Abstract
We have previously shown that canine signaling lymphocyte activation molecule (SLAM; also known as CD150) acts as a cellular receptor for canine distemper virus (CDV). In this study, we established Vero cells stably expressing canine SLAM (Vero.DogSLAMtag cells). Viruses were isolated in Vero.DogSLAMtag cells one day after inoculation with spleen samples from five out of seven dogs with distemper. By contrast, virus isolation with reportedly sensitive marmoset B95a cells was only successful from three diseased animals at 7 to 10 days after inoculation, and no virus was recovered from any dogs when Vero cells were used for isolation. The CDV strain isolated in Vero.DogSLAMtag cells did not cause cytopathic effects in B95a and human SLAM-expressing Vero cells, whereas the strain isolated in B95a cells from the same dog did so in canine or human SLAM-expressing Vero cells as well as B95a cells. There were two amino acid differences in the hemagglutinin sequence between these strains. Cell fusion analysis after expression of envelope proteins and vesicular stomatitis virus pseudotype assay showed that their hemagglutinins were responsible for the difference in cell tropism between them. Site-directed mutagenesis indicated that glutamic acid to lysine substitution at position 530 of the hemagglutinin was required for the adaptation to the usage of marmoset SLAM. Our results indicate that Vero cells stably expressing canine SLAM are highly sensitive to CDV in clinical specimens and that only a single amino acid substitution in the hemagglutinin can allow the virus to adapt to marmoset SLAM.
Canine distemper virus (CDV) is a member of the Morbillivirus genus in the family Paramyxoviridae, which also includes measles virus (MV), rinderpest virus, peste des petits ruminants virus, and emerging viruses of aquatic mammals (phocine, dolphin, and porpoise distemper viruses) (12, 22, 41). Morbilliviruses are enveloped viruses with nonsegmented negative-strand RNA genomes. There are two envelope glycoproteins, the hemagglutinin (H) and fusion (F) protein, mediating receptor binding and membrane fusion, respectively. Morbilliviruses are highly contagious pathogens that cause devastating diseases in humans and animals, producing fever, coryza, conjunctivitis, gastroenteritis, and pneumonia in their respective natural hosts. The major sites of viral propagation are lymphoid tissues, and acute diseases are usually accompanied by profound lymphopenia and immunosuppression, leading to secondary infections (12, 22).
In addition to these manifestations, CDV often affects the central nervous system of the host, unlike MV and rinderpest virus (39). Dogs and many other species of the order Carnivora are susceptible to CDV infection, and the mortality rates vary greatly among species. In recent years, CDV has also emerged as a significant pathogen of new host species, causing outbreaks in lions in the Serengeti National Park, Tanzania, in leopards and other large cats in zoos, and in seals in Lake Baikal, Russia (3, 40).
CDV is usually isolated by cocultivation of lymphocytes from suspect dogs with mitogen-stimulated dog lymphocytes (2). Field isolates of CDV are also reported to replicate in dog and ferret macrophages (5, 28). Cell lines such as Vero (African green monkey kidney) cells do not allow the propagation of field isolates, whereas cell culture-adapted CDV strains such as the Onderstepoort vaccine strain are able to replicate in many cell lines (4, 13, 29). It is known that virulence for the natural host may be lost when CDV is adapted to cell culture (14, 20). Recently, the marmoset B-cell line B95a was shown to be a good host for CDV isolation (18).
Cellular receptors are one of the major determinants of the host range and tissue tropism of a virus. We and others have previously shown that human signaling lymphocyte activation molecule (SLAM; also known as CD150), a membrane glycoprotein expressed on cells of the immune system (9, 31), is a cellular receptor for MV (11, 17, 25, 35, 43). The common tropism and pathology of morbilliviruses prompted us to examine whether SLAM also acts as receptors for morbilliviruses other than MV, and we demonstrated that it is indeed the case, at least for several strains of CDV and rinderpest virus (36, 37). Vaccine strains of CDV, which had been passaged on SLAM-negative cells, were found to use an alternative receptor(s) besides canine SLAM, probably because of in vitro adaptation (36).
In this study, we show that canine SLAM-expressing Vero cells are highly sensitive to wild strains of CDV, much more so than B95a cells. Viruses were readily isolated in canine SLAM-expressing Vero cells from the majority of clinical samples as early as one day after inoculation, whereas the isolation rate was lower and 7 to 10 days were required for successful isolation in B95a cells. Thus, it is likely that by using canine SLAM-expressing Vero cells, we are able to recover viruses more representative of those replicating in vivo without selection and/or adaptation. Furthermore, we found that a single amino acid substitution in the H protein, the receptor-binding envelope protein of CDV, conferred on the virus the ability to use marmoset SLAM as a cellular receptor. These results indicate that the cell line we established is a desirable host for CDV isolation and support the contention that canine SLAM is the principal cellular receptor for CDV in vivo.
MATERIALS AND METHODS
Cells.
Vero, B95a, and 293T cells were grown as previously described elsewhere (34). The Vero cell clone stably expressing canine SLAM (Vero.DogSLAMtag) was generated by transfecting Vero cells with pCXN2 (23) and pCAGDogSLAMtag (36). It was grown in Dulbecco modified Eagle medium supplemented with 7% heat-inactivated fetal bovine serum, 0.15% sodium carbonate, and 0.4 mg of G418 per ml. CHO.Neo, CHO.B95aSLAM and CHO.DogSLAMtag are CHO cell clones stably expressing Neo, marmoset SLAM and canine SLAM, respectively (36). CHO cell clones were grown in RPMI 1640 medium supplemented with 7% heat-inactivated fetal bovine serum, 0.15% sodium carbonate, and 0.5 mg of G418 per ml.
Virus isolation and infection.
Single cell suspensions were prepared from spleens (about 0.5 g each) of autopsied dogs with distemper and sonicated in 10 ml of RPMI 1640 medium. The solutions were clarified by centrifugation. Vero.DogSLAMtag, B95a, and Vero cells were plated in 24-well plates, and infected with sonicated spleen samples. Viruses isolated in Vero.DogSLAMtag and B95a cells inoculated with the sample of dog 5 were named the 5VD and 5B strains, respectively. The 5VD strain was passaged in Vero.DogSLAMtag cells, and the 5B strain was passaged in B95a cells. Vero.DogSLAMtag, B95a, and Vero cells were plated in 24-well plates and were infected with the 5VD and 5B strains.
Immunofluorescence staining.
Vero cell clones were stained with mouse anti-influenza virus H monoclonal antibody 12CA5 (Boehringer Mannheim), and then with fluorescein isothiocyanate (FITC)-labeled secondary antibody. The stained cells were analyzed on a FACScan machine (Becton Dickinson). Vero.DogSLAMtag cells inoculated with viral samples were fixed with acetone-methanol (1:1) and stained with mouse anti-CDV N protein monoclonal antibody (VMRD Inc.) and then with FITC-labeled secondary antibody. The stained cells were observed under a fluorescence microscope.
Molecular cloning of CDV H protein cDNAs.
Total RNA was extracted from infected cells (Vero.DogSLAMtag and B95a cells infected with the 5VD and 5B strains, respectively) at 48 h after infection and used for reverse transcription (RT) with oligo(dT) primer. We then performed PCR using the primers 5′-TTGGTACCAACTTAGGGCTCAGGTAGTCC-3′ and 5′-TTTAGCATGCTGGAGATGGTTTAATTCAATCG-3′ (restriction sites are underlined) (36) and KOD-Plus polymerase (TOYOBO Biochemicals). H protein cDNAs of the 5VD and 5B strains were subcloned into the eukaryotic expression vector pCAGGS (23), and the resulting constructs were named pCAG5VDH and pCAG5BH, respectively. The nucleotide sequences of the H protein cDNAs were determined. Site-directed mutagenesis was performed by using gene splicing by overlap extension with primers in which desired mutations were introduced, as described in detail elsewhere (26, 38). The mutant constructs were verified by DNA sequencing.
Transfection.
CHO cell clones were plated in 24-well plates, and transfected with expression plasmids encoding CDV envelope proteins using Lipofectamine 2000 reagent (Invitrogen). Development of cytopathic effect (CPE) in transfected cells was observed under a microscope.
Pseudotype assay.
The vesicular stomatitis virus (VSV) pseudotypes were prepared by infecting with VSVΔ*G-G (bearing the VSV envelope G protein) 293T cells which had been transfected with appropriate expression plasmids encoding envelope proteins using Lipofectamine 2000 reagent, as previously described (33, 34, 36). To prepare VSVΔG*-5VDHF, VSVΔG*-5BHF, VSVΔG*-5VDH530F, and VSVΔG*-5VDH548F, 293T cells were transfected with pCAG5VDH, pCAG5BH, pCAG5VDH530, and pCAG5VDH548, respectively, together with pCAGOPF (36). CHO cell clones were plated in 96-well plate and infected with 100 μl of serially diluted solutions of each pseudotype stock. Infectious titers of the pseudotypes were determined by counting the number of green fluorescent protein (GFP)-expressing cells and expressed as infectious units per milliliter.
Nucleotide sequence accession number.
The GenBank accession numbers for the H protein sequences of the 5VD and 5B strains are AY297454 and AY297453, respectively.
RESULTS
Establishment of a Vero cell clone stably expressing canine SLAM.
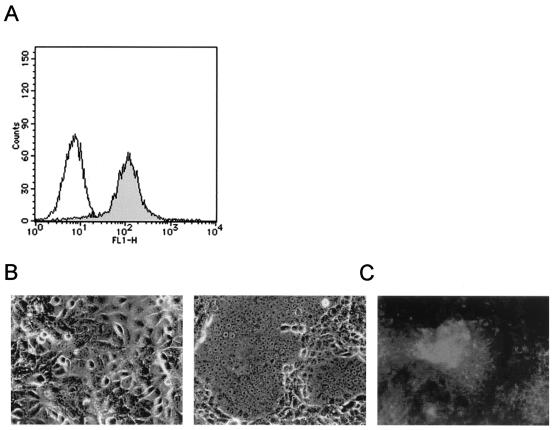
We previously constructed the expression plasmid encoding the membrane-bound form of canine SLAM fused to the influenza virus H tag at the N terminus (pCAGDogSLAMtag) (36). Although the H tag is located at the ectodomain of the molecule, our previous study demonstrated that it does not interfere with the binding of CDV H proteins (36). Vero cells were transfected with pCAGDogSLAMtag plus pCXN2 containing the neo gene and selected in the presence of G418. The resulting stable clones were examined by immunofluorescence staining with anti-H epitope monoclonal antibody (Fig. 1A). We selected and used the clone expressing the highest level of canine SLAM (Vero.DogSLAMtag) in the following experiments.
FIG. 1.
CDV isolation in canine SLAM-expressing Vero cells. (A) Vero.DogSLAMtag cells were stained with anti-influenza virus H epitope monoclonal antibody (solid profile) or mouse control IgG (empty profile), followed by staining with FITC-labeled anti-mouse IgG. (B) Vero.DogSLAMtag cells were inoculated with spleen sample from a dog diagnosed as having canine distemper (right) or left uninoculated (left) and were then observed at 24 h after inoculation. (C) Vero.DogSLAMtag cells infected with isolated virus were stained with anti-CDV N protein monoclonal antibody, followed by staining with FITC-labeled anti-mouse IgG. The stained cells were observed under a fluorescence microscope. Uninfected cells treated in the same way did not show any fluorescence.
Isolation of CDV from dogs with distemper.
Virus isolation was attempted from autopsy samples of seven dogs clinically diagnosed as having canine distemper. Vero.DogSLAMtag, B95a, and Vero cells were inoculated with spleen cells from these dogs and observed daily for the development of CPE (Table 1). Within 24 h after inoculation, samples from five dogs caused CPE in Vero.DogSLAMtag cells, which was characterized by syncytium formation (Fig. 1B). Samples from three out of these five dogs also caused CPE in B95a cells, although CPE became recognizable only at day 7 or later. No samples tested caused CPE in Vero cells during 21 days of the observation period. For all samples that caused CPE, the presence of CDV was confirmed by staining cells with anti CDV N protein monoclonal antibody (Fig. 1C).
TABLE 1.
Isolation of CDV in Vero.DogSLAMtag, B95a, and Vero cells
| Dog no. | Age (mo) | Virus isolationa
|
||
|---|---|---|---|---|
| Vero.DogSLAMtag | B95a | Vero | ||
| 1 | 2 | 24 h | —b | — |
| 2 | 3 | 24 h | — | — |
| 3 | 2 | — | — | — |
| 4 | 3 | 24 h | 7 days | — |
| 5 | 2 | 24 h | 10 days | — |
| 6 | 3 | — | — | — |
| 7 | 3 | 24 h | 7 days | — |
The time when CPE was first detected is shown.
—, unsuccessful virus isolation during 21 days of the observation period after inoculation.
Difference in cell tropism between viruses isolated in Vero.DogSLAMtag and B95a cells.
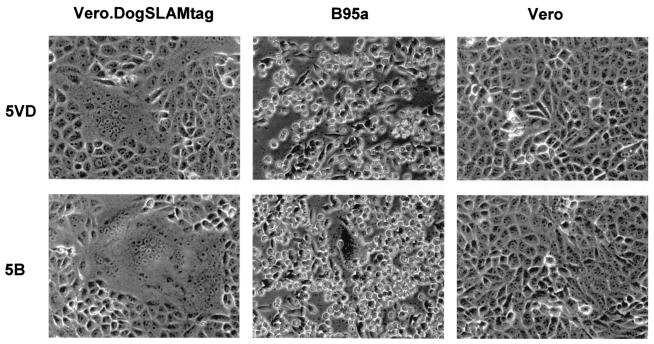
We examined cell tropism of viruses isolated in Vero.DogSLAMtag cells (5VD strain) and B95a cells (5B strain) from dog 5 (Table 1). Vero.DogSLAMtag, B95a, and Vero cells were infected with the 5VD and 5B strains (Fig. 2). We used the 5VD strain passaged once in Vero.DogSLAMtag cells and the 5B strain passaged twice in B95a cells after isolation. The 5VD strain produced syncytia in Vero.DogSLAMtag cells but not in B95a cells at 24 h after infection. By contrast, the 5B strain caused CPE both in Vero.DogSLAMtag cells and in B95a cells at 24 h after infection. Further, the 5B strain, but not the 5VD strain, caused syncytium formation in human SLAM-expressing Vero cells (data not shown). No CPE was found in Vero cells infected with either strain. Longer incubation (up to 96 h) did not affect the presence or absence of CPE in these cells, although the observed CPE became stronger. Thus, CDV strains isolated in Vero.DogSLAMtag and B95a cells from the same dog exhibited different abilities to cause CPE in different cell lines.
FIG. 2.
Infection of cells with the 5VD and 5B strains of CDV. Vero.DogSLAMtag, B95a, and Vero cells were infected with the indicated CDV strains isolated from dog 5. The cells were observed at 24 h after infection.
Properties of H proteins of CDV strains isolated in Vero.DogSLAMtag and B95a cells.
We isolated cDNA clones encoding the H proteins of the 5VD and 5B strains by using the RT-PCR. There were two amino acid differences (at positions 530 and 548) in the predicted H protein sequences between the two strains (Table 2). Analysis with databases in GenBank showed that the H proteins of the 5VD and 5B strains had 99% identities at the amino acid level to those of recent CDV isolates (HM-3 and 98-002 strains) (16) and 90% identity to that of the Onderstepoort vaccine strain.
TABLE 2.
Amino acid differences in the H protein between CDV strains
| Position | Amino acid in strain
|
||
|---|---|---|---|
| 5VD | 5B | Onderstepoort | |
| 21 | S | —a | T |
| 30 | Q | — | H |
| 56 | A | — | T |
| 61 | R | — | H |
| 68 | V | — | M |
| 71 | G | — | S |
| 76 | D | — | E |
| 78 | L | — | M |
| 89 | M | — | I |
| 146 | I | — | V |
| 155 | D | — | E |
| 156 | A | — | S |
| 159 | V | — | I |
| 162 | S | — | A |
| 180 | G | — | S |
| 186 | Y | — | H |
| 197 | R | — | K |
| 212 | K | — | R |
| 218 | S | — | N |
| 238 | Y | — | D |
| 247 | K | — | E |
| 298 | D | — | E |
| 309 | N | — | S |
| 314 | S | — | G |
| 324 | G | — | W |
| 329 | N | — | D |
| 330 | Q | — | H |
| 331 | V | — | I |
| 342 | V | — | M |
| 344 | R | — | K |
| 358 | V | — | I |
| 367 | V | — | A |
| 370 | Q | — | K |
| 373 | G | — | E |
| 376 | N | — | G |
| 386 | S | — | T |
| 401 | V | — | R |
| 417 | I | — | V |
| 442 | N | — | S |
| 446 | D | — | N |
| 460 | L | — | V |
| 467 | S | — | G |
| 475 | T | — | L |
| 484 | R | — | W |
| 500 | M | — | I |
| 502 | K | — | R |
| 506 | T | — | I |
| 510 | L | — | I |
| 517 | N | — | S |
| 522 | V | — | I |
| 530 | E | K | S |
| 531 | N | — | D |
| 548 | T | M | T |
| 549 | Y | — | H |
| 572 | D | — | N |
| 583 | S | — | A |
| 586 | T | — | A |
| 594 | D | — | N |
| 605b | S | — | |
| 606 | K | — | |
| 607 | P | — | |
—, identity with the corresponding amino acid residue in the 5VD strain.
The three residues at the C terminus (positions 605 to 607) are missing in the H protein of the Onderstepoort strain.
The H and F proteins of CDV can cause cell fusion when expressed together in susceptible cell lines (32, 42). We prepared expression plasmids encoding the H protein of the 5VD strain (pCAG5VDH) and that of the 5B strain (pCAG5BH). CHO cells expressing the neo gene product (CHO.Neo), canine SLAM (CHO.DogSLAMtag) or marmoset SLAM (CHO.B95aSLAM) were transfected with pCAG5VDH or pCAG5BH plus pCAGOPF expressing the F protein of the Onderstepoort strain (Fig. 3, top 2 rows). Transfection with pCAG5VDH plus pCAGOPF induced syncytium formation in CHO.DogSLAMtag cells but not in CHO.Neo and CHO.B95aSLAM cells, whereas transfection with pCAG5BH plus pCAGOPF caused CPE in both CHO.DogSLAMtag and CHO.B95aSLAM cells but not in CHO.Neo cells. These results were consistent with the developments of CPE that the parental CDV strains (from which the respective H protein genes were derived) exhibited in cell lines expressing canine SLAM, marmoset SLAM or no receptor molecule.
FIG. 3.
Syncytium formation in CHO cell clones after transfection with expression plasmids encoding CDV envelope proteins. CHO.Neo, CHO.DogSLAMtag and CHO.B95aSLAM cells were transfected with pCAG5VDH, pCAG5BH, pCAG5VDH530 or pCAG5VDH548, together with pCAGOPF. The transfected cells were observed at 24 h after transfection.
To further confirm that the abilities of the respective H proteins to interact with cellular receptors determine cell tropism of these CDV strains, we used the VSV pseudotype system in which the H protein of each of the 5VD and 5B strains and the common F protein (of the Onderstepoort strain) were expressed on the surface of the virion in the absence the VSV G envelope protein (33, 36). The infectivity titer of the VSV pseudotype bearing the H protein of the 5VD strain and the F protein of the Onderstepoort strain (VSVΔG*-5VDHF) was more than 10,000 times higher on CHO.DogSLAMtag cells than that on CHO.Neo cells, but its infectivity titer on CHO.B95aSLAM cells was not higher than that on CHO.Neo cells (Fig. 4A). On the other hand, VSV pseudotype bearing the H protein of the 5B strain and the F protein of the Onderstepoort strain (VSVΔG*-5BHF) was more than 1,000 times higher on CHO.DogSLAMtag cells and about 100 times higher on CHO.B95a cells than that on CHO.Neo cells. Infectivity titers of VSV pseudotypes bearing the VSV G protein (VSVΔG*-G), the H and F proteins of the MV Edmonston strain (VSVΔG*-EdHF) and no envelope protein (VSVΔG*) were also shown as controls in Fig. 4.
FIG. 4.
Infectivities of pseudotype viruses on CHO cell clones. The indicated CHO cell clones were infected with VSVΔG*-G, VSVΔG*-EdHF, VSVΔG*-5VDHF, VSVΔG*-5BHF, or VSVΔG* (A) or with VSVΔG*-EdHF, VSVΔG*-5VDH530F, VSVΔG*-5VDH548F, or VSVΔG* (B), and infectious titers were measured by counting the number of GFP-expressing cells.
Taken together, these results indicate that the 5VD strain can use canine SLAM but not marmoset SLAM as a cellular receptor, whereas the 5B strain can use both canine SLAM and marmoset SLAM as receptors, and that their H proteins are responsible for the difference in receptor usage.
Amino acid residue responsible for the adaptation to marmoset SLAM.
In order to determine which amino acid residue(s) is responsible for the adaptation to the use of marmoset SLAM as a cellular receptor, we performed site-directed mutagenesis of the H gene of the 5VD strain, constructing expression plasmids (pCAG5VDH530 and pCAG5VDH548) encoding the H proteins that had glutamic acid to lysine substitution at position 530 and threonine to methionine substitution at position 548, respectively. CHO cell clones were transfected with expression plasmids encoding the F and mutant H proteins (Fig. 3, bottom 2 rows). Transfection with pCAG5VDH530 plus pCAGOPF induced syncytium formation in CHO.DogSLAMtag and CHO.B95aSLAM cells, whereas transfection with pCAG5VDH548 plus pCAGOPF did so in CHO.DogSLAMtag cells but not in CHO.B95aSLAM cells. The pseudotype VSVΔG*-5VDH530F bearing the Onderstepoort F protein and the 5VD H protein whose residue at position 530 was mutated had about 40 times higher infectivity titer on CHO.B95aSLAM cells than that on CHO.Neo cells (Fig. 4B). On the other hand, the pseudotype VSVΔG*-5VDH548F bearing the Onderstepoort F protein and the 5VD H protein whose residue at position 548 was changed exhibited 10 times higher infectivity titer on CHO.B95aSLAM cells than on CHO.Neo cells. Taken together, these results indicate that although methionine at position 548 may play some role, lysine at position 530 was more critical for the ability of the H protein to interact with marmoset SLAM.
DISCUSSION
In this study, we showed that Vero cells stably expressing canine SLAM, a cellular receptor for CDV, are highly sensitive to clinical CDV specimens. CDV isolation is usually done by cocultivation of lymphocytes from suspect dogs with mitogen-stimulated dog lymphocytes (2). Although CPE is usually difficult to detect in these cells, CDV may be demonstrated in them by immunofluorescence staining 3 to 6 days later. By contrast, the present study showed that Vero.DogSLAMtag cells developed apparent syncytia as early as 24 h after inoculation with samples. Their adherent nature makes it extremely easy to detect the development of syncytia. Furthermore, by using this cell line we do not have to prepare lymphocyte cultures from healthy dogs. The marmoset B-cell line B95a is also recently used for isolating CDV, and CPE is easily detected in B95a cells (18). We used Vero.DogSLAMtag and B95a cells to recover CDV from seven dogs with distemper. Viruses were isolated in Vero.DogSLAMtag cells from 5 dogs (71%), whereas the isolation rate (43%) was lower, and longer cell culture (7 to 10 days) was required with B95a cells. Thus, we believe that we can most easily and efficiently isolate CDV in Vero.DogSLAMtag cells from clinical specimens. Besides the expression of the cellular receptor for CDV, the defect of beta interferon production in the parental Vero cells (10) may also contribute to the high sensitivity of Vero.DogSLAMtag cells to CDV. Furthermore, this cell line will allow us to easily titrate neutralizing antibodies in dog sera against various strains of CDV.
In humans and mice, SLAM is reportedly expressed on immature thymocytes, activated T and B cells, activated monocytes and macrophages, and mature dendritic cells (6-9, 19, 21, 24, 27, 30). Although expression of canine SLAM has not yet been examined in dogs, its distribution is likely to be similar to those in humans and mice. The fact that we were able to isolate CDV at 24 h after inoculation at the high rate may indicate that viruses within clinical specimens replicated in Vero.DogSLAMtag cells without adaptation and/or selection. It in turn strongly suggests that canine SLAM acts as the principal cellular receptor for CDV replicating in vivo. This is consistent with the lymphotropism and immunosuppressive nature of CDV. On the other hand, CDV may also infect epithelial cells in lung, stomach, intestinal and bladder tissues, and neuronal cells in the central nervous system (12, 22), where SLAM has not been detected in other species. Like MV, it is not known at this moment how CDV infects cells other than those of the immune system (43). The presence of other cellular receptors has been proposed for MV (1, 15).
We analyzed in detail CDV strains isolated in Vero.DogSLAMtag and B95a cells from the same dog. The 5VD strain isolated in Vero.DogSLAMtag cells could use dog SLAM but not marmoset and human SLAMs as a receptor, whereas the 5B strain isolated in B95a cells after 10 days of culture was able to use all of dog, marmoset and human SLAMs. This suggests that the 5VD strain is more representative of viruses in the body, while the 5B strain is the virus that had adapted to marmoset SLAM during in vitro culture. There were two amino acid differences in the receptor-binding H protein between the two strains. Cell fusion analysis and VSV pseudotype assay clearly demonstrated that virus entry (or interaction with the cellular receptor) determines cell tropism of these strains. A single amino acid substitution at position 530 conferred on the H protein of the 5VD strain the ability to interact with marmoset SLAM. Thus, it is likely that the virus in dog 5 spleen sample first infected and replicated in B95a cells at a very low efficiency as reported for wild-type MV infecting SLAM-negative cells (15). Then, a mutation at position 530 of the H protein had occurred during culture, and the virus containing such a mutation with an additional mutation at position 548 was selected in B95a cells. It should be noted that the 5B strain had acquired the ability to utilize not only marmoset SLAM but also human SLAM. The same holds true for two other B95a-isolated strains, HA7 and 851, that were previously studied (36). Thus, virus isolation in B95a cells may select CDV strains that can use human SLAM as a receptor.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science, and Culture and the Ministry of Health, Labor, and Welfare of Japan and from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Andres, O., K. Obojes, K. S. Kim, V. ter Meulen, and J. Schneider-Schaulies. 2003. CD46- and CD150-independent endothelial cell infection with wild-type measles viruses. J. Gen. Virol. 84:1189-1197. [DOI] [PubMed] [Google Scholar]
- 2.Appel, M. J., S. Pearce-Kelling, and B. A. Summers. 1992. Dog lymphocyte cultures facilitate the isolation and growth of virulent canine distemper virus. J. Vet. Diagn. Investig. 4:258-263. [DOI] [PubMed] [Google Scholar]
- 3.Appel, M. J., and B. A. Summers. 1995. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet. Microbiol. 44:187-191. [DOI] [PubMed] [Google Scholar]
- 4.Appel, M. J. G., and J. H. Gillespie. 1972. Canine distemper virus. Virol. Monogr. 11:1-96.
- 5.Appel, M. J. G., and O. R. Jones. 1967. Use of alveolar macrophages for cultivation of canine distemper virus. Proc. Soc. Exp. Biol. Med. 126:571-574. [DOI] [PubMed] [Google Scholar]
- 6.Aversa, G., C.-C. Chang, J. M. Carballido, B. G. Cocks, and J. E. de Vries. 1997. Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J. Immunol. 158:4036-4044. [PubMed] [Google Scholar]
- 7.Bleharski, J., K. Niazi, P. Sieling, G. Cheng, and R. Modlin. 2001. Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated dendritic cells and directly augments production of inflammatory cytokines. J. Immunol. 167:3174-3181. [DOI] [PubMed] [Google Scholar]
- 8.Castro, A. G., T. M. Hauser, B. G. Cocks, J. Abrams, S. Zurawski, T. Churakova, F. Zonin, D. Robinson, S. G. Tangye, G. Aversa, K. E. Nichols, J. E. de Vries, L. L. Lanier, and A. O'Garra. 1999. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in Th1 and Th2 cells. J. Immunol. 163:5860-5870. [PubMed] [Google Scholar]
- 9.Cocks, B. G., C.-C. J. Chang, J. M. Carballido, H. Yssel, J. E. de Vries, and G. Aversa. 1995. A novel receptor involved in T-cell activation. Nature 376:260-263. [DOI] [PubMed] [Google Scholar]
- 10.Emeny, J. M., and M. J. Morgan. 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 43:247-252. [DOI] [PubMed] [Google Scholar]
- 11.Erlenhoefer, C., W. J. Wurzer, S. Loffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2001. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 75:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin, D. E. 2001. Measles virus, p. 1401-1441. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 13.Haig, D. A. 1956. Canine distemper-immunization with avianized virus. Onderstepoort J. Vet. Res. 17:19-53. [Google Scholar]
- 14.Harrison, M. J., D. T. Oxer, and F. A. Smith. 1968. The virus of canine distemper in cell culture. II. Effect of serial passage in ferret kidney cell cultures and BS-C-1 cell cultures on the virulence of canine distemper virus. J. Comp. Pathol. 78:133-139. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto, K., N. Ono, H. Tatsuo, H. Minagawa, M. Takeda, K. Takeuchi, and Y. Yanagi. 2002. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 76:6743-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto, M., Y. Une, and M. Mochizuki. 2001. Hemagglutinin genotype profiles of canine distemper virus from domestic dogs in Japan. Arch. Virol. 146:149-155. [DOI] [PubMed] [Google Scholar]
- 17.Hsu, E., C. Iorio, F. Sarangi, A. Khine, and C. Richardson. 2001. CDw150(SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology 279:9-21. [DOI] [PubMed] [Google Scholar]
- 18.Kai, C., F. Ochikubo, M. Okita, T. Iinuma, T. Mikami, F. Kobune, and K. Yamanouchi. 1993. Use of B95a cells for isolation of canine distemper virus from clinical cases. J. Vet. Med. Sci. 55:1067-1070. [DOI] [PubMed] [Google Scholar]
- 19.Kruse, M., E. Meinl, G. Henning, C. Kuhnt, S. Berchtold, T. Berger, G. Schuler, and A. Steinkasserer. 2001. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1β. J. Immunol. 167:1989-1995. [DOI] [PubMed] [Google Scholar]
- 20.Metzler, A. E., R. J. Higgins, S. Krakowka, and A. Koestner. 1980. Virulence of tissue culture-propagated canine distemper virus. Infect. Immun. 29:940-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minagawa, H., K. Tanaka, N. Ono, H. Tatsuo, and Y. Yanagi. 2001. Induction of the measles virus receptor (SLAM) on monocytes. J. Gen. Virol. 82:2913-2917. [DOI] [PubMed] [Google Scholar]
- 22.Murphy, F. A., E. P. J. Gibbs, M. C. Horzinek, and M. J. Studdert. 1999. Veterinary virology, 3rd ed., p. 411-428. Academic Press, San Diego, Calif.
- 23.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants by a novel eukaryotic vector. Gene 108:193-200. [DOI] [PubMed] [Google Scholar]
- 24.Ohgimoto, S., K. Ohgimoto, S. Niewiesk, I. Klagge, J. Pfeuffer, I. Johnston, J. Schneider-Schaulies, A. Weidmann, V. ter Meulen, and S. Schneider-Schaulies. 2001. The haemagglutinin protein is an important determinant of measles virus tropism for dendritic cells in vitro. J. Gen. Virol. 82:1835-1844. [DOI] [PubMed] [Google Scholar]
- 25.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ono, N., H. Tatsuo, K. Tanaka, H. Minagawa, and Y. Yanagi. 2001. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J. Virol. 75:1594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polacino, P. S., L. M. Pinchuk, S. P. Sidorenko, and E. A. Clark. 1996. Immunodeficiency virus cDNA synthesis in resting T lymphocytes is regulated by T cell activation signals and dendritic cells. J. Med. Primatol. 25:201-209. [DOI] [PubMed] [Google Scholar]
- 28.Poste, G. 1971. The growth and cytopathogenicity of virulent and attenuated strains of canine distemper virus in dog and ferret macrophages. J. Comp. Pathol. 81:49-54. [DOI] [PubMed] [Google Scholar]
- 29.Rockborn, G. 1958. Canine distemper virus in tissue culture. Arch. Gesamte Virusforsch. 8:485-492. [Google Scholar]
- 30.Sidorenko, S. P., and E. A. Clark. 1993. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J. Immunol. 151:4614-4624. [PubMed] [Google Scholar]
- 31.Sidorenko, S. P., and E. A. Clark. 2003. The dual-function CD150 receptor subfamily: the viral attraction. Nat. Immunol. 4:19-24. [DOI] [PubMed] [Google Scholar]
- 32.Stern, L. B., M. Greenberg, J. M. Gershoni, and S. Rozenblatt. 1995. The hemagglutinin envelope protein of canine distemper virus (CDV) confers cell tropism as illustrated by CDV and measles virus complementation analysis. J. Virol. 69:1661-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takada, A., C. Robinson, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94:14764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatsuo, H., K. Okuma, K. Tanaka, N. Ono, H. Minagawa, A. Takade, Y. Matsuura, and Y. Yanagi. 2000. Virus entry is a major determinant of cell tropism of Edmonston and wild-type strains of measles virus as revealed by vesicular stomatitis virus pseudotypes bearing their envelope proteins. J. Virol. 74:4139-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 36.Tatsuo, H., N. Ono, and Y. Yanagi. 2001. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 75:5842-5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatsuo, H., and Y. Yanagi. 2002. The morbillivirus receptor SLAM (CD150). Microbiol. Immunol. 46:135-142. [DOI] [PubMed] [Google Scholar]
- 38.Vallejo, A. N., R. J. Pogulis, and L. R. Pease. 1995. Mutagenesis and synthesis of novel recombinant genes using PCR, p. 603-612. In C. W. Dieffenbach and G. S. Dveksler (ed.), PCR primer: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 39.Vandevelde, M., and A. Zurbriggen. 1995. The neurobiology of canine distemper virus infection. Vet. Microbiol. 44:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser, I. K., V. P. Kumarev, C. Orvell, P. de Vries, H. W. Broeders, M. W. van de Bildt, J. Groen, J. S. Teppema, M. C. Burger, F. G. UytdeHaag, and A. D. M. E. Osterhaus. 1990. Comparison of two morbilliviruses isolated from seals during outbreaks of distemper in north west Europe and Siberia. Arch. Virol. 111:149-164. [DOI] [PubMed] [Google Scholar]
- 41.Visser, I. K., M. F. van Bressem, T. Barrett, and A. D. Osterhaus. 1993. Morbillivirus infections in aquatic mammals. Vet. Res. 24:169-178. [PubMed] [Google Scholar]
- 42.von Messling, V., G. Zimmer, G. Herrler, L. Haas, and R. Cattaneo. 2001. The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J. Virol. 75:6418-6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanagi, Y., N. Ono, H. Tatsuo, K. Hashimoto, and H. Minagawa. 2002. Measles virus receptor SLAM (CD150). Virology 299:155-161. [DOI] [PubMed] [Google Scholar]