Abstract
The dengue virus type 3 (DENV-3) vaccine candidate, rDEN3Δ30, was previously found to be under-attenuated in both SCID-HuH-7 mice and rhesus monkeys. Herein, two strategies have been employed to generate attenuated rDEN3 vaccine candidates which retain the full complement of structural and nonstructural proteins of DENV-3 and thus are able to induce humoral or cellular immunity to each of the DENV-3 proteins. First, using the predicted secondary structure of the 3’ untranslated region (3’-UTR) of DENV-3 to design novel deletions, nine deletion mutant viruses were engineered and found to be viable. Four of nine deletion mutants replicated efficiently in Vero cells and were genetically stable. Second, chimeric rDENV-3 viruses were generated by replacement of the 3’-UTR of the rDENV-3 cDNA clone with that of rDENV-4 or rDEN4Δ30 yielding the rDEN3-3’D4 and rDEN3-3’D4Δ30 viruses, respectively. Immunization of rhesus monkeys with either of two deletion mutant viruses, rDEN3Δ30/31 and rDEN3Δ86, or with rDEN3-3’D4Δ30 resulted in infection without detectable viremia, with each virus inducing a strong neutralizing antibody response capable of conferring protection from DENV-3 challenge. The rDEN3Δ30/31 virus showed a strong host range restriction phenotype with complete loss of replication in C6/36 mosquito cells despite robust replication in Vero cells. In addition, rDEN3Δ30/31 had reduced replication in Toxorynchites mosquitoes following intrathoracic inoculation. The results are discussed in the context of vaccine development and the physical structure of the DENV 3’-UTR.
Introduction
The four dengue virus serotypes (DENV-1, DENV-2, DENV-3, and DENV-4) circulate in tropical and subtropical regions of the world inhabited by more than 2.5 billion people [1]. The DENV are endemic in at least 100 countries and cause more disease in humans than any other arbovirus. Annually, an estimated 50–100 million dengue infections result in hundreds of thousands of cases of dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), with children bearing the brunt of the disease burden [2, 3]. DHF/DSS remains a leading cause of hospitalization and death of children in at least eight southeast Asian countries [2]. The dramatic increase in both the incidence and severity of disease caused by the four DENV serotypes over the past two decades is due in large part to the geographic expansion of the primary vectors, the peridomestic mosquito species Aedes aegypti and Ae. albopictus, and to the increased prevalence and co-circulation of the four DENV serotypes [1, 4]. In urban settings, DENV are maintained in a cycle of transmission between Aedes species and humans, with no other apparent viral reservoir [5].
The DENV, members of the Flaviviridae family, have a spherical shape of approximately 40 to 60 nm diameter that contain a single-stranded positive-sense RNA genome [6]. A single viral polypeptide is co-translationally processed by viral and cellular proteases generating three structural proteins (capsid C, membrane M, and envelope E) and at least seven non-structural (NS) proteins. The genome organization of the DENV is 5’-UTR-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-UTR-3’ (UTR – untranslated region, prM – membrane precursor) [7].
In response to the increasing incidence and severity of DENV infection, an economical vaccine that prevents disease caused by the DENV has become a global public health priority. The cost-effectiveness, safety, and long-term efficacy associated with the live attenuated vaccine against yellow fever (YF) virus, another mosquito-borne flavivirus, serves as a model for the feasibility of developing a live attenuated DENV vaccine [8]. An effective DENV vaccine should confer protection for each serotype since all four serotypes commonly circulate in endemic regions, and secondary infection with a heterologous serotype is associated with increased disease severity [9]. Unfortunately, previous attempts to develop a tetravalent, live attenuated vaccine against the DENV have found that one or more vaccine components exhibited either under- or over-attenuation resulting in unacceptable reactogenicity or poor immunogenicity, respectively [10, 11]. Modification of the concentration of one of the serotypes in a tetravalent vaccine has not been able to reliably correct problems of reactogenicity or over-attenuation [12–14]. Therefore, it appears that the path towards generation of an efficacious, live attenuated tetravalent DENV vaccine will include the development and evaluation of multiple vaccine candidates for each serotype to identify four individual viruses that can be combined into a formulation that successfully balances attenuation and immunogenicity. Access to a “menu” of vaccine candidates for each serotype will provide the flexibility to optimize a tetravalent formulation that is minimally reactogenic and that induces strong immunity to each serotype in humans.
Previously, we have employed two strategies for generating monovalent live attenuated vaccine candidates for each serotype that can then be combined into tetravalent formulations [15]. First, reverse genetics has been used to introduce an attenuating 30 nucleotide (nt) deletion (Δ30) mutation into the 3’-UTR of cDNA clones of each DENV serotype [16–21]. In initial studies, the rDEN4Δ30 vaccine candidate was found to be attenuated in rhesus monkeys and phase I/II clinical trials in humans have demonstrated that infection with vaccine virus results in low viremia, is strongly immunogenic, and exhibits minimal reactogenicity without serious adverse events [20, 22]. Recently, the rDEN1Δ30 vaccine candidate, which was also attenuated in rhesus monkeys, has been found to share a similar set of properties in clinical trials as that observed for rDEN4Δ30: low viremia, strong immunogenicity, and minimal reactogenicity in 20 volunteers [21]. Unfortunately, the rDEN2Δ30 and rDEN3Δ30 vaccine candidates did not appear to be satisfactorily attenuated in rhesus monkeys during pre-clinical testing and will not be tested in humans [16, 17]. Consequently, as an alternative strategy for vaccine development for DENV-2 and DENV-3, antigenic chimeric viruses have been generated by replacement of the structural proteins of the attenuated rDEN4Δ30 vaccine candidate with those from DENV-2 or DENV-3 yielding the rDEN2/4Δ30 and rDEN3/4Δ30 vaccine candidates, respectively [16, 23]. The rDEN2/4Δ30 vaccine virus has been tested in humans and appears safe and strongly immunogenic [24], and clinical evaluation of the rDEN3/4Δ30 virus is currently underway. However, the immune response to DENV-2 or DENV-3 would be directed predominantly against the M and E proteins or to cross-reactive determinants of DENV-4 proteins.
Here, we describe three additional vaccine candidates for the DENV-3 serotype generated by genetic modification of the 3’-UTR of the DENV-3 cDNA clone [16]. Development of these DENV-3 vaccine candidates, which possess the full complement of wild type DENV-3 proteins, is important for two reasons. First, the present vaccine candidate for DENV-3, rDEN3/4Δ30, may be found to be under- or over-attenuated in clinical trials as a monovalent vaccine or as a component of a tetravalent formulation. Second, an optimal vaccine for conferring protection from disease caused by DENV-3 may require induction of T cell responses against the entire set of DENV-3 proteins, rather than just the M and E which are the only DENV-3 sequences present in the rDEN3/4Δ30 chimeric virus. To generate additional DENV-3 vaccine candidates, nine novel deletions which encompass or supplement the original Δ30 deletion in the 3’-UTR were introduced into the rDENV-3 cDNA clone. Additionally, chimeric viruses were generated in which the 3’-UTR of the rDENV-3 cDNA clone was replaced with that of rDENV-4 or rDEN4Δ30. Viable viruses were analyzed for growth in Vero cells, attenuation in SCID mice transplanted with HuH-7 cells, and replication and immunogenicity in rhesus monkeys. Three mutant viruses (rDEN3Δ30/31, rDEN3Δ86, and rDEN3-3’D4Δ30) have preclinical phenotypes that suggest that they may be safe and immunogenic in humans.
Materials and Methods
Cells and Viruses
Vero cells (African green monkey kidney) were maintained in OptiPro SFM (Invitrogen, Grand Island, NY) supplemented with 4 mM L-glutamine (Invitrogen). HuH-7 cells (human hepatoma) were maintained in D-MEM/F-12 (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 1 mM L-glutamine and 0.05 mg/ml gentamicin (Invitrogen). C6/36 cells (Aedes albopictus mosquito cells) were maintained at 32°C in Minimal Essential Medium (MEM) containing Earle’s salts and 25 mM HEPES buffer (Invitrogen) and supplemented with 10% FBS, 2 mM L-glutamine, and 0.1 mM non-essential amino acids (Invitrogen).
A recombinant DENV-3 (rDENV-3) virus strain was previously generated by reverse genetics from the biological isolate, Sleman/78, provided by Dr. Duane Gubler (John Burns School of Medicine, University of Hawaii) [16]. The virus was isolated during a 1978 dengue outbreak characterized by mild illness in the Sleman area of Central Java, Indonesia [25].
Genetic construction of rDEN3 deletion mutations
We sought to generate additional deletion mutations in the 3’-UTR that include the original Δ30 (nt 173–143, numbered from the 3’ terminus) mutation previously introduced into rDENV-3 [16]. Figure 1B lists seven deletion mutations which encompass the original Δ30 mutation including Δ50, Δ61, Δ80, Δ86, Δ116A, Δ116B, and Δ146. In addition, the Δ30/31 mutation includes the original Δ30 mutation and an additional non-contiguous 31 nt deletion. The Δ31 mutation was also generated alone to discern the contribution of either Δ30 or Δ31 in the combined Δ30/31 deletion mutation. The positions in the predicted secondary structure of the DENV-3 3’-UTR of the nucleotides that border each deletion are indicated in Figure 1A.
Figure 1.
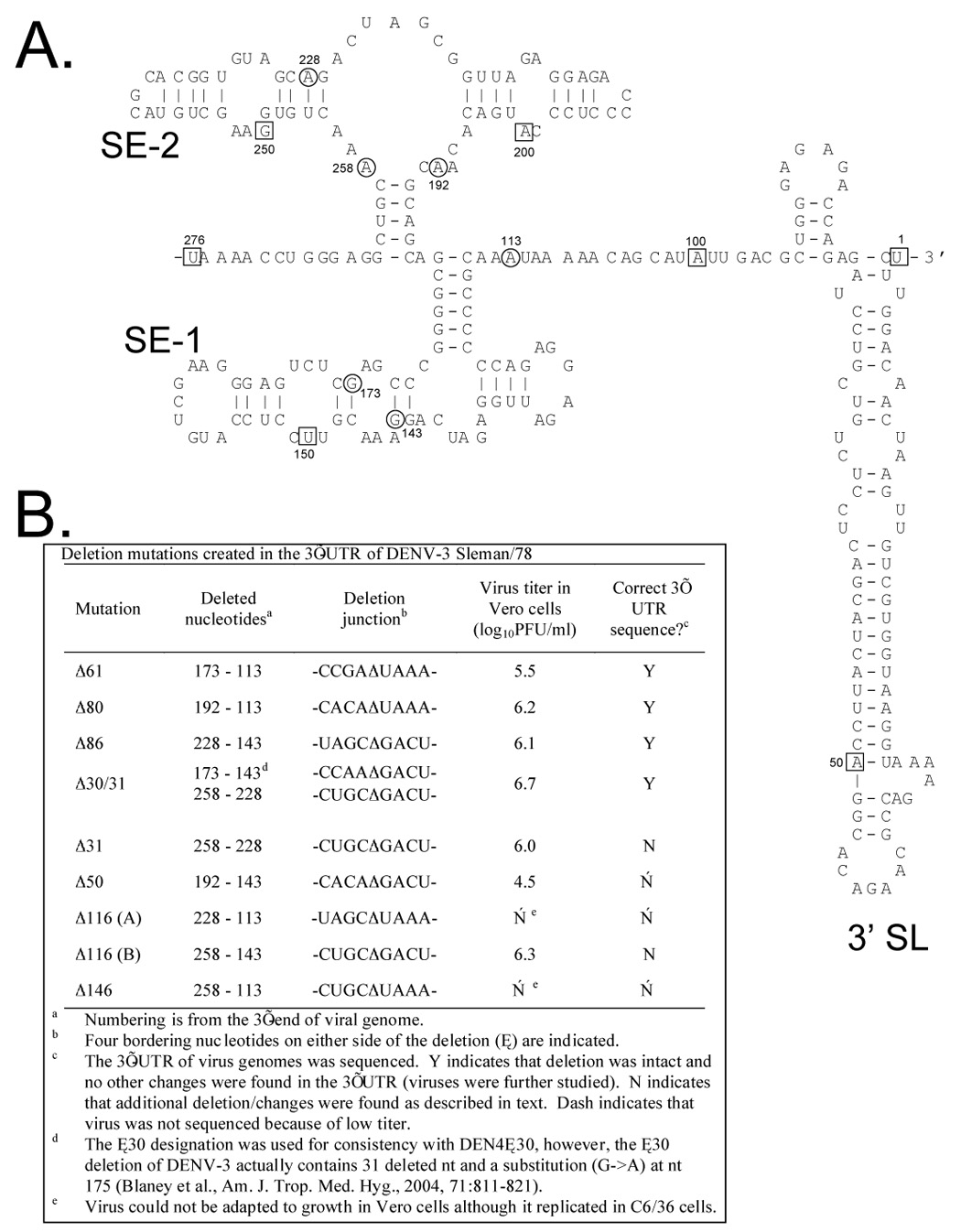
A. The predicted secondary structure for the 3’-UTR of wt DENV-3 Sleman/78. The sequence used for construction of the secondary structure model is the last 276 nucleotides of DENV-3 (nucleotides 10432 – 10707 from GenBank accession number AY648961). The M-fold program [42, 43] was used and nucleotides 267–276 and 95–104 were constrained to be single stranded in order to prevent circularization of the structure and loss of the conserved terminal 3’ hairpin stem loop (designated 3’ SL). Nucleotides are boxed at every 50 bases for reference to indicate position relative to the 3’ end. Circled nucleotides represent borders of the described deletions. The two stem-loop structural elements, designated SE-1 and SE-2, correspond approximately to the previously described TL2 and TL1, DB1 and DB2, and A3 and A2 structural elements [36, 37, 41]. B. Summary of the recovery and properties of the nine mutant viruses with deletions introduced into the 3’-UTR.
PCR mutagenesis was used to introduce the nine new deletion mutations into the DENV-3 Sleman/78 cDNA plasmid, p3, which was previously used to generate the rDEN3Δ30 vaccine candidate [16]. The p3-frag.4 cDNA subclone (encompassing DENV-3 nucleotides 9749–10,707) was used as the template for PCR reactions with mutagenic oligonucleotides. To generate full-length DENV-3 cDNA plasmids containing the deletion mutations, the PstI-KpnI fragments (963 nt) from the mutated p3-frag.4 cDNA subclones were introduced into the p3-7164 cDNA plasmid, and the presence of the appropriate deletion mutation was confirmed by sequence analysis. The p3-7164 plasmid encodes the 7164 Vero cell adaptation mutation (amino acid 115 in NS4B Val→Ala) which had previously been shown to enhance growth and transfection efficiency in Vero cells [16].
Genetic construction of rDENV-3 chimeric viruses with the 3’-UTR derived from rDENV-4 or rDEN4Δ30
Another strategy was used to generate novel rDENV-3 vaccine candidates that involved the replacement of the 3’-UTR of the rDENV-3 cDNA clone with that of rDENV-4 or rDEN4Δ30 (Figure 2). The p3-3’D4Δ30 plasmid was generated as follows. First, PCR mutagenesis was used to introduce a HpaI restriction site into the p3-frag.4 cDNA subclone (Figure 2B). To introduce the rDEN4Δ30 3’-UTR into the p3-frag.4(HpaI) cDNA subclone, a 364 nt fragment encompassing the p4Δ30 3’-UTR was amplified by PCR using a forward primer (5’-AACAACAACAAACACCAAAGGCTATTG-3’) and reverse primer (5’-CCTACCGGTACCAGAACCTGTTG-3’). To generate the p3-frag.4-3’D4Δ30 cDNA subclone, the HpaI-KpnI fragment was removed from p3-frag.4(HpaI) and replaced with the p4Δ30 3’-UTR PCR fragment which had been cleaved by KpnI. The PstI-KpnI fragment of p3-frag.4-3’D4Δ30 was introduced into the p3 plasmid to make the full length cDNA clone, p3-3’D4Δ30. The sequence of the 3’-UTR and NS5 junction were confirmed to be correct.
Figure 2.
Chimerization of rDEN3 with the 3’-UTR of rDENV-4 or rDEN4Δ30. A. Recombinant 3’-UTR chimeric dengue viruses were constructed by replacing the 3’-UTR of rDENV-3 with regions derived from either rDENV-4 or rDEN4Δ30. The relative location of the Δ30 mutation in the 3’-UTR is indicated by an arrow. The junctions between the ORF and UTR for rDENV-3 and rDENV-4 are indicated as junctions 1 and 2, respectively. Intertypic junction 3 is also indicated for the resulting chimeric viruses. B. Nucleotide and amino acid sequence of the junction regions are shown. For junction 3, nucleotide substitutions used to introduce a unique HpaI restriction enzyme recognition site are shown in lower case.
To generate p3-3’D4, the 30 deleted nucleotides of the Δ30 deletion mutation were introduced into the p3-frag.4-3’D4Δ30 subclone. Briefly, the MluI – KpnI fragment of p3-frag.4-3’D4Δ30, which encompasses the Δ30 region, was replaced with the corresponding fragment of p4 to make the plasmid, p3-frag.4-3’D4. To generate a full-length chimeric genome, the PstI – KpnI fragment of p3 was replaced with the corresponding fragment of p3-frag.4-3’D4. The 3’-UTR sequence of the p3-3’D4 plasmid was determined to be correct as the missing 30 nt of the Δ30 mutation was replaced by wild type DENV-4 sequence.
Recovery and propagation of rDENV-3 viruses
For recovery of viruses, 5’-capped RNA transcripts were synthesized in vitro from cDNA plasmids and transfected into either Vero cells or C6/36 cells. Because of genetic instability in E coli, the p3 plasmid requires a linker with redundant stop codons flanked by SpeI restriction sites [16]. Prior to transcription and generation of infectious virus, these linker sequences were removed from mutant p3 cDNA plasmids by digestion with SpeI. Plasmids were then recircularized by ligation, linearized with Acc65I (isoschizomer of KpnI which cleaves leaving only a single 3’ nucleotide), and transcribed in vitro using SP6 polymerase. Purified transcripts were then transfected into Vero or C6/36 cells using DOTAP liposomes (Roche, Indianopolis, IN). After passage in Vero cells to reach a minimum virus titer of approximately 106.0 PFU/ml, viruses were biologically cloned by two or three terminal dilutions before experimental stocks were prepared in Vero cells.
For analysis of replication in tissue culture, growth curves were performed in Vero cells and C6/36 cells. Tissue culture flasks (75 cm²) of confluent cells were infected at a multiplicity of infection of 0.01. Aliquots of 0.5 ml were removed from flasks daily for seven days. After addition of SPG [22] to a concentration of 1X, samples were frozen on dry ice and stored at −80°C. Virus titer was determined by plaque assay on Vero cells for all samples. The limit of detection was 101.0 PFU/ml.
Animal models of DEN virus infection
For analysis of virus replication in SCID-HuH-7 mice, four to six week-old SCID mice (Tac:Icr:Ha(ICR)-Prkdcscid) (Taconic, Germantown, NY) were injected intraperitoneally with 0.1 mL of phosphate-buffered saline containing 107 HuH-7 cells which had been propagated in tissue culture [26]. Tumors were detected in the peritoneum five to six weeks after transplantation, and tumor-bearing mice were infected by direct inoculation into the tumor with 104 PFU of virus in 50 µ1 Opti-MEM I (Invitrogen). Serum was collected from infected mice on day 7 post-infection and frozen at −80°C. The virus titer was determined by plaque assay in Vero cells.
Viruses were evaluated for replication and immunogenicity in rhesus macaques using established methods [22]. DENV-seronegative monkeys were injected subcutaneously with 105 PFU of virus diluted in L-15 medium (Invitrogen) or with a mock inoculum. Serum was collected on days 0–6, 8, 10 and 28 after inoculation and stored at −80°C. Virus titer in serum was determined for each day by plaque assay in Vero cells, and serum neutralizing antibody titer was determined for days 0 and 28 by plaque reduction neutralization test [22]. On day 35 post-infection, all monkeys were challenged by subcutaneous infection with 105 PFU of DENV-3 Sleman/78 wild type virus. Serum was collected on days 0–6, 8, and 10, frozen at −80°C, and the virus titer in serum samples was determined by plaque assay in Vero cells.
Replication in mosquitoes
Replication of rDENV-3 and rDEN3Δ30/31 was studied in Toxorynchites amboinenesis mosquitoes. Intrathoracic inoculation of serial ten-fold dilutions of test virus was performed as described previously [27]. After a 14 day incubation, heads were separated and homogenized in diluent. Virus titer in head homogenates was determined by plaque assay in Vero cells.
Results
Generation of rDENV-3 with 3’-UTR deletions
Transfections in Vero cells and C6/36 cells were performed with cDNA clones representing each of the nine deletion mutations listed in Figure 1B. Viruses with Δ30/31, Δ31, Δ50, Δ61, Δ80, Δ86, Δ116A, Δ116B, and Δ146 mutations were successfully recovered in C6/36 cells, whereas rDEN3Δ31 was the only virus that could also be recovered in Vero cells. The rDENV-3 deletion mutant viruses were then passaged in Vero cells before biological cloning by two terminal dilutions in Vero cells. Cloned viruses were then passaged two to seven times in Vero cells in an attempt to reach a titer of approximately 106.0 PFU/ml which is considered sufficient to allow for cost-effective manufacture. Three recombinant viruses (rDEN3Δ50, rDEN3Δ116A, and rDEN3Δ146) were found to be excessively restricted for replication in Vero cells, despite being viable (Figure 1B), and were not studied further. The genetic sequence of the 3’-UTR was determined for the six remaining deletion mutant viruses that reached peak virus titers of approximately 106.0 PFU/ml. The intended 3’-UTR sequence with the appropriate deletion was found for rDEN3Δ61, rDEN3Δ80, rDEN3Δ86 and rDEN3Δ30/31 (Figure 1B). However, two mutant viruses were found to contain additional deletions or mutations and were deemed to have potentially unstable genotypes. First, rDEN3Δ31 had the engineered 3’-UTR deletion of nt 258-228 but also contained a 25 nt deletion of nt 222–198. Second, rDEN3Δ116B had the engineered 3’-UTR deletion of nt 258-143 but also contained a 8 nt deletion of nt 430–423 and a single A->G substitution at nt 265. The observed genetic instability with these two viruses makes manufacturing a homogeneous suspension of virus a significant challenge so they were not studied further. For the nine original deletions constructed, four mutant viruses were found to replicate efficiently in Vero cells and to contain the engineered 3’-UTR sequence, and were studied further; rDEN3Δ61, rDEN3Δ80, rDEN3Δ86 and rDEN3Δ30/31 (Figure 1B).
Generation of rDENV-3 chimeric viruses with the 3’-UTR derived from rDENV-4 or rDEN4Δ30
The 3’-UTR chimeric virus, rDEN3-3’D4Δ30, was designed to be a vaccine candidate for inclusion in tetravalent formulations which share the Δ30 deletion mutation among all four serotypes (Figure 2). Sharing the Δ30 mutation amongst all serotypes is important since it precludes generation of a wild type recombinant virus during manufacture or use in vaccinees although recombination is viewed as an unlikely event [28]. The rDEN3-3’D4 virus was also generated to identify the contribution of the 3’-UTR chimerization and the Δ30 mutation to a phenotype observed for rDEN3-3’D4Δ30. rDEN3-3’D4 was recovered in C6/36 cells and Vero cells, whereas rDEN3-3’D4Δ30 was only recovered in Vero cells. Mutant viruses were then passaged once in Vero cells followed by biological cloning by two terminal dilutions in Vero cells. rDEN3-3’D4 and rDEN3-3’D4Δ30 were then passaged four or six times in Vero cells, respectively, and reached virus titers of greater than 106.5 PFU/ml. The genetic sequence of the NS5 – 3’-UTR junction and the entire 3’-UTR was found to be as engineered for rDEN3-3’D4 and rDEN3-3’D4Δ30. Therefore, both viruses were studied further.
Replication of DENV-3 mutant viruses in SCID-HuH-7 mice
The four deletion mutant viruses (rDEN3Δ30/31, rDEN3Δ61, rDEN3Δ80, and rDEN3Δ86) and the rDEN3-3’D4 and rDEN3-3’D4Δ30 chimeric viruses were evaluated for level of replication in SCID-HuH-7 mice. This mouse model provided the original evidence that the rDEN3Δ30 virus was not attenuated compared to parent virus rDENV-3, while the antigenic chimeric virus, rDEN3/4Δ30, was approximately 100-fold restricted in replication in the mice when compared to wild type parent viruses [16].
As indicated in Table 1, wild type DENV-3 Sleman/78 replicated to a mean peak virus titer of 106.9 PFU/ml. However, rDEN3Δ86 and rDEN3-3’D4Δ30 were more than 10-fold restricted in replication compared to wild type DENV-3 virus whereas the replication of rDEN3Δ30/31 was slightly less than 10-fold restricted. On the basis of this arbitrary cut-off, these three viruses were selected for further evaluation. It is important to note that the rDEN4Δ30 virus which has a well-characterized, non-reactogenic phenotype in humans was found to be only 6-fold restricted in replication in SCID-HuH-7 mice compared to wild type DENV-4[29].
Table 1.
Replication of mutant DENV-3 viruses in SCID-HuH-7 mice.
| Virusa | Analysis group | No. of mice | Mean peak virus titer (log10PFU/ml ± SE) | Mean log10 reduction from DEN3 virus titer |
|---|---|---|---|---|
| DENV-3 (Sleman/78) | 8 | 6.9 ± 0.1 | ||
| rDEN3Δ30/31 | 1 | 8 | 6.0 ± 0.3 | 0.9 |
| rDEN3Δ61 | 1 | 9 | 6.3 ± 0.2 | 0.6 |
| rDEN3Δ80 | 1 | 9 | 6.3 ± 0.3 | 0.6 |
| rDEN3Δ86 | 1 | 10 | 5.6 ± 0.4b | 1.3 |
| rDEN3-3’D4 | 2 | 11 | 6.5 ± 0.4 | 0.4 |
| rDEN3-3’D4Δ30 | 2 | 9 | 5.7 ± 0.2b | 1.2 |
Groups of SCID-HuH-7 mice were inoculated into the tumor with 104 PFU of the indicated virus. Serum was collected on day 7 and virus titer was determined in Vero cells.
Analysis groups 1 and 2 were independently compared to wild type DENV-3. Mean virus titers are significantly different from DENV-3 virus titer as determined by Tukey-Kramer post-hoc test (P < 0.05).
Replication of DEN3 mutant viruses in tissue culture
The level of virus replication in both Vero cells and C6/36 mosquito cells was assessed for the rDEN3Δ30/31 and rDEN3Δ86 deletion mutant viruses and the rDEN3-3’D4 viruses with and without Δ30. Replication in Vero cells was analyzed because these cells are the substrate for manufacture, while growth in C6/36 cells was assessed because attenuation phenotypes in these mosquito cells may be associated with restricted replication in Aedes mosquitoes, which serve as the vector for DENV transmission [30].
The replication kinetics of each virus in both cell lines is shown in Figure 3. In Vero cells, rDEN3Δ30/31, rDEN3Δ86, and rDEN3-3’D4Δ30 replicated to a peak level that approximated that of wild type DENV-3 with similar kinetics. These three vaccine candidates reached peak virus titers of 106.5 to 106.7 PFU/ml which demonstrates the feasibility of manufacture for each of these viruses. In Vero cells, the rDEN3-3’D4 virus replicated to a peak titer of 107.8 PFU/ml which is nearly 100-fold higher than that observed for wild type DENV-3 indicating that inclusion of the DENV-4 3’-UTR may augment replication in Vero cells. rDENV-4 replicates to a peak titer of approximately 108.0 PFU/ml which indicates that the chimeric virus achieved a peak titer that does not exceed that of either of its parent viruses [18].
Figure 3.
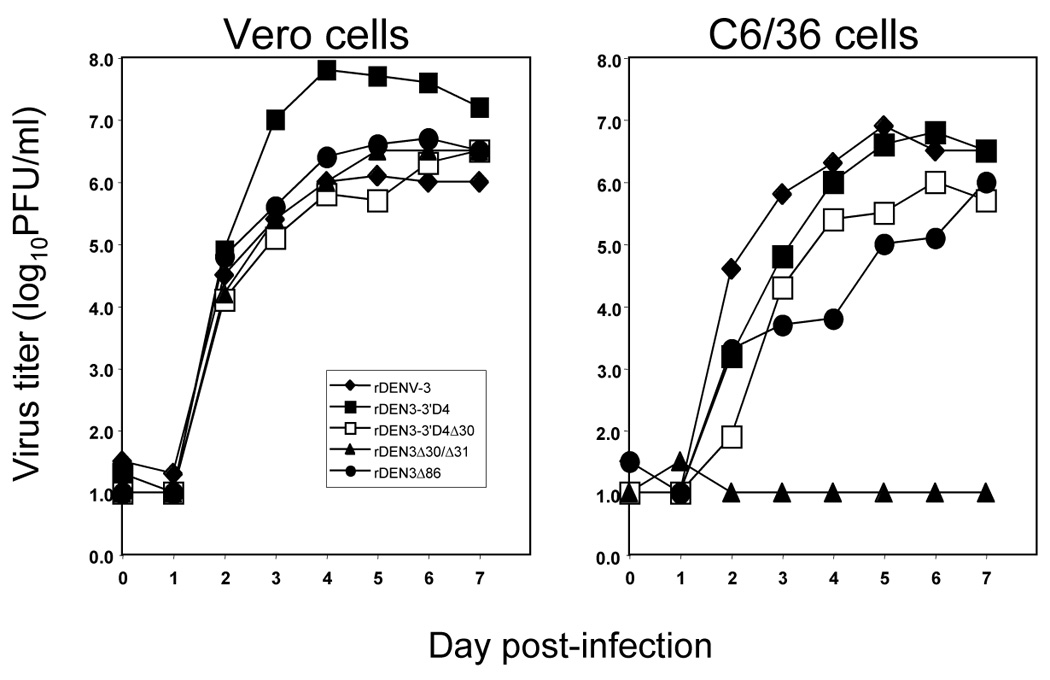
Replication of rDENV-3 in Vero cells and C6/36 cells. Cells were infected at an MOI of 0.01. Virus titer of supernatants was determined by plaque assay in Vero cells for all samples. The limit of detection is 10¹ PFU/ml.
Analysis of virus replication in C6/36 cells demonstrated that rDEN3Δ86 and rDEN3-3’D4Δ30 reached peak titers approximately 10-fold lower than the peak virus titer of wild type DENV-3 virus, 106.9 PFU/ml (Figure 3). The rDEN3-3’D4 virus replicated to a peak titer similar to that observed for wild type DENV-3. The most striking result was the lack of replication of rDEN3Δ30/31 in C6/36 cells. After day 1, virus was not detected in culture medium from C6/36 cells infected with rDEN3Δ30/31 virus despite the efficient replication observed in Vero cells. These results were confirmed in a second independent growth curve experiment and indicate a host range attenuation phenotype in tissue culture.
Sequence analysis of rDENV-3 mutant viruses
The genomes of rDEN3Δ30/31, rDEN3Δ86, and rDEN3-3’D4Δ30 were fully sequenced to check for adventious mutations which may have arisen during passage in Vero cells and likely contribute to enhanced growth and adaptation in Vero cells (Table 2). Each virus was found to possess the 7164 Vero cell adaptation mutation that had been engineered into the cDNA plasmids. The rDEN3Δ30/31 virus was found to contain a single coding change in NS4B while rDEN3-3’D4Δ30 lacked coding changes but did contain a single nucleotide substitution in the 3’-UTR. Sequence analysis of rDEN3Δ86 revealed a coding change in M and a single nucleotide substitution in the 3’-UTR. Interestingly, a mixed population at nt 10267 (A→A/U) of rDEN3Δ86 was found that changes the stop codon (UAA) at the end of NS5 to UAU which encodes Tyr. This would serve to extend NS5 by only 2 amino acids (Tyr-Thr-End) since an inframe stop codon remains at nts 10271–10273.
Table 2.
Adventitious mutations that were identified in rDEN3Δ30/31, rDEN3Δ86, and rDEN3-3’D4Δ30
| Virusa | Gene | Nucleotide position | Nucleotide substitution | Amino acid position | Amino acid change |
|---|---|---|---|---|---|
| rDEN3Δ30/31 | NS4B | 7398 | C → U | 193 | Ala → Val |
| rDEN3Δ86 | M | 512 | A → G | 26 | Lys → Glu |
| NS3 | 6076 | C → U | 521 | silent | |
| NS5 | 8623 | U → C | 353 | silent | |
| NS5 | 10267b | A → U | END | end → Tyr | |
| 3’ UTR | 10455 | G → C | — | — | |
| rDEN3-3’D4Δ30 | C | 250 | U → C | 52 | silent |
| NS3 | 5899 | U → C | 462 | silent | |
| 3’ UTR | 10534 | A →G | – | – |
rDEN3Δ30/31 and rDEN3Δ86 were compared to the DENV-3 p3 plasmid cDNA (Genbank # AY656169). rDEN3-3’D4Δ30 was compared to the DENV-3 p3 plasmid cDNA clone (5’ UTR and genes) and the DEN4 p4 cDNA clone (Genbank # AY648301) for the 3’ UTR.
There is a mixed population at this nt position (A→A/U) that changes the stop codon (UAA) at the end of NS5 to UAU which encodes Tyr. This would serve to extend NS5 by only 2 amino acids (Tyr-Thr-End) since an inframe stop codon remains at nts 10271–10273.
Replication, immunogenicity, and protective efficacy of DENV-3 mutants in rhesus monkeys
Based on the attenuation in SCID-HuH-7 mice and efficient growth in Vero cells, rDEN3Δ30/31, rDEN3Δ86, and rDEN3-3’D4Δ30 were evaluated in rhesus monkeys. The mutant viruses were compared with wild type DENV-3 for level and duration of viremia, neutralizing antibody induction, and the ability to confer protection from wild type DENV-3 virus challenge. The rDEN3-3’D4 virus was also evaluated to identify a contribution of chimerization to attenuation with and without the Δ30 mutation.
Groups of four rhesus monkeys were inoculated subcutaneously with 105 PFU of the indicated virus (Table 3). Wild type DENV-3 Sleman/78 virus replicated in rhesus monkeys to a mean peak virus titer of 101.8 PFU/ml serum with all monkeys developing viremia. These results parallel previous studies of DENV-3 in rhesus monkeys [16]. Viremia was not detected in any monkey infected with any of the three vaccine candidates, namely rDEN3Δ30/31, rDEN3Δ86, or rDEN3-3’D4Δ30, demonstrating a clear attenuation phenotype for each of these viruses in rhesus monkeys. Interestingly, the rDEN3-3’D4 virus was detected in 75% of monkeys with a mean peak virus titer of 1.3 log10PFU/ml serum suggesting that the presence of the Δ30 mutation is critical for attenuation of the rDEN3-3’D4Δ30 chimeric virus. Despite the lack of detectable viremia, mean neutralizing antibody levels in monkeys infected with rDEN3Δ30/31 and rDEN3Δ86 reached levels similar to that of wild type DENV-3, 1:253 (Table 3). In contrast, the rDEN3-3’D4Δ30 virus induced mean neutralizing antibody levels approximately three-fold lower than wild type DENV-3. However, 100% of monkeys immunized with each vaccine candidate seroconverted as defined by a four-fold or greater rise in serum neutralizing antibody levels after inoculation. Thus all monkeys were infected by each of the vaccine candidates despite the lack of detectable viremia. Determination of virus titer in serum after challenge with DENV-3 virus indicated that immunization with each of the vaccine candidates induced complete protection from detectable viremia as would be expected given the observed neutralizing antibody levels.
Table 3.
Replication and immunogenicity of rDENV-3 mutant viruses in rhesus monkeys.
| Virusa | No. of monkeys | % of monkeys with viremia | Mean no. of viremic days per monkey | Mean peak virus titerb (log10PFU/ml ± SE) | Geometric mean serum neutralizing antibody titer (reciprocal dilution)c |
Post-challengedd |
||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 | % of monkeys with viremia | Mean peak virus titerb (log10PFU/ml ± SE) | |||||
| DENV-3 (Sleman/78) | 4 | 100 | 3.5 | 1.8 ± 0.1 | < 5 | 253 | 0 | < 1.0 |
| rDENΔ330/31 | 4 | 0 | 0 | < 1.0 | < 5 | 304 | 0 | < 1.0 |
| rDEN3Δ86 | 4 | 0 | 0 | < 1.0 | < 5 | 224 | 0 | < 1.0 |
| rDEN3-3’D4 | 4 | 75 | 1.5 | 1.3 ± 0.2 | < 5 | 229 | 0 | <1.0 |
| rDEN3-3’D4Δ30 | 4 | 0 | 0 | < 1.0 | < 5 | 77 | 0 | < 1.0 |
| mock infected | 2 | 0 | 0 | < 1.0 | < 5 | < 5 | 100 | 1.8 ± 0.2 |
Groups of rhesus monkeys were inoculated subcutaneously on day 0 with 105 PFU of the indicated virus in a 1 ml dose. Serum was collected daily for 10 days and on day 28.
Virus titer in serum was determined by plaque assay in Vero cells. Mean peak virus titer of DENV-3-infected rhesus monkeys was significantly different from that of other groups as determined by Tukey-Kramer post-hoc test (P < 0.05).
Plaque reduction (60%) neutralizing antibody titers were determined using DENV-3 (Sleman/78) virus on Vero cells.
Monkeys were challenged after 35 days with DENV-3 (Sleman/78) administered subcutaneously in a 1 ml dose containing 105 PFU. Serum was collected daily for 10 days.
Replication of rDEN3Δ30/31 in Toxorynchites mosquitoes
Based on the attenuation of rDEN3Δ30/31 in rhesus monkeys and its restricted replication in C6/36 mosquito cells, rDENΔ30/31 was compared to wild type rDENV-3 for infectivity and level of replication in highly sensitive Toxorynchites amboinensis mosquitoes (Table 4). Ten-fold serial dilutions of virus were inoculated intrathoracically, and the ability to infect head tissues was evaluated by performing a plaque assay on mosquito head homogenates after a 14 day incubation. The infectivity of rDENV-3 and rDENΔ30/31 was very similar as the 50% mosquito infectious dose was approximately 101.3 PFU for both viruses (Table 4). However, the level of replication of rDENΔ30/31 in the heads of infected mosquitoes was approximately 5- to 30-fold reduced. This reduction was significant at the 102.3 and 101.3 PFU doses tested. This finding indicates that although rDENΔ30/31 has infectivity for Toxorynchites by intrathoracic infection similar to that of wild type rDENV-3, there is a statistically significant restriction in the level of replication in mosquitoes afforded by the Δ30/31 mutation.
Table 4.
Replication of rDENV-3 and rDEN3Δ30/31 in Toxorynchites amboinensis
| Virus | Dosea (log10PFU) | No tested | % infectedb | Mean virus titerc (log10PFU/head) | Reduction (log10) compared to same dose of wt virus |
|---|---|---|---|---|---|
| rDENV-3 wt | 2.3 | 20 | 90 | 4.2 ± 0.1d | |
| 1.3 | 19 | 53 | 4.2 ± 0.1e | ||
| 0.3 | 17 | 18 | 4.3 ± 0.3 | ||
| rDEN3Δ30/31 | 2.3 | 12 | 83 | 2.7 ± 0.3d | 1.5 |
| 1.3 | 16 | 44 | 3.1 ± 0.3e | 1.1 | |
| 0.3 | 8 | 13 | 3.6 ± 0.0 | 0.7 |
Virus titer administered intrathoracically in a 0.2 ul inoculum.
Percentage of mosquitoes with detectable virus at day 14 post-inoculation was determined by plaque assay on mosquito head homogenates in Vero cells.
Calculated using only values of virus-positive heads.
For 102.3 PFU dose of rDENV-3 and rDEN3Δ30/31, mean virus titers were significantly different as determined by a Tukey-Kramer post-hoc test (P < 0.001).
For 101.3 PFU dose of rDENV-3 and rDEN3Δ30/31, mean virus titers were significantly different as determined by a Tukey-Kramer post-hoc test (P < 0.005).
Discussion
Reverse genetics has proven to be a very valuable tool to introduce attenuating mutations into dengue viruses to produce live attenuated DENV vaccine candidates [18, 31]. Specifically, modification of the 3’-UTR as a means to attenuate DENV has resulted in the development of several promising live attenuated vaccine candidates [19, 22, 32, 33]. The introduction of deletion mutations into untranslated regions is an attractive strategy for development of live attenuated DENV vaccines for two reasons. First, deletions have the potential for a high level of genetic stability and are unlikely to revert to wild type sequence. Second, attenuating deletion mutations in the 3’-UTR do not affect the sequence of translated gene products, and thus, the authentic wild type proteins encoded by the vaccine virus should induce the full spectrum of humoral and cell-mediated immune response in vaccinees. In contrast, chimeric vaccines viruses, such as the DEN3/4Δ30(ME) vaccine candidate [16], induce DENV-3-specific immunity to only the M and E proteins. The present study defines novel deletions and a DEN3/4 chimeric virus with a 3’-UTR swap and deletion that appear to be promising vaccine candidates for induction of protective immunity to the full set of DEN3 antigenic targets.
The DENV 3’-UTRs range in length from approximately 400–450 nucleotides and contain three defined regions, namely, the variable region which abuts the NS5 gene stop codon, the core region, and an essential 3’ terminal stem loop structure which is well-conserved among the flaviviruses [34]. The DENV 3’-UTR is believed to play a key role in the regulation of viral replication by virtue of controlling RNA synthesis [35]. Both viral and cellular factors are known to bind to the 3’-UTR, and these interactions appear to regulate the efficiency of RNA replication [34]. There is some evidence suggesting that the attenuating effects of deletion mutations in the 3’-UTR are mediated by a disruption in secondary structure that results in a decrease in the efficiency of RNA replication [35]. Studies by Men et al. first characterized a panel of deletion mutations of varying length in the 3’-UTR of DENV-4 [33]. Deletions in the 3’-UTR from 30 to 262 nt in length were found to be viable and, with the exception of a single deletion (243–183), genetically stable. Various deletions including nt 172–143 (Δ30) were found to confer reduced replication in tissue culture and signs of attenuation in rhesus monkeys [33]. In our laboratory, the Δ30 deletion mutation that interrupts Structural Element 1 (SE-1) (Figure 1) in the 3’-UTR has been utilized for the development of vaccine candidates for each DENV serotype [16, 17, 19, 22]. Although M-fold predictions suggest that elements of the secondary structure of the DENV 3’-UTRs are conserved, differences in the impact of Δ30 among the different DENV serotype suggests that such predictions fail to detect important structural variation [36, 37]. RNAse mapping of the core region of the 3’-UTR of rDENV-4 has also pointed to important variation in secondary structure among the DEN serotypes [38]. For example, as initially predicted by Shurtleff et al. [36], RNAse mapping corroborates the prediction that the SE-1 (CS2) sequence of rDENV-4 folds into a single stem and loop “turret” rather than the double-branched dumbbell structure of rDENV-3. Moreover, because SE1 in rDENV-4 is composed of only a single branch, incorporation of Δ30 results in a decrease in length, rather than branching complexity, of SE1 in this serotype. Whether this putative variation in the structure of SE1 in rDENV-4 and rDENV-3 is borne out by RNAse mapping, and whether it may explain the variation in the phenotypic impact of Δ30 among the viruses, is currently under investigation in our laboratories.
Due to the wild type phenotype of rDEN3Δ30, which contains a deletion in SE-1, it was hypothesized that the presence of the unaltered reciprocal structure, SE-2, might be sufficient for efficient structure and function of the overall DENV-3 3’-UTR [16]. Therefore, the additional deletions described here were generated that extend into SE-2. In addition, the Δ30/31 deletion mutation was generated to disrupt both of the reciprocal structures, SE-1 and SE-2. All of the deletions were found to be viable and therefore, may be useful for basic studies of the structure and function of the DENV-3 3’-UTR. However, only deletion viruses that had adequate replication in Vero cells and that maintained the introduced deletion without major modification were considered in this study since these are characteristics required for vaccine candidates. Nonetheless, it is intriguing that while Δ30 in SE1 is highly stable in all four DENV serotypes [16, 17, 19, 22], Δ31 in SE2 triggered an additional large deletion within the same structural element. Although previous studies have found the structure of these two elements to be almost identical [37], this discrepancy in their tolerance of a homologous deletion suggests differences in either secondary or tertiary structure.
Of the nine DENV-3 viruses with 3’-UTR deletions recovered and evaluated in the present study, two viruses, rDEN3Δ86 and rDEN3Δ30/31, appear to be suitable vaccine candidates. Both rDEN3Δ86 and rDEN3Δ30/31 were mildly attenuated in SCID-HuH-7 mice, but in rhesus monkeys, the viruses were highly attenuated as indicated by the absence of viremia in immunized animals. These results suggest that the disruption of both SE-2 and SE-1 is necessary for attenuation of DENV-3. These findings further demonstrate that while the general secondary structure of the DENV 3’-UTRs is believed to be conserved, there are significant differences between the serotypes that are not currently understood. Despite the absence of viremia in rhesus monkeys immunized with rDEN3Δ86 and rDEN3Δ30/31, a robust neutralizing antibody response developed suggesting that these deletions confer strong attenuation while still allowing a level of replication sufficient for development of an antibody response. The antibody response in monkeys immunized with rDEN3Δ86 and rDEN3Δ30/31 reached levels similar to that of monkeys infected with wild type DEN3 which makes these vaccine candidates particularly attractive for study in humans.
In addition to a balanced level of attenuation and immunogenicity, a particular concern for live attenuated vaccines for arboviruses to be used in humans is the potential for unwanted replication and transmission in mosquito vectors. Ideally, a live attenuated DENV vaccine candidate should have two characteristics to block transmission. First, vaccine candidates should replicate to low levels in humans, which serves to decrease the likelihood of a feeding mosquito becoming infected. In the case of rDEN3Δ86 and rDEN3Δ30/31, the lack of detectable viremia in rhesus monkeys suggests that they would indeed replicate to low levels in humans similar to other DENV vaccine candidates studied in our laboratory [18]. Second, live attenuated DENV vaccine candidates should have an inherent restriction for growth in mosquitoes, as observed for the rDEN4Δ30 virus [27]. Therefore, the DENV-3 described here were assayed for replication in C6/36 cells which are derived from Aedes albopictus. A single virus, rDEN3Δ30/31, was found to be defective for replication in C6/36 cells while capable of efficient replication in Vero cells. Since previous studies have demonstrated that wild type DENV-3 Sleman/78 has low infectivity for orally fed Aedes aegyptii mosquitoes [16], rDEN3Δ30/31 was tested for infectivity and replication in intrathoracically-infected Toxorynchites mosquitoes. In this highly sensitive mosquito, rDEN3Δ30/31 was found to have a small but statistically significant decrease in replication. Therefore, the rDEN3Δ30/31 vaccine candidate appears to have several factors which would prevent transmission of the virus from vaccinee to mosquito: (1) development of low or no viremia in vaccinees, (2) the natural low infectivity of DENV-3 Sleman/78 for Aedes [16], and (3) attenuation conferred by the Δ30/31 mutation.
An alternative method for developing a DENV-3 vaccine candidate was also pursued by swapping the 3’-UTR of DENV-3 with that of rDENV-4 or rDEN4Δ30. This is a new strategy for attenuation of a flavivirus, although chimeric live oral poliovirus vaccines have been generated by swapping the 5’ UTR of a modified Sabin type 3 strain with that of type 1 and 2 in an effort to increase genetic stability [39]. In addition, Markoff and colleagues previously constructed chimeric West Nile virus/DENV-2 viruses with swaps confined to the 3’ terminal stem loop structure that were found to be attenuated for replication in tissue culture [40]. Alignment of DENV-3 Sleman/78 and rDENV-4 reveals that the DENV-3 3’-UTR contains an approximately 60 nt extension in the variable region near the beginning of the 3’-UTR. Excluding this additional sequence in the DENV-3 3’-UTR, the nucleotide homology of the DENV-3 and DENV-4 3’-UTR is only 75%. However, the linear order of specific structural elements of the 3’-UTR appears to be conserved among the four serotypes [36, 37, 41] even though as discussed above, the configuration of individual elements may differ among the serotypes. Therefore, the ability of the DENV-4 3’-UTR to function normally in the context of the rest of the DENV-3 genome was not difficult to envision. In fact, the rDEN3-3’D4 virus showed no signs of attenuation when compared to wild type DENV-3 in tissue culture, SCID-HuH-7 mice, or rhesus monkeys. Surprisingly, the rDEN3-3’D4 virus appeared to replicate better than wild type DENV-3 in Vero cells, and this virus might be useful to achieve the high virus yields required for manufacture of an inactivated DENV-3 vaccine. When the Δ30 mutation was introduced into the DENV-4 3’-UTR (rDEN3-3’D4Δ30), significant attenuation was observed in C6/36 cells, Vero cells, SCID-HuH-7 mice, and rhesus monkeys. The impact of Δ30 in rDEN3-3’D4 is generally consistent with its impact in rDEN4, save that in the latter case the addition of Δ30 does not cause attenuation in Vero cells [27]. This finding further indicates that the context of the Δ30 deletion dictates whether the mutation will confer attenuation upon a given virus. The present studies do not elucidate why the Δ30 mutation fails to attenuate DENV-3 in the context of the DENV-3 3’-UTR. The only insight into the molecular mechanism by which the Δ30 mutation confers attenuation upon the DENV comes from a study of DENV replicons. Alvarez et al. found that a replicon containing the 3’-UTR with the Δ30 deletion had 50- to 100-fold decreased RNA replication in BHK or C6/36 cells [35]. Further studies are needed to discern the structural basis for the divergent effects observed for deletion mutations in DENV-3 versus DENV-4.
Based on the restricted replication and strong immunogenicity in rhesus monkeys observed for rDEN3Δ30/31, rDEN3Δ86, and rDEN3-3’D4Δ30 as well as the mosquito restriction of rDEN3Δ30/31, the viruses were re-derived to generate Clinical Lots suitable for administration in humans. Seed stocks of rDEN3Δ30/31 and rDEN3-3’D4Δ30 were successfully generated and found to retain the correct modified 3’ UTR sequences along with differing adventitious mutations (data not shown). However, the rederived rDEN3Δ86 virus was found to contain an expanded deletion mutation (346–108) and this genetic instability will preclude further evaluation of this virus as a vaccine candidate. Therefore, only Clinical Lots of rDEN3Δ30/31 and rDEN3-3’D4Δ30 are currently being manufactured and will be tested in humans. At present, the rDEN3/4Δ30 antigenic chimeric virus is currently being evaluated in a clinical trial and will most likely be the DENV-3 component of the first tetravalent formulation that we will test in humans [16]. Should the rDEN3/4Δ30 virus be over- or under-attenuated in humans, then rDEN3Δ30/31 or rDEN3-3’D4Δ30 may be a suitable reserve vaccine candidate. The fact that rDEN3Δ30/31 and rDEN3-3’D4Δ30 possess the full complement of DENV-3 antigenic targets instead of only the M and E genes (such as rDEN3/4Δ30) suggests that they might be preferable to the antigenic chimeric virus.
Acknowledgements
These studies were supported with funds from the NIAID Division of Intramural Research. TR is supported by the NMSU RISE program (NIH grant GM61222); KH is supported by NIH-NM-INBRE (P20 RR016480-05) and NSF-ADVANCE (SBE-123690).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Geneva: WHO; Dengue haemorrhagic fever: diagnosis, treatment prevention and control. (2nd ed.) 1997
- 3.Gubler DJ, Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv Virus Res. 1999;53:35–70. doi: 10.1016/s0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- 4.Gubler DJ. The global pandemic of dengue/dengue haemorrhagic fever: current status and prospects for the future. Ann Acad Med Singapore. 1998;27(2):227–234. [PubMed] [Google Scholar]
- 5.Burke DS, Monath TP. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Fourth ed. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 1043–1125. [Google Scholar]
- 6.Rice CM. Flaviviridae: The viruses and their replication. In: Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, et al., editors. Fields Virology. Third ed. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 931–959. [Google Scholar]
- 7.Lindenbach BD, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Fourth ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 1043–1125. [Google Scholar]
- 8.Monath TP. Yellow fever. In: Plotkin SA, Orenstein WA, editors. Vaccines. 3 ed. Philadelphia: W.B. Saunders Co.; 1999. pp. 815–879. [Google Scholar]
- 9.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature Medicine. 2004 Dec;10 (12 Suppl):S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 10.Kanesa-thasan N, Sun W, Kim-Ahn G, Van Albert S, Putnak JR, King A, et al. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine. 2001;19:3179–3188. doi: 10.1016/s0264-410x(01)00020-2. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, Edelman R, Kanesa-thasan N, Eckels KH, Putnak JR, King AD, et al. Vaccination of human volunteers with monovalent and tetravalent live-attenuated dengue vaccine candidates. Am J Trop Med Hyg. 2003 December 1;69(90060):24–31. doi: 10.4269/ajtmh.2003.69.6_suppl.0690024. [DOI] [PubMed] [Google Scholar]
- 12.Kitchener S, Nissen M, Nasveld P, Forrat R, Yoksan S, Lang J, et al. Immunogenicity and safety of two live-attenuated tetravalent dengue vaccine formulations in healthy Australian adults. Vaccine. 2006 Feb 27;24(9):1238–1241. doi: 10.1016/j.vaccine.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Edelman R, Wasserman SS, Bodison SA, Putnak RJ, Eckels KH, Tang D, et al. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg. 2003 Dec;69 (6 Suppl):48–60. doi: 10.4269/ajtmh.2003.69.48. [DOI] [PubMed] [Google Scholar]
- 14.Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, et al. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am J Trop Med Hyg. 2002 Mar;66(3):264–272. doi: 10.4269/ajtmh.2002.66.264. [DOI] [PubMed] [Google Scholar]
- 15.Blaney JE, Jr., Matro JM, Murphy BR, Whitehead SS. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol. 2005 May;79(9):5516–5528. doi: 10.1128/JVI.79.9.5516-5528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaney JE, Jr., Hanson CT, Firestone CY, Hanley KA, Murphy BR, Whitehead SS. Genetically modified, live attenuated dengue virus type 3 vaccine candidates. Am J Trop Med Hyg. 2004;71(6):811–821. [PubMed] [Google Scholar]
- 17.Blaney JE, Jr., Hanson CT, Hanley KA, Murphy BR, Whitehead SS. Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect Dis. 2004 Oct 4;4(1):39. doi: 10.1186/1471-2334-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaney JE, Jr., Durbin AP, Murphy BR, Whitehead SS. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 2006 Spring;19(1):10–32. doi: 10.1089/vim.2006.19.10. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead SS, Falgout B, Hanley KA, Blaney JE, Jr., Markoff L, Murphy BR. A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J Virol. 2003;77:1653–1657. doi: 10.1128/JVI.77.2.1653-1657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durbin AP, Whitehead SS, McArthur J, Perreault JR, Blaney J, J E, Thumar B, et al. rDEN4Δ30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis. 2005;191(5):710–718. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 21.Durbin AP, McArthur J, Marron JA, Blaney JE, Jr., Thumar BR, Wanionek K, et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Human Vaccines. 2006 Jul–Aug;2(4):167–173. doi: 10.4161/hv.2.4.2944. [DOI] [PubMed] [Google Scholar]
- 22.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65(5):405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 23.Whitehead SS, Hanley KA, Blaney JE, Gilmore LE, Elkins WR, Murphy BR. Substitution of the structural genes of dengue virus type 4 with those of type 2 results in chimeric vaccine candidates which are attenuated for mosquitoes, mice, and rhesus monkeys. Vaccine. 2003 Oct 1;21(27–28):4307–4316. doi: 10.1016/s0264-410x(03)00488-2. [DOI] [PubMed] [Google Scholar]
- 24.Durbin AP, McArthur JH, Marron JA, Blaney JE, Thumar B, Wanionek K, et al. rDEN2/4Delta30(ME), A Live Attenuated Chimeric Dengue Serotype 2 Vaccine Is Safe and Highly Immunogenic in Healthy Dengue-Naive Adults. Human Vaccines. 2006 Nov 5;2(6):255–260. doi: 10.4161/hv.2.6.3494. [DOI] [PubMed] [Google Scholar]
- 25.Gubler DJ, Suharyono W, Lubis I, Eram S, Gunarso S. Epidemic dengue 3 in central Java, associated with low viremia in man. Am J Trop Med Hyg. 1981;30(5):1094–1099. doi: 10.4269/ajtmh.1981.30.1094. [DOI] [PubMed] [Google Scholar]
- 26.Blaney JE, Jr., Johnson DH, Manipon GG, Firestone CY, Hanson CT, Murphy BR, et al. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology. 2002 Aug 15;300(1):125–139. doi: 10.1006/viro.2002.1528. [DOI] [PubMed] [Google Scholar]
- 27.Troyer JM, Hanley KA, Whitehead SS, Strickman D, Karron RA, Durbin AP, et al. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg. 2001 Nov;65(5):414–419. doi: 10.4269/ajtmh.2001.65.414. [DOI] [PubMed] [Google Scholar]
- 28.Monath TP, Kanesa-Thasan N, Guirakhoo F, Pugachev K, Almond J, Lang J, et al. Recombination and flavivirus vaccines: a commentary. Vaccine. 2005 Apr 27;23(23):2956–2958. doi: 10.1016/j.vaccine.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 29.Hanley KA, Manlucu LR, Manipon GG, Hanson CT, Whitehead SS, Murphy BR, et al. Introduction of mutations into the non-structural genes or 3′ untranslated region of an attenuated dengue virus type 4 vaccine candidate further decreases replication in rhesus monkeys while retaining protective immunity. Vaccine. 2004;22:3440–3448. doi: 10.1016/j.vaccine.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Hanley KA, Manlucu LR, Gilmore LE, Blaney JE, Jr., Hanson CT, Murphy BR, et al. A trade-off in replication in mosquito versus mammalian systems conferred by a point mutation in the NS4B protein of dengue virus type 4. Virology. 2003 Jul 20;312(1):222–232. doi: 10.1016/s0042-6822(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 31.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nature Rev Microbiol. 2007;5(7):518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 32.Markoff L, Pang X, Houng HS, Falgout B, Olsen R, Jones E, et al. Derivation and characterization of a dengue type 1 host range-restricted mutant virus that is attenuated and highly immunogenic in monkeys. J Virol. 2002 Apr;76(7):3318–3328. doi: 10.1128/JVI.76.7.3318-3328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Men R, Bray M, Clark D, Chanock RM, Lai CJ. Dengue type 4 virus mutants containing deletions in the 3′ noncoding region of the RNA genome: analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J Virol. 1996;70(6):3930–3937. doi: 10.1128/jvi.70.6.3930-3937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markoff L. 5′- and 3′-noncoding regions in flavivirus RNA. Advances in Virus Research. 2003;59:177–228. doi: 10.1016/S0065-3527(03)59006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez DE, De Lella Ezcurra AL, Fucito S, Gamarnik AV. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology. 2005 Sep 1;339(2):200–212. doi: 10.1016/j.virol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Shurtleff AC, Beasley DW, Chen JJ, Ni H, Suderman MT, Wang H, et al. Genetic variation in the 3′ non-coding region of dengue viruses. Virology. 2001;281(1):75–87. doi: 10.1006/viro.2000.0748. [DOI] [PubMed] [Google Scholar]
- 37.Olsthoorn RC, Bol JF. Sequence comparison and secondary structure analysis of the 3′ noncoding region of flavivirus genomes reveals multiple pseudoknots. RNA. 2001 Oct;7(10):1370–1377. [PMC free article] [PubMed] [Google Scholar]
- 38.Romero TA, Tumban E, Jun J, Lott WB, Hanley KA. Secondary structure of dengue virus type 4 3′ untranslated region: impact of deletion and substitution mutations. J Gen Virol. 2006 Nov;87(Pt 11):3291–3296. doi: 10.1099/vir.0.82182-0. [DOI] [PubMed] [Google Scholar]
- 39.Macadam AJ, Ferguson G, Stone DM, Meredith J, Knowlson S, Auda G, et al. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. Journal of virology. 2006 Sep;80(17):8653–8663. doi: 10.1128/JVI.00370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng L, Falgout B, Markoff L. Identification of specific nucleotide sequences within the conserved 3′- SL in the dengue type 2 virus genome required for replication. J Virol. 1998;72(9):7510–7522. doi: 10.1128/jvi.72.9.7510-7522.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proutski V, Gould EA, Holmes EC. Secondary structure of the 3′ untranslated region of flaviviruses: similarities and differences. Nucleic Acids Res. 1997 Mar 15;25(6):1194–1202. doi: 10.1093/nar/25.6.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003 Jul 1;31(13):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathews DH, Sabina J, Zuker DH. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol. 1999 May 21;228(5):911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]


