Abstract
We previously demonstrated that hormone treatments which stimulate female-directed singing increased levels and turnover of dopamine (DA) in brain areas controlling the motor patterning of song. To help determine how DA affects singing, we quantified the effects of treating adult male finches with the D1/D2 receptor antagonist cis-flupenthixol. Adult males were given subcutaneous silastic implants of androgen, in case drug treatment interfered with androgen secretion. One week later, they were tested with females. Males were divided into three groups matched for levels of courtship singing. Males were then subcutaneously implanted with osmotic minipumps containing either saline, a low, or a high dose of cis-flupenthixol. Each male was tested with a different female 5 and 10 days after implantation to determine how this D1/D2 receptor antagonist affected behavior. Both drug doses affected female-directed singing 5 days after initiation of treatment. High-dose males sang to females significantly less often than males in the other two groups. Low-dose males showed fewer high-intensity courtship displays in which males dance towards females as they sing. These effects on courtship singing were not seen at day 10, though other behavioral effects were seen at this time. Male beak wipes, rocks, following females and female withdrawals from males were also affected by drug treatment. General activity in the home cage was decreased by day 11. These data demonstrate that singing and several other female-directed behaviors are sensitive to perturbations in DA receptor function.
Keywords: Dopamine, cisflupenthixol, sexual behavior, singing, songbird, D1/D2 receptors, activity
INTRODUCTION
Dopaminergic function plays an important role in modulating motivation and reward as well as motor control. Dopamine (DA) has been implicated in the control of many species-specific behaviors, including male sexual behavior. In mammals, DA has been implicated in the anticipatory responses made prior to initiation of sexual behavior [36], in the motor aspects of copulation [28], and in response to engaging in copulation (i.e., it is thought to serve a reward function in this regard [37].
DA has also been implicated in the control of sexual behavior in male birds. The importance of DA in regulating male sexual behavior in birds has been most thoroughly documented in Japanese quail. In this species, DA is involved both in approach to females and in copulation [15]. For passerine birds, singing is an important component of a male's sexual repertoire. In these species, DA appears to modulate courtship singing. Singing is controlled by a series of well-defined, interconnected nuclei known as the vocal control system [34]. Immunocytochemical studies using antibodies to tyrosine hydroxylase, the rate-limiting enzyme in the synthesis of DA and the other catecholaminergic neurotransmitters, showed the vocal control system is strongly innervated by catecholaminergic neurons [4,14]. Such strong catecholaminergic innervation is not seen in comparable forebrain areas in species of birds that do not sing [7,33]. The catecholaminergic innervation of the vocal control system is also sexually differentiated in species in which females sing less than males. For example, the catecholaminergic innervation of the vocal control system is much stronger in male zebra finches and canaries than in females [4,14]. When female canaries are treated with testosterone, the frequency and complexity of their singing behavior increases. This increase in singing behavior is preceded by an increase in the strength of the catecholaminergic innervation of the vocal control system [5]. In zebra finches, castration decreased levels and turnover of DA in vocal control nuclei on the motor pathway that controls song production [11]. We have also shown that hormone treatments that increase singing behavior in zebra finches increased levels and/or turnover of DA in vocal control nuclei on the motor pathway [12]. High turnover rates are typically associated with high rates of neurotransmitter synthesis and release [6,18,30,43]. These data suggest that the increases in singing behavior caused by gonadal hormone treatment might be mediated, at least in part, by increased DA function in brain areas controlling singing behavior.
To determine if DA is involved in the modulation of female-directed singing inzebra finches, we administered the mixed D1/D2 receptor antagonist cis-flupenthixol. This drug has been widely used to investigate the involvement of DA in the control of male sexual behavior in mammals [1,35,42]
MATERIALS AND METHODS
All methodology was approved by the Institutional Animal Care & Use Committee of Hunter College and met all local and federal guidelines for animal research.
Animals
Adult zebra finches (Taenopygia guttata) were purchased from Canary Bird Farm (Englishtown, NJ). Upon arrival, birds were treated with ivermectin to kill parasites and banded for identification. Birds were housed in our standard stainless steel cages (56 × 56 × 56cm). Males were housed individually, females were housed in groups of 3–4. They were given a one-week adaptation period before the experiment began. Animal rooms were temperature controlled (24°C ± 2°C) with a 14:10 light:dark cycle. Relative humidity was kept above 65% to help maintain birds in breeding condition. A vitamin-supplemented commercial finch seed mix, water, grit, and cuttlebone were available ad libitum. This diet was supplemented with fresh greens and oranges.
Experimental design
In a previous study in which males were randomly assigned to treatment groups, we discovered after the experiment was completed that the mean courtship levels of the experimental groups differed significantly before birds received their treatments, making it more difficult to demonstrate the effects of experimental treatment [13]. Therefore in this study, we assigned males to groups so that the mean frequency (± SEM) of courtship displays and copulatory behavior of the three groups on the first 15-min test were as similar as possible. Males were run in blocks of 8–9 males at a time. Males which courted less than 6 times were eliminated from the experiment, because drug treatment was expected to lower sexual behavior. One male showing extremely high levels of courtship, over 8 standard deviations above the mean, was also eliminated. Males received androgen implants at least one week before their first test for sexual behavior. This assured adequate androgen levels, in case later treatment with cis-flupenthixol interfered with androgen secretion. Following their first test, each male received a subcutaneous osmotic minipump containing the mixed D1/D2 DA receptor antagonist cis-flupenthixol (0, 0.5 or 5 μg/g/day). The second test occurred five days after implantation, and the third test followed five days later.
Drug and hormone implantation
At the beginning of the experiment, males were anesthetized with Metofane (Pitman-Moore, Mundelien, IL) and implanted subcutaneously between the wings with silastic implants of androstenedione (5mm of packed hormone in silastic tubing 0.76mm ID, 1.65mm OD [Fisher Scientific, Somerville, NJ] sealed with silastic adhesive [Factor II, Lakeside Arizona] See [22] for further details). This size implant maintains androgen levels in the range normally seen in breeding male finches [32]. Androstenedione was used because it activates the highest levels of courtship singing of any androgen tested in castrated male finches [22]. Following the first behavioral test, males were anesthetized with Metofane and implanted subcutaneously between the wings with 100 μl osmotic minipumps (Model 1002, Alza, Palo Alto, CA) providing the mixed D1/D2 DA receptor antagonist cis-flupenthixol (0, 0.5 or 5 μg/g/day). The birds were anesthetized and their minipumps removed after the third test, and some males were eliminated because the skin over the implant was not intact. Final Ns were SAL = 9, LOW = 13, HIGH = 10).
Behavioral tests
Following the basic behavioral testing paradigm we have used for the past 25 years (See [22,41] for additional details], males were tested three times for sexual behavior. Observers were blind to the males’ treatments. Mock observations were conducted two to three days before the first observation to acclimate the birds to testing conditions. For each test, a different female was placed in a male’s cage for 15min and their behavior recorded onto data sheets divided into 15sec intervals. All tests were done between 3:30 and 7:00 pm. A Radio Shack Model 3001 unidirectional microphone was placed immediately in front of the male's cage and songs recorded on a Marantz PMD 201 cassette recorder for later analysis. A Sony Digital Handicam DCR-TRV 330 on a tripod was used to record observations for later verification. The camera was about 35 inches from the cage. Four categories of courtship singing were recorded. Distant singing occurred when a male sang to a female perched in a different area of the cage. In low-intensity courtship, the male sat in a relaxed posture on the same perch as the female, turned towards her, and sang. Males showing high-intensity displays typically landed on the same perch, assumed a more vertical posture, and approached the female while singing and performing a pivoting dance display. These three categories were summed to provide the fourth measure, total female-directed singing. Latency to the first song was recorded to the nearest 15 seconds. While tests focused on courtship singing and copulation, the occurrence of many other common behavior patterns was recorded, including those with a primarily aggressive function (e.g., peck, chase), grooming behaviors (e.g., preen, scratch), and other social activities (e.g., follow, heteropreen). Females solicited copulation by assuming a horizontal posture and vibrating their tails up and down rapidly. Rocking was a frequent behavior which involved tilting from an upright to a horizontal posture. If the birds tilted beyond horizontal, that was scored as a bow.
After the first two blocks of birds were run, we became interested in whether drug treatment was affecting general activity. So for the remaining blocks, we quantified the birds’ movements in their home cages. Males were observed for 15 min on the day of their first test and the days after the second and third tests for sexual behavior. During each observation, the number of times the birds landed on each of the four perches, the floor, and the feeder were counted. (SAL = 6, LOW = 8, HIGH = 5).
Statistical analyses
Data were analyzed using Graphpad Prism Version 4.2 for Windows (Graphpad Software, San Diego, CA). Since many data violated the assumptions underlying parametric statistics, nonparametric Kruskal Wallis analyses of variance were used. When a significant effect was found, differences between groups were examined using Dunn’s Multiple Comparison tests. Social behaviors were compared by calculating difference scores for each behavior by subtracting each bird’s score on his first test for sexual behavior (i.e., the pre-drug test) from his score on the second test following 5 days of vehicle or drug treatment. This procedure was repeated for the third test. The difference scores of the three groups were then compared. Using difference scores allowed us to analyze how each bird’s behavior changed with control or drug treatment and repeated testing. Because we had previously found that 12 days of treatment with cis-flupenthixol during development caused striking reductions in courtship singing and sexual behavior when the males reached adulthood [21]. and because treatment with this drug reduced male sexual behavior in other species [1,35,42], we predicted that drug treatment woulddecrease courtship singing and copulatory behavior. These comparisons were done with one-tailed tests. Analyses of all other behaviors used two-tailed tests. Activity scores for each observation were evaluated using Kruskal-Wallis ANOVAs, followed by Dunn’s Multiple Comparison Tests.
RESULTS
Sexual Behavior Test 1 (pre-drug test)
There were no significant differences between groups on any of the behaviors on their initial test for sexual behavior (Kruskal Wallis ANOVAs).
Sexual Behavior Test 2 (5 days of drug treatment)
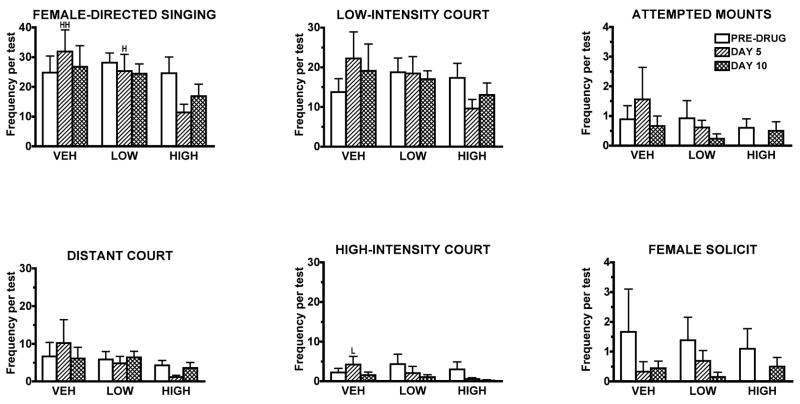
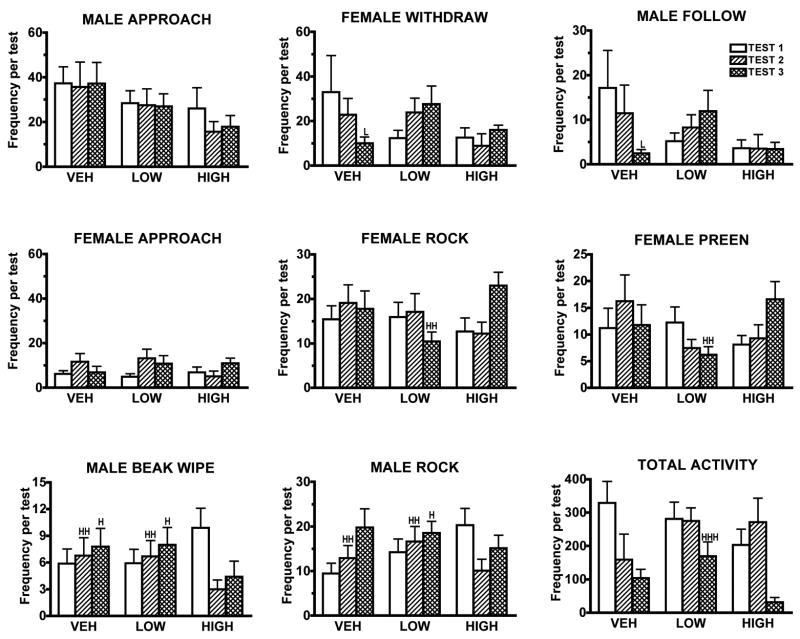
At the doses used, treatment with cis-flupenthixol had no obvious effects on gross motor behavior. Drug-treated males flew about their cages, vocalized, and perched normally. Drug-treated males were as difficult to catch as controls. Treatment with the high dose of cis-flupenthixol caused deficits in courtship singing from the pre-drug Test 1 to Test 2. Total courtship singing differed significantly across the three groups (P = 0.005). While total singing increased from Test 1 to Test 2 in vehicle-treated males and decreased slightly in low-dose males, it declined over 50% in high-dose males. (See Fig. 1 for all comparisons. This figure presents the mean frequencies of behavior, not the difference scores.) The frequency of high-intensity courtship displays was significantly different across groups (P = 0.04). Males treated with the low dose of cis-flupenthixol showed a significant decrease in high-intensity displays from Test 1 to Test 2 compared to control males. The frequencies of distant and low-intensity courtship singing did not differ across groups. Levels of female solicitation and male copulatory behavior were very low, and there were no significant differences across groups. However, no high-dose males attempted to mount and no females solicited them to copulate on Test 2. This was the only instance in which there was no copulatory behavior in all of the tests. Drug treatment affected beak wiping and rocking behavior (P = 0.0047, P = 0.0035, respectively. See Fig. 2), with high-dose males showing a significant decline in these two behaviors compared to males in the other two groups. Despite its effects on courtship, treatment with cis-flupenthixol did not affect male approaches to or following of females on this test.
Fig. 1.
The effects of 0, 5 or 10 days treatment with vehicle (VEH), 0.5 (LOW) or 5 μg/g/day (HIGH) of the mixed D1/D2 receptor antagonist, cis-flupenthixol (N = 9, 13, 10 respectively) on mean courtship singing and copulatorybehavior (± SEM) in zebra finches. While these graphs present mean behavioral frequencies (± SEM) for each test, the analyses were run on difference scores, subtracting each bird’s score on its initial pre-drug test from its scores on each drug test (See Data Analysis). HSignificantly different from high-dose males, P < 0.05. HHSignificantly different from high-dose males, P < 0.01. VSignificantly different from vehicle-treated males, P < 0.05
Fig. 2.
The effects of 0, 5 or 10 days treatment with vehicle (VEH), 0.5 (LOW) or 5 μg/g/day(HIGH) of the mixed D1/D2 receptor antagonist, cis-flupenthixol on social behavior and activity (Mean ± SEM) in zebra finches. While these graphs present mean behavioral frequencies (± SEM) for each test, the social behavior analyses were run on difference scores, subtracting each bird’s score on its initial pre-drug test from its scores on each drug test (See Data Analysis). Note that the total frequencies on the Y axes differ between some of the graphs. HSignificantly different from high-dose males, P < 0.05. HHSignificantlydifferent from high-dose males, P < 0.01, HHHSignificantly different from high-dose males, P < 0.001. vSignificantly different from vehicle-treated males, P < 0.05
Sexual Behavior Test 3 (10 days of drug treatment)
No significant differences in courtship singing were found in the difference scores between the third and first tests (See Fig. 1). Male beak wiping and rocking behavior still differed significantly across groups (P = 0.0096,and 0.0352 respectively, See Fig. 2). High-dose males were still beakwiping less than they had on their first test, while both control and low-dose males beakwiped more. High-dose males were still rocking less than on their first tests while low-dose males rocked more. Drug treatment affected several female behavior patterns on this test compared to Test 1, withdrawals from males, female rock, and female preen (P = 0.05, 0.01, 0.01, respectively). Females tested with low-dose males increased their withdrawal rate from Test 1 to Test 3, while females tested with control males withdrew from males less frequently on Test 3. Females tested with high-dose males, rocked and preened significantly more than those tested with low-dose males. Drug treatment also affected male following behavior on the third test (P = 0.023). Low-dose males increased their following behavior from Test 1 to Test 3 while control males followed females less on their third test.
Activity Tests
Because the activitytests were initiated after two blocks of males were run, the N for these tests was small. However, males showed significantly different activity patterns on their third activity test (P = 0.047, See Fig. 2). High-dose males were significantly less active than low-dose males on the third test.
DISCUSSION
These results suggest that DA plays an important role in activating courtship singing and reproductive behavior in male songbirds. Short-term treatment with a D1/D2 DA receptor antagonist caused striking deficits in adult reproductive behavior which were not reversed by androgen treatment. Since the data were analyzed by calculating difference scores between birds’ post-implant drug tests and the pre-drug test for each individual and then comparing difference scores across the three groups, these comparisons reflect different patterns of response across tests in the three groups of birds. Five days of treatment with cis-flupenthixol significantly lowered courtship singing in male zebra finches. Low-dose males showed a significant reduction in high-intensity courtship displays, in which males dance towards a female as they sing. High-dose males showed a significant reduction in total female-directed singing. While high-dose males showed decreased levels of all three types of courtship displays which make up this category (i.e., low-intensity, high-intensity, and distant displays), the reductions in the individual measures were not significant. The reductions in courtship singing were not attributable to reduced androgen secretion, since all males were given androgen implants prior to testing. The effects were also not attributable to a change in activity. The high dose of cis-flupenthixol did affect activity levels measured in the home cage, but this effect was first seen at day 11. In fact, the drug-treated males were more active than control males on the sixth day of drug treatment, although this effect was not significant. The effects of drug treatment on courtship singing were short lived, since levels of female-directed singing were returning to those shown by vehicle-treated males by the tenth day of drug treatment. This suggests homeostatic mechanisms were probably compensating for the drug’s effects. DA antagonist treatment typically upregulates DA receptors [17], and tolerance to flupenthixol has been found after as little as seven days of treatment [24].
Levels of copulatory behavior in these sexually inexperienced birds were low and were not significantly affected by treatment with cis-flupenthixol. Interestingly however, no females solicited high-dose males to copulate on day 5, though they solicited some of them before drug treatment and at day 10. Eleven percent of control and 31% of low-dose males were solicited on day 5.. Similarly, no high-dose males attempted to mount on day 5, though some of them attempted to mount on their pre-drug and day 10 tests. Twenty-two percent of control males and 38% of low-dose males attempted to mount on the day five test. These data suggest that lowered DA transmission lowered copulatory behavior.
Six additional social behavior patterns were significantly affected by cis-flupenthixol treatment. High-dose males beak wiped and rocked significantly less than males in the other two groups on both days 5 and 10. While wiping the beak on the perch is commonly associated with cleaning the beak after feeding, this behavior is also frequent during interactions with females [44]. Typically, the first behavior a male finch shows when a female is placed in his cage is to sing or to beak wipe. The function of rocking in which the bird goes from a vertical to a horizontal posture is unclear. Two possibilities are that rocking is incipient beak wiping or preliminary to withdrawal. Males often show incomplete beak wipes in which they bend towards the perch, but do not actually touch the perch with their beaks. Similarly, birds bend forward before flying off a perch. Although the patterns of male beak wiping and rocking appear almost identical, these two behaviors were not correlated. Neither were rocking and withdrawals.
Courtship in zebra finches typically consists of the male approaching the female and singing. The female typically flies away, and the male follows and sings again. The pattern of male follows typically mirrors the pattern of female withdrawals–about 50% of the time, males follow females when they withdraw. While females also approach males, they do this much less frequently than males approach them. Males treated with the high dose of cis-flupenthixol approached females slightly less during drug treatment, but this decline was not significant. Females tested with control males withdrew less over tests while those tested with low-dose males withdrew more over tests, showing a significant increase compared to females tested with controls on the day-10 test. In response, the low-dose males were significantly more likely to follow females to their next perch within 15 seconds than vehicle-treated males. Females tested with low-dose males on day 10 also showed a significant decrease in rocking and preening compared to females tested with high-dose males. Although we did not find any significant changes in the behavior of low-dose males on their day 10 tests aside from following behavior, these data suggest that females detected something different about the behavior of the low-dose males on the third test. The increase in following behavior indicates that low-dose males were clearly motivated to maintain contact with females.
Treatment with the high dose of cis-flupenthixol also lowered male activity levels by the third observation, the day after their third test for sexual behavior. Cis-flupenthixol‘s effect on activity occurred at a time that males appeared to be recovering from the drug’s initial effects on sexual behavior, suggesting that different mechanisms are involved. One possibility is that these effects are controlled by different receptor types. Studies in mammals have found that cis-flupenthixol treatment increased levels of D2, but not D1 receptors [16,27]. In any case, the effects on sexual behavior appeared to be unrelated to the effects on activity.
The effects of the two doses of cis-flupenthixol on behavior do not follow the typical dose-response pattern. The effects of treatment with DA antagonists are, however, dose dependent. Behavioral, biochemical and electrophysiological studies demonstrate that DA autoreceptors are 5–10 times more sensitive to the effects of non-selective dopaminergic drugs like cis-flupenthixol than postsynaptic receptors [17]. Thus, low doses of receptor antagonist result in higher levels of DA being released into synapses. With higher doses of antagonist, while more d DA is released into synapses, sufficient receptor antagonist is present to block DA’s effects on postsynaptic cells. Thus, DA antagonist treatment does not cause normal dose-response curves. Sometimes both low and high doses cause similar effects, perhaps indicating that any perturbation of dopaminergic function disrupts the behavior. In this regard, it is important to remember that in addition to its effects on DA release and receptor binding, receptor antagonist treatment blunts the ability of the neurotransmitter system to provide strong differential responses to changing stimuli (e.g., the appearance of a female). Receptor antagonist treatment appears to be more effective in disrupting than in stimulating behavior. In the current study, the low and high doses had differential effects, indicating that differential stimulation of postsynaptic receptors has differential effects on behavior. As expected, the high dose of cis-flupenthixol had greater effects on male behavior, lowering overall rates of courtship singing on the second test, beak wiping and rocking on the second and third tests and total activity on the third test. The low dose affected high-intensity courtship displays on the second test and behavior of female partners on the third test, including withdrawals, rocking, and preening. While male following in this group was increased on this test, this was driven by the increase in female withdrawals.
How did cis-flupenthixol treatment cause deficits in female-directed singing and other sexual behavior patterns? Research in mammals has demonstrated that DA plays an important role in activating male sexual behavior. Stimuli from a receptive female and/or the act of copulation stimulate the release of DA in brain areas modulating motivation, motor coordination, and sexual behavior [26]. Studies in Japanese quail [8,9,15] have demonstrated the importance of DA in coordinating sexual behavior in male birds as well. Administering cis-flupenthixol caused dramatic deficits in sexual motivation and performance in rats [10,26,42] and rabbits [1,2] without affecting motor performance.
Since cis-flupenthixol was administered systemically, it should have affected dopaminergic function throughout the brain. In rats, three neural systems have been implicated in DA’s modulation of male sexual behavior: 1) the nigrostriatal system which promotes somatomotor activity; 2) the mesolimbic system which is involved in numerous types of motivation; and 3) the MPOA which appears to focus motivation onto sexual targets, increase copulatory rates, and coordinate genital reflexes [26]. Stimuli from a receptive female rat and/or the act of copulation stimulate the release of DA in all three areas [26]. Microinjections of cis-flupenthixol into the MPOA in rats decreased the percent of males copulating, the percent males ejaculating, and the frequencies of intromissions and ejaculations among males who did copulate [35,42], demonstrating the MPOA’s importance in coordinating male sexual behavior.
The available evidence suggests that the MPOA is involved in female-directed singing and male copulatory behavior in birds. Previously, we showed that hormone treatments which stimulated high rates of courtship singing and copulation attempts significantly increased DA levels and turnover in the MPOA in male finches [11,12]. In European starlings, the size of the medial preoptic nucleus was positively related to rates of courtship singing and the length of song bouts directed at females [38]. In this species, lesioning the medial preoptic nucleus significantly reduced courtship singing without affecting nestbox occupancy or the tendency to associate with females [3]. Examination of the activity of immediate-early genes in European starlings and house sparrows suggests that the ventral tegmental area, one of the primary sources of DA production, is also involved in the control of courtship singing and mounting behavior [23,39].
Auditory and vocal control nuclei (VCN) in finches also receive strong dopaminergic innervations. Hormone treatments which stimulate high rates of female-directed singing consistently increased DA turnover in two VCN, NIf and RA (nucleus interfacialis of the nidopallium, robust nucleus of the archipallium, respectively) on the efferent pathway controlling the motor patterning of song [11,12]. These hormone treatments also modulated dopaminergic function in an auditory area, field L, and two VCN, Area X and lMAN (lateral magnocellular nucleus of the nidopallium), on the anterior forebrain circuit involved in song learning. In these nuclei, hormone treatments which stimulate singing decreased DA levels and/or turnover rates in adult males [11,12]. In the past, discussions of DA’s effects on singing usually centered on Area X, since this nucleus is part of the songbird basal ganglia. It receives a very strong dopaminergic projection from the ventral tegmental area [31] and has extremely high levels and turnover of DA [11,12]. However, Area X does not appear to be active during female-directed singing [25,29]. Thus, altered dopaminergic function in Area X appears unlikely to be involved in the deficits in male courtship singing found in this study. DA is also known to be involved in auditory gating [19]. Manipulation of dopaminergic function may have made male finches less responsive to auditory stimuli from females.
In an earlier study, we used the same treatment regime on juvenile male finches, treating them from day 45 to day 57 and then quantified their sexual behavior as adults, starting about six weeks later [21]. Treatment of juveniles had much more profound effects on courtship and copulatory behavior. In juveniles, the low dose of cis-flupenthixol had significantly greater effects than the high dose. Low-dose males rarely sang in the presence of females and never attempted to mount. Low-dose males were, however, interested in females and approached them as often as control males. We hypothesize that the profound decrements in directed singing and copulatory behavior in birds treated as juveniles were caused by the low dose of cis-flupenthixol causing increased DA release during a sensitive period of development [20,40] with resultant long-term down regulation in DA receptors [17]. Decreased availability of DA receptors in adulthood would explain the low levels of sexual behavior in these birds. In the current study, homeostatic mechanisms in adult males appeared better able to cope with drug treatment and to moderate its effects over time. Another interesting finding of our juvenile study was that undirected singing appeared to be unaffected. Athough treatment of juveniles with the low-dose of cis-flupenthixol almost eliminated female-directed singing, these males were often observed to sing undirected song [21]. These data suggest that directed and undirected song are regulated independently and that the latter is much less sensitive to manipulations of DA function.
This current results suggest that DA plays an important role in activating courtship singing and reproductive behavior in male songbirds. Short-term treatment with a DA receptor antagonist caused striking deficits in adult reproductive behavior which were not reversed by androgen treatment. How this antagonist treatment affected neurotransmitter function, and which brain areas are responsible for the deficits in adult reproductive behavior remain to be determined.
Acknowledgments
Sharon Rauceo carried out the observations and analysis of sexual behavior. Cheryl Harding was responsible for study design, matching the treatment groups, surgeries, and data interpretation. Alex Maldonado coordinated the study. The remaining authors carried out the activity observations and analysis. This research was supported by NIH Grant GM 60654 to Hunter College. Support from the Research Centers in Minority Institutions Award RR-03037 from the National Center for Research Resources of the National Institutes of Health, which supports the infrastructure of the Biopsychology & Behavioral Neuroscience Doctoral Program at Hunter, is also acknowledged. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agmo A, Paredes RG, Ramos JI, Contreras JL. Dopamine and sexual behavior in the male rabbit. Pharmacol Biochem Behav. 1996;55:289–95. doi: 10.1016/s0091-3057(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 2.Agmo A, Picker Z. Catecholamines and the initiation of sexual behavior in male rats without sexual experience. Pharmacol Biochem Behav. 1990;35:327–34. doi: 10.1016/0091-3057(90)90164-d. [DOI] [PubMed] [Google Scholar]
- 3.Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav Neurosci. 2006;120:1326–36. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nulcei. Cell Tissue Res. 2001;304:237–59. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- 5.Appeltants D, Ball GF, Balthazart J. Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neurosci. 2003;121:801–14. doi: 10.1016/s0306-4522(03)00496-2. [DOI] [PubMed] [Google Scholar]
- 6.Arbuthnott GW, Crow TJ, Fuxe K, Ungerstedt U. Depletion of catecholamines in vivo induced by electrical stimulation of central monoamine pathways. Brain Res. 1970;24:471–83. doi: 10.1016/0006-8993(70)90186-1. [DOI] [PubMed] [Google Scholar]
- 7.Ball GF, Casto JM, Balthazart J. Autoradiographic localization of D1-like dopamine receptors in the forebrain of male and female Japanese quail and their relationship with immunoreactive tyrosine hydroxylase. J Chem Neuroanat. 1995;9:121–33. doi: 10.1016/0891-0618(95)00075-i. [DOI] [PubMed] [Google Scholar]
- 8.Balthazart J, Baillien M, Ball GF. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:37–55. doi: 10.1016/s1096-4959(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 9.Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–80. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- 10.Pehek EA, Warner RK, Bazzet TJ, Bitman D, Band LC, Eaton RC, Hull EM. Microinjections of cis-flupenthixol, a dopamine antagonist, into the medial preoptic area impairs male sexual behavior in rats. Brain Res. 1988;443:70–76. doi: 10.1016/0006-8993(88)91599-5. [DOI] [PubMed] [Google Scholar]
- 11.Barclay SR, Harding CF. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: Steroid effects on brain monoamines. Brain Res. 1988;459:333–43. doi: 10.1016/0006-8993(88)90649-x. [DOI] [PubMed] [Google Scholar]
- 12.Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–62. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- 13.Barclay SR, Harding CF, Waterman SA. Central DSP-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol Biochem Behav. 1996;53:213–20. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 14.Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivityin the brains of male and female zebra finches. J Neurobiol. 1992;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- 15.Castagna C, Ball GF, Balthazart J. Effects of dopamine agonists on appetitive and consummatory male sexual behavior in Japanese quail. Pharmacol Biochem Behav. 1997;58:403–14. doi: 10.1016/s0091-3057(97)00243-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen AC, Gurling HM. D2 dopamine receptor but not AMPA and kainate glutamate receptor genes show altered expression in response to long term treatment with trans- and cis-flupenthixol in the rat brain. Brain Res Mol Brain Res. 1999;68:14–21. doi: 10.1016/s0169-328x(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 17.Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. New York: Oxford University Press; 2003. p. 405. [Google Scholar]
- 18.Costa E, Neff NH. Estimation of turnover rates to study the metabolic regulation of the steady state level of neuronal monoamines. In: Lajda A, editor. Handbook of Neurochemistry. New York: Plenum Press; 1977. pp. 45–90. [Google Scholar]
- 19.de Bruin NM, van Luijtelaar EL, Cools AR, Ellenbroek BA. Auditory information processing in rat genotypes with different dopaminergic properties. Psychopharmacology (Berl) 2001;156:352–9. doi: 10.1007/s002130100785. [DOI] [PubMed] [Google Scholar]
- 20.Dermon CR, Kouvelas ED. Quantitative analysis of the localization of adrenergic binding sites in chick brain. J Neurosci Res. 1989;23:297–303. doi: 10.1002/jnr.490230308. [DOI] [PubMed] [Google Scholar]
- 21.Harding CF. Brief alteration in dopaminergic function during development causes deficits in adult reproductive behavior. J Neurobiol. 2004;61:301–8. doi: 10.1002/neu.20039. [DOI] [PubMed] [Google Scholar]
- 22.Harding CF, Sheridan K, Walters MJ. Hormonal specificityand activationof sexual behavior in male zebra finches. Horm Behav. 1983;17:111–33. doi: 10.1016/0018-506x(83)90021-1. [DOI] [PubMed] [Google Scholar]
- 23.Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–24. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- 24.Hess EJ, Norman AB, Creese I. Chronic treatment with dopamine receptor antagonists: behavioral and pharmacologic effects on D1 and D2 dopamine receptors. J Neurosci. 1988;8:2361–70. doi: 10.1523/JNEUROSCI.08-07-02361.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci. 1999;19:10461–81. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105–16. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- 27.Hull EM, Warner RK, Bazzett TJ, Eaton RC, Thompson JT, Scaletta LL. D2/D1 ratio in the medial preoptic area affects copulation of male rats. J Pharmacol Exp Ther. 1989;251:422–7. [PubMed] [Google Scholar]
- 28.Hull EM, Weber MS, Eaton RC, Dua R, Markowski VP, Lumley L, Moses J. Dopamine receptors in the ventral tegmental area affect motor, but not motivational or reflexive, components of copulation in male rats. Brain Res. 1991;554:72–6. doi: 10.1016/0006-8993(91)90173-s. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–88. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- 30.Korf J, Grasdijk L, Westerink BHC. Effects of electrical stimulation of the nigrostriatal pathway of the rat on dopamine metabolism. J Neurochem. 1976;26:579–84. doi: 10.1111/j.1471-4159.1976.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196:347–54. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- 32.Luine V, Nottebohm F, Harding C, McEwen BS. Androgen affects cholinergic enzymes in syringeal motor neurons and muscle. Brain Res. 1980;192:89–107. doi: 10.1016/0006-8993(80)91011-2. [DOI] [PubMed] [Google Scholar]
- 33.Moons L, Van Gils J, Ghijsels E, Vandesande F. Immunocytochemical localization of L-DOPA and dopamine in the brain of the chicken (Gallus domesticus) J Comp Neurol. 1994;346:97–118. doi: 10.1002/cne.903460107. [DOI] [PubMed] [Google Scholar]
- 34.Nottebohm F. The neural basis of birdsong. PLoS Biol. 2005;3:e164. doi: 10.1371/journal.pbio.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pehek EA, Warner RK, Bazzett TJ, Bitran D, Band LC, Eaton RC, Hull EM. Microinjection of cis-flupenthixol, a dopamine antagonist, into the medial preoptic area impairs sexual behavior of male rats. Brain Res. 1988;443:70–6. doi: 10.1016/0006-8993(88)91599-5. [DOI] [PubMed] [Google Scholar]
- 36.Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–43. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- 37.Pleim ET, Matochik JA, Barfield RJ, Auerbach SB. Correlation of dopamine release in the nucleus-accumbens with masculine sexual behavior in rats. Brain Res. 1990;524:160–3. doi: 10.1016/0006-8993(90)90507-8. [DOI] [PubMed] [Google Scholar]
- 38.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–61. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 39.Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–18. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 41.Walters MJ, Harding CF. The effects of an aromatization inhibitor on the reproductive behavior of male zebra finches. Horm Behav. 1988;22:207–18. doi: 10.1016/0018-506x(88)90067-0. [DOI] [PubMed] [Google Scholar]
- 42.Warner RK, Thompson JT, Markowski VP, Loucks JA, Bazzett TJ, Eaton RC, Hull EM. Microinjection of the dopamine antagonist cis-flupenthixol into the MPOA impairs copulation, penile reflexes and sexual motivation in male rats. Brain Res. 1991;540:177–82. doi: 10.1016/0006-8993(91)90505-p. [DOI] [PubMed] [Google Scholar]
- 43.Weiner N. A critical assessment of methods for the determination of monoamine synthesis turnover rates in vivo. In: Usdin E, editor. Neuropsychopharmacology of Monoamines and Their Regulatory Enzymes. New York: Raven Press; 1974. pp. 143–58. [Google Scholar]
- 44.Zann RA. The zebra finch: A synthesis of field and laboratory studies. New York: Oxford University Press; 1996. p. 352. [Google Scholar]