Short abstract
The reticulon family is a diverse group of proteins that mostly localize to the endoplasmic reticulum and may be important in neurodegenerative diseases.
Abstract
The reticulon family is a large and diverse group of membrane-associated proteins found throughout the eukaryotic kingdom. All of its members contain a carboxy-terminal reticulon homology domain that consists of two hydrophobic regions flanking a hydrophilic loop of 60-70 amino acids, but reticulon amino-terminal domains display little or no similarity to each other. Reticulons principally localize to the endoplasmic reticulum, and there is evidence that they influence endoplasmic reticulum-Golgi trafficking, vesicle formation and membrane morphogenesis. However, mammalian reticulons have also been found on the cell surface and mammalian reticulon 4 expressed on the surface of oligodendrocytes is an inhibitor of axon growth both in culture and in vivo. There is also growing evidence that reticulons may be important in neurodegenerative diseases such as Alzheimer's disease and amyotrophic lateral sclerosis. The diversity of structure, topology, localization and expression patterns of reticulons is reflected in their multiple, diverse functions in the cell.
Gene organization and evolutionary history
Proteins of the reticulon family are present in all eukaryotic organisms examined and range in size from 200 to 1,200 amino acids. The vertebrate proteins of this family are called reticulons (RTNs) and those found in other eukaryotes are called reticulon-like proteins (RTNLs). All family members contain the reticulon homology domain (RHD), a conserved region at the carboxy-terminal end of the molecule consisting of two hydrophobic regions flanking a hydrophilic loop. Reticulons have been identified in the genomes of Homo sapiens, Mus musculus, Danio rerio, Xenopus laevis, Drosophila melanogaster, Caenorhabditis elegans, Arabidopsis thaliana, Saccharomyces cerevisiae and many other eukaryotes, but not in archaea or bacteria [1-6]. The ubiquity of reticulons in the eukaryotic kingdom is consistent with a highly conserved function and/or a diversity of functions.
Nearly all reticulon genes contain multiple introns and exons, and most are alternatively spliced into multiple isoforms [1]. Intron losses and gains over the course of evolution have given rise to the large, diverse reticulon family. The presence of reticulons in eukaryotic but not prokaryotic organisms and their close association with the endoplasmic reticulum (ER) suggest that reticulons evolved along with the eukaryotic endomembrane system.
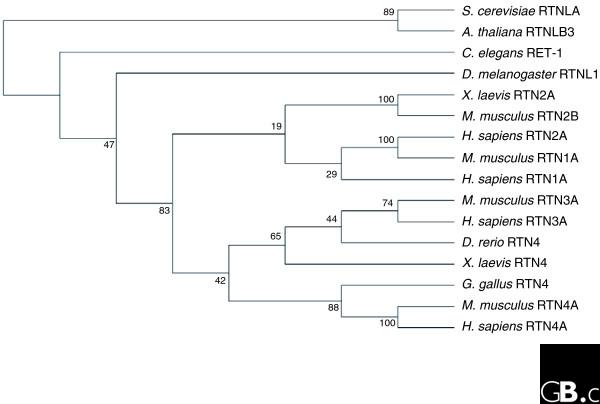
Across phyla, the second hydrophobic region of the RHD is the most highly conserved, followed by the first hydrophobic region, with the carboxyl terminus the least conserved [7]. In mammals, there are four reticulon genes encoding reticulon proteins RTN1-4. The RHDs of RTN1, 3 and 4 share the highest sequence identity at the amino-acid level (average 73%), whereas RTN2 has only 52% identity with human RTN4 (Figure 1). The amino-acid sequence identity between RHDs of C. elegans and S. cerevisiae drops to 15-50%.
Figure 1.
Phylogenetic analysis of the reticulon homology domains (RHDs) of selected RTNs and RTNLPs. Alignments were created using the ClustalW2 program [99] and the tree was generated with Phylo_win software [100]. Bootstrap numbers are shown; the number of repetitions was 1,000. The tree was generated using the maximum likelihood method. GenBank accession numbers are as follows: H. sapiens RTN1A, NP_066959; H. sapiens RTN2A, NP_005610; H. sapiens RTN3A, NP_006045; H. sapiens RTN4A, NP_065393; M. musculus RTN1A, NP_703187; M. musculus RTN2B, NP_038676; M. musculus RTN3A, NP_001003934; M. musculus RTN4A, NP_918943; G. gallus RTN4, NP_989697; X. laevis RTN2A, NP_001089014; X. laevis RTN4, NP_001083238; D. rerio RTN4, NP_001018620; D. melanogaster Rtnl1A, NP_787987; C. elegans RET-1, NP_506656; S. cerevisiae RTNLA, NP_010077; A. thaliana RTNLB3, NP_176592.
In contrast to the highly conserved carboxy-terminal RHD, the amino-terminal regions of reticulons display no sequence similarity at all, even among paralogs within the same species [8]. Furthermore, the expression patterns of different reticulons and their splice isoforms can be variable, even within the same organism [9-11]. This divergence in sequence and expression is consistent with evolution of species- and cell-type-specific roles for reticulons [12]. This is particularly clear in the mammalian RTN family, in which the longest isoform of RTN4, RTN4A, also known as Nogo-A, has been shown to inhibit neurite outgrowth and axon regeneration in models of injury [8,13-18]. Interestingly, RTN4A was found to be absent in fishes, organisms in which there is extensive regeneration of the CNS after injury [4]. Divergent results for genetic knockouts of different regions and isoforms of RTN4 suggest that the amino-terminal domain might contribute to the inhibition of nerve regeneration after injury [12]. Thus, the divergent reticulon amino-terminal domains appear to carry out species- and cell-specific roles, whereas the RHD may carry out more basic cellular functions.
Characteristic structural features
The RHD consists of two hydrophobic regions, each 28-36 amino acids long, which are thought to be membrane-embedded regions, separated by a hydrophilic loop of 60-70 amino acids, and followed by a carboxy-terminal tail about 50 amino acids long (Figure 2a). Although much amino-acid identity has been lost over the course of evolution, the overall structure of the RHD has been preserved from plants to yeasts to humans. This suggests that three-dimensional protein structure is of greater importance than individual residues for RHD function. The RHD hydrophobic regions are unusually long for transmembrane domains: each spans approximately 30-35 amino acids, whereas most transmembrane domains are about 20 amino acids in length. This raises the interesting question of whether this longer length has significance for reticulon function. The topology of these hydrophobic regions within membranes is so far only partially defined.
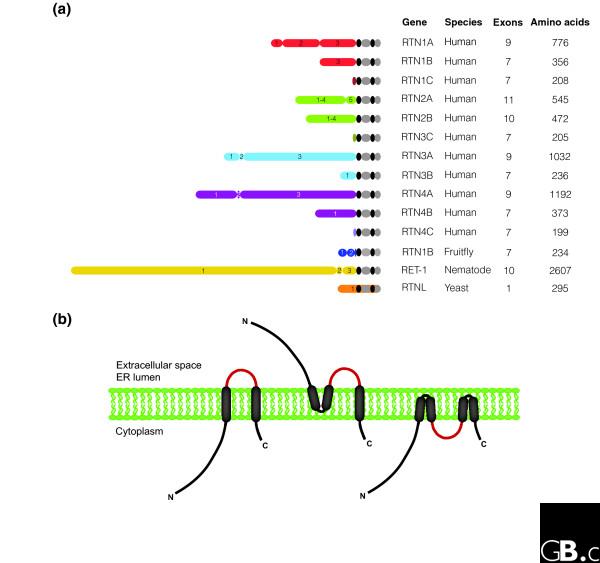
Figure 2.
The structure and membrane topology of reticulons. (a) Structure of reticulon proteins. Numbers refer to the exons that encode the protein regions. Black ovals represent hydrophobic regions. GenBank accession numbers are as in Figure 1. (b) Possible topologies of reticulon proteins in membranes. Although eight or more conformations are possible, only those for which evidence exists are depicted. Different topologies in different cell types and different membranes may enable reticulons to carry out diverse roles in the cell.
Reticulon topology
The RHD loop region has been detected both on the surface of cells and intracellularly, and it has been suggested that the RHD hydrophobic regions might either span the ER membrane or plasma membrane completely or might double back on themselves to form a hairpin (Figure 2b). Antibodies against the amino-terminal domain of RTN4 bind to the surface of chick oligodendrocytes in live spinal cord explants [8] and cultured oligodendrocytes interact specifically with both amino-terminal domain-specific antibodies and antibodies directed against the RTN4 66-amino-acid loop (66-loop) [16]. These findings suggest that the amino terminus and the 66-loop project into extracellular space, and therefore that the first RHD hydrophobic region must double back on itself in the membrane. However, other data suggest that the amino-terminal domain is intracellular. Antibodies against the 66-loop region of RTN4 detect small amounts of this epitope on the surface of live COS-7 cells, but antibodies against c-Myc tags fused to either the amino or the carboxy terminus do not bind to live cells [8].
More recent data from non-neuronal cells in which RTN4 is overexpressed strongly support a third model, in which most of both the amino-terminal domain and the 66-loop are cytoplasmic. In COS cells treated with maleimide polyethylene glycol, cysteines in the amino-terminal domain and the loop regions of ER-associated RTN4 were found to be modified by the reagent after detergent disruption of the plasma membrane but not the ER membrane [6]. Cysteines in the carboxy-terminal region were only partially modified. All these results suggest that mammalian reticulons might have different topologies in the ER and plasma membranes; such multiple conformations may enable them to carry out multiple roles in the cell. Another protein with multiple membrane topologies is the mammalian prion protein (PrP); overexpression of a certain transmembrane form of the prion protein, CtmPrP, causes neurodegenerative disease distinct from that caused by the natural pathogenic prion form PrPSc [19,20]. Another possibility is that reticulons assume different topologies in different cell types: the reticulon amino-terminal region has been detected only in the cytoplasm in COS-7 cells, but has been found on both the surface and in the cytoplasm of oligodendrocytes. Again, this may reflect the diverse roles of reticulon proteins in different cell types.
Reticulon tertiary structure
The solution structure of the RHD loop of RTN4, known as Nogo66, has recently been probed by circular dichroism (CD) and nuclear magnetic resonance (NMR). Nogo66 is soluble in pure water and consists of three alpha helices, two short flanking one long, spanning residues 6-15, 21-40 and 45-53, followed by the unstructured residues 55-60 [21,22]. The Nogo66 loop is involved in several RTN4-specific signaling cascades, including interaction with the Nogo receptor (NogoR) to inhibit neurite outgrowth [23], and with the cell adhesion molecule contactin-associated protein (Caspr) [24] to mediate the localization of potassium channels at axonal paranodes. The human RTN1 and RTN3 66-loops share 71% and 63% identity with the RTN4 loop; mouse RTN1 and RTN3 identity with human RTN4 is 67% and 59%. Despite this high degree of identity, the RTN1 66-loop does not bind to NogoR, and the function of the 66-loops in RTN1 and RTN3 is unknown in both mammals and lower organisms.
As mentioned above, the amino-terminal regions of different reticulons are highly divergent in sequence. The amino-terminal domains of the human RTN4 isoforms appear to be highly unstructured, even under physiological conditions. In silico analysis and measurements by CD and NMR of the human isoforms RTN4A and RTN4B reveal a high degree of disorganization, with only short alpha helices and beta sheets that exist transiently [25]. Recent studies have shown that intrinsically unstructured proteins (IUPs) are more likely to form multiprotein complexes than are proteins with stable tertiary structure [26], are better able to 'moonlight' - carry out alternative functions [25] - and may fold upon binding to their partners [27]. It has been shown that up to 33% of eukaryotic proteins contain long disordered regions, compared with 2% of archeal proteins [25]. The characterization of RTN4 as an intrinsically unstructured/disordered protein may explain its involvement in many physiological processes, as explained below.
Localization and function
The first known reticulon protein, RTN1, was identified from a cDNA in neural tissue [28] and subsequently characterized as an antigen specific to neuroendocrine cells [29]. This so-called neuroendocrine-specific protein (NSP) was later renamed reticulon when it was discovered by both immunohistochemical and biochemical methods to be associated with the ER in COS-1 cells [30]. Reticulons do not contain an ER localization sequence per se, but a single RHD hydrophobic region is sufficient to target an enhanced green fluorescent protein-RTN fusion protein to the ER, whereas deletion of the RHD abolishes association with the ER [13,31]. Reticulons have been shown to localize to the ER in yeast, Arabidopsis, C. elegans, Xenopus, Drosophila and mammals [2,3,5,6,32-34]. Most reticulon research has focused on RTN4 in the CNS and its effects on neurite outgrowth and axonal regeneration after spinal cord injury. However, the presence of reticulons in all eukaryotic organisms and their ubiquitous ER-associated expression indicate a more general role. We shall focus on three areas of reticulon localization and function: ER-associated roles, oligodendrocyte-associated roles in inhibition of neurite outgrowth, and the role of reticulons in neurodegenerative diseases.
ER-associated reticulons and their function
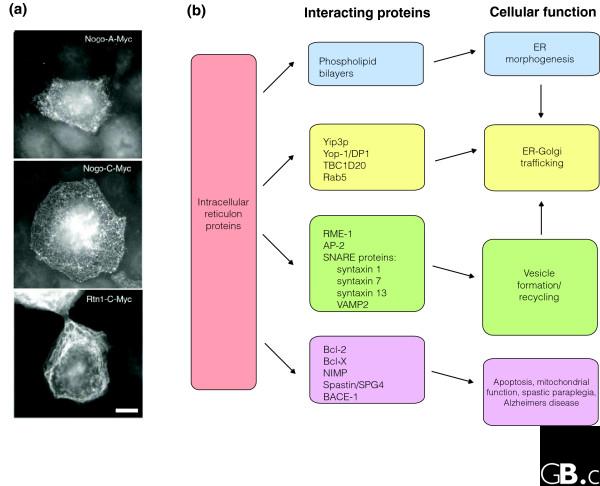
There is growing evidence that reticulons are involved in bending and shaping the ER membrane, in trafficking of material from the ER to the Golgi apparatus, and in apoptosis (Figure 3). Antibody-mediated inhibition of RTN4A in mammalian cells prevents GTP-induced formation of elongated membrane tubules in vitro [6], and knocking out both the C. elegans RTNL RET-1 and its associated protein YOP-1 interferes with ER formation during mitosis in the worm [33]. RTN4A also localizes to subdomains of the Xenopus nuclear envelope, and its inhibition by anti-RTN4A antibodies limits nuclear envelope assembly [35]. C. elegans RET-1 also interacts with the protein RME-1, a regulator of endocytic recycling [36-38]. In a yeast two-hybrid screen, the mammalian RTN1 isoforms RTN1A and RTN1B were found to interact with a component of the mammalian endocytosis adaptor complex AP-2. RTN1C, in contrast, may be involved in exocytosis. It associates with calreticulin-negative regions of the ER and co-immunoprecipitates with the SNARE proteins syntaxin 1, syntaxin 7, syntaxin 13 and VAMP2 [39]. Overexpression of a fragment of RTN1C increases the rate of exocytosis in PC12 cells. RTN1, RTN2, and RTN4 were all identified in a yeast two-hybrid screen using the vesicle fusion protein chaperone β-SNAP as bait, although these results were not confirmed by co-immunoprecipitation [40]. In summary, reticulons interact with proteins involved in vesicular formation and fusion such as SNAREs and SNAPs. Reticulons also appear to play a role in ER morphogenesis; nevertheless, cells lacking reticulon expression do not have major defects in ER, endosomal or microsomal structure.
Figure 3.
The expression and function of reticulons. (a) Myc-tagged reticulon constructs transfected into COS-7 cells show a reticular expression pattern. Scale bar, 70 μm. (b) Proteins known to interact with ER-associated and intracellular reticulons and their possible intracellular roles. Some classes of proteins may overlap in their cellular functions.
Reticulons are also involved in intracellular trafficking - a close cousin of vesicle formation and recycling. Overexpression of RTN3 in HeLa cells prevents retrograde transport of proteins from the Golgi complex to the ER [41]. In yeast, RTNL1B forms complexes with Yip3p, the yeast ortholog of the mammalian Rab-GDI displacement factor (GDF). Small GTPases of the Rab family facilitate vesicle trafficking between organelles, and are regulated by GDFs [42]. In C. elegans, inhibition of RET-1 and YOP-1 disrupted nuclear envelope assembly, and of 29 Rabs screened, depletion of Rab5 mimicked this phenotype closely [33]. In a screen in human cells for GTPase-activating proteins (GAPs), which inhibit Rab function, the protein TBC1D20 was found to be a GAP for Rab1 and Rab2, and in the same study, interaction between RTN1C and TBC1D20 was identified in a yeast two-hybrid screen, a further argument for a role for reticulons as regulators of Rab-regulated intracellular trafficking [43].
In mammalian cells, reticulons may also play a role in apoptosis. Both RTN1C and what is now known to be RTN4A were identified in a screen for interactors with Bcl-XL, a powerful inhibitor of apoptosis [44]. RTN1C was found to inhibit Bcl-XL, and RTN4A was found to inhibit both Bcl-XL and another apoptosis inhibitor, Bcl-2, demonstrating a pro-apoptotic role for reticulons. More recently, RTN1C was shown to modulate apoptosis by upregulating the sensitivity of the ER to stressors in neuroblastoma cells [45]. Several labs have shown that RTN3 also enhances apoptosis via interaction with Bcl-2 [46-48]. Although these and other data indicate that reticulons may have a role in tumor suppression via upregulation of apoptosis, this topic is not without controversy [49].
Oligodendrocyte reticulon and its role in neurite outgrowth inhibition
The longest isoform of RTN4, RTN4A, has been extensively characterized in the mammalian CNS (recently reviewed by Liu et al. [50]). It had long been known that in contrast to the myelin of the peripheral nervous system, myelin from the CNS appeared to prevent neuronal regeneration after injury [51]. In 1988, by size fractionation of rat CNS myelin, Caroni and Schwab discovered a 250 kDa inhibitor of neurite outgrowth [52]. This protein was later identified as a novel reticulon (RTN4A) and also named Nogo-A after its inhibitory effect on neuronal regeneration [8,13,14]. As the protein is generally called by this name in neuronal regeneration studies, we shall use that name in the following discussion. GrandPré et al. [8] showed that the extracellular/ER luminal portion of Nogo-A, the 66-loop termed Nogo66, is a potent inhibitor of neurite outgrowth. The receptor for Nogo66 was subsequently identified and termed NogoR [23]. Inhibition of NogoR using the antagonist peptide NEP1-40 releases myelin-mediated inhibition of neurite outgrowth in culture, and both the acute intrathecal delivery and delayed systemic delivery of NEP1-40 promotes axonal regeneration of corticospinal tract fibers after dorsal hemisection in rats [18,53-55]. Work on the amino-terminal domain of Nogo-A demonstrated its capacity to induce growth-cone collapse independent of NogoR via a region now called Δ20 [16,23], whereas another amino-terminal region, termed Nogo-A-24, is known to enhance the binding affinity of Nogo66 for NogoR when fused to Nogo66 [15]. Interestingly, the RHD region common to all isoforms of Nogo (RTN4) is alone sufficient to delay nerve regeneration after sciatic nerve crush [56].
Numerous in vivo studies in animals have found that either genetic ablation or pharmacological inhibition of the Nogo-A-NogoR interaction promotes axon growth and behavioral recovery after spinal cord injury [17,18,53,54,57-62], and significant improvement of recovery after similar prevention of Nogo-A action is also seen after stroke injury [63-65]. The field is not free from controversy, however [66,67]. The genetic background can alter the effects of Nogo inhibition [68], and studies of spinal cord injury in Nogo-knockout animals generated in different laboratories have yielded variable results [69-71]. The weight of evidence for a role for Nogo-A as an inhibitor of neurite outgrowth and a limitor of axon growth in spinal cord injury, however, make it a prime target for therapeutic intervention. Indeed, clinical trials of anti-Nogo antibodies are already under way.
Although the mechanism of action of reticulon in the ER remains to be elucidated, the mechanism by which Nogo-A inhibits neurite outgrowth and axon regeneration in the CNS is well characterized (Figure 4, Table 1). The receptor - NogoR - for the Nogo66 region was identified in 2001 [21], but this receptor lacks a transmembrane and signaling domain and so must interact with a co-receptor or other signal transducer. Several candidates for this role have been discovered: the neurotrophin receptor p75, the transmembrane protein LINGO-1 and the orphan tumor necrosis factor family member TAJ/TROY have all been shown to bind NogoR and participate in the inhibition of neurite outgrowth in vitro [72-78] (see Figure 4, Table 1). The epidermal growth factor receptor (EGFR) may indirectly be a signal transducer for NogoR - the kinase activity of EGFR has been shown to be required for the inhibitory action of Nogo66 in culture - but EGFR does not directly bind NogoR [78]. A crystal structure of the ligand-binding domain of the NogoR receptor has been determined [79,80].
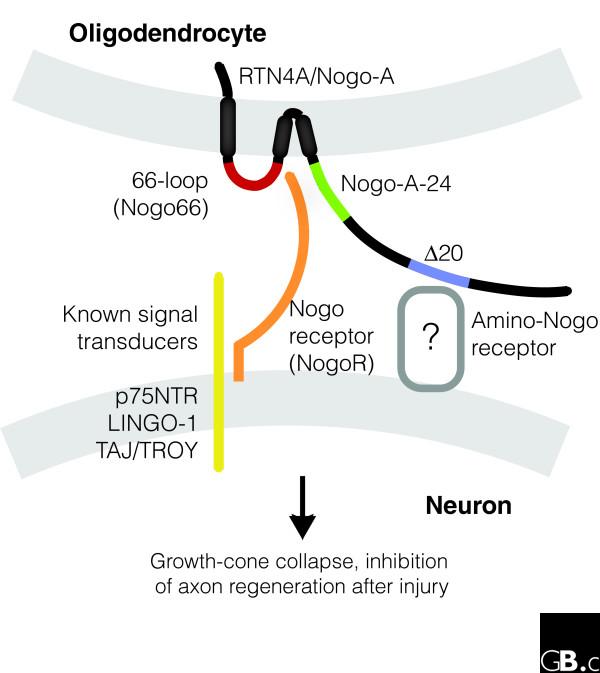
Figure 4.
Interaction of Nogo-A with Nogo receptor. The interaction of Nogo-A (RTN4A) on oligodendrocytes and the Nogo receptor (NogoR) on neurons results in inhibition of axon regeneration after injury via Rho signaling [17,23,55,59,61-63,79]. The different regions of Nogo-A are colored as follows: red, 66-loop (Nogo66); green, Nogo-A-24; blue, Δ20. NogoR is in orange. The 66-loop interacts with NogoR to mediate growth-cone collapse and neurite outgrowth in vitro and to inhibit axon regeneration after injury [21,23,25,53,56,58,60,62]. The amino-terminal region Nogo-A-24 increases the binding affinity of Nogo-A to NogoR and also binds NogoR directly [15]. The amino-terminal region Δ20 can mediate fibroblast and growth-cone collapse independently of NogoR [16]. Some known co-receptors and signal transducers are listed beside the yellow symbol and are described in more detail in Table 1.
Table 1.
Co-receptors and signal transducers in the Nogo-A-NogoR interaction
| Molecular region/interactor | Function/interaction | Reference(s) |
| NogoA-24 | Increases binding affinity of Nogo-A to Nogo receptor (NogoR); binds NogoR directly | [15] |
| Δ20 | Mediates fibroblast and growth cone collapse independently of NogoR | [16] |
| p75NTR | Neurotrophin receptor; binds NogoR and mediates inhibition of neurite outgrowth via myelin-associated inhibitors | [72,73] |
| LINGO-1 | Binds NogoR; activates Rho in complex with p75 and NogoR; mediates Nogo66-induced neurite outgrowth inhibition | [74,75] |
| TAJ/TROY | Binds NogoR, activates Rho in complex with LINGO-1 and NogoR; absence attenuates myelin inhibition of neurite outgrowth | [76,77] |
| EGFR | Kinase activity required for neurite outgrowth but EGFR does not bind NogoR | [78] |
NogoR and all its putative co-receptors rely on the small GTPase RhoA for their downstream effects. Upon RhoA activation as a result of NogoR signaling, Rho-activated kinase (ROCK) stimulates actinomyosin activity, causing growth-cone collapse [50,81]. Blocking Rho activity either pharmacologically or with dominant-negative RhoA releases Nogo66-mediated neurite outgrowth inhibition in vitro [82-85].
Frontiers
Many aspects of our knowledge of the reticulon protein family remain incomplete. There is no consensus on the mechanism(s) underlying the ER-associated function of reticulons, and debate continues over the role of mammalian Nogo-A in the inhibition of neurite outgrowth. The most exciting frontier of reticulon research, however, is in the field of neurodegenerative disease. There is growing evidence that reticulons may have a role in amyotrophic lateral sclerosis (ALS), Alzheimer's disease, multiple sclerosis and perhaps hereditary spastic paraplegia.
In 2004 it was found that all four human reticulon proteins interact with the enzyme that produces the pathologic agent in Alzheimer's disease. He et al. [86] showed that BACE1, the δ-secretase that cleaves amyloid precursor protein (APP) into β-amyloid peptide (Aβ), co-immunoprecipitates with RTN1, RTN2, RTN3 and RTN4 [86]. In vitro, overexpression of a single RTN reduced the levels of Aβ produced by HEK-293 cells expressing the Swedish mutant of APP, and conversely, knockdown of RTN3 by RNA interference increased Aβ levels [54]. More recently, Murayama and colleagues screened for proteins that interact with BACE1 and identified RTN3 and RTN4 [87]. These authors also demonstrated decreased Aβ production in cells expressing the Swedish mutant [87]. Notably, in a subtractive hybridization screen, Yokota and colleagues [88] found that human RTN3 was downregulated in the temporal lobes of Alzheimer's patients. Although these data are intriguing, the exact role of reticulons in Alzheimer's disease remains unknown, and further investigation is needed to confirm whether these proteins may be potential therapeutic targets in Alzheimer's disease.
Reticulons have also been found to be involved in ALS. In an ALS mouse model expressing human superoxide dismutase (SOD) containing a disease-causing dominant mutation, Dupuis et al. [89] found differential up- and downregulation of RTN4A and RTN4C mRNA compared with wild-type mice. Jokic et al. [90] demonstrated that levels of RTN4 in muscle biopsies of ALS patients correlated with disease severity. Pradat et al. [91] found that expression of RTN4A in lower motor neuron syndromes was prognostic of ALS, but Wojcik and colleagues [92] have found recently that RTN4A expression is not unique to ALS. Genetic analysis of RTN4 in the SOD mouse model of ALS shows that it has a significant impact on survival [93]. Importantly, this effect on survival does not seem to be due to a direct effect on mutant SOD levels (YSY and SMS, unpublished data), and may instead be related to the roles of RTN4A in vesicle formation and trafficking. It is of note that RTN4A levels in muscle increase in surgically denervated wild-type mice [94], and as mentioned above, other groups have found that changes in RTN4A expression are not necessarily specific to ALS [92]. Considering the impact of RTN4 in the mouse model of ALS, however, this protein remains a possible candidate drug target for the disease.
Lastly, RTN4 may have a role in multiple sclerosis and hereditary spastic paraplegia. Autoantibodies against the isoform A-specific region of RTN4 have been found in serum and cerebrospinal fluid of patients with multiple sclerosis [95]. Interestingly, administration of exogenous anti-RTN4A antibodies protects against demyelination in the experimental autoimmune encephalitis mouse model of multiple sclerosis [96]. Spastin, the most commonly mutated protein in hereditary spastic paraplegia, was found to interact with RTN1 and RTN3 via yeast two-hybrid screening; the interaction between spastin and RTN1 was further confirmed by co-immunoprecipitation and co-localization of the two proteins in transfected HeLa cells [97,98].
Questions remain regarding all aspects of the reticulon family, from its most basic characteristics such as membrane topology to its partners in intracellular trafficking, to the downstream signaling molecules that effect the reticulons' influence on human disease. Despite the lack of consensus about the mechanism of action of reticulons in normal cellular function and in neurodegenerative disease, their involvement in several disease processes makes them important targets for therapeutic development.
Acknowledgments
Acknowledgements
This work was supported by an NIH Institutional Medical Scientist Training award to YSY and by grants to SMS from the NIH, the Wings for Life Foundation, the Falk Medical Research Trust and the Christopher Reeve Paralysis Foundation.
References
- Oertle T, Klinger M, Stuermer CA, Schwab ME. A reticular rhapsody: phylogenic evolution and nomenclature of the RTN/Nogo gene family. FASEB J. 2003;17:1238–1247. doi: 10.1096/fj.02-1166hyp. [DOI] [PubMed] [Google Scholar]
- Nziengui H, Bouhidel K, Pillon D, Der C, Marty F, Schoefs B. Reticulon-like proteins in Arabidopsis thaliana: structural organization and ER localization. FEBS Lett. 2007;581:3356–3362. doi: 10.1016/j.febslet.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Wakefield S, Tear G. The Drosophila reticulon, Rtnl-1, has multiple differentially expressed isoforms that are associated with a sub-compartment of the endoplasmic reticulum. Cell Mol Life Sci. 2006;63:2027–2038. doi: 10.1007/s00018-006-6142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann H, Klinger M, Oertle T, Heinz D, Pogoda HM, Schwab ME, Stuermer CA. Analysis of the reticulon gene family demonstrates the absence of the neurite growth inhibitor Nogo-A in fish. Mol Biol Evol. 2005;22:1635–1648. doi: 10.1093/molbev/msi158. [DOI] [PubMed] [Google Scholar]
- Moreira EF, Jaworski CJ, Rodriguez IR. Cloning of a novel member of the reticulon gene family (RTN3): gene structure and chromosomal localization to 11q13. Genomics. 1999;58:73–81. doi: 10.1006/geno.1999.5807. [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Yan R, Shi Q, Hu X, Zhou X. Reticulon proteins: emerging players in neurodegenerative diseases. Cell Mol Life Sci. 2006;63:877–889. doi: 10.1007/s00018-005-5338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D, Coffin RS, Prinjha RK, Campbell G, Anderson PN. Nogo-A expression in the intact and injured nervous system. Mol Cell Neurosci. 2003;24:1083–1102. doi: 10.1016/j.mcn.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Di Scala F, Dupuis L, Gaiddon C, De Tapia M, Jokic N, Gonzalez de Aguilar JL, Raul JS, Ludes B, Loeffler JP. Tissue specificity and regulation of the N-terminal diversity of reticulon 3. Biochem J. 2005;385:125–134. doi: 10.1042/BJ20040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Hu F, Liu BP, Budel S, Liao J, Chin J, Fournier A, Strittmatter SM. Nogo-A interacts with the Nogo-66 receptor through multiple sites to create an isoform-selective subnanomolar agonist. J Neurosci. 2005;25:5298–5304. doi: 10.1523/JNEUROSCI.5235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kim JE, Budel S, Hampton TG, Strittmatter SM. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde RS, Mastrianni JA, Scott MR, DeFea KA, Tremblay P, Torchia M, DeArmond SJ, Prusiner SB, Lingappa VR. A transmembrane form of the prion protein in neurodegenerative disease. Science. 1998;279:827–834. doi: 10.1126/science.279.5352.827. [DOI] [PubMed] [Google Scholar]
- Stewart RS, Piccardo P, Ghetti B, Harris DA. Neurodegenerative illness in transgenic mice expressing a transmembrane form of the prion protein. J Neurosci. 2005;25:3469–3477. doi: 10.1523/JNEUROSCI.0105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Liu J, Song J. Nogo goes in the pure water: solution structure of Nogo-60 and design of the structured and buffer-soluble Nogo-54 for enhancing CNS regeneration. Protein Sci. 2006;15:1835–1841. doi: 10.1110/ps.062306906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander H, Hettich E, Greiff K, Chatwell L, Skerra A. Biochemical characterization of the recombinant human Nogo-A ectodomain. FEBS J. 2007;274:2603–2613. doi: 10.1111/j.1742-4658.2007.05796.x. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Nie DY, Zhou ZH, Ang BT, Teng FY, Xu G, Xiang T, Wang CY, Zeng L, Takeda Y, Xu TL, et al. Nogo-A at CNS paranodes is a ligand of Caspr: possible regulation of K(+) channel localization. EMBO J. 2003;22:5666–5678. doi: 10.1093/emboj/cdg570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Song J. The N- and C-termini of the human Nogo molecules are intrinsically unstructured: bioinformatics, CD, NMR characterization, and functional implications. Proteins. 2007;68:100–108. doi: 10.1002/prot.21385. [DOI] [PubMed] [Google Scholar]
- Meszaros B, Tompa P, Simon I, Dosztanyi Z. Molecular principles of the interactions of disordered proteins. J Mol Biol. 2007;372:549–561. doi: 10.1016/j.jmb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- Wieczorek DF, Hughes SR. Developmentally regulated cDNA expressed exclusively in neural tissue. Brain Res. 1991;10:33–41. doi: 10.1016/0169-328X(91)90053-Z. [DOI] [PubMed] [Google Scholar]
- Roebroek AJ, van de Velde HJ, Van Bokhoven A, Broers JL, Ramaekers FC, Van de Ven WJ. Cloning and expression of alternative transcripts of a novel neuroendocrine-specific gene and identification of its 135-kDa translational product. J Biol Chem. 1993;268:13439–13447. [PubMed] [Google Scholar]
- van de Velde HJ, Roebroek AJ, Senden NH, Ramaekers FC, Van de Ven WJ. NSP-encoded reticulons, neuroendocrine proteins of a novel gene family associated with membranes of the endoplasmic reticulum. J Cell Sci. 1994;107:2403–2416. doi: 10.1242/jcs.107.9.2403. [DOI] [PubMed] [Google Scholar]
- Iwahashi J, Hamada N, Watanabe H. Two hydrophobic segments of the RTN1 family determine the ER localization and retention. Biochem Biophys Res Commun. 2007;355:508–512. doi: 10.1016/j.bbrc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Senden NH, van de Velde HJ, Broers JL, Timmer ED, Kuijpers HJ, Roebroek AJ, Van de Ven WJ, Ramaekers FC. Subcellular localization and supramolecular organization of neuroendocrine-specific protein B (NSP-B) in small cell lung cancer. Eur J Cell Biol. 1994;65:341–353. [PubMed] [Google Scholar]
- Audhya A, Desai A, Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. J Cell Biol. 2007;178:43–56. doi: 10.1083/jcb.200701139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EC, Shim S, Han JK. Identification and expression of XRTN2 and XRTN3 during Xenopus development. Dev Dyn. 2005;233:240–247. doi: 10.1002/dvdy.20327. [DOI] [PubMed] [Google Scholar]
- Kiseleva E, Morozova KN, Voeltz GK, Allen TD, Goldberg MW. Reticulon 4a/NogoA locates to regions of high membrane curvature and may have a role in nuclear envelope growth. J Struct Biol. 2007;160:224–235. doi: 10.1016/j.jsb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi J, Kawasaki I, Kohara Y, Gengyo-Ando K, Mitani S, Ohshima Y, Hamada N, Hara K, Kashiwagi T, Toyoda T. Caenorhabditis elegans reticulon interacts with RME-1 during embryogenesis. Biochem Biophys Res Commun. 2002;293:698–704. doi: 10.1016/S0006-291X(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- Grant B, Zhang Y, Paupard MC, Lin SX, Hall DH, Hirsh D. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol. 2001;3:573–579. doi: 10.1038/35078549. [DOI] [PubMed] [Google Scholar]
- Steiner P, Kulangara K, Sarria JC, Glauser L, Regazzi R, Hirling H. Reticulon 1-C/neuroendocrine-specific protein-C interacts with SNARE proteins. J Neurochem. 2004;89:569–580. doi: 10.1111/j.1471-4159.2004.02345.x. [DOI] [PubMed] [Google Scholar]
- Martin HG, Henley JM, Meyer G. Novel putative targets of N-ethylmaleimide sensitive fusion protein (NSF) and alpha/beta soluble NSF attachment proteins (SNAPs) include the Pak-binding nucleotide exchange factor betaPIX. J Cell Biochem. 2006;99:1203–1215. doi: 10.1002/jcb.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana Y, Koyama S, Nakajima K, Hatsuzawa K, Nagahama M, Tani K, Hauri HP, Melancon P, Tagaya M. Reticulon 3 is involved in membrane trafficking between the endoplasmic reticulum and Golgi. Biochem Biophys Res Commun. 2005;334:1198–1205. doi: 10.1016/j.bbrc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Geng J, Shin ME, Gilbert PM, Collins RN, Burd CG. Saccharomyces cerevisiae Rab-GDI displacement factor ortholog Yip3p forms distinct complexes with the Ypt1 Rab GTPase and the reticulon Rtn1p. Eukaryotic Cell. 2005;4:1166–1174. doi: 10.1128/EC.4.7.1166-1174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AK, Yoshimura S, Stephens DJ, Preisinger C, Fuchs E, Barr FA. Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J Cell Sci. 2007;120:2997–3010. doi: 10.1242/jcs.014225. [DOI] [PubMed] [Google Scholar]
- Tagami S, Eguchi Y, Kinoshita M, Takeda M, Tsujimoto Y. A novel protein, RTN-XS, interacts with both Bcl-XL and Bcl-2 on endoplasmic reticulum and reduces their anti-apoptotic activity. Oncogene. 2000;19:5736–5746. doi: 10.1038/sj.onc.1203948. [DOI] [PubMed] [Google Scholar]
- Di Sano F, Fazi B, Tufi R, Nardacci R, Piacentini M. Reticulon-1C acts as a molecular switch between endoplasmic reticulum stress and genotoxic cell death pathway in human neuroblastoma cells. J Neurochem. 2007;102:345–353. doi: 10.1111/j.1471-4159.2007.04479.x. [DOI] [PubMed] [Google Scholar]
- Zhu L, Xiang R, Dong W, Liu Y, Qi Y. Anti-apoptotic activity of Bcl-2 is enhanced by its interaction with RTN3. Cell Biol Int. 2007;31:825–830. doi: 10.1016/j.cellbi.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Wan Q, Kuang E, Dong W, Zhou S, Xu H, Qi Y, Liu Y. Reticulon 3 mediates Bcl-2 accumulation in mitochondria in response to endoplasmic reticulum stress. Apoptosis. 2007;12:319–328. doi: 10.1007/s10495-006-0574-y. [DOI] [PubMed] [Google Scholar]
- Kuang E, Wan Q, Li X, Xu H, Liu Q, Qi Y. ER Ca2+ depletion triggers apoptotic signals for endoplasmic reticulum (ER) overload response induced by overexpressed reticulon 3 (RTN3/HAP). J Cell Physiol. 2005;204:549–559. doi: 10.1002/jcp.20340. [DOI] [PubMed] [Google Scholar]
- Oertle T, Merkler D, Schwab ME. Do cancer cells die because of Nogo-B? Oncogene. 2003;22:1390–1399. doi: 10.1038/sj.onc.1206278. [DOI] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kim JE, Bonilla IE, Qiu D, Strittmatter SM. Nogo-C is sufficient to delay nerve regeneration. Mol Cell Neurosci. 2003;23:451–459. doi: 10.1016/S1044-7431(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Fouad K, Klusman I, Schwab ME. Regenerating corticospinal fibers in the marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur J Neurosci. 2004;20:2479–2482. doi: 10.1111/j.1460-9568.2004.03716.x. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/S0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Cheatwood JL, Bollnow MR, Kolb BE, Schwab ME, Kartje GL. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb Cortex. 2006;16:529–536. doi: 10.1093/cercor/bhi132. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Alsbiei T, O'Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Banos K, Yee KM. Response to: Kim et al., "Axon regeneration in young adult mice lacking Nogo-A/B." Neuron 38, 187-199. Neuron. 2007;54:191–195. doi: 10.1016/j.neuron.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Kim JE, Lee JK, Strittmatter SM. Response to correspondence: Kim et al., "axon regeneration in young adult mice lacking Nogo-A/B." Neuron 38, 187-199. Neuron. 2007;54:195–199. doi: 10.1016/j.neuron.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Schnell L, Montani L, Duncan C, Simonen M, Schneider R, Liebscher T, Gullo M, Schwab ME. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/S0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. No Nogo: now where to go? Neuron. 2003;38:153–156. doi: 10.1016/S0896-6273(03)00233-2. [DOI] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, Cate R, Strittmatter SM, Nikolov DB. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 2003;22:3291–3302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Bazan JF, G. M, Park JB, Wang K, Tessier-Lavigne M, He Z, Garcia KC. Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron. 2003;38:177–185. doi: 10.1016/S0896-6273(03)00232-0. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Kalb RG, Strittmatter SM. Rho GTPases and axonal growth cone collapse. Methods Enzymol. 2000;325:473–482. doi: 10.1016/s0076-6879(00)25467-0. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellezam B, Dubreuil C, Winton M, Loy L, Dergham P, Selles-Navarro I, McKerracher L. Inactivation of intracellular Rho to stimulate axon growth and regeneration. Prog Brain Res. 2002;137:371–380. doi: 10.1016/s0079-6123(02)37028-6. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Lu Y, Qahwash I, Hu XY, Chang A, Yan R. Reticulon family members modulate BACE1 activity and amyloid-beta peptide generation. Nat Med. 2004;10:959–965. doi: 10.1038/nm1088. [DOI] [PubMed] [Google Scholar]
- Murayama KS, Kametani F, Saito S, Kume H, Akiyama H, Araki W. Reticulons RTN3 and RTN4-B/C interact with BACE1 and inhibit its ability to produce amyloid beta-protein. Eur J Neurosci. 2006;24:1237–1244. doi: 10.1111/j.1460-9568.2006.05005.x. [DOI] [PubMed] [Google Scholar]
- Yokota T, Mishra M, Akatsu H, Tani Y, Miyauchi T, Yamamoto T, Kosaka K, Nagai Y, Sawada T, Heese K. Brain site-specific gene expression analysis in Alzheimer's disease patients. Eur J Clin Invest. 2006;36:820–830. doi: 10.1111/j.1365-2362.2006.01722.x. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Gonzalez de Aguilar JL, di Scala F, Rene F, de Tapia M, Pradat PF, Lacomblez L, Seihlan D, Prinjha R, Walsh FS, et al. Nogo provides a molecular marker for diagnosis of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10:358–365. doi: 10.1006/nbdi.2002.0522. [DOI] [PubMed] [Google Scholar]
- Jokic N, Gonzalez de Aguilar JL, Pradat PF, Dupuis L, Echaniz-Laguna A, Muller A, Dubourg O, Seilhean D, Hauw JJ, Loeffler JP, et al. Nogo expression in muscle correlates with amyotrophic lateral sclerosis severity. Ann Neurol. 2005;57:553–556. doi: 10.1002/ana.20420. [DOI] [PubMed] [Google Scholar]
- Pradat PF, Bruneteau G, Gonzalez de Aguilar JL, Dupuis L, Jokic N, Salachas F, Le Forestier N, Echaniz-Laguna A, Dubourg O, Hauw JJ, et al. Muscle Nogo-A expression is a prognostic marker in lower motor neuron syndromes. Ann Neurol. 2007;62:15–20. doi: 10.1002/ana.21122. [DOI] [PubMed] [Google Scholar]
- Wojcik S, Engel WK, Askanas V. Increased expression of Noga-A in ALS muscle biopsies is not unique for this disease. Acta Myol. 2006;25:116–118. [PubMed] [Google Scholar]
- Jokic N, Gonzalez de Aguilar JL, Dimou L, Lin S, Fergani A, Ruegg MA, Schwab ME, Dupuis L, Loeffler JP. The neurite outgrowth inhibitor Nogo-A promotes denervation in an amyotrophic lateral sclerosis model. EMBO Rep. 2006;7:1162–1167. doi: 10.1038/sj.embor.7400826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson C, Libelius R, Tagerud S. Nogo (Reticulon 4) expression in innervated and denervated mouse skeletal muscle. Mol Cell Neurosci. 2003;22:298–307. doi: 10.1016/S1044-7431(02)00036-2. [DOI] [PubMed] [Google Scholar]
- Reindl M, Khantane S, Ehling R, Schanda K, Lutterotti A, Brinkhoff C, Oertle T, Schwab ME, Deisenhammer F, Berger T, et al. Serum and cerebrospinal fluid antibodies to Nogo-A in patients with multiple sclerosis and acute neurological disorders. J Neuroimmunol. 2003;145:139–147. doi: 10.1016/j.jneuroim.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Karnezis T, Mandemakers W, McQualter JL, Zheng B, Ho PP, Jordan KA, Murray BM, Barres B, Tessier-Lavigne M, Bernard CC. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat Neurosci. 2004;7:736–744. doi: 10.1038/nn1261. [DOI] [PubMed] [Google Scholar]
- Mannan AU, Krawen P, Sauter SM, Boehm J, Chronowska A, Paulus W, Neesen J, Engel W. ZFYVE27 (SPG33), a novel spastin-binding protein, is mutated in hereditary spastic paraplegia. Am J Hum Genet. 2006;79:351–357. doi: 10.1086/504927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan AU, Boehm J, Sauter SM, Rauber A, Byrne PC, Neesen J, Engel W. Spastin, the most commonly mutated protein in hereditary spastic paraplegia interacts with Reticulon 1 an endoplasmic reticulum protein. Neurogenetics. 2006;7:93–103. doi: 10.1007/s10048-006-0034-4. [DOI] [PubMed] [Google Scholar]
- ClustalW2 http://www.ebi.ac.uk/Tools/clustalw2
- Phylo_win http://pbil.univ-lyon1.fr/software/phylowin.html