Verbal Memory, F(1,62) = 6.3, p < .05; in both cases, the EO group demonstrated improvement following surgery, whereas the LO group demonstrated decline. On one other measure, Visual Memory, the effect of group approached significance, F(1,63) = 3.4, p = .069; again, the direction favored the EO group, which showed improvement on this measure, compared to the LO group which showed no change. None of the other measures demonstrated significant between-group differences (all ps > .1).
Figure 1.
Neuropsychological change scores (post-surgery minus pre-surgery) by age of onset [early onset (EO) vs. late onset (LO)]. The bars on the x-axis are ordered from left to right to correspond with the top-to-bottom ordering of the key on the right.
The data in Figure 1 support the first hypothesis. We found that age of onset had a significant effect on neuropsychological outcome, whereby earlier age of onset was associated with relatively better outcome, compared to later age of onset, on at least some neuropsychological measures. In fact, in the EO group, there was a positive change on every one of the 8 neuropsychological composite measures; moreover, on 4 of the 8 measures, the magnitude of change was non-trivial (more than 0.3 z-score units, in the small-to-medium effect size range). By contrast, in the LO group, 3 measures showed decline post-surgically, and 1 very significantly so (verbal memory); 1 measure was unchanged (visual memory); and 4 measures showed improvement. The data indicate that the between-group differences are not overwhelming, and in fact, if one applies an alpha-correction to the one-way ANOVAs reported above, none of the outcomes would reach the required level of significance (p < .006 using a Bonferroni correction). Nonetheless, the pattern of outcome is consistent, especially in the memory domain, with a trend favoring better outcome in the EO group. Taking the data all together, it can be concluded that there is support for the notion that the EO group demonstrated relatively better neuropsychological outcome following surgery, whereas the LO group demonstrated relatively poorer neuropsychological outcome.
Functional organization
Most of the patients (n = 58) underwent presurgical intracarotid amobarbital testing (i.e., Wada testing) to assist in identifying language and memory laterality (Wada & Rasmussen, 1960). Cognitive testing during the procedure was conducted by a trained neuropsychologist, and included assessment of arousal/attention, written and spoken receptive and expressive language capacity, and motor function. Prior to amobarbital injection, subjects were given a brief introduction (using sample stimuli) to the types of questions they would be asked during the procedure. Stimuli were presented on cards that were held within the subject’s intact visual field. Naming was assessed by asking subjects to name line drawings of common items. Reading capacity was evaluated by asking subjects to read aloud a sentence and several written words. Response to commands was used to assess language comprehension. Motor function was assessed using ratings of grip strength. Additionally, free recall memory for verbal (i.e., the sentence and the written words) and visual (i.e., the line drawings) stimuli presented during the procedure were assessed after the effects of amobarbital were determined to have dissipated (i.e., 15 or more minutes after injection). Recognition memory was also assessed by asking the subject to chose the target item from several distractors that were presented on cards, in a format comparable to that used during the procedure. Subjects underwent bilateral injections on the same day, starting with the hemisphere thought to contain the seizure focus.
Subjects were classified as having traditional functional organization (TFO; n = 23) if Wada testing indicated primarily left hemisphere mediation of language and verbal memory and primarily right hemisphere mediation of visual memory. This determination was made based on the conclusions contained within the written report of the neuropsychologist conducting the Wada assessment. In order to classify a subject as TFO, a statement indicating that language and verbal memory were mediated exclusively by the left hemisphere and that visual memory was mediated exclusively by the right hemisphere was required. In cases where the right hemisphere appeared to play some role in language and verbal memory functions, a statement indicating that the left hemisphere clearly played a more dominant role was required in order to classify the subject as TFO; the same criterion was applied in cases of left hemisphere contribution to visual memory functions. Any deviation from this pattern (e.g., greater right hemisphere mediation of language) resulted in a classification of nontraditional functional organization (NTFO; n = 35). Table 4 contains demographic data for the TFO and NTFO groups. In our view, the greater proportion of subjects classified as nontraditionally functionally organized in this sample, compared to some previously published reports, is likely due to several factors: (1) functional organization (i.e., “dominance”) is often characterized with respect to lateralization of language capacities; we examined both language and memory functions in order to more broadly classify functional organization; and (2) as discussed earlier, the sample as a whole (and in particular, the EO group) has a relatively higher proportion of left-handed subjects when compared with the typical baserate. It has been well described (see Satz, Strauss, Wada, and Orsini, 1988, for review) that left-handers, particularly those with early lesions to the left hemisphere, are more likely than not to demonstrate reorganization of language functions to the right hemisphere.
Table 4.
Demographics as a function of functional organization classification
| Group | Age1 mean (SD) | Onset age2 mean (SD) | Gender1 M:F | Education* mean (SD) | Handednessa R:L |
|---|---|---|---|---|---|
| Traditional (n = 23) | 38.4 (10.2) | 14.6 (12.0) | 14:9 | 12.2 (1.3) | 20:3 |
| Nontraditional (n = 35) | 31.0 (9.7) | 8.4 (7.3) | 15:20 | 13.0 (2.0) | 29:6 |
Note. t(56) = 2.8, p < .01
t(56) = 2.4, p < .05
Independent samples t-test revealed no differences between groups
Chi-square test indicated no differences between groups.
Relationship between age of onset and functional organization
Two findings supported our prediction that earlier age of seizure onset would be associated with a higher degree of nontraditional functional organization. First, as shown in Table 4, the average age of continuous seizure onset was significantly greater in subjects classified as having TFO (M = 14.6 years) than in subjects classified as having NTFO (M = 8.4 years). Second, a marginally significantly greater number of early onset subjects (70%) were classified as having NTFO, as compared with 50% of late onset subjects, χ² = 2.4, p = .099.
Effect of functional organization on neuropsychological outcome
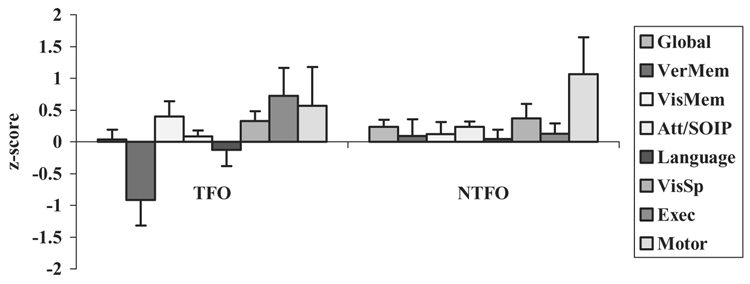
Pre- and post-surgical neuropsychological scores for the TFO and NTFO groups are presented in Table 5. To test the prediction that nontraditional functional organization would be associated with better neuropsychological outcome than traditional functional organization, a series of one-way ANOVAs with group (TFO versus NTFO) as the independent variable were conducted on the 8 neuropsychological measures (7 composite scores plus 1 global score). (As before, we used one-way ANOVAs rather than MANOVA due to the fact that not all participants received all tests.) The data are plotted in Figure 2. There was a significant group effect for Verbal Memory, F(1, 56) = 4.8, p = .03, with the NTFO group demonstrating significantly better outcome than the TFO group. None of the other measures demonstrated significant between-group differences (all ps > .1).
Table 5.
Pre- and postsurgical neuropsychological scores for TFO v. NTFO patients
| TFO pre mean (SD) | NTFO pre mean (SD) | TFO post mean (SD) | NTFO post mean (SD) | |
|---|---|---|---|---|
| WAIS Digit Span scaled score | 8.1 (3.1) n = 20 | 7.9 (2.7) n = 33 | 8.6 (2.9) n = 21 | 8.9 (2.8) n = 33 |
| WAIS Arithmetic scaled score | 9.1 (2.3) n = 15 | 8.6 (3.3) n = 23 | 9.4 (2.9) n = 21 | 8.8 (3.2) n = 30 |
| WAIS Similarities scaled score | 8.3 (2.2) n = 18 | 8.5 (3.5) n = 21 | 8.3 (2.0) n = 17 | 8.3 (3.0) n = 25 |
| WAIS Block Design scaled score | 9.4 (2.5) n = 23 | 9.2 (2.9) n = 34 | 10.1 (2.9) n = 23 | 9.8 (3.1) n = 33 |
| WAIS Digit Symbol scaled score | 7.7 (2.4) n = 20 | 8.6 (2.8) n = 29 | 8.5 (2.7) n = 23 | 10.1 (3.5) n = 31 |
| AVLT Trial 5 raw score | 10.8 (2.1) n = 22 | 10.8 (2.9) n = 34 | 9.1 (3.0) n = 22 | 11.1 (2.6) n = 35 |
| AVLT Delay raw score | 8.2 (3.1) n = 22 | 8.0 (3.5) n = 34 | 6.9 (3.7) n = 22 | 8.6 (3.1) n = 35 |
| AVLT Recognition Correct raw score | 13.9 (1.7) n = 20 | 13.8 (1.7) n = 33 | 12.3 (3.4) n = 22 | 13.8 (1.6) n = 34 |
| CFT Copy raw score | 30.6 (3.2) n = 21 | 29.6 (5.9) n = 34 | 32.3 (2.5) n = 22 | 31.0 (4.1) n = 35 |
| CFT Recall raw score | 13.5 (5.1) n = 21 | 15.0 (5.4) n = 34 | 18.2 (6.4) n = 22 | 16.1 (7.0) n = 35 |
| BVRT Correct raw score | 6.4 (1.9) n = 20 | 6.7 (1.9) n = 30 | 6.1 (1.8) n = 23 | 6.6 (2.1) n = 35 |
| BVRT Errors raw score | 6.2 (3.6) n = 20 | 5.1 (3.6) n = 30 | 5.4 (3.7) n = 23 | 5.1 (4.2) n = 35 |
| RMT Words raw score | 42.9 (4.8) n = 11 | 43.8 (6.0) n = 21 | 42.2 (5.3) n = 14 | 45.0 (4.7) n = 20 |
| RMT Faces raw score | 38.6 (3.1) n = 11 | 37.3 (3.8) n = 20 | 39.1 (4.2) n = 13 | 37.8 (5.5) n = 20 |
| BNT raw score | 41.8 (13.4) n = 13 | 44.2 (10.4) n = 19 | 42.4 (12.3) n = 19 | 45.6 (9.9) n = 27 |
| COWAT age corrected raw score | 35.4 (11.2) n = 21 | 31.9 (11.8) n = 35 | 35.4 (11.9) n = 21 | 34.0 (10.9) n = 34 |
| Benton Facial Discrimination Test age corrected raw score | 44.3 (3.4) n = 17 | 43.1 (6.4) n = 28 | 43.8 (3.9) n = 16 | 43.3 (4.6) n = 26 |
| Grooved Pegboard test dominant hand raw score (sec) | 95.3 (66.4) n = 13 | 87.4 (47.4) n = 25 | 92.2 (57.5) n = 16 | 72.6 (18.7) n = 25 |
| Grooved Pegboard test nondominant hand raw score (sec) | 84.3 (21.8) n = 13 | 90.2 (35.8) n = 25 | 80.4 (13.5) n = 16 | 78.0 (16.9) n = 25 |
| Trailmaking Test A raw score (sec) | 31.3 (14.9) n = 16 | 32.0 (20.6) n = 33 | 31.4 (13.3) n = 19 | 28.6 (15.1) n = 33 |
| Trailmaking Test B raw score (sec) | 128.0 (84.0) n = 16 | 85.7 (78.0) n = 33 | 91.6 (44.7) n = 19 | 75.7 (51.1) n = 33 |
Figure 2.
Neuropsychological change scores (post-surgery minus pre-surgery) by functional organization [traditional functional organization (TFO) vs. nontraditional functional organization (NTFO)]. The bars on the x-axis are ordered from left to right to correspond with the top-to-bottom ordering of the key on the right.
The data in Figure 2 support the third hypothesis. That is, we found that nontraditional functional organization was associated with relatively better neuropsychological outcome, compared to traditional functional organization. In the NTFO group, there was a positive change on every one of the 8 neuropsychological composite measures following surgery. In the TFO group, by contrast, there was post-surgical decline on 2 of 8 composite measures, and for one (Verbal Memory), the decline was notable (nearly one SD unit). As before, the data indicate that the between-group differences are not overwhelming, and the difference in Verbal Memory does not reach statistical significance after a Bonferroni correction (alpha = .006).
Follow-up analysis
A variable that may also play an important role in this line of investigation is hemispheric laterality, that is, the side of the temporal lobectomy. We did not incorporate this into the design of the study a priori. Nonetheless, laterality is clearly relevant to the neuropsychological measurements, especially in domains that tend to be strongly lateralized in conventional normal cerebral organization. Thus, we conducted a follow-up analysis aimed at exploring how laterality played a role in postoperative changes in neuropsychological performance, as a function of age of seizure onset. We tested two specific hypotheses in this regard: (1) Subjects with early age of seizure onset (EO) and left temporal lobectomy (LTL) would demonstrate poorer outcome on measures of verbal memory and language, compared to all of the other groups; and (2) Subjects with EO and right temporal lobectomy (RTL) would demonstrate poorer outcome on measures of visual memory and visuospatial skills, compared to all other groups.
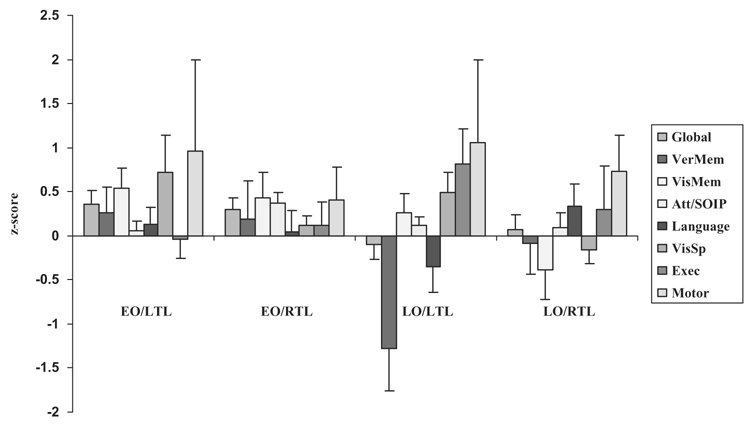
A series of two-way ANOVAs (lobectomy hemisphere x age of onset) was conducted on the 8 neuropsychological domains. The data are plotted in Figure 3. A main effect of lobectomy hemisphere was observed for visuospatial skills, F(1, 64) = 5.3, p = .03. Following temporal lobectomy, LTL subjects demonstrated improvement on measures of visuospatial functioning, whereas RTL subjects, as a whole, demonstrated no statistically significant change in performance when compared with pre-surgical assessment (in fact, analysis of the interaction, although not statistically significant, indicates that EO RTL subjects demonstrated modest improvement in visuospatial skills, whereas LO RTL subjects demonstrated modest deterioration in this domain). The main effect for age of onset findings mirror the results described previously (see Effect of Age of Onset on Neuropsychological Outcome). In particular, analysis revealed a main effect of age of onset for global neuropsychological outcome, F(1, 66) = 4.4, p = .04, verbal memory, F(1, 63) = 5.2, p = .03, and visual memory, F(1, 64) = 4.7, p = .04.
Figure 3.
Neuropsychological change scores (post-surgery minus pre-surgery) by onset classification and lobectomy hemisphere [early onset/left temporal lobectomy (EO/LTL) vs. early onset/right temporal lobectomy (EO/RTL) vs. late onset/left temporal lobectomy (LO/LTL) vs. late onset/right temporal lobectomy (LO/RTL)]. The bars on the x-axis are ordered from left to right to correspond with the top-to-bottom ordering of the key on the right.
While there were no statistically significant interaction effects (likely due to the decrease in power resulting from smaller cell sizes), several trends were observed. Specifically, LO subjects, and particularly LO/LTL subjects, demonstrated significant deterioration on measures of verbal memory following temporal lobectomy, when compared with EO/LTL and EO/RTL subjects, who demonstrated improvement, F(1, 63) = 2.5, p = .1. There was also a trend, F(1, 63) = 2.5, p = .1, indicating deterioration on language measures in LO LTL subjects, whereas all other groups improved in this domain.
In total, these results support our follow-up hypotheses, and suggest that age of onset effects interact with laterality of surgery to produce different patterns of change. Examination of the data in Figure 3 indicates that subjects with early onset seizure disorder who undergo either right or left temporal lobectomy demonstrate generally stable or improved neuropsychological performances on post-surgical assessment. Specifically, only one domain out of 8 in the EO/LTL group (i.e., executive functioning) and none out of 8 in the EO/RTL group declined following surgery. In contrast, subjects with late onset seizure disorder demonstrate generally poorer outcomes following surgery, but the nature of the changes depends on the side of lobectomy. In particular, in the LO/LTL group, 3 out of 8 domains demonstrated deterioration on post-surgical assessment (i.e., global outcome, verbal memory, and language). In the LO/RTL group, verbal memory also deteriorated following surgery, as did visual memory and visuospatial functions.
DISCUSSION
The overall results of our study are generally confirmatory of previous findings from the literature, and indicate three main conclusions: (1) Early onset of seizure disorder (operationalized in our study as before age 9) is associated with relatively better neuropsychological outcome following anterior temporal lobectomy, compared to late onset; (2) There is a higher incidence of nontraditional functional organization in patients with early onset seizure disorder; and (3) Nontraditional functional organization is associated with relatively better neuropsychological outcome following anterior temporal lobectomy, compared to traditional functional organization. Our study has several strengths which help put these findings on firmer empirical footing: a wide range of neuropsychological functions was sampled, the groups had sizeable Ns and were well matched on a variety of demographic and seizure-related variables, and the post-surgical follow-up testing was completed in the chronic phase of recovery, 3 or more months post-surgery (with a mean of nearly two years). Our findings lend additional support to previous conclusions reached by other epilepsy research groups, including Powell et al. (1985), Saykin and colleagues (Saykin et al., 1989; Stafiniak et al., 1990), and Hermann and colleagues (Hermann et al., 1995).
Many individuals with seizure disorders have a history of a risk factor that is known or suspected to have played an etiological role in the development of the seizure disorder (e.g., early head injury, early CNS infection, high fever). The onset of the risk factor incident, in fact, rather than the onset of the actual seizure disorder, could represent the earliest genesis for cerebral functional reorganization. For this reason, some investigators (e.g., Stafiniak et al., 1990; Saykin et al., 1995) have used age of risk for later development of seizure disorder rather than age of onset of continuous seizures as a classification factor. When we re-ran our analyses using age of first risk for seizures as the classification variable for determining groups (i.e., less than or equal to 5 years = early onset; greater than 5 years = late onset, as in prior studies, see Saykin et al., 1995; Stafiniak et al., 1990), our results were essentially unchanged, and entirely consistent with the pattern described previously. In the end, we are more confident of age at onset of continuous seizures as the classification variable, because we have more reliable data for this than we do for the age at first risk variable.
A wrinkle in this literature, alluded to in the Introduction, is that earlier age of seizure onset has been associated with poorer neuropsychological test performance (irrespective of surgical status), compared to later onset seizure disorder (e.g., Dikmen et al., 1975, 1977; Hermann et al., 2002; Lespinet et al., 2002), which opens the possibility that early onset patients have relatively “less to lose” than late onset patients. It was possible to examine this possibility in some detail in our dataset. Comparing the pre-operative performances of the early onset group and late onset group (specifically, columns 1 and 3 in Table 3), it can be seen that there are almost no meaningful differences between the groups. In fact, on only one measure (Grooved Pegboard test, dominant hand) was there a sizeable difference (favoring the late onset group); on all of the other measures, the differences between the groups were trivial. Thus, our data provide no indication that the early onset group had a lower baseline level of performance that could have contributed to the finding that this group showed relatively better post-surgical outcome.
The results of our study also speak to the notion that functional organization might act as an explanatory variable in how age of onset affects outcome following temporal lobectomy. We found a marginally significantly higher rate of nontraditional functional organization in the early onset group, and we also found that the patients with nontraditional functional organization had an earlier age of seizure onset (less than 9 years old, on average). Together with the finding that the patients with nontraditional functional organization had a relatively better neuropsychological outcome following anterior temporal lobectomy than did patients with traditional functional organization, these data are consistent with the idea that “reorganization” of function can have a protective effect. This is the gist of what Powell et al. (1985) suggested, and it is also reminiscent of the ideas set forth by Stafiniak et al. (1990) and Pataraia et al. (2004).
Our study has important qualifications. The most obvious one concerns the magnitude of the effects we obtained. None of the main outcomes was overwhelming in size, although the patterns tended to be quite consistent. Not surprisingly, given the nature of the resection, which typically included part of the mesial temporal region, the domain of memory yielded the largest effect, and verbal memory, in particular, showed a clear between-group difference for both the early onset v. late onset and nontraditional functional organization v. traditional functional organization contrasts (and clearly contributed to the effect we observed on the aggregate “global” measure, as well). But overall, the differences between the groups were small in magnitude. Hence, our conclusions regarding the more positive outcomes in the early onset and nontraditional functional organization groups have to be taken with the qualification that the differences are small and relative. By no means can it be concluded that these factors confer a large protective effect on neuropsychological outcome following anterior temporal lobectomy. Nonetheless, our findings are consistent with the general patterns reported previously by epilepsy researchers, and as such, would appear to fit well into the general picture that emerges from this literature.
Acknowledgments
Supported by Program Project Grant NINDS NS19632
Footnotes
We are following the consensus in the field in regard to our determination of a cutoff for classifying participants as early versus late onset. For some seizure populations, e.g., patients with febrile seizures, there is evidence for a trimodal distribution of age of epilepsy onset (Janszky, Janszky & Ebner, 2004). For our purposes, though, the dichotomous approach was in line with previous studies of similar populations of patients.
References
- Dikmen S, Matthews CG, Harley JP. The effect of early versus late onset of major motor epilepsy upon cognitive-intellectual performance. Epilepsia. 1975;16:73–81. doi: 10.1111/j.1528-1157.1975.tb04723.x. [DOI] [PubMed] [Google Scholar]
- Dikmen S, Matthews CG, Harley JP. Effect of early versus late onset of major motor epilepsy on cognitive-intellectual performance: Further considerations. Epilepsia. 1977;18:31–36. doi: 10.1111/j.1528-1157.1977.tb05584.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Wiebe S, French J, Sperling M, Williamson P, Spender D, et al. Practice parameter: Temporal lobe and localized neocortical resections for epilepsy. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy: A study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87–95. doi: 10.1046/j.1528-1157.2002.24101.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Hesdorffer DC. Incidence and prevalence. In: Hauser WA, Hesdorffer DC, editors. Epilepsy: frequency causes, and consequences. New York: Demos Press; 1990. [Google Scholar]
- Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, et al. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–1071. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Relationship of age at onset, chronological age, and adequacy of preoperative performance to verbal memory change after anterior temporal lobectomy. Epilepsia. 1995;36:137–145. doi: 10.1111/j.1528-1157.1995.tb00972.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Somes G, Dohan FC, Berry AD, Clement L. Declarative memory following anterior temporal lobectomy in humans. Behavioral Neuroscience. 1994;108:3–10. doi: 10.1037//0735-7044.108.1.3. [DOI] [PubMed] [Google Scholar]
- Janszky J, Janszky I, Ebner A. Age at onset in mesial temporal lobe epilepsy with a history of febrile seizures. Neurology. 2004;63:1296–1298. doi: 10.1212/01.wnl.0000140701.40447.88. [DOI] [PubMed] [Google Scholar]
- Lespinet V, Bresson C, N’Kaoua B, Rougier A, Claverie B. Effect of age of onset of temporal lobe epilepsy on the severity and the nature of preoperative memory deficits. Neuropsychologia. 2002;40:1591–1600. doi: 10.1016/s0028-3932(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Loring DW. Neuropsychological evaluation in epilepsy surgery. Epilepsia. 1997;38 (Suppl. 4):S18–S23. doi: 10.1111/j.1528-1157.1997.tb04535.x. [DOI] [PubMed] [Google Scholar]
- Novelly RA, Augustine EA, Mattson RH, Glaser GH, Williamson PD, Spencer DD, et al. Selective memory improvement and impairment in temporal lobectomy for epilepsy. Annals of Neurology. 1984;15:64–67. doi: 10.1002/ana.410150112. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Dodrill CB. Verbal memory deficits after left temporal lobectomy for epilepsy. Journal of Neurosurgery. 1985;62:101–107. doi: 10.3171/jns.1985.62.1.0101. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Leary S, Seidenberg M, Berent S, Boll TJ. Effects of age of onset of tonic-clonic seizures on neuropsychological performance in children. Epilepsia. 1981;22:197–204. doi: 10.1111/j.1528-1157.1981.tb04102.x. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Billingsley-Marshall RL, Castillo EM, Breier JI, Simos PG, Sarkari S, Fitzgerald M, Clear T, Papanicolaou AC. Organization of receptive language-specific cortex before and after left temporal lobectomy. Neurology. 2005;64:481–487. doi: 10.1212/01.WNL.0000150900.71773.E6. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Simos PG, Castillo EM, Billingsley-Marshall RL, McGregor AL, Breier JI, Sarkari S, Papanicolaou AC. Reorganization of language-specific cortex in patients with lesions or mesial temporal epilepsy. Neurology. 2004;63:1825–1832. doi: 10.1212/01.wnl.0000144180.85779.9a. [DOI] [PubMed] [Google Scholar]
- Powell GE, Polkey CE, McMillan T. The new Maudsley series of temporal lobectomy. I: Short-term cognitive effects. British Journal of Clinical Psychology. 1985;24:109–124. doi: 10.1111/j.2044-8260.1985.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Satz P, Strauss E, Wada J, Orsini DL. Some correlates of intra- and interhemispheric speech organization after left focal brain injury. Neuropsychologia. 1988;26:345–350. doi: 10.1016/0028-3932(88)90087-5. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Sussman NM, O’Connor MJ, Gur RE. Memory deficits before and after temporal lobectomy: Effect of laterality and age of onset. Brain and Cognition. 1989;9:191–200. doi: 10.1016/0278-2626(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Stafiniak P, Robinson LJ, Flannery KA, Gur RC, O’Connor MJ, Sperling MR. Language before and after temporal lobectomy: Specificity of acute changes and relation to early risk factors. Epilepsia. 1995;36:1071–1077. doi: 10.1111/j.1528-1157.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Stafiniak P, Saykin AJ, Sperling MR, Kester DB, Robinson LJ, O’Connor MJ, et al. Acute naming deficits following dominant temporal lobectomy: Prediction by age at 1st risk for seizures. Neurology. 1990;40:1509–1512. doi: 10.1212/wnl.40.10.1509. [DOI] [PubMed] [Google Scholar]
- Stroup E, Langfitt J, Berg M, McDermott M, Pilcher W, Como P. Predicting verbal memory decline following anterior temporal lobectomy. Neurology. 2003;60:1266–1273. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- Tranel D. The Iowa-Benton school of neuropsychological assessment. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatric disorders. 2nd edition. New York: Oxford University Press; 1996. pp. 81–101. [Google Scholar]
- Tranel D. Theories of clinical neuropsychology and brain-behavior relationships: Luria and beyond. In: Morgan JE, Ricker JH, editors. Handbook of clinical neuropsychology. Amsterdam: Swets & Zeitlinger; (In press) [Google Scholar]
- Trenerry MR, Jack CR, Ivnick RJ, Sharbrough FW, Cascino GD, Hirschorn KA, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–1805. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance: Experimental and clinical observations. Journal of Neurosurgery. 1960;17:266–282. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal lobe epilepsy. New England Journal of Medicine. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wolf RL, Ivnik RJ, Hirschorn KA, Sharbrough FW, Cascino GD, Marsh WR. Neurocognitive efficiency following left temporal lobectomy: Standard versus limited resection. Journal of Neurosurgery. 1993;79:76–83. doi: 10.3171/jns.1993.79.1.0076. [DOI] [PubMed] [Google Scholar]