Abstract
Background
In patients with extensive myocardial bridging, evaluation of its clinical significance remains a challenge.
Hypothesis
Sequential invasive testing is feasible and gives more insight into the pathophysiological mechanism of bridging-related angina.
Methods
Twelve patients with chest pain, proven ischaemia and extensive myocardial bridging were assessed. Myocardial bridging was evaluated at rest, during intracoronary acetylcholine infusion, through coronary flow velocity and flow reserve measurements, and during dobutamine stress.
Results
The mean length of the bridging segment was 24.9 mm (QCA; range 8.4-48.0 mm). Acetylcholine infusion caused severe vasospasm in two patients. In these two patients anginal symptoms were related to vasospasm and sequential testing was discontinued. In the remaining ten patients sequential testing was continued. Coronary flow reserve was normal in all patients: 3.3±0.6. In six patients reliable quantitative measurements could be performed during dobutamine stress. The mean systolic diameter of the bridging segment was 1.6±0.4 at baseline and 1.3±0.3 during dobutamine stress (mean of differences 0.38 (95% CI 0.1-0.7)). The difference between the diastolic and systolic diameter in the bridging segment increased from 0.3±0.2 mm at baseline to 1.0±0.5 mm during dobutamine infusion (mean of differences 0.6 (95% CI 0.3 to 0.9)).
Conclusion
Sequential testing for bridging is feasible and may disclose endothelial dysfunction or spasm as an underlying mechanism in a minority of patients. Coronary flow reserve was preserved. Dobutamine stress unmasked further lumen reduction and may give further insight into the clinical significance of myocardial bridging in individual patients. (Neth Heart J 2008;16:10-5.18317538)
Keywords: acetylcholine, coronary flow reserve, coronary spasm, dobutamine stress, endothelium, myocardial bridging
The prevalence of myocardial bridging varies between 15 and 86% in anatomical studies and between 0.5 and 2.5% in angiographic studies.1-3 In general, myocardial bridging is considered harmless. Nevertheless, in case reports myocardial bridging has been described as the sole abnormality on angiography in patients with angina pectoris, myocardial infarction, left ventricular rupture and sudden cardiac death.1-7 Although coronary stenting may normalise coronary haemodynamics, its long-term effect is poor.8 In addition, myocardial bridging can be found in a small group of patients presenting with complaints of angina and confirmed ischaemia. This clinical presentation may fuel the continuing debate on whether bridging may cause the symptoms and signs of myocardial ischaemia and whether this finding should subsequently be treated. As bridging may result in ischaemia by different pathophysiological mechanisms, we hypothesised that sequential functional evaluation during a single catheterisation procedure may unravel the operating mechanism in individual cases.
Methods
Patient selection
Patients presenting with angina between January 1996 and March 2003 and in whom myocardial bridging was found as the sole abnormality on a diagnostic coronary in whom bridging was suspected to be the cause of the angina were included. Severe bridging was defined as a bridging segment with a length of at least 20 mm or a lumen reduction of at least 60% of the reference diameter during diastole.
Quantitative coronary angiography
Coronary angiography was performed using standard procedures. The angiograms were recorded on film (until 2000) or on CD ROM. All drug administrations were noted on the film or CD-ROM. Quantitative coronary angiography (QCA) was performed with the CAAS II software (films) or with the Siemens Quanticor system (CD-ROM). By manual selection of the bridging segment, the computer automatically computed the minimal and mean diameter, the length and mean diameter reduction. The mean proximal and distal diameters were measured similarly.
Intracoronary Doppler flow measurement
The flow velocity proximal, distal and within the bridging segment was obtained by intracoronary Doppler flow measurement. The heart rate and blood pressure were measured continuously. The average peak-flow velocity (AVP), the ratio between proximal and distal flow velocity (P/D ratio), the coronary flow reserve (CFR; 12 μg adenosine by intracoronary injection) and the diastolic-systolic velocity ratio (DSVR) were obtained. The measurements were continuously recorded on videotapes. Administration of a drug (dobutamine) was marked.
Procedure
Patients in whom myocardial bridging was found at previous coronary angiography underwent a stepwise evaluation of the bridging segment of the target vessel. First an angiogram was taken at rest for a baseline assessment. If no other coronary abnormality was seen except for bridging, a spasm provocation test with a stepwise acetylcholine infusion was performed. First, a baseline coronary angiogram was done, visualising the bridging segment. Then acetylcholine-chloride (concentration 0.16 μg/ml; Clinalfa AG, Läufelfingen, Switzerland) was infused through the catheter for at least three minutes. This results in an infusion of 120x10-8 mmol/min, resulting in a final concentration in the coronary blood of 1x10-8 mol/l (with the assumption that the blood flow in the left main coronary artery was 120 ml/min). This procedure was repeated using 10-7 mol/l and 10-6 mol/l respectively. Finally, the response to nitroglycerin was recorded one minute after an intracoronary bolus of 0.5 mg nitroglycerin. The response to both stimuli was measured by automatic contour detection technique (QCA) in the bridging segment as well as proximal and distal of this segment. QCA was performed by a validated automatic contour detection technique (CMS, Medis Co., Nuenen, the Netherlands). A positive spasm provocation test was defined as a vessel lumen reduction to 90% or less in diastole following administration of acetylcholine.9
A negative spasm provocation test was followed by a ten-minute resting period. Subsequently, the APV, DSVR, P/D ratio and CFR were obtained. Then, dobutamine was administered in increasing concentrations. The starting dose was 5 μg/kg/min, after five and ten minutes the dosage was increased to 10 μg/kg/min and 20 μg/kg/min. The dose was increased to 30 μg/kg/min after 13 minutes and finally to 40 μg/kg/min after 16 minutes. If the target heart rate was not reached, 0.5 mg/kg atropine was given in addition. During the dobutamine stress APV, DSVR, heart rate, blood pressure and ECG were recorded continuously. A continuous 12-lead electrocardiogram was recorded in order to detect arrhythmias or ischaemia. During the highest dobutamine dose, angiography was performed. Beta-blockers were discontinued at least 24 hours before testing.
Statistics
The results are expressed as mean and standard deviation (SD). If two projections were available the view in which the length of the bridging segment appeared the longest was chosen to measure the length of the segment. The segments proximal and distal to the bridging were considered to be circular. Therefore, the mean of both directional views was assessed. The view in which the diameter of the bridging segment was the smallest at baseline was compared with the diameter of the segment in the same direction during the consecutive tests. The APV and the DSVR during dobutamine stress testing were compared with the APV and DSVR at baseline by the Student’s t-test for paired groups. P<0.05 was regarded as significant. The results of the Student’s t-tests are expressed as mean of the differences (95%).
Results
Twelve patients in whom myocardial bridging was found during angiography were enrolled in this study. Demographic and clinical data are shown in table 1. The median age was 54 years (range 33-66) and ten of the twelve subjects were men. All patients had proven ischaemia, either by electrocardiography (n=1), exercise test (n=8) or myocardial scintigraphy (n=4). In one patient myocardial bridging was found on admission following cardiac arrest. Two patients were referred with myocardial bridging on diagnostic coronary angiograms from other hospitals. The interval between the first presentation of symptoms and the diagnosis of myocardial bridging was on average 12 months (range 0-120). The median number of hospital admissions was 2 (range 1-6). One patient had a cardiac arrest, one ventricular tachycardia, six angina at rest and five exertional chest pain.
Table 1.
Patient and angiographic characteristics.
| Patient No. | Age(yrs) | Sex(M/F) | Interval(months) | Ischaemia | Medication | Minimaldiameter baseline | Length of bridge | Spasm Y/N | CFR | Dobu(Y/N) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C ACE | N | S | ||||||||||
| 1 | 39 | M | 18 | 1 | + | + | -- | + | + | 0.82 | 29.24 | Y | 2.6 | N |
| 2 | 49 | F | 16 | 1 | + | - | -+ | - | + | 1.06 | 19.63 | N | 3.1 | Y |
| 3 | 55 | M | 2 | 1 | + | + | -- | - | + | 0.92 | 28.69 | N | 4.5 | Y |
| 4 | 55 | M | 12 | 2 | + | + | -- | - | + | 0.77 | 34.79 | N | 3.3 | N |
| 5 | 60 | M | 6 | 2 | + | + | -- | + | + | 0.93 | 27.55 | N | 3.7 | Y |
| 6 | 55 | M | 9 | 2 | - | - | + + | + | + | 1.08 | 18.28 | N | 2.9 | Y |
| 7 | 33 | M | 120 | 2 | - | - | + - | - | - | 1.33 | 14.06 | N | Y | |
| 8 | 37 | M | 0 | 2 | + | + | + + | - | + | 1.03 | 8.32 | N | 3.7 | Y |
| 9 | 66 | M | ? | 1 | + | + | + - | + | + | 1.25 | 33.51 | N | 3.5 | Y |
| 10 | 38 | M | ? | 2 | + | + | + - | - | - | N | 2.8 | Y | ||
| 11 | 44 | F | 15 | 3 | - | - | -- | - | + | 1.34 | 17.13 | Y | N | |
| 12 | 54 | M | 0 | 2 | - | - | -- | - | - | 1.37 | 42.25 | N | Y |
Dobu=dobutamine, CFR=coronary flow velocity reserve, interval=interval between presentation of symptoms and test. Ischaemia: 1=positive exercise test, 2=positive myocardial scintigraphy, 3= ischaemia on rest electrocardiogram. Medication: A = acetylsalicylic acid, B=β-blocker, C=calcium receptor antagonist, ACE=ACE inhibitor, N=long-acting nitrate, S=statin. Spasm: in patient no. 1 diastolic diameter decreased from 1.11 mm to 0.3 mm and in patient no. 11 an occlusive spasm during the lowest acetylcholine dose was observed.
Quantitive coronary angiography
In all patients the bridging segment was located in the left anterior descending artery (LAD). No other coronary vessel disease was present to attribute the symptoms to. Coronary angiograms were taken from the most optimal angle at baseline, during intracoronary infusion of increasing acetylcholine doses, after intracoronary nitroglycerin and at the peak of dobutamine stress (figure 1). Both the systolic and diastolic mean proximal, mean distal, mean and minimal diameters of the bridging segment are shown in table 2. The difference between the mean systolic and mean diastolic diameter was 0.3 (0.2) mm. In four patients the minimal diameter at baseline was less than 1 mm. No patients had a minimal systolic diameter less than 0.5 mm. The mean length of the bridging segment was 24.9 mm (range 8.4-48.0 mm).
Figure 1.
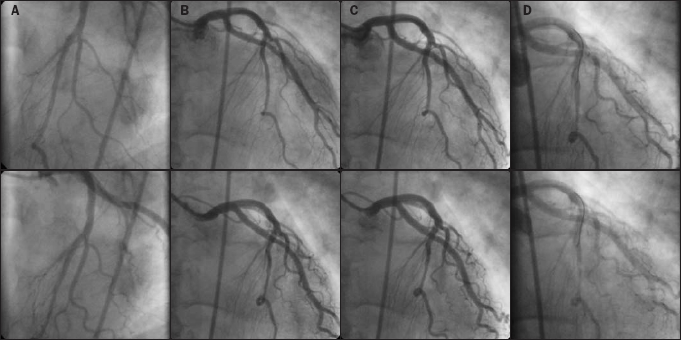
Coronary angiographic diastolic and systolic images. Diastolic (upper panels) and systolic frame (lower panels) of coronary angiography A) at baseline, B) after the highest dose of intracoronary acetylcholine, C) after intracoronary nitroglycerin, D) during dobutamine stress.
Table 2.
Quantitative coronary angiography.
| Baseline(n=11) | ACT1(n=9) | ACT2(n=8) | ACT3(n=8) | NTG(n=10) | Dobu(n=6) | |
|---|---|---|---|---|---|---|
| Proximal | ||||||
| - Diastole | 2.5 (0.6) | 2.2 (1.0) | 2.6 (0.8) | 2.5 (0.7) | 2.9 (0.7) | 2.9 (0.7) |
| - Systole | 2.5 (0.6) | 2.2 (1.0) | 2.6 (0.7) | 2.5 (0.7) | 2.9 (0.8) | 3.0 (0.8) |
| Distal | ||||||
| - Diastole | 1.7 (0.2) | 1.4±0.6 | 1.7 (0.2) | 1.7 (0.4) | 1.9 (0.3) | 1.8 (0.2) |
| - Systole | 1.7 (0.3) | 1.4±0.6 | 1.7 (0.2) | 1.6 (0.3) | 1.9 (0.3) | 1.7 (0.1) |
| Mean in bridge | ||||||
| - Diastole | 1.9 (0.5) | 1.6±0.8 | 2.0 (0.6) | 1.9 (0.6) | 2.2 (0.6)** | 2.3 (0.6) |
| - Systole | 1.6 (0.4) | 1.5±0.7 | 1.7 (0.4) | 1.6 (0.5) | 1.4 (0.4)* | 1.3 (0.3)* |
| Minimal in bridge | ||||||
| - Diastole | 1.5 (0.4) | 1.2±0.6 | 1.4 (0.6) | 1.4 (0.6) | 1.7 (0.5)* | 1.5 (0.4) |
| - Systole | 1.1 (0.2) | 1.0±0.5 | 1.0 (0.4) | 1.0 (0.4) | 0.8 (0.3)*** | 0.6 (0.2)*** |
*p<0.05, **p<0.01,***p<0.005 vs. baseline. Diameters are expressed as mean (SD) in millimetres. ACT1,2,3 = acetylcholine 10-8, 10-7 and 10-6 mol/l respectively, NTG=nitroglycerin, dobu=dobutamine.
Acetylcholine induced coronary spasm in two patients. In the other subjects the diameters after acetylcholine infusion were similar to baseline measurements. In one patient (no. 11) vasospasm of the entire LAD was induced after the administration of 10-8 mol/l of acetylcholine. This patient also experienced anginal pain at rest accompanied by electrocardiographic signs of ischaemia. The test was discontinued and intracoronary nitroglycerin as well as atropine were required. In patient no. 1 the minimal diastolic diameters decreased from 1.1 at baseline to 0.8, 0.8 and 0.3 mm following increasing doses of acetylcholine. The minimal systolic diameter was less than 1.0 mm in two patients following 10-8 mol/l, in three following 10-7 mol/l and in two following 10-6 mol/l of acetylcholine.
Nitroglycerin was given after the acetylcholine test. In eight of ten patients the minimal systolic diameter after nitroglycerin administration was less than 1.0 mm. After nitroglycerin, the mean difference between the diastolic and systolic diameter was 0.7 (0.5) and differed significantly from baseline (mean of differences 0.5 (95% CI 0.3 to 0.7)). The mean as well as minimal systolic diameter decreased significantly compared with baseline (mean of differences -0.2 (95% CI -0.4 to - 0.02) and -0.3 (95% CI -0.5 to -0.1) respectively), whereas the mean and minimal diastolic diameter increased (mean of differences 0.2 (95% CI 0.1 to 0.4) and 0.2 (95% CI 0.1 to 0.4) respectively).
Dobutamine testing was performed in the ten patients without spasm. Due to technical problems (inadequate digital format (n=2) or insufficient quality (n=2) of the angiogram) this was evaluable in six patients. During dobutamine stress the minimal systolic diameter was less than 1.0 mm in all six patients and less than 0.50 mm in five of them. The mean difference of the diastolic and systolic diameter was 1.0 (0.5) and was higher than at baseline (mean of differences 0.6 (95% CI 0.3 to 0.9)). The mean and minimal systolic diameter decreased significantly compared with baseline (mean of differences -0.4 (95% CI -0.7 to -0.08) and -0.6 (95% CI -0.8 to -0.3) respectively), whereas the mean and minimal diastolic diameter were similar to baseline. Two out of ten patients complained of angina pectoris during dobutamine stress. These patients also experienced exercise-related angina in daily life. One patient, a survivor of sudden death, developed nonsustained ventricular tachycardias during dobutamine testing.
Intracoronary Doppler flow measurement
Using coronary Doppler flow measurements, CFR was assessed in the artery with myocardial bridging. In addition flow measurements were continued during dobutamine infusion (figure 2). The mean CFR was 3.3 (0.6). In nine patients the APV was also measured during dobutamine stress testing. At rest, APV was 13.9 (6.9) cm/s and during maximal dobutamine stress testing 30.9 (11.2) cm/s. In six patients atropine was added to maximal dobutamine stress testing, and APV further increased to 43.3 (27.0) cm/s. The increase in APV was significant at 20, 30 and 40 μg/kg/min (p<0.004, p<0.0003 and p<0.0005 respectively). Blood pressure increased from 110±20 / 67±12 mmHg to 122±11 / 67±15 mmHg. The heart rate increased from 68±11 to 117±33. The P/D ratio was 1.1. The DSVR was 2.9±2.4 at baseline and did not change during dobutamine stress testing. Volumetric flow was calculated and increased from 29±5 to 91±32 ml/min during dobutamine infusion with a concomitant increase in rate pressure product from 7250±750 to 15,400±2250 mmHg/min.
Figure 2.
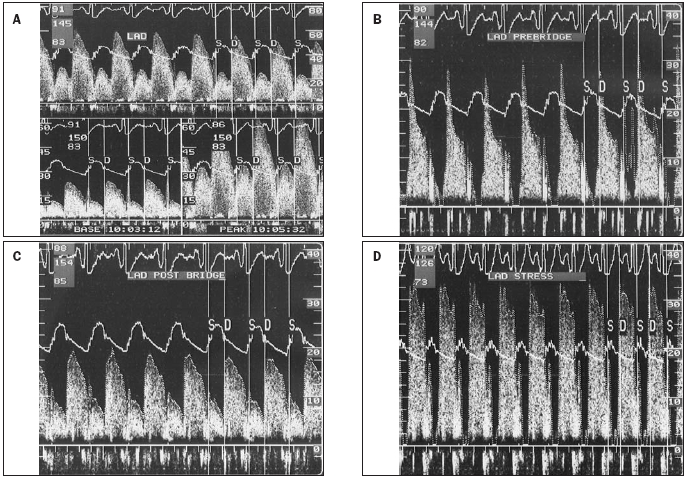
Coronary Doppler flow measurements. A) coronary flow reserve, B) in the segment immediately proximal to the bridging, note the typical spike and dome configuration, C) at rest, D) during dobutamine stress, note the difference with the registration at rest: during stress a complete absence of systolic antegrade flow was observed.
Discussion
This study assessed the clinical impact of sequential functional testing for myocardial bridging during coronary angiography. The results support the clinical conception that sequential testing is feasible. The implemented strategic approach enables the evaluation of the different possible underlying pathophysiological entities such as decreased perfusion either due to vasospasm, or mechanical obstruction in combination with an increased demand (increased heart rate and contractility).
The overall validity of the results is limited due to the small number of patients. However, other studies have comparable sample sizes. In these earlier reports, standard methods to diagnose bridging were used. As the cross-sectional intraluminal area during systolic narrowing in a bridging segment is flattened and not round, Ge et al. used intravascular ultrasound (IVUS) to visualise the abnormal segment.2,4 However, in more severe cases the IVUS catheter is too large. In such cases, QCA from multiple views is a good alternative for the evaluation of myocardial bridging. It should be mentioned that the more elliptical intraluminal shape in significant bridging may be visualised in one direction and missed from another angle.3 Therefore, we used the view from which the systolic narrowing was largest, to approximate the true maximal systolic narrowing of the bridging segment. In addition, mean segmental as well as minimal diameters were evaluated.
In contrast to a previous report, we found a low incidence of spasm.10 This may have been related to racial differences. The previous study was conducted in a Japanese population, which is known to have a high incidence of coronary spasm. A positive spasm provocation test provides a rationale for drug therapy aimed at improvement of endothelial function and vasodilatation. We found an aggravation of myocardial bridging following nitroglycerin administration comparable with results found in a previous study.11 Despite this mechanical impairment, the beneficial mechanistic effect of nitroglycerin may be found in reduction of preload.8 Schwarz et al. have demonstrated that the β-blocker esmolol improves coronary perfusion by its negative inotropic and chronotropic action.8 On the other hand, the use of β-blockers may increase the risk of coronary spasm, especially in susceptible patients.
In contrast to previous reports,4,8,13,14 we found a normal CFR distal to the bridging segment in most patients. This discrepancy may in part be related to the fact that in most previous reports papaverine instead of adenosine was given for flow reserve measurements. It is known that papaverine targets a different vessel size in contrast to adenosine.15 This may have obscured the normal CFR as found in our study. Based on our results, the clinical utility of CFR measurements in myocardial bridging patients without coronary spasm may be questioned. Haemodynamic evaluation of myocardial bridging with pressure measurements, especially diastolic pressure gradients during dobutamine infusion, may be a better approach.16
Dobutamine stress angiography may elucidate anatomical changes during higher heart rates and inotropy. An ample increase was found in systolic and to a lesser extent in diastolic diameter narrowing. Intensification of systolic narrowing may further impair diastolic flow, by a delayed gain in luminal dimensions during early diastole.8 Two patients had angina pectoris during the test and one (a survivor of near sudden death) nonsustained ventricular tachycardia.
The importance of dobutamine testing for clinical decision-making remains unclear. In individual cases it might shed a clear light on the haemodynamic importance of the bridging segment, and identify the need for a coronary intervention.7 In order to establish the diagnostic and prognostic value of dobutamine testing in patients with myocardial bridging, a larger group of patients should be evaluated.
Monotherapy may be inadequate in some patients. Therefore, a larger study group is required to assess whether the outcome of a spasm provocation test and dobutamine stress angiography support a treatment strategy with drugs such as calcium antagonists, β-blockers, mechanical/surgical intervention, or a combination. Finally, the future use of drug-eluting stents in this group of patients is challenging.17
Conclusion
These results show that sequential testing for bridging is feasible and may disclose endothelial dysfunction or spasm as an underlying mechanism in a subgroup of patients. The CFR was normal in patients without spasm. In search of a tailored approach for individual patients, dobutamine stress angiography may give further insight into the clinical significance of myocardial bridging.
Acknowledgments
The authors wish to thank Mrs D. Amo of DIAGRAM in Zwolle, the Netherlands, for her assistance with the QCA measurements.
References
- 1.Bourassa MG, Butnaru A, Lespérance J, Tardif JC. Symptomatic Myocardial Bridges: overview of Ischemic Mechanisms and Current Diagnostic and Treatment Stategies. J Am Coll Cardiol 2003; 41:351-9. [DOI] [PubMed] [Google Scholar]
- 2.Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Gorge G, et al. Comparison of Intravasular Ultrasound and Angiography in the Assessment of Myocardial Bridging. Circulation 1994;89:1725-32. [DOI] [PubMed] [Google Scholar]
- 3.Angelini P, Trivellato M, Donis J, Leachman RD. Myocardial Bridges: A Review. Prog Cardiovasc Dis 1983;26:75-88. [DOI] [PubMed] [Google Scholar]
- 4.Ge J, Jeremias A, Rupp M, Abels M, Baumgart D, Liu F, et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J 1999;20:1707-16. [DOI] [PubMed] [Google Scholar]
- 5.Möhlenkamp S, Hort W, Ge J, Erbel E. Update on Myocardial Bridging. Circulation 2002;106:2616-22. [DOI] [PubMed] [Google Scholar]
- 6.Tio RA, Ebels T. Ventricular Septal Rupture Caused by Myocardial Bridging. Ann Thorax Surg 2001;72:1369-70. [DOI] [PubMed] [Google Scholar]
- 7.Tio RA, Van Gelder IC, Boonstra PW, Crijns HGJM. Myocardial bridging in a survivor of sudden cardiac near-death?: Role of intracoronary Doppler flow measurements and angiography during dobutamine stress in the clinical evaluation. Heart 1997;77:280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haager PK, Schwarz ER, Vom Dahl J, Klues HG, Reffelmann T, Hanrath P. Long term angiographic and clinical follow up in patients with stent implantation for symptomatic myocardial bridging. Heart 2000;84:403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murase Y, Yamada Y, Hirashiki A, Ichihara S, Kanda H, Watarai M, et al. Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur Heart J 2004;25:970-7. [DOI] [PubMed] [Google Scholar]
- 10.Teragawa H, Fukuda Y, Matsuda K, Hirao H, Higashi Y, Yamagata T, et al. Myocardial bridging increases the risk of coronary spasm. Clin Cardiol 2003;26:377-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hongo Y, Tada H, Ito K, Yasumura Y, Miyatake K, Yamagishi M. Augmentation of vessel squeezing at coronary-myocardial bridge by nitroglycerin: study by quantitative coronary angiography and intravascular ultrasound. Am Heart J 1999;138:345-50. [DOI] [PubMed] [Google Scholar]
- 12.Klues HG, Schwarz ER, Vom Dahl J. Disturbed Intracoronary Hemodynamics in Myocardial Bridging, Early Normalization by Intracoronary Stent Placement. Circulation 1997;96:2905-13. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz ER, Klues HG, Vom Dahl J, Klein I, Krebs W, Hanrath P. Functional, Angiographic and Intracoronary Doppler Flow Characteristics in Symptomatic Patients With Myocardial Bridging: Effect of Short-Term Intravenous Beta-Blocker Medication. J Am Coll Cardiol 1996;27:1637-45. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional characteristics of myocardial bridging. A combined angiographic and intracoronary Doppler flow study. Eur Heart J 1997;18:434-42. [DOI] [PubMed] [Google Scholar]
- 15.Uren NG, Crake T. Resistive vessel function in coronary artery disease. Heart 1996;76:299-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escaned J, Cortes J, Flores A, Goicolea J, Alfonso F, Hernandez R, et al. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol 2003;42:226-33. [DOI] [PubMed] [Google Scholar]
- 17.Ng E, Jilaihawi H, Gershlick AH. Symptomatic myocardial bridging-a niche indication for drug-eluting stents? Int J Cardiol 2005;99:463-4. [DOI] [PubMed] [Google Scholar]


