Abstract
The anterior cingulate cortex presumptively regulates blood pressure reactions to behavioral stressors. There is little evidence in humans, however, that stressor-evoked changes in blood pressure correlate with concurrent changes in anterior cingulate activity. Using fMRI, we tested whether changes in mean arterial blood pressure correlate with ongoing changes in blood oxygen level dependent (BOLD) activation in 9 women and 11 men who completed a stressful Stroop color-word interference task. Higher mean arterial pressure during the Stroop task correlated with greater BOLD activation in two regions of the cingulate cortex (perigenual and mid-anterior) and in other networked brain regions, including the insula, thalamus, and periaqueductal gray. These results support the hypothesis that the anterior cingulate cortex regulates blood pressure reactions to behavioral stressors in humans.
Keywords: Blood pressure, Cardiovascular reactivity, Functional magnetic resonance imaging, Stress
Behavioral stressors prompt cardiovascular reactions that provide metabolic support for adaptive action (Cannon, 1928; Obrist, 1981). One form of stressor-evoked cardiovascular reactivity—blood pressure reactivity—is a particular focus of psychophysiological research on cardiovascular health. This research focus is driven by increasing empirical support for the hypothesis that large-magnitude (or exaggerated) increases in blood pressure, which exceed the metabolic demands of behavioral stressors, may signal or promote cardiovascular disease (Krantz & Manuck, 1984; Treiber et al., 2003). A long-standing view is that both cortical and subcortical brain regions regulate blood pressure and other cardiovascular reactions to behavioral stressors (e.g., Lovallo & Gerin, 2003; Soufer, Arrighi, & Burg, 2002). In humans, these brain regions include the mid-anterior, perigenual, and subgengual areas of the cingulate cortex (Brodmann’s areas 32, 24, and 25), orbital and medial areas of the prefrontal cortex (Brodmann’s areas 9, 10, and 11), insula, hypothalamus, periaqueductal gray matter, cerebellum, and subcortical cell groups that regulate neuroendocrine and autonomic nervous system control over the cardiovascular system (for a review, see Benarroch, 1997).
An emerging view is that the cingulate cortex may play an integrative role in initiating and representing blood pressure and other cardiovascular reactions to behavioral stressors (Critchley, Good, et al., 2003; Critchley, Mathias, et al., 2003; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004). Expressly, the cingulate cortex is held to initiate descending efferent commands for autonomic, neuroendocrine, and cardiovascular reactions that are calibrated to meet the contextual and metabolic demands of cognitive, emotional, and behavioral challenges or stressors. More broadly, the cingulate cortex is also held to integrate ascending afferent visceral sensory information (provided by changes in blood pressure and other peripheral physiological adjustments) with ongoing cognitive and emotional processes in order to guide future adaptive behaviors (Critchley, Mathias, et al., 2003). By this integrative view of the cingulate cortex, blood pressure and other cardiovascular reactions to behavioral stressors should thus correlate with indicators of concurrent functional neural activation in the cingulate cortex and in other brain regions that presumptively regulate cardiovascular reactivity.
To date, a number of functional neuroimaging studies have demonstrated that stressor-evoked changes in heart rate and heart rate variability correlate with the concurrent activation of the cingulate cortex and other brain regions that regulate cardiovascular reactivity (Critchley, Mathias, et al., 2003; Gianaros, Van Der Veen, & Jennings, 2004; Lane, Reiman, Ahern, & Thayer, 2001). As yet, however, there is limited evidence that activation in these brain regions correlates with stressor-evoked changes in blood pressure. More precisely, only one prior positron emission tomography study tested whether stressor-evoked changes in mean arterial blood pressure correlate with concurrent changes in functional neural activation (Critchley, Corfield, Chandler, Mathias, & Dolan, 2000). Results from that study showed that in 6 participants who completed two behavioral stressors, a mental arithmetic task and an isometric handgrip task, increased mean arterial pressure during the performance of the two stressors correlated with increased regional cerebral blood flow to the perigenual and mid-anterior areas of the cingulate cortex (Brodmann’s areas 32 and 24), the orbitofrontal cortex (area 10), postcentral gyrus (area 2), insula, and cerebellum—providing preliminary support for the view that these brain regions initiate or represent increases in blood pressure to behavioral stressors in humans.
In the present functional magnetic resonance imaging (fMRI) study, we questioned whether we could replicate and extend these prior findings by observing similar patterns of correlation between mean arterial pressure and functional neural activation (as estimated by the amplitude of the blood oxygen-level dependent [BOLD] response) in participants who completed a modified Stroop color-word interference task, which has been used in prior studies of blood pressure reactivity and cardiovascular disease risk (Debski et al., 1991; Gianaros et al., 2002; Jennings et al., 2004; Kamarck et al., 1992, 1997). To answer this question, the Stroop task was modified from the way it has been administered in prior studies of cardiovascular risk so that it could be administered in a blocked fMRI design. In prior cardiovascular reactivity studies, this version of the Stroop task was administered as a single block of Stroop color-word identification trials, which lasted for several minutes. During this extended block of Stroop trials, the individual’s performance (accuracy at color word identification) is maintained at ~ 60% to experimentally equate the behavioral performance and subjective demand (or challenge) of the task across individuals (Debski et al., 1991). During this block of performance-adjusted trials, the individual’s blood pressure is also measured approximately every 90 s. To facilitate the concurrent assessment of mean arterial pressure and the fMRI BOLD response in the present study, individuals completed alternating 90-s blocks of the demanding and performance-adjusted Stroop task condition—which increases blood pressure—and a minimally demanding control condition that was matched for stimulus and motor response characteristics. Such a design parallels prior functional neuroimaging studies that have used similarly demanding versions of the Stroop task to engage the cingulate cortex and other limbic brain systems that presumptively regulate cardiovascular and cardiac autonomic reactivity (e.g., Critchley et al., 2003; Matthews, Paulus, Simmons, Nelesen, & Dimsdale, 2004). With this fMRI adaptation of a task that has been used in prior studies of cardiovascular disease risk, we thus tested whether a stressor-evoked increase in mean arterial pressure would correlate with greater fMRI BOLD response amplitudes in cortical or subcortical brain systems that are held to regulate cardiovascular reactions to behavioral stressors in humans.
Method
Participants
Twenty individuals (9 men, 11 women; M age = 64.2 years, SD = 6.18) from the Pittsburgh, Pennsylvania, community participated. Participants were screened to exclude those with a current or prior history of cardiovascular disease, hypertension, a cerebrovascular accident, stroke, or myocardial infarction, congestive heart failure, atrial or ventricular arrhythmias, coronary bypass, carotid artery, or peripheral vascular surgery, hepatitis, cirrhosis, diabetic neuropathy, Type 1 or 2 diabetes, a pulmonary or respiratory disease, cancer, a substance abuse or psychiatric disorder, use of psychotropic medication, claustrophobia, or metallic implants. All participants provided informed consent and were paid $40.00 (US). We previously reported results from 16 of these participants on between-person differences in functional neural activation to the Stroop color-word interference task (Gianaros, May, Siegle, & Jennings, 2005); however, we did not examine within-person correlations between blood pressure and functional neural activation—as we report here. The University of Pittsburgh Institutional Review Board approved all of the study procedures that the participants completed.
Materials
Stroop color-word interference stimuli were projected onto a 15 × 30 cm transparent screen that was positioned at the foot of the magnetic resonance scanner bed. The Stroop stimuli that were projected onto the screen were viewed by the participant with a 6 × 10 cm mirror that was affixed to a standard birdcage radiofrequency head coil and that was positioned approximately 3 cm away from the participant’s eyes. The Stroop stimuli were presented on the screen in single trials. For each trial, a target word was displayed in the center of the screen, and four identifier words were displayed in a single row along the bottom of the screen. Target and identifier words included red, blue, yellow, and green. The task of the participant was to identify the color in which the target word was shown by selecting (as quickly and as accurately as possible) one of the four identifier words that named the color of the target word. Target and identifier words were shown in the colors red, blue, yellow, or green (see below). Participants selected an identifier word by pressing one of four corresponding buttons on a Fiber Optic Button Response System (Psychology Software Tools, Inc., Pittsburgh, PA). On each trial, the identifier word that the participant selected was outlined, and the correct identifier word was highlighted.
Stroop trials were presented in two alternating 90-s blocks of trials. In one block, the incongruent block, the target word was presented in a color that differed from the color that the target word named, and all of the identifier words were presented in a color that differed from the color that the identifier word named. In the other block, the congruent block, the target word was presented in the same color that the target word named, and all of the identifier words were presented in the same color as the target word. To increase the challenge of the incongruent block and to equate performance across participants for this block, each participant’s accuracy at target word identification was adaptively maintained at 60% by shortening and lengthening trial presentation times, such that more accurate performance within an incongruent block led to shorter response time windows in which the participant could select an identifier word before the next trial; conversely, less accurate performance led to longer response time windows before the next trial. Response time windows ranged from 1 to 5 s and could shorten or lengthen from one trial to the next by 500 ms (Debski et al., 1991).
To match the total number of trials shown to each participant in each block and to control for the possible confounding effects of motor response differences between the incongruent and congruent blocks, the total number of trials presented in each congruent block was yoked to the total number of trials that were completed in the preceding incongruent block. Across participants, the average number of trials completed during the incongruent block was 22.98 (SD = 4.27), and the average number of trials completed in the congruent block was 22.91 (SD = 4.20). Post hoc analyses further confirmed that the performance titration procedure was successful: Mean accuracy at target word identification was 60.82% during the incongruent condition. In contrast, mean accuracy at target word identification was 95.95% during the congruent condition. Thus, the Stroop task consisted of alternating blocks of congruent color word identification trials that were minimally challenging and were matched for stimulus and response characteristics to more challenging incongruent trials, which were performance titrated and performance equated across participants. The incongruent block was expected (on the basis of prior studies that have used this task) to increase mean arterial pressure compared to the congruent block.
Procedure
Each participant abstained from eating, exercising, and from consuming caffeinated and tobacco products for 3 h and from drinking alcoholic beverages for 12 h before testing. When she or he arrived for testing, the participant was given a verbal description of the study procedures, a consent form to complete, and an opportunity to practice one to three blocks of the incongruent and congruent Stroop task trials. After practice, the participant was led into the magnetic resonance scanner room and was seated on the scanner bed. The participant then had a blood pressure cuff placed over her or his brachial artery and was laid supine, such that her or his head was positioned into a radiofrequency head coil. The head coil was filled with foam padding to reduce head movements. The participant was then moved into the bore of the scanner. Once inside the scanner, structural magnetic resonance images were obtained for approximately 8 min and functional images were obtained over four functional imaging runs, each run lasting 6 min 10 s. In each of the four runs, the participant fixated on a crosshair that was positioned in the middle of the screen for the first 10 s, and then she or he completed two 90-s blocks of incongruent trials and two 90-s blocks of congruent trials in a fixed and alternating sequence that began with an incongruent block. Across the four runs, the participant thus completed a total of 16 (8 incongruent, 8 congruent) blocks of trials. After the four runs, the participant was removed from the scanner, debriefed, and paid.
Blood Pressure Measurement
One automated oscillometric measure of mean arterial pressure was obtained during each of the sixteen 90-s blocks with an Omega 1400 MR-compatible blood pressure monitor (In Vivo Research, Orlando, FL).
Structural and Functional Magnetic Resonance Image Acquisition
Structural and functional magnetic resonance images were obtained with a 3-Tesla Signa scanner (General Electric Medical Systems, Milwaukee, WI) and a standard birdcage radiofrequency head coil. Prior to functional imaging, structural images were obtained with a spoiled gradient recalled acquisition pulse sequence (TE = 5 s, TR = 25 ms, flip angle = 40°). With this sequence, we obtained 124 axial slices that were 1.5 mm thick (0 mm spacing between slices) and that covered a whole brain volume of 256 × 192 mm with a 24 × 18 cm field of view. These structural images were later used to coregister the participant’s functional images to her or his own anatomical space.
Functional images were obtained over each functional run with a reverse-spiral pulse sequence (TR = 1500 ms, TE = 25 ms, 60° flip angle), which attenuates losses in BOLD signal intensity (signal dropout) at air–tissue interfaces that are prone to susceptibility artifacts (Stenger, Boada, & Noll, 2000). The data from the first 10 s of each functional run were discarded from subsequent analyses to allow the net magnetization (and fMRI BOLD signal intensity) to stabilize after the first excitation pulse. The remaining functional images for each functional run consisted of 34 axial and contiguous T2*-weighted images, which were parallel to the plane of the anterior and posterior commissures (3.2 mm slice thickness, 20 cm field of view). Thus, with a 1500-ms TR, we obtained 60 whole brain volumes of T2*-weighted images for each of the sixteen 90-s blocks of incongruent and congruent trials. From these whole-brain T2*-weighted images, we estimated the voxel-by-voxel BOLD hemodynamic response.
Image Preprocessing
Functional and structural images were preprocessed and analyzed with statistical parametric mapping software (SPM2; Wellcome Trust Centre for the Study of Cognitive Neurology; http://www.fil.ion.ucl.ac.uk/spm/spm2.html; Friston et al., 1995). Image preprocessing consisted of four steps. First, the T2*-weighted images that were obtained during each of the four functional imaging runs were realigned to the first image of that run to correct for the effects of head movements on image alignment. Second, the realigned images from each participant were coregistered to the same anatomical space of her or his structural image. Third, realigned and coregistered images were spatially normalized to the anatomical space of the brain template provided by the Montreal Neurological Institute in SPM2 (“T1.img”). Fourth, realigned, coregistered, and normalized T2*-weighted images were smoothed in the X, Y, and Z dimensions with a 6-mm full width at half-maximum isotropic Gaussian kernel to increase their signal-to-noise ratio and to accommodate between-participant differences in brain morphology.
fMRI and Blood Pressure Data Analysis
After image preprocessing, a two-level mixed-effects parametric modulation analysis (Buchel, Holmes, Rees, & Friston, 1998) was used to examine the correlation between the amplitude of the fMRI BOLD hemodynamic response (estimated from the preprocessed T2*-weighted images) and the concurrent level of mean arterial pressure across the eight congruent and eight incongruent blocks of the Stroop task. In the first level of this mixed-effects analysis, each participant’s 16 observed BOLD responses for the eight congruent and eight incongruent task blocks were correlated with 16 canonical hemodynamic response functions that were convolved with the corresponding block levels of mean arterial pressure. This first-level analysis is comparable to a within-participant correlation analysis that examines, with a single design matrix, the voxel-wise relationships between the 16 estimates of BOLD response amplitudes for each congruent and incongruent block and the 16 corresponding measurements of mean arterial pressure. This approach is also comparable to the analytical approach that we used in a prior report that examined the within-person correlations between positron emission tomography estimates of rCBF and heart period and heart period variability across a series of working memory tasks (Gianaros et al., 2004).
In each first-level analysis for each participant, a high-pass filter of 360 s was applied to the T2*-weighted images from each run to remove the effects of scanner drift on BOLD signal intensity; the motion correction parameters that were used for image realignment during preprocessing were also used as covariates to correct for the potential confounding effects of image realignment on BOLD signal intensity. Thus, for each participant, these first-level analyses provided voxel-by-voxel coefficients that reflected the relationship between BOLD response amplitude and the concurrent level of mean arterial pressure across the eight congruent and eight incongruent Stroop task blocks.
In the second level of the mixed-effects analysis, the coefficients for each participant from the above individual (first-level) analyses were aggregated across participants to perform a one-sample t test (df=N [20]−1 = 19). This second level t test tested the null hypothesis that the voxel-by-voxel BOLD response amplitudes did not correlate with mean arterial pressure across the conditions of the Stroop task (i.e., the coefficients did not differ from zero). The False Discovery Rate procedure was used to correct for conducting these voxel-by-voxel tests across the entire brain volume (Genovese, Lazar, & Nichols, 2002). The t statistics from this second level analysis are shown as color-scaled statistical parametric maps in Figure 1. A corresponding figure that illustrates a first-level analysis for a single participant is shown in the left panel of Figure 2. These color-scaled maps indicate brain regions in which greater BOLD response amplitudes (taken as indicators of greater functional neural activation) correlated with a concurrently higher level of mean arterial pressure.
Figure 1.
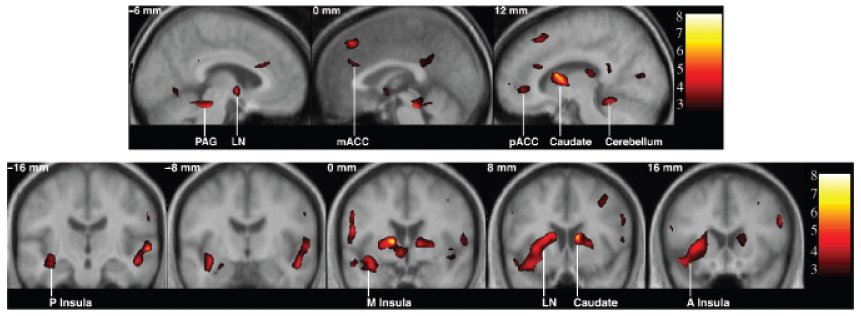
Profiled are brain regions where a greater hemodynamic BOLD response amplitude correlated with a concurrently higher level of mean arterial pressure during a Stroop color-word interference task. Colored regions correspond to t values (scaled to the color bars at right) that were derived from mixed-effects analyses that examined the correlation between BOLD response amplitudes and mean arterial pressure. Coordinate values in the sagittal images at top refer to the relative distance (in millimeters) from the midline (+: right; −: left); values in the coronal images at bottom refer to the relative distance (in millimeters) from the anterior commissure (+: anterior; −: posterior). PAG: periaqueductal gray matter; LN: lenticular nucleus; mACC: mid-anterior cingulate; pACC: perigenual cingulate; P: posterior; M: mid; A: anterior.
Figure 2.
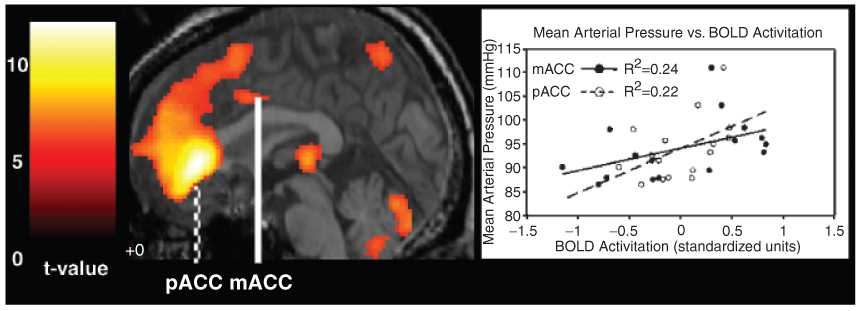
Shown at left are brain regions in which greater hemodynamic BOLD response amplitudes correlated with concurrently greater levels of mean arterial pressure during a Stroop color-word interference task for a single individual. Overlaid on a medial section of this individual’s brain are colored regions that correspond to t values (scaled to the color bar at far left) that were derived from a parametric modulation analysis, which examined the correlation between BOLD response amplitudes and mean arterial pressure. The perigenual (pACC) and mid-anterior (mACC) regions of the cingulate cortex are labeled. Standardized BOLD activation in these two regions are plotted along the x-axis of the scatter plot at right; mean arterial pressure as a function of BOLD activation in these two cingulate regions are plotted along the y-axis.
To assess the average change in mean arterial pressure from the congruent to the incongruent task blocks, the mean arterial pressures that were obtained for each of the eight congruent and eight incongruent blocks were averaged separately and then compared with a 95% confidence interval.
Results
Mean Arterial Pressure Increased during the Incongruent Block of the Stroop Task
Mean arterial pressure increased by an average of 4.82 mmHg from the congruent block (M = 95.36 ± SD = 8.69 mmHg) to the incongruent block (M = 100.18 ± 9.26 mmHg) of the Stroop task (95% confidence interval of the MAP increase = 3.03, 6.62 mmHg). Across the four functional runs, mean arterial pressure showed a similar increase from the congruent to the incongruent blocks, with the increase being smallest for the final run (Table 1). Overall, these results indicate that the performance titrated version of the Stroop color-word interference task evoked an increase in blood pressure from the control (congruent) condition to the demanding (incongruent) condition.
Table 1.
Average Mean Arterial Pressure during Congruent and Incongruent Conditions of a Stroop Color-Word Interference Task
| Functional imaging run |
||||||||
|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
|||||
| Condition |
Condition |
Condition |
Condition |
|||||
| Congruent | Incongruent | Congruent | Incongruent | Congruent | Incongruent | Congruent | Incongruent | |
| MAP (SD) | 99.1 (10.9) | 103.1 (11.8) | 97.5 (11.5) | 101.9 (11.9) | 96.4 (9.7) | 99.7 (12.1) | 96.2 (11.0) | 99.6 (12.4) |
Note: Congruent and incongruent conditions were completed twice during each functional imaging run; mean arterial pressure (MAP) values shown are the average of the two repetitions of each condition for each functional imaging run.
Increased Mean Arterial Pressure Correlated with Greater fMRI BOLD Response Amplitudes
Increased mean arterial pressure during the Stroop task correlated with greater fMRI BOLD response amplitudes in both cortical and subcortical brain regions that are held to regulate cardiovascular reactions to behavioral stressors. Cortical regions included the perigenual and mid-anterior cingulate cortex (Brodmann’s areas 24 and 32), the bilateral anterior and mid-insular cortex, and the medial and bilateral prefrontal cortex (areas 9 and 10), extending into the area of the frontal eye field (area 8). Other cortical regions in which a greater BOLD response amplitude correlated with increased mean arterial pressure included the dorsolateral prefrontal cortex, encompassing areas 44, 45, and 46, the supplementary motor cortex (area 6), and sectors of the temporal (area 22), inferior parietal (areas 39, 40), and occipital cortex (areas 18, 19). Subcortical regions in which greater BOLD response amplitudes correlated with increased mean arterial pressure included two regions of the basal ganglia, the lenticular nucleus and the caudate; the anterior and posterior thalamus; the left periaqueductal grey matter; and the right cerebellum. Listed in Table 2 are brain regions in which greater fMRI BOLD response amplitudes correlated with increased mean arterial pressure during the Stroop task at a brain-volume corrected level of statistical significance; profiled in Figure 1 are a representative subset of these regions.
Table 2.
Brain Regions in Which a Greater Hemodynamic BOLD Response Amplitude Correlated with a Concurrently Greater Level of Mean Arterial Pressure during a Stroop Color-Word Interference Task
| Brodmann area | Coordinates |
Number of voxels in region | ||||||
|---|---|---|---|---|---|---|---|---|
| Side | Region | X | Y | Z | t Value | p Value | ||
| R | Anterior cingulate | 32 | 8 | 28 | 24 | 15 | 4.11 | 0.04 |
| L | Anterior cingulate | 32 | −8 | 26 | 24 | 7 | 4.10 | 0.04 |
| R | Perigenual cingulate | 32/24 | 12 | 44 | −2 | 6 | 3.72 | 0.04 |
| L | Anterior inferior insula | −48 | 12 | −12 | 9 | 4.98 | 0.03 | |
| R | Mid-insula | 54 | 4 | 10 | 9 | 3.88 | 0.04 | |
| R | Lateral prefrontal cortex | 10 | 26 | 56 | 2 | 18 | 5.22 | 0.03 |
| L | — | 9, 10 | −30 | 48 | 28 | 40 | 4.91 | 0.03 |
| R | — | 9 | 52 | 20 | 30 | 59 | 5.38 | 0.02 |
| R | Frontal eye fields | 8 | 6 | 28 | 48 | 143 | 5.13 | 0.03 |
| L | Dorsolateral prefrontal cortex | 44, 45, 46 | −44 | 36 | 12 | 103 | 5.13 | 0.03 |
| L | Inferior parietal cortex | 39, 40 | −56 | −46 | 42 | 18 | 5.08 | 0.03 |
| R | — | 39, 40 | 54 | −38 | 44 | 22 | 4.91 | 0.03 |
| L | Premotor/supplementary motor cortex | 6 | −52 | 0 | 18 | 53 | 4.95 | 0.03 |
| R | Temporal cortex | 22 | 60 | −16 | 0 | 182 | 6.31 | 0.02 |
| R | Occipital cortex | 19 | 50 | −74 | 16 | 99 | 6.64 | 0.02 |
| R | — | 18, 19 | 6 | −80 | 18 | 30 | 4.17 | 0.04 |
| L | — | 18, 19 | −18 | −68 | −4 | 228 | 7.38 | 0.01 |
| L | Anterior thalamus | −14 | 0 | 8 | 22 | 8.05 | 0.01 | |
| R | Posterior thalamus | 16 | −24 | 16 | 63 | 4.95 | 0.03 | |
| R | Caudate | 12 | 10 | 14 | 150 | 6.98 | 0.01 | |
| R | Lenticular nucleus | 24 | 4 | 4 | 150 | 4.10 | 0.04 | |
| R | Cerebellum | 28 | −42 | −12 | 265 | 4.98 | 0.03 | |
| L | Periaqueductal gray matter | −2 | −32 | −20 | 265 | 6.42 | 0.02 | |
Note: Listed next to left (L) and right (R) brain regions are approximate Brodmann areas, the Montreal Neurological Institute X, Y, and Z coordinates for the voxel within a region that had the highest t score from mixed-effects analyses, the total number of voxels that defined a region, and the False Discovery Rate corrected p value for the listed voxel.
For an individual participant, Figure 2 illustrates the relationship between mean arterial pressure and BOLD activation in the perigenual and mid-anterior regions of the cingulate cortex. For this figure, we extracted the uncorrected BOLD signal time series for each of the congruent and incongruent task conditions for all voxels within a 5-mm radius of the coordinates for the areas of peak correlation between mean arterial pressure and BOLD activation in the periguenial and mid-anterior cingulate cortex. Each time series was then normalized, such that each data point in the time series was mean centered for each region of interest and then standardized by dividing the mean of the centered values by their average standard deviation across the four functional runs. After normalization, the BOLD time series in the eight congruent and eight incongruent task blocks were averaged to obtain 16 mean values of BOLD activation. These 16 mean BOLD values were then correlated with the 16 corresponding measurements of mean arterial pressure for each individual. Across individuals, BOLD activation in these two regions of the cingulate cortex accounted for a moderate percentage of the variance in mean arterial pressure across the Stroop task conditions (median perigenual cingulate r² = .069, range = .0008 − .552; median mid-anterior cingulate r² = .098, range = .0005 − .418).
In supplementary analyses, we did not observe negative correlations between BOLD activation and mean arterial pressure at corrected or uncorrected levels of voxel-wise statistical significance. These null results indicate that lesser regional BOLD activity did not correlate with greater mean arterial pressure in this version of the Stroop task. Further, we did not observe gender differences in second-level analyses in the strength of the correlation between mean arterial pressure and BOLD activation at corrected or uncorrected levels of statistical significance.
Discussion
The present study demonstrates that increased mean arterial pressure to a modified version of a Stroop color-word interference task correlates with greater fMRI BOLD response amplitudes in a set of cortical and subcortical brain regions that are held to regulate cardiovascular reactions to behavioral stressors. These particular regions included the perigenual and mid-anterior cingulate cortex (Brodmann’s areas 24 and 32), medial and lateral regions of the prefrontal cortex (areas 8, 9, 10), the insula, periaqueductal gray matter, and the cerebellum. Increased mean arterial pressure also correlated with greater BOLD response amplitudes in sensory, motor, and multimodal association areas of the cortex, in two areas of the basal ganglia (caudate and lenticular nucleus), and in the anterior and posterior thalamus (Table 1; Figure 1). These cortical and subcortical brain regions in which BOLD response amplitude correlated with increased mean arterial pressure during the Stroop task overlap considerably with those reported in a prior positron emission tomography study, which also examined the correlation between mean arterial pressure and functional neural activation during two different behavioral stressors (Critchley et al., 2000). These regions of correlation also overlap with those reported in a number of other functional neuroimaging studies that have examined the correlation between other indicators of cardiovascular and cardiac autonomic activity, such as changes in heart rate and heart rate variability, and stressor-evoked and emotion-related functional neural activation (Critchley, Mathias, et al., 2003; Gianaros et al., 2004; Lane et al., 2001). Collectively, the results of these prior studies and those of the present study thus provide a converging characterization of the cortical and subcortical brain systems that regulate (initiate and represent) cardiovascular reactions to behavioral stressors in humans.
It has long been hypothesized that cortical brain systems initiate and represent blood pressure and other cardiovascular reactions to behavioral stressors that are widely used in human psychophysiological research (the “cortical irradiation hypothesis” by Obrist, 1981, is one example). In many earlier psychophysiological models of cardiovascular reactivity, however, the particular cortical brain systems that were hypothesized to regulate cardiovascular reactions to behavioral stressors were largely unspecified or they were extrapolated from invasive work in nonhuman animals. A more recent conceptual framework (Critchley, Mathias, et al., 2003) holds that one particular cortical brain system, the cingulate cortex, plays a key role in calibrating blood pressure and other cardiovascular reactions with contextually adaptive behavior to meet the metabolic demands of cognitive and emotional challenges. This framework is supported by human functional neuroimaging studies that have shown that behaviorally evoked changes in cardiovascular (e.g., heart rate) and cardiac autonomic (e.g., heart rate variability) activity are correlated with concurrent changes in the functional neural activation of several regions within the cingulate, including the perigenual and mid-cingulate regions (Critchley, Mathias, et al., 2003; Gianaros et al., 2004; Lane et al., 2001). Moreover, there is evidence that patients with circumscribed lesions to the anterior cingulate cortex show blunted cardiovascular reactions to behavioral stressors, such as the Stroop task (Critchley, Mathias, et al., 2003). The present results that Stroop stressor-evoked increases in mean arterial pressure correlated with greater activation in the perigenual and mid-anterior cingulate cortex are thus in line with prior research and with the proposed role of the cingulate cortex in regulating cardiovascular reactivity.
The cingulate cortex has also been proposed to initiate and represent stressor-evoked cardiovascular reactions through its reciprocal connections with other brain systems that play a primary role in representing and relaying afferent visceral information (insula, thalamus), in generating contextually appropriate motor responses (motor cortex, basal ganglia, and cerebellum), in appraising an event’s motivational salience and meaning to guide adaptive behavior (ventromedial and orbitofrontal cortex), and in performing the cognitive operations that are necessary to processes and to meet the demands of a given cognitive or emotional challenge (lateral prefrontal and parietal cortices; for reviews, see Barbas, 2000; Benarroch, 1997; Ongur, Ferry, & Price, 2003; Thayer & Lane, 2000; Verbene & Owens, 1998). These propositions are broadly consistent with the present findings that in addition to correlating with greater activation in the cingulate cortex, stressor-evoked increases in mean arterial pressure also correlated with greater BOLD response amplitudes in the dorsolateral prefrontal, inferior parietal, temporal, and occipital cortices and in insular, thalamic, basal ganglia, and cerebellar regions—regions that support a range of cognitive, motor, and multimodal sensory processes that were likely engaged by the Stroop task. It should be noted, however, that although these patterns of correlation are consistent with the presumptive role of the cingulate cortex and these brain systems in regulating cardiovascular reactivity and although they are consistent with similar patterns of correlation that have been reported in prior functional neuroimaging studies, these patterns of correlation underscore a primary limitation of the present study.
Namely, the correlational results of this descriptive study do not permit us to dissociate the patterns of functional neural activation that uniquely reflected the efferent initiation from the afferent representation of increased mean arterial pressure. Future studies that are designed to resolve the temporal onset of a stressor-evoked fMRI BOLD response in relation to the onset of an increase in blood pressure may be better able to dissociate those brain systems that initiate descending commands for increases in mean arterial pressure from those that represent the afferent information provided by increases in mean arterial pressure. It should also be noted that our imaging parameters limited our ability to spatially localize BOLD activation in smaller brain regions or subnuclei within brain regions that regulate mean arterial pressure, such as the hypothalamus and brain stem. This limitation could be addressed in future studies by using slice acquisitions that are thinner than 3.2 mm, thus allowing for a more fine-grained spatial characterization of the relationship between functional neural activation and cardiovascular reactivity.
Another limitation of the present study was that an increase in mean arterial pressure was taken as an indicator of cardiovascular reactivity. This was done to follow the methods used in the one prior positron emission tomography study of blood pressure reactivity. Increases in mean arterial pressure are the aggregate product of underlying changes in cardiac output and vascular resistance (Obrist, 1981). It is well known that individuals may show equivalent increases in mean arterial pressure despite showing differences in their underlying changes in cardiac output and vasoconstriction. Moreover, these underlying changes in stressor-evoked cardiac and vascular reactions may vary as a function of whether an individual appraises a stressor as a threat or a challenge—appraisals that were not assessed in the present study (Quigley, Feldman, & Weinstein, 2002; Tomaka, Blascovich, Kibler, & Ernst, 1997). Thus, new questions for future research are whether different patterns of functional neural activation correlate with the underlying cardiac and vascular components that comprise such aggregate endpoints as increased blood pressure and whether such functional neural activation patterns correlate with an individual’s appraisal of a stressor.
It should also be noted that the present study used a single task, a performance-titrated Stroop color-word interference task, to elicit both regional brain activation and concurrent changes in mean arterial pressure. This task can be considered as a frustrating and nonspecific stressor that elicits increases in blood pressure, in part, because it provides negative performance feedback, requires continuous task engagement, and makes sustained demands on attention, working memory, the cognitive control over resolving stimulus and response conflicts (Critchley, Tang, Glaser, Butterworth, & Dolan, 2005). These cognitive processes are known to engage such brain systems as the cingulate cortex, dorsolateral prefrontal cortex, parietal cortex, and other cortical and subcortical regions that were related to blood pressure reactivity in the present study (for reviews, see Paus, Koski, Caramanos, & Westbury, 1998; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). By only using a single, nonspecific stressor, it is thus difficult to isolate the particular aspects of the Stroop task (e.g., negative performance feedback, mental effort, task engagement, etc.) that played a primary (or unique) role in engaging the regional brain activation that was associated with concurrent changes in mean arterial pressure. Along a similar line, it is also difficult to dissociate which patterns of brain activation were independently related to performing the Stroop task and which were independently related to blood pressure reactivity. This dissociation is difficult because the Stroop task was used to elicit blood pressure reactivity, which necessarily conflates task performance and blood pressure as correlates of brain activation. In addition to more comprehensive assessments of subjective task appraisals, cardiac and vascular reactions, and cardiovascular response timing, future studies should also use a number of different behavioral stressors to elicit regional brain activation and concurrent changes in cardiovascular activity. By using more than a single task, researchers could determine common patterns of regional brain activation that are uniquely associated with changes in cardiovascular activity—regardless the specific task used.
We did not find a correlation between lesser regional BOLD activity and greater mean arterial pressure. One explanation of this null result is that the congruent and incongruent conditions of the Stroop color-word interference task were not compared with a resting baseline or a crosshairs fixation condition—as is common in fMRI studies. For this reason, it is possible that the two conditions of the Stroop task did not elicit relative decreases in functional neural activation (deactivations) that could have been correlated with concurrent changes in mean arterial pressure. Thus, to determine whether lesser regional BOLD activation is correlated with mean arterial pressure, future studies should not only use different experimental tasks, but also comparison conditions, such as resting baselines or fixation conditions, that may elicit relative decreases in BOLD activation.
One question raised by the present study is whether stressor-evoked functional neural activation cortical and subcortical brain systems relates to the risk for cardiovascular disease. Large-magnitude (exaggerated) increases in blood pressure that presumptively exceed the metabolic demands of behavioral stressors are hypothesized to raise the risk for hypertension and atherosclerosis (Krantz & Manuck, 1984). It has also been hypothesized that the cingulate cortex may be one brain system that calibrates the magnitude of blood pressure and other cardiovascular reactions to behavioral stressors in order to provide appropriate metabolic support for contextually adaptive behavior (Critchley, Mathias, et al., 2003). Functional subdivisions of the cingulate cortex, such as the perigenual and mid-anterior regions, may support this calibration processes through their reciprocal connections with multimodal associational, motor, and visceral sensory cortical brain systems and with midbrain and hindbrain cell groups that regulate neuroendocrine and autonomic nervous system control of the cardiovascular system (Ongur et al., 2003; Vogt, Berger, & Derbyshire, 2003; Vogt, Nimchinsky, Vogt, & Hof, 1995). In a previous report on a subset of participants from the present study, we found that those individuals who showed larger-magnitude blood pressure reactions to the Stroop task on two testing occasions also showed greater Stroop task-evoked activation of the posterior cingulate cortex than their lesser blood-pressure reactive counterparts (Gianaros et al., 2005). These preliminary findings suggested that greater posterior cingulate activation to a behavioral stressor could be a functional neural correlate of an individual’s tendency to show exaggerated blood pressure reactivity. In the present study, however, mean arterial pressure did not correlate with functional neural activation of the posterior cingulate cortex. This particular finding is consistent with neuroantomical tracing studies showing that the posterior cingulate cortex has sparse connections with cortical and subcortical brain regions that play a primary role in regulating neuroendocrine and autonomic control over the cardiovascular system (Ongur et al., 2003; Vogt, Absher, & Bush, 2000; Vogt et al., 1995, 2003). The posterior cingulate cortex does, however, have dense reciprocal connections with the perigenual and mid-anterior divisions of the cingulate cortex and with medial temporal regions (Vogt, Vogt, & Laureys, in press). Thus, it is possible that stressor-evoked activation patterns of the posterior cingulate cortex may indirectly modulate cardiovascular reactivity or that they may be related to another functional dimension of individual differences in cardiovascular reactivity, such as vigilance for threat (Gianaros et al., 2005). Further neuroimaging studies that experimentally manipulate such functional processes are thus needed to delineate the role of the cingulate cortex in the central regulation of cardiovascular reactivity to behavioral stressors.
To summarize, the experimental approach taken in the present study (modifying a standardized cardiovascular reactivity task for a functional neuroimaging design) allowed us to further characterize the cortical and subcortical brain regions that may initiate and represent blood pressure reactions to behavioral stressors in humans. Whether stressor-evoked patterns of functional neural activation in these brain systems relate to specific functional processes of blood pressure reactivity (initiation or representation), to individual differences in underlying patterns of cardiac and vascular reactivity, to threat and challenge appraisal processes, or to cardiovascular health outcomes are questions that should be addressed in future research.
Acknowledgments
We thank V. Andrew Stenger, who programmed the reverse spiral pulse sequence for fMRI data acquisition; Michael J. Eddy, who helped to design, develop, and implement the Stroop color-word interference task; and Hugo D. Critchley, who commented on a draft of the manuscript. This research was supported by the National Institutes of Health Pittsburgh Mind-Body Center (NHLBI 65111 and 65112), by NHLBI-NRSA 1-F32-71333-01, and by NIMH 1-K01-070616-01.
REFERENCES
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin. 2000;52:319. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Central autonomic network: Functional organization and clinical correlations. Armonk, NY: Futura Publishing Company; 1997. [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The mechanism of emotional disturbance of bodily functions. New England Journal of Medicine. 1928;198:877–884. [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. Journal of Physiology. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Good CD, Ashburner J, Frackowiak RS, Mathias CJ, Dolan RJ. Changes in cerebral morphology consequent to peripheral autonomic denervation. NeuroImage. 2003;18:908–916. doi: 10.1016/s1053-8119(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;26:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Debski TT, Kamarck TW, Jennings JR, Young LW, Eddy MJ, Zhang YX. A computerized test battery for the assessment of cardiovascular reactivity. International Journal of Biomedical Computing. 1991;27:277–289. doi: 10.1016/0020-7101(91)90068-p. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Bleil ME, Muldoon MF, Jennings JR, Sutton-Tyrrell K, McCaffery JM, et al. Is cardiovascular reactivity associated with atherosclerosis among hypertensives? Hypertension. 2002;40:742–747. doi: 10.1161/01.hyp.0000035707.57492.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, May JC, Siegle GJ, Jennings JR. Is there a functional neural correlate of individual differences in cardiovascular reactivity? Psychosomatic Medicine. 2005;67:31–39. doi: 10.1097/01.psy.0000151487.05506.dc. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Everson SA, Kaplan GA, Manuck SB, Jennings JR, Salonen R. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men: Findings from the Kuopio Ischemic Heart Disease Study. Circulation. 1997;96:3842–3848. doi: 10.1161/01.cir.96.11.3842. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy MJ. Reliable measures of behaviorally-evoked cardiovascular reactivity from a PC-based test battery: Results from student and community samples. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodologic critique. Psychological Bulletin. 1984;96:435–464. [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Thayer JF. Activity in the medial prefrontal cortex correlates with vagal component of heart rate variability. Brain and Cognition. 2001;47:97–100. [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological reactivity: Mechanisms and pathways to cardiovascular disease. Psychosomatic Medicine. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular psychophysiology: A perspective. New York: Plenum Press; 1981. [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage. 2004;22:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: A review of 107 pet activation studies. NeuroReport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- Quigley KS, Feldman LB, Weinstein S. Cardiovascular patterns associated with threat and challenge appraisals: A within-subjects analysis. Psychophysiology. 2002;39:292–302. doi: 10.1017/s0048577201393046. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Soufer R, Arrighi JA, Burg MM. Brain, behavior, mental stress, and the neurocardiac interaction. Journal of Nuclear Cardiology. 2002;9:650. doi: 10.1067/mnc.2002.129884. [DOI] [PubMed] [Google Scholar]
- Stenger V, Boada F, Noll D. Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T2*-weighted functional MRI. Magnetic Resonance Medicine. 2000;44:525–531. doi: 10.1002/1522-2594(200010)44:4<525::aid-mrm5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kibler J, Ernst JM. Cognitive and physiological antecedents of threat and challenge appraisal. Journal of Personality & Social Psychology. 1997;73:63–72. doi: 10.1037//0022-3514.73.1.63. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Verbene AJM, Owens NC. Cortical modulation of the cardiovascular system. Progress in Neurobiology. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Absher JR, Bush G. Human retrosplenial cortex: Where is it and is it involved in emotion? Trends in Neuroscience. 2000;23:195–197. doi: 10.1016/s0166-2236(00)01579-4. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. Journal of Comparitive Neurology. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. doi: 10.1016/j.neuroimage.2005.07.048. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


