Abstract
Splicing of nuclear precursors of mRNA (pre-mRNA) involves dynamic interactions between the RNA constituents of the spliceosome. The rearrangement of RNA–RNA interactions, such as the unwinding of the U4/U6 duplex, is believed to be driven by ATP-dependent RNA helicases. We recently have shown that spliceosomal U5 small nuclear ribonucleoproteins (snRNPs) from HeLa cells contain two proteins, U5–200kD and U5–100kD, which share homology with the DEAD/DEXH-box families of RNA helicases. Here we demonstrate that purified U5 snRNPs exhibit ATP-dependent unwinding of U4/U6 RNA duplices in vitro. To identify the protein responsible for this activity, U5 snRNPs were depleted of a subset of proteins under high salt concentrations and assayed for RNA unwinding. The activity was retained in U5 snRNPs that contain the U5–200kD protein but lack U5–100kD, suggesting that the U5–200kD protein could mediate U4/U6 duplex unwinding. Finally, U5–200kD was purified to homogeneity by glycerol gradient centrifugation of U5 snRNP proteins in the presence of sodium thiocyanate, followed by ion exchange chromatography. The RNA unwinding activity was found to reside exclusively with the U5–200kD DEXH-box protein. Our data raise the interesting possibility that this RNA helicase catalyzes unwinding of the U4/U6 RNA duplex in the spliceosome.
Keywords: helicase, precursors of mRNA, RNA processing, spliceosome
The spliceosome, which catalyzes splicing of nuclear precursor mRNA (pre-mRNA), is formed by an ordered recruitment of the small nuclear ribonucleoproteins (snRNPs) U1, U2, U5 and U4/U6, and numerous non-snRNP proteins onto the pre-mRNA (1, 2). During spliceosome assembly a complex and highly dynamic network of both small nuclear RNA (snRNA)–snRNA and snRNA–pre-mRNA interactions is formed (1, 3, 4). Initially, U1 snRNA hybridyzes with the 5′ splice site, and U2 snRNA interacts with the branch site region of a pre-mRNA. Although the latter interaction is likely to persist through all steps of splicing (3), U1 snRNA must be displaced from the intron in an ATP-dependent reaction when the [U4/U6.U5] tri-snRNP enters the pre-spliceosome (5). Before or concomitant with this step, the phylogenetically conserved duplex between U4 and U6 snRNA, which exists in the [U4/U6.U5] tri-snRNP, dissociates and U6 snRNA interacts simultaneously with U2 snRNA and the 5′ splice site (reviewed in refs. 1, 3, and 4). The unwinding of the U4/U6 RNA duplex thus is essential for establishing an RNA network in the catalytic center of the spliceosome. What triggers this or other rearrangements of RNA is presently unknown. Yet it is generally thought that one or more spliceosomal proteins of the DEAD/DEXH-box family of ATP-dependent RNA helicases (6, 7) play a major role in these events (4, 8, 9). In the yeast Saccharomyces cerevisiae, the precursor RNA processing proteins Prp2p, Prp5p, Prp16p, Prp22p, Prp28p, and Prp43p have been identified as putative DEAD/DEXH-box RNA helicases required for pre-mRNA splicing (10–15), and potential mammalian homologs of some are known (16–19). Indirect evidence that they may function as ATP-dependent RNA helicases was obtained for Prp2p, Prp5p, and Prp16p, which have been shown to exhibit RNA-dependent ATPase activity (20–22) and to induce structural changes in the spliceosome in an ATP-dependent manner (21, 23, 24). However, the RNA targets of these proteins are unknown and bona fide RNA helicase activity remains to be demonstrated.
We previously reported that the HeLa U5 snRNP proteins U5–200kD and U5–100kD (the human homolog of Prp28p) contain either two DEXH-box domains or one DEAD-box domain, respectively (17, 25). The S. cerevisiae homolog of U5–200kD, Snu246p (25) [also termed Brr2p (26), Slt22p (27), or Rss1p (28)] is also an integral U5 snRNP protein (25) and is essential for pre-mRNA splicing in yeast (25–28). Consistent with a putative function as ATP-dependent RNA helicases, the human U5–200kD and U5–100kD proteins have been shown to bind ATP in vitro (29). The presence of these U5 proteins also in the [U4/U6.U5] tri-snRNP (17, 25) raises the interesting possibility that one or both could be involved in the dissociation of the U4/U6 duplex.
The first goal of this study was to investigate whether the proteins of HeLa U5 snRNPs can mediate the unwinding of a U4/U6 RNA duplex in vitro. We demonstrate here that intact U5 snRNPs efficiently unwind this duplex in an ATP-dependent manner and provide evidence that the activity resides with the group of U5 snRNP-specific proteins. After salt-induced dissociation of U5 snRNPs and glycerol gradient centrifugation of its proteins, the activity was found to fractionate only in company of the U5–200kD RNA helicase, but was completely absent in any other protein fractions (including the second helicase, U5–100kD). By additional ion exchange chromatography, the U5–200kD protein was isolated from all other U5 proteins. Again, the unwinding of RNA occurred only where U5–200kD was present. These data strongly indicate that the U5–200kD DEXH-box RNA helicase is the U5 snRNP protein that catalyzes U4/U6 RNA unwinding in vitro. Moreover, our observations support the idea that U4/U6 could be the natural substrate of U5–200kD in the spliceosome.
MATERIALS AND METHODS
Purification of U5 snRNPs.
A mixture of 20S U5, 12S U1, 12S U2, and 25S [U4/U6.U5] snRNPs was obtained by α-m3G-immunoaffinity chromatography of HeLa nuclear extract and subsequently fractionated by glycerol gradient centrifugation in G-buffer (0.15 M KCl/20 mM Hepes/KOH pH 7.9/1.5 mM MgCl2/0.5 mM dithiothreitol/0.5 mM phenylmethylsulfonyl fluoride) (30). Fractions containing U5 snRNPs were subjected to chromatography on an FPLC MonoQ ion exchange column (Pharmacia) and again centrifuged on a glycerol gradient (30). For a partial dissociation of [U4/U6.U5] snRNPs in 0.7 M KCl, tri-snRNPs obtained from a glycerol gradient fractionation of α-m3G-immunoaffinity eluates (see above) were concentrated by ultracentrifugation (265,000 × g, 10 h, 4°C) and the pellet was resuspended in G-buffer containing 0.7 M KCl (G700). Subsequently, the proteins were centrifuged on a 10–30% (wt/wt) glycerol gradient in G700 (7 h, 118,000 × g, 4°C) and analyzed by SDS/PAGE. The isolation of pure U5–200kD protein from U5 snRNPs was essentially as described for the high-salt treatment of [U4/U6.U5] snRNPs, except that the concentrated U5 snRNPs were resuspended and centrifuged on a 5–20% (wt/wt) glycerol gradient in the presence of 0.4 M sodium thiocyanate (NaSCN) rather than KCl (118,000 × g, 23 h, 4°C). MonoQ chromatography of U5–200kD protein was performed after a 3-fold dilution of the U5–200kD-gradient fractions in 20 mM Hepes/KOH, pH 7.9, 1.5 mM MgCl2, on a SMART MonoQ column (Pharmacia). The proteins were eluted by a 0.15 to 1 M NaSCN gradient (4°C). Before analysis of RNA unwinding activity, the collected fractions were dialyzed against G-buffer containing 100 mM KCl and 5% (vol/vol) glycerol. Immunoblot analyses were carried out with an ECL RPN2106 reaction kit (Amersham) according to the manufacturer’s instructions. Antigens used to induce polyclonal rabbit antisera against the U5–200kD and U5–100kD proteins were described previously (9, 10).
Preparation of RNA and DNA Duplices.
Plasmids used for in vitro transcription of the S. cerevisiae U4 and U6 snRNA sequences were generated and kindly provided by Patrizia Fabrizio (University of Marburg, Germany) (31). The plasmids encoding C4 or MO Ia RNA were a generous gift from Hans Stahl (University of Homburg/Saar, Germany) (32). For each RNA duplex prepared, one strand was radiolabeled with [α-32P]-UTP (specific activity 3,000 Ci/mmol), and the RNA products were purified by denaturing gel electrophoresis. The amount of RNA transcript was determined according to the radioactivity incorporated and by UV spectrophotometry of the nonlabeled product. Radiolabeled RNA (0.3 pmol) was incubated with a 5- to 10-fold excess of nonlabeled RNA in a volume of 20 μl in 400 mM NaCl, 1.5 mM MgCl2, and 2 mM EDTA. After heating at 90°C for 3 min, the samples were slowly cooled to room temperature. The DNA duplex was generated from a 33-mer universal primer, which was extended by four nucleotides in the presence of [α-32P]-dATP and dCTP, using Taq polymerase and an M13 mp7 DNA template, according to Stahl et al. (33). Before RNA unwinding assays, the efficiency of duplex formation was monitored by native gel electrophoresis as described below.
RNA Unwinding Assay.
In a standard assay, 10 fmol of duplex was incubated with either 100 fmol of U5 snRNP or 1 pmol of U5–200kD protein, in the presence of 200 μM ATP, 50 mM NaCl, 30 mM Tris/Cl (pH 7.0), 1.2 mM MgCl2, 1.5 mM DTT, 0.5 mM EDTA, 20 units of RNasin, and 1.2 μg of acetylated BSA. After incubation at 40°C for 1 h, the reactions were stopped by the addition of 150 mM EDTA, 50% (vol/vol) glycerol, 2% (wt/vol) SDS, and 0.25% (wt/vol) xylene cyanol. The duplex was separated from single-stranded products by standard SDS/11% PAGE at 4°C. The molar amount of U5 snRNPs was calculated from the apparent molecular weights of U5 proteins and the U5 snRNA (34).
RESULTS
ATP-Dependent RNA Unwinding Activity of Purified HeLa U5 snRNPs.
To investigate whether U5 snRNP proteins could function as RNA helicases, we initially tested highly purified U5 snRNPs for an RNA unwinding activity in vitro. As a substrate we used an RNA duplex formed by annealing in vitro transcribed U4 and U6 RNAs from S. cerevisiae. The yeast U4 and U6 RNAs are highly homologous to their human counterparts (35), yet duplices formed from the yeast RNAs in vitro are more homogenous (36) and exhibit the correct Y-shaped secondary structure in solution (C. Branlant, personal communication).
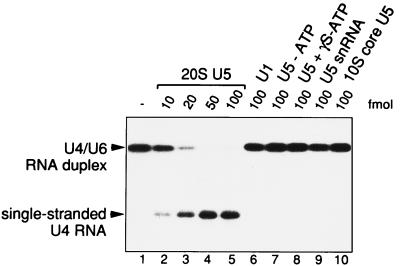
As shown in Fig. 1, purified 20S U5 snRNPs unwound the U4/U6 RNA duplex in the presence of 200 μM ATP. ATP hydrolysis was required as no RNA unwinding activity was observed either in the absence of ATP or with the nonhydrolyzable ATP analog γS-ATP (Fig. 1). The reaction was saturable with a 5- to 10-fold molar excess of U5 snRNPs over the U4/U6 duplex (50–100 fmol snRNP). As a control, no RNA unwinding activity was observed with purified U1 snRNPs, isolated U5 snRNA, or, most significantly, the 10S core U5 snRNP, which contains the Sm proteins but lacks specific U5 proteins (Fig. 1). These data suggested that the U5-specific proteins are responsible for the RNA unwinding activity observed with 20S U5 snRNPs.
Figure 1.
ATP-dependent RNA unwinding activity of MonoQ-purified mammalian 20S U5 snRNPs. The unwinding of a [32P]-U4/U6 snRNA duplex (10 fmol) by 20S U5 snRNPs was assayed in the presence of ATP (lanes 2–5), without ATP (lane 7), or with the nonhydrolyzable analog γS-ATP (lane 8). Unwinding of the U4/U6 RNA duplex also was monitored with U5 snRNA (lane 9), 10S core U5 snRNPs (lane 10), and 12S U1 snRNPs (lane 6), in the presence of ATP. The concentrations of snRNPs or U5 snRNA used are indicated. The [32P]-U4/U6 snRNA duplex in the absence of protein is shown in lane 1. The positions of duplex and single-stranded U4 RNA are indicated. Details concerning the analysis of RNA unwinding are described in Materials and Methods.
RNA Unwinding Activity of U5 snRNPs Depleted from a Subset of Proteins.
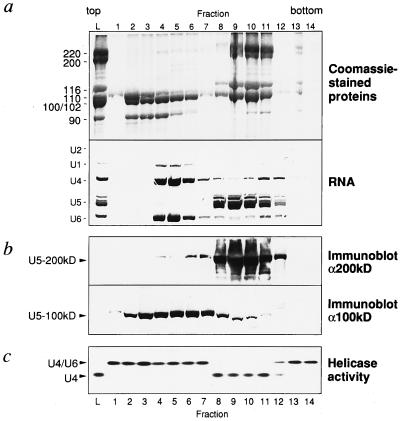
We next tested whether RNA unwinding activity required intact U5 snRNP or also could be observed with individual U5 snRNP proteins. To obtain U5 snRNPs partially depleted of a subset of proteins, a mixture of 20S U5 and [U4/U6.U5] tri-snRNPs was incubated in the presence of 0.7 M KCl and subjected to centrifugation on glycerol gradients. Under these conditions, the [U4/U6.U5] tri-snRNPs were almost completely dissociated into U5 and U4/U6 snRNPs (as judged by RNA analysis of the gradient fractions, Fig. 2a, Lower), and proteins known to be bound to the tri-snRNP in a salt-sensitive manner (e.g., the 90-kDa U4/U6-specific protein) (34) were found in the top fractions of the gradient (Fig. 2a, Upper). In addition, at least two U5-specific proteins with molecular masses in the 100- to 110-kDa range also sedimented at the top of the glycerol gradient (Fig. 2a, Upper). One of these was identified by immunoblotting as the DEAD-box protein U5–100kD (Fig. 2b, Lower); only minor amounts of U5–100kD remained associated with the U5 snRNP particle (Fig. 2b, Lower, compare lanes 2–7 with 9–11). In contrast, dissociation of the U5–200kD protein was not observed under these conditions (Fig. 2b, Upper). Significantly, gradient fractions of U5 snRNPs that lacked the U5–100kD DEAD-box protein (Δ100kD-U5 snRNPs) efficiently unwound the U4/U6 duplex (Fig. 2c). In comparison, the activity was absent in fractions 2–7, which contain the U5–100kD protein and lack U5–200kD (Fig. 2c). It also should be noted that similar extents of U4/U6 duplex unwinding were observed with equimolar amounts of both Δ100kD-U5 snRNPs or intact 20S U5 snRNPs, indicating that the U5–100kD protein does not play a major role in the dissociation of U4/U6 RNA duplices under our assay conditions (data not shown).
Figure 2.
Fractionation of 20S U5 and [U4/U6.U5] snRNPs under high salt conditions yields a Δ100-kDa U5 snRNP active in RNA unwinding. (a) The composition of proteins (Upper; for clarity, only high molecular weight proteins are shown) and snRNAs (Lower) after centrifugation in high salt (0.7 M KCl) glycerol gradients. (b) Immunodetection of the putative RNA helicases U5–200kD (Upper) and U5–100kD (Lower) from fractions displayed in a. (c) RNA unwinding activity of gradient fractions. An equivalent volume of each gradient fraction was assayed for RNA unwinding activity. Lane L, a mixture of 20S U5 and [U4/U6.U5] snRNPs before dissociation in 0.7 M KCl. The positions of proteins and snRNAs are indicated to the left.
Copurification of RNA Unwinding Activity with the U5 snRNP-Specific 200-kDa Protein (U5–200kD).
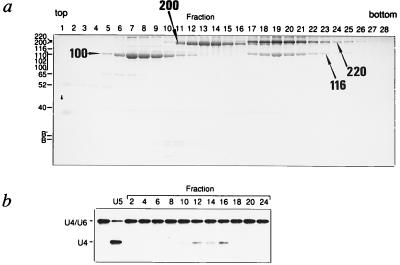
The data presented thus far suggest that U5–200kD may be responsible for the RNA unwinding activity of U5 snRNPs. To provide direct evidence for this hypothesis, the U5–200kD protein was dissociated from purified 20S U5 snRNPs by incubation with 0.4 M NaSCN and centrifugation through a NaSCN-containing glycerol gradient. This procedure dissociates the 20S U5 snRNP into 10S U5 snRNP core particles and free U5-specific proteins. Fig. 3a shows the distribution of proteins after glycerol gradient fractionation of NaSCN-dissociated U5 snRNPs. 10S core U5 snRNPs were found in fractions 9–13 as indicated by the presence of the B/B′ Sm proteins (Fig. 3a) and U5 snRNA in these fractions (data not shown). Significantly, the U5–200kD protein, which migrates in fractions 11–16 of the gradient, is well separated from all other high molecular weight U5 snRNP proteins (Fig. 3a). Only minor amounts of the other U5-specific proteins are present in the U5–200kD-containing fractions; two of the high molecular weight contaminants are in fact degradation products of U5–200kD, as revealed by immunoblotting (data not shown). After removal of NaSCN by dialysis, gradient fractions were monitored for RNA unwinding activity (Fig. 3b). Only those fractions that contained significant amounts of U5–200kD protein (fractions 11–16) were active in unwinding the U4/U6 duplex (note that differences in activity displayed by the 200-kDa-containing fractions may be caused by variances in protein concentration after dialysis). Thus, our observations are consistent with the idea that U5–200kD might contain an intrinsic RNA unwinding activity.
Figure 3.
Isolation of the U5–200kD protein from purified 20S U5 snRNPs. (a) U5 snRNP proteins fractionated by glycerol gradient centrifugation in the presence of 0.4 M NaSCN. The positions of proteins are indicated to the left. The most prominent U5 snRNP proteins U5–100kD, U5–200kD, U5–220kD, and U5–116kD are indicated on the gel by arrows. The 10S core U5 snRNP is indicated by the presence of the Sm proteins B/B′ (additional Sm proteins are not shown in this figure). The 100- to 110-kDa band contains in addition to U5–100kD the U5-specific 102-kDa and 110-kDa proteins. The U5-specific 40-kDa protein sedimenting across the gradient is a novel WD40-repeat protein and does not encompass an RNA helicase-like domain (T.A. and R.L., unpublished data). (b) RNA unwinding activity of individual fractions from the gradient shown in a. For U4/U6 RNA unwinding analysis, equivalent volumes were taken from the gradient fractions and dialyzed against G-buffer containing 5% glycerol. The amount of U5–200kD protein provided in this assay was ca. 100 ng for a peak fraction (fractions 11–16).
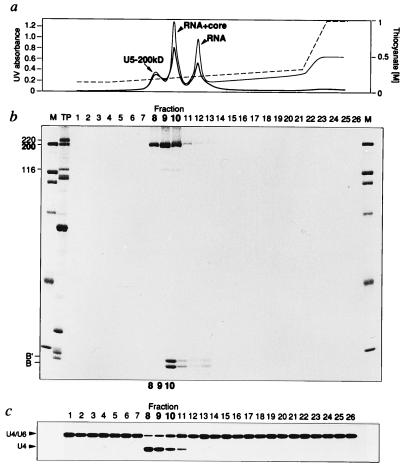
To further purify the U5–200kD protein, we subjected the U5–200kD containing fractions of an identically prepared glycerol gradient to MonoQ chromatography. Elution with a NaSCN gradient yielded three peaks (Fig. 4a). The analysis of the protein composition from each fraction showed that U5–200kD, the major protein component of the first peak, was eluted in highest purity in fraction 8 (Fig. 4b). In addition to U5–200kD, fraction 9 contained trace amounts of the U5–116kD protein, a G domain protein that lacks any RNA helicase domain (37). In fraction 10, U5–200kD coeluted with Sm core proteins (Fig. 4b) and U5 snRNA (not shown), whereas fractions 12–14 contained predominantly snRNA (data not shown). As shown in Fig. 4c, significant unwinding activity was observed only in fractions 8–10, all of which contain U5–200kD.
Figure 4.
Highly purified U5–200kD protein successively fractionated on NaSCN-glycerol gradients and by MonoQ chromatography is still active in U4/U6 RNA duplex unwinding. (a) Elution profile from the MonoQ column. The absorbance of the eluted fractions is measured at 280 nm (thick line) and 260 nm (thin line). The content of the peak eluates is indicated. The NaSCN concentrations are shown to the right, and the step gradient for elution is displayed by a broken line. (b) Coomassie-staining of the proteins present in the eluted fractions. M, molecular mass standards of 200, 116, 97, 66, 45, and 34 kDa. TP, total proteins from a mixture of spliceosomal snRNPs. (c) RNA unwinding activity of the eluted fractions. After dialysis against G-buffer with 5% glycerol, equivalent volumes of the eluted fractions were assayed for U4/U6 RNA unwinding in the presence of 200 μM ATP.
Most importantly, the specific activity was highest in fraction 8, which contains essentially pure U5–200kD protein and no detectable contaminants. A somewhat lower specific activity is observed in fractions 9 and 10, which could be interpreted as either an inhibitory effect of the proteins copurifying in these fractions, or that fractions 8–10 differ with respect to their content of inactive U5–200kD protein.
In the highly pure fraction 8, a ca. 100-fold molar excess of U5–200kD (200 ng) over the RNA duplex was required to observe almost complete duplex unwinding. The increased amount of purified U5–200kD required for RNA unwinding, as compared with U5 snRNPs (100-fold vs. 5-fold molar excess over duplex), could be caused by partial denaturation of the purified U5–200kD protein during fractionation. In addition, the unwinding activity could be assisted by protein–protein interactions within the U5 snRNP.
Investigation of the Substrate Requirements of U5–200kD in Vitro.
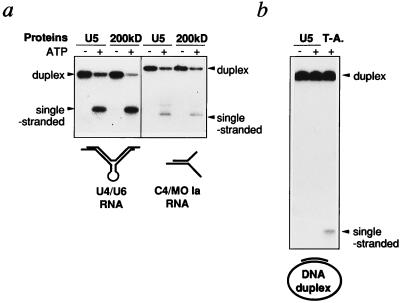
As a first step toward understanding the mechanism of RNA unwinding by U5–200kD, we investigated its specificity for duplex substrates in vitro. Interestingly, isolated 20S U5 snRNPs and highly purified U5–200kD protein (fraction 8, Fig. 4b) are not only active in dissociating U4/U6 RNA duplices but both also unwind a 17-bp-long RNA duplex (C4/MO Ia RNA) (32) unrelated to U4 and U6 RNAs (Fig. 5a). U5 snRNPs, however, do not unwind a DNA duplex (33), which under the same conditions is separated by incubation with simian virus 40 T antigen (Fig. 5a). This finding indicates that 2′ hydroxyl groups in the sugar backbone are critical for substrate recognition by U5–200kD, yet not the base sequence. Apparently, it is not the peculiar structure of U4/U6 RNA that U5–200kD recognizes, but rather the base-paired nature of the RNA.
Figure 5.
Substrate specificity of the ATP-dependent RNA unwinding activity. The reactions were incubated with ≈10 fmol of the corresponding duplex in the presence or absence of 200 μM ATP, as indicated. The position of the duplices and radiolabeled single-stranded RNA are indicated. The secondary structures of each substrate are represented schematically at the bottom. (a) RNA unwinding activity of 20S U5 snRNPs or isolated U5–200kD protein with U4/U6 RNA (Left) or the nonspliceosomal C4/MO Ia RNA (Right). The unwinding activity was investigated with 100 fmol U5 snRNPs or 1 pmol U5–200kD. (b) A 37-bp DNA duplex was incubated with ≈100 fmol 20S U5 snRNPs or 20 ng of hexameric large T antigen (denoted T-A.).
DISCUSSION
In this study, we have provided direct evidence that purified U5 snRNPs catalyze an ATP-dependent unwinding of U4/U6 RNA duplices in vitro. The salt-induced dissociation of proteins from U5 snRNPs revealed that U4/U6 RNA unwinding activity appears only in a protein complex that contains the putative DEXH-box RNA helicase U5–200kD. The second putative RNA helicase present in U5 snRNPs, U5–100kD, does not display such activity. U5–200kD finally was isolated from all other proteins by glycerol gradient centrifugation in the presence of NaSCN, combined with ion exchange chromatography (Fig. 4, fraction 8). U5–200kD is the single U5 snRNP protein isolated that unwinds U4/U6 RNA.
In view of the high purity of the final U5–200kD preparation and the exclusive appearance of RNA unwinding activity with U5–200kD through all fractionation steps, our data strongly suggest that this activity is an intrinsic property of the U5–200kD protein and not caused by an as yet unidentified contaminant. In this context it also is interesting to note that U5 snRNPs and highly purified U5–200kD protein both share the same nucleoside triphosphate requirement, in that RNA unwinding activity is observed only in the presence of ATP or dATP and, less efficiently, with CTP/dCTP, but not with GTP or UTP (data not shown). The NTP requirement of U5–200kD is thus more specific than that of spliceosomal proteins that exhibit an RNA-dependent NTPase activity, such as Prp2p (20) and Prp16p (23).
The mechanism by which U5–200kD mediates the dissociation of RNA is at present not understood. This understanding will require the expression and isolation of soluble recombinant U5–200kD protein, which thus far has proven problematic. Yet our observation that, in vitro, U5–200kD unwinds not only U4/U6 RNA but also nonrelated RNA duplices indicates that this reaction is not a fortuitous event relying on the particular structure of U4/U6 RNA. On the contrary, because low substrate specificity in vitro appears to be a general characteristic of RNA helicases (ref. 7, and references therein), these data support the idea that U5–200kD functions like a genuine RNA helicase.
Because U4/U6 RNA is not the sole substrate of U5–200kD in vitro, the questions arise whether this duplex is the natural target of U5–200kD in the spliceosome and what confers specificity in vivo. U5–200kD could modulate RNA structures either before or subsequent to the entry of the [U4/U6.U5] snRNP into the spliceosome. Within the precatalytic spliceosome, several RNA duplices may be envisaged as potential substrates for U5–200kD, such as the interaction of U1 snRNA with the 5′ splice site of the pre-mRNA, the unwinding of internal helices in U2 snRNA, or the U4/U6 RNA duplex shown here to be unwound by U5–200kD in vitro.
Indeed, several observations from this and other studies suggest that U4/U6 RNA may indeed be unwound by U5–200kD in vivo. Unlike helicases that interact transiently with the spliceosomal components (20, 22), U5–200kD is stably associated with the [U4/U6.U5] tri-snRNP (25). It is reasonable to think that the molecular environment of U5–200kD in the snRNP determines substrate specificity, and therefore its close proximity to U4/U6 RNA makes it a likely candidate for mediating the release of U4 from U6 RNA in the spliceosome. Although it is difficult to prove this idea in intact spliceosomes, one might expect, however, that isolated [U4/U6.U5] tri-snRNPs should exhibit ATP-dependent unwinding of the endogenous U4/U6 duplex. This so far has not been observed in highly purified HeLa tri-snRNPs, which may be explained by the lack of subsequent destabilizing interactions (such as U2/U6 duplex formation) that occur in the spliceosome after U4/U6 dissociation. Interestingly, P. Ragunathan and C. Guthrie recently have shown the ATP-dependent dissociation of free U4 and U6 snRNAs from a large snRNP complex containing U2 and [U4/U6.U5] tri-snRNPs from S. cerevisiae (C. Guthrie, personal communication). Their observation that U4/U6 dissociation did not occur when the multi-snRNP complex contained a mutant form of Brr2p (the yeast homolog of U5–200kD) demonstrates the essential role of Brr2p in this event. Furthermore, Xu et al. (27) reported previously that the slt22–1 allele of the same protein is synthetically lethal with mutations in U2 RNA that destabilize the U2/U6 base-pairing interactions, and Slt22p was proposed to proofread this base-pairing interaction. The results obtained from U5–200kD, together with the genetic studies of its homolog in yeast, suggest that this protein plays an important role in dissociation of the U4/U6 duplex and subsequent U2/U6 duplex formation. In this respect, it will be interesting to investigate in future studies whether the unique occurrence of two DEXH-box helicase domains in U5–200kD is important for the function of this protein.
To date, the function of the second RNA helicase in U5 snRNPs, U5–100kD (17), remains enigmatic. Our observation that, in contrast to U5–200kD, the U5–100kD protein did not unwind U4/U6 RNA simply may indicate that this protein requires conditions we have not provided in the assay. For example, the RNA unwinding activity of U5–100kD may depend on interactions with other spliceosomal proteins (which could be mediated by its Arg/Ser-rich domain). Nonetheless, the existence of U5–100kD in the [U4/U6.U5] tri-snRNP, together with the potential U4/U6 unwinding protein U5–200kD, suggests that its function may be logically connected to this reaction. For example, U5–100kD may induce disruption of the duplex formed between U1 snRNA and pre-mRNA, which occurs concomitant with or subsequent to unwinding of the U4/U6 duplex. The two helicases U5–200kD and U5–100kD in U5 snRNPs thus might act in a highly regulated and sequential way toward formation of a catalytic spliceosome.
Acknowledgments
We thank Pratima Ragunathan and Christine Guthrie for communicating results before publication. We are grateful to Hans Stahl (University of Homburg/Saar, Germany) and Patrizia Fabrizio for plasmids and helpful discussions, and to Irene Öchsner, Hero Brahms, and Claudia Schneider for snRNPs. We thank Nicholas Watkins and Cindy Will for a critical review of the manuscript. This study was funded by the Deutsche Forschungsgemeinschaft. B.L. was supported by the Fonds der Deutschen Chemischen Industrie.
ABBREVIATIONS
- pre-mRNA
precursors of mRNA
- snRNA
small nuclear RNA
- snRNP
small nuclear ribonucleoprotein
- Prp
precursor RNA processing
- NaSCN
sodium thiocyanate
References
- 1.Moore M J, Query C C, Sharp P A. In: The RNA World. Gesteland R F, Atkins J F, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 303–357. [Google Scholar]
- 2.Will C L, Lührmann R. Curr Opin Cell Biol. 1997;9:320–327. doi: 10.1016/s0955-0674(97)80003-8. [DOI] [PubMed] [Google Scholar]
- 3.Nilsen T W. Cell. 1994;78:1–4. doi: 10.1016/0092-8674(94)90563-0. [DOI] [PubMed] [Google Scholar]
- 4.Madhani H D, Guthrie C. Annu Rev Genet. 1994;28:1–26. doi: 10.1146/annurev.ge.28.120194.000245. [DOI] [PubMed] [Google Scholar]
- 5.Konforti B B, Koziolkiewicz M J, Konarska M M. Cell. 1993;75:863–873. doi: 10.1016/0092-8674(93)90531-t. [DOI] [PubMed] [Google Scholar]
- 6.Schmid S R, Linder P. Mol Microbiol. 1992;6:283–91. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 7.Gorbalenya A E, Koonin E V. Curr Opin Struct Biol. 1993;3:419–429. [Google Scholar]
- 8.Wassarman D A, Steitz J A. Nature (London) 1991;349:463–464. doi: 10.1038/349463a0. [DOI] [PubMed] [Google Scholar]
- 9.Beggs J D. In: Pre-mRNA Processing. Lamond A I, editor. Austin, TX: Landes; 1995. pp. 79–95. [Google Scholar]
- 10.Lee M G, Lane D P, Beggs J D. Yeast. 1986;2:59–67. doi: 10.1002/yea.320020105. [DOI] [PubMed] [Google Scholar]
- 11.Dalbadie-McFarland G, Abelson J. Proc Natl Acad Sci USA. 1990;87:4236–4240. doi: 10.1073/pnas.87.11.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couto J R, Tamm J, Parker R, Guthrie C. Genes Dev. 1987;1:445–455. doi: 10.1101/gad.1.5.445. [DOI] [PubMed] [Google Scholar]
- 13.Company M, Arenas J, Abelson J. Nature (London) 1991;349:487–493. doi: 10.1038/349487a0. [DOI] [PubMed] [Google Scholar]
- 14.Strauss E J, Guthrie C. Genes Dev. 1991;5:629–641. doi: 10.1101/gad.5.4.629. [DOI] [PubMed] [Google Scholar]
- 15.Arenas J E, Abelson J N. Proc Natl Acad Sci USA. 1997;94:11798–11802. doi: 10.1073/pnas.94.22.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono Y, Ohno M, Shimura Y. Mol Cell Biol. 1994;14:7611–7620. doi: 10.1128/mcb.14.11.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teigelkamp S, Mundt C, Achsel T, Will C, Lührmann R. RNA. 1997;3:1313–1326. [PMC free article] [PubMed] [Google Scholar]
- 18.Gee S, Krauss S W, Miller E, Aoyagi K, Arenas J, Conboy J. Proc Natl Acad Sci USA. 1997;94:11803–11807. doi: 10.1073/pnas.94.22.11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleckner J, Zhang M, Valcarcel J, Green M R. Genes Dev. 1997;11:1864–1872. doi: 10.1101/gad.11.14.1864. [DOI] [PubMed] [Google Scholar]
- 20.Kim S H, Smith J, Claude A, Lin R J. EMBO J. 1992;11:2319–2326. doi: 10.1002/j.1460-2075.1992.tb05291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Day C L, Dalbadie-McFarland G, Abelson J. J Biol Chem. 1996;271:33261–33267. doi: 10.1074/jbc.271.52.33261. [DOI] [PubMed] [Google Scholar]
- 22.Schwer B, Guthrie C. Nature (London) 1991;349:494–499. doi: 10.1038/349494a0. [DOI] [PubMed] [Google Scholar]
- 23.Schwer B, Guthrie C. EMBO J. 1992;11:5033–5039. doi: 10.1002/j.1460-2075.1992.tb05610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S-H, Lin R-J. Mol Cell Biol. 1996;16:6810–6819. doi: 10.1128/mcb.16.12.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauber J, Fabrizio P, Teigelkamp S, Lane W S, Hartmann E, Lührmann R. EMBO J. 1996;15:4001–4015. [PMC free article] [PubMed] [Google Scholar]
- 26.Noble S M, Guthrie C. Genetics. 1996;143:67–80. doi: 10.1093/genetics/143.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu D, Nouraini S, Field D, Tang S-J, Friesen J D. Nature (London) 1996;381:709–713. doi: 10.1038/381709a0. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Rossi J Y. RNA. 1996;2:835–848. [PMC free article] [PubMed] [Google Scholar]
- 29.Laggerbauer B, Lauber J, Lührmann R. Nucleic Acids Res. 1996;24:868–875. doi: 10.1093/nar/24.5.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Will C L, Kastner B, Lührmann R. In: RNA Processing. Higgins S J, Hames B D, editors. II. Oxford: IRL; 1994. pp. 141–177. [Google Scholar]
- 31.Fabrizio P, McPheeters D S, Abelson J. Genes Dev. 1989;3:2137–2150. doi: 10.1101/gad.3.12b.2137. [DOI] [PubMed] [Google Scholar]
- 32.Scheffner M, Knippers R, Stahl H. Cell. 1989;57:955–963. doi: 10.1016/0092-8674(89)90334-6. [DOI] [PubMed] [Google Scholar]
- 33.Stahl H, Dröge P, Knippers R. EMBO J. 1986;5:1939–1944. doi: 10.1002/j.1460-2075.1986.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauber J, Plessel G, Prehn S, Will C L, Fabrizio P, Gröning K, Lane W S, Lührmann R. RNA. 1997;3:926–941. [PMC free article] [PubMed] [Google Scholar]
- 35.Brow D A, Guthrie C. Nature (London) 1988;334:213–218. doi: 10.1038/334213a0. [DOI] [PubMed] [Google Scholar]
- 36.Brow D A, Vidaver R M. RNA. 1995;1:122–131. [PMC free article] [PubMed] [Google Scholar]
- 37.Fabrizio P, Laggerbauer B, Lauber J, Hartmann E, Lührmann R. EMBO J. 1997;16:4092–4106. doi: 10.1093/emboj/16.13.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]