Abstract
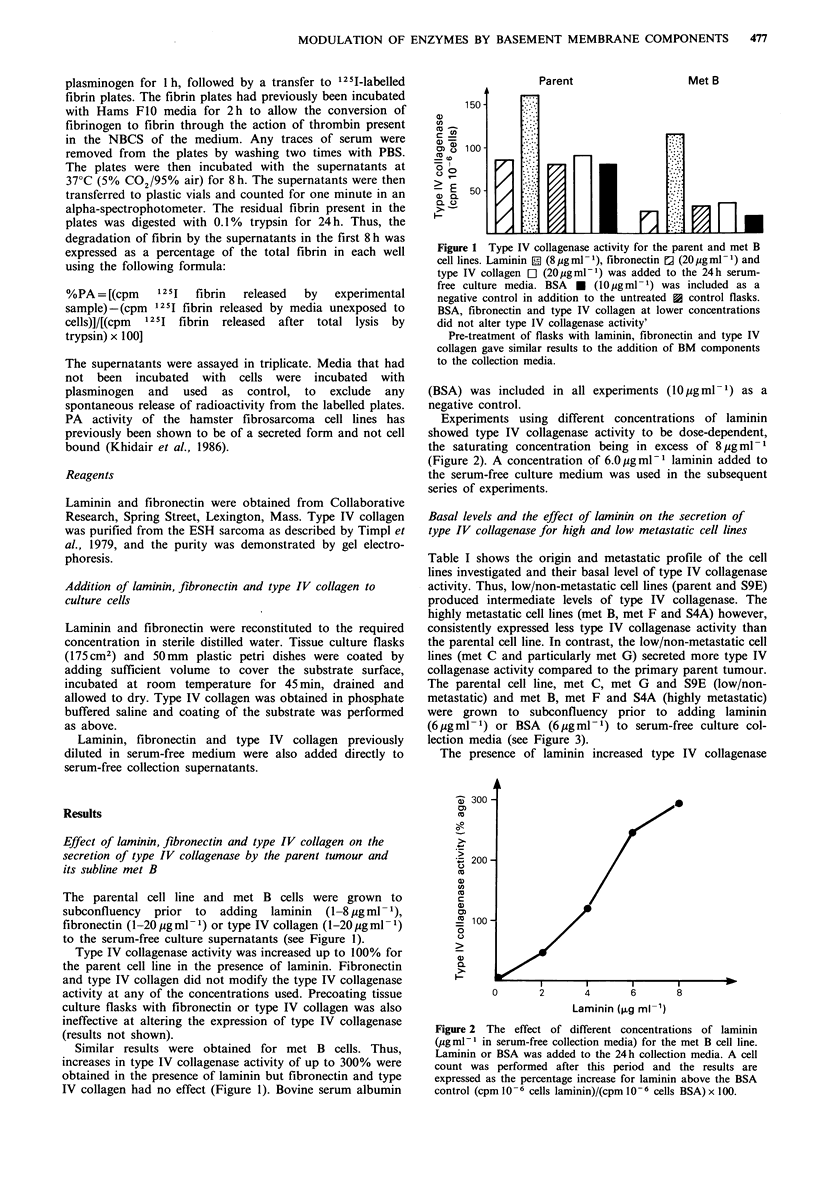
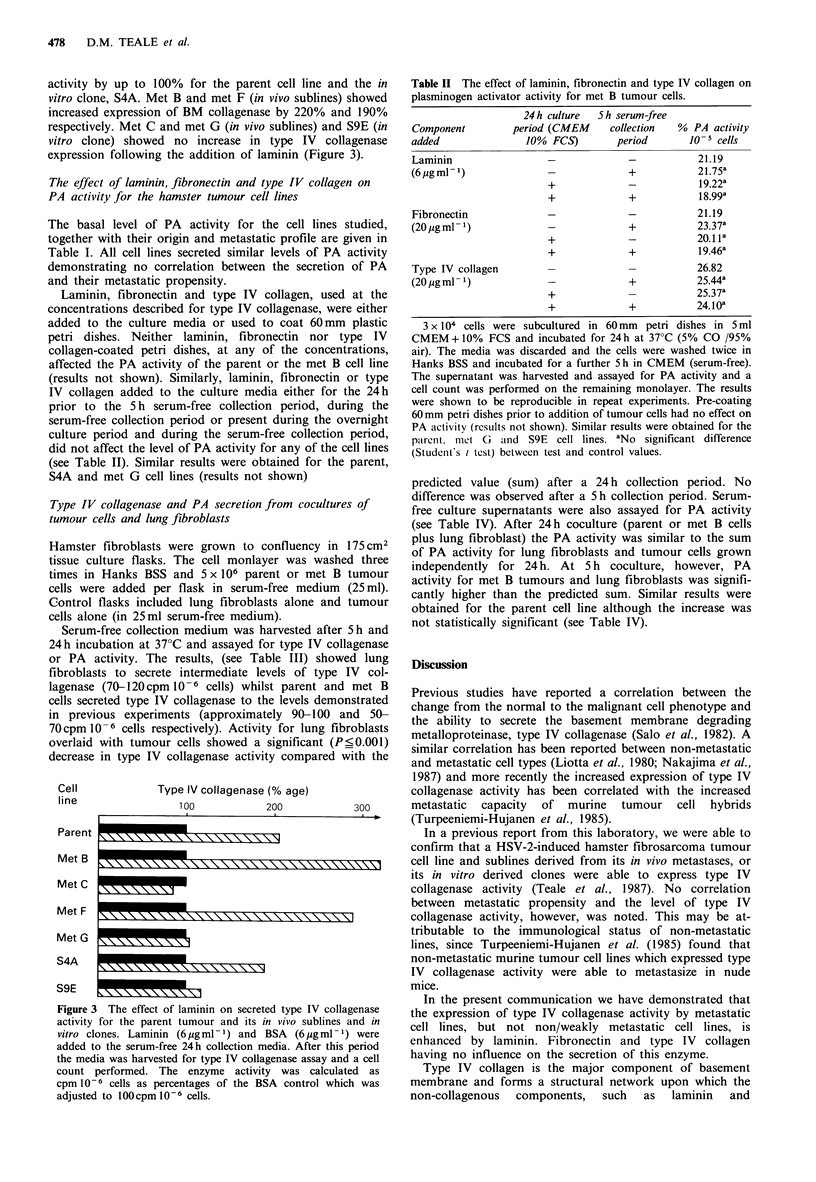
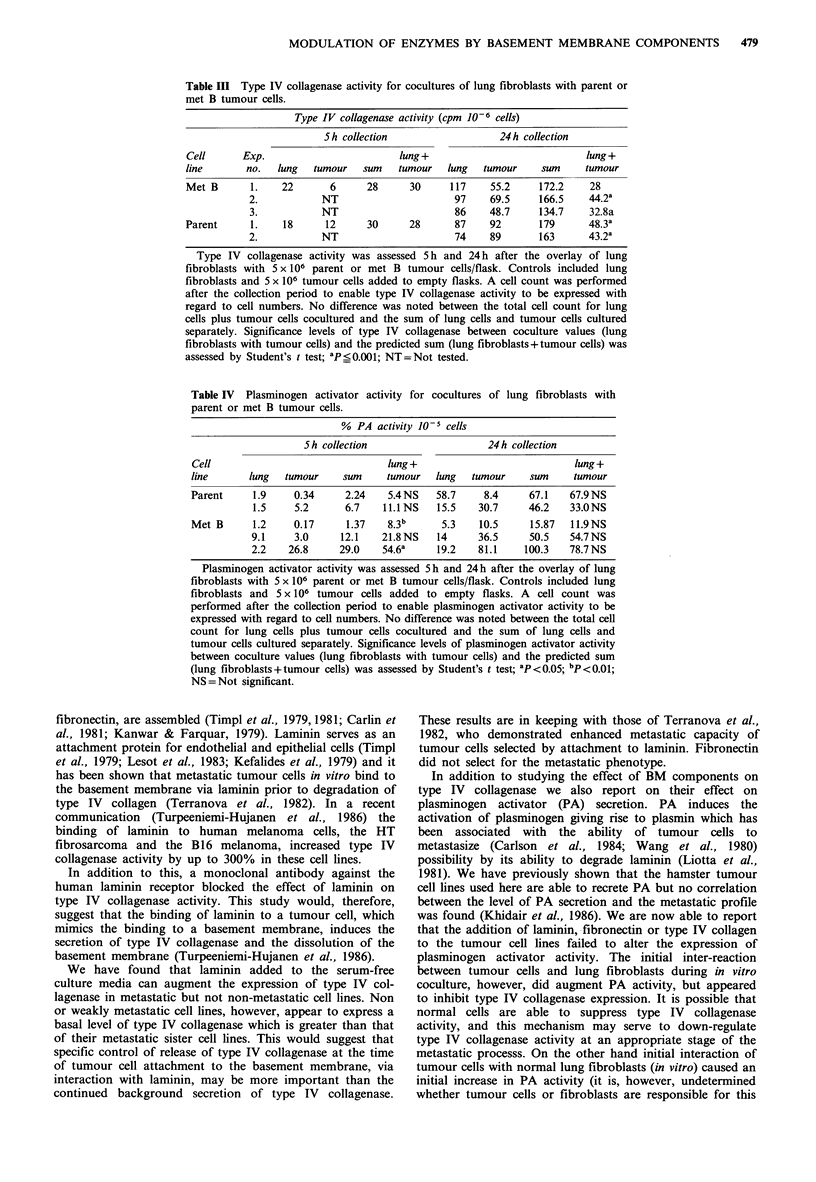
The effect of basement membrane components (laminin, fibronectin and type IV collagen) and lung fibroblasts on type IV collagenase and plasminogen activator activity was investigated in a primary HSV-2-induced hamster fibrosarcoma, and its in vivo derived sublines and in vitro derived clones of varying metastatic potential. Fibronectin and type IV collagen were ineffective at influencing the expression of either type IV collagenase or plasminogen activator activity. Laminin, however, at concentrations of 1-10 micrograms ml-1 added to the serum-free culture supernatants, increased the release of type IV collagenase by up to 100% for the parental cell line. Three highly metastatic sublines (two from in vivo origin and one from in vitro cloning) showed increases of up to 300%. Non-metastatic sublines (two from in vivo origin and one from in vitro cloning), however, showed no increase in type IV collagenase activity. Plasminogen activator release from either the parental line cell or its metastatic sublines and clones, was unaffected by the addition of laminin. Addition of tumour cells to lung fibroblast monolayers resulted in an increased expression of PA activity in the supernatant, whilst type IV collagenase activity was reduced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlin B., Jaffe R., Bender B., Chung A. E. Entactin, a novel basal lamina-associated sulfated glycoprotein. J Biol Chem. 1981 May 25;256(10):5209–5214. [PubMed] [Google Scholar]
- Carlsen S. A., Ramshaw I. A., Warrington R. C. Involvement of plasminogen activator production with tumor metastasis in a rat model. Cancer Res. 1984 Jul;44(7):3012–3016. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A., Alper R., Clark C. C. Biochemistry and metabolism of basement membranes. Int Rev Cytol. 1979;61:167–228. doi: 10.1016/s0074-7696(08)61998-1. [DOI] [PubMed] [Google Scholar]
- Khidair I. A., Teale D. M., Potter C. W., Rees R. C. Production of plasminogen activator by a primary HSV-2-induced hamster fibrosarcoma and its in vivo derived sublines. Cancer. 1986 Apr 15;57(8):1522–1527. doi: 10.1002/1097-0142(19860415)57:8<1522::aid-cncr2820570814>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Laug W. E., Jones P. A., Benedict W. F. Relationship between fibrinolysis of cultured cells and malignancy. J Natl Cancer Inst. 1975 Jan;54(1):173–179. doi: 10.1093/jnci/54.1.173. [DOI] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Terranova V. P. Cleavage of laminin by thrombin and plasmin: alpha thrombin selectively cleaves the beta chain of laminin. Thromb Res. 1981 Mar 15;21(6):663–673. doi: 10.1016/0049-3848(81)90268-1. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Kleinerman J., Catanzaro P., Rynbrandt D. Degradation of basement membrane by murine tumor cells. J Natl Cancer Inst. 1977 May;58(5):1427–1431. doi: 10.1093/jnci/58.5.1427. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Robey P. G., Abe S. Partial purification and characterization of a neutral protease which cleaves type IV collagen. Biochemistry. 1981 Jan 6;20(1):100–104. doi: 10.1021/bi00504a017. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Nakajima M., Welch D. R., Belloni P. N., Nicolson G. L. Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clones of differing metastatic potentials. Cancer Res. 1987 Sep 15;47(18):4869–4876. [PubMed] [Google Scholar]
- Ossowski L., Quigley J. P., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Requirement of plasminogen for correlated changes in cellular morphology, colony formation in agar, and cell migration. J Exp Med. 1973 Nov 1;138(5):1056–1064. doi: 10.1084/jem.138.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Keski-Oja J., Turpeenniemi-Hujanen T., Tryggvason K. Secretion of basement membrane collagen degrading enzyme and plasminogen activator by transformed cells--role in metastasis. Int J Cancer. 1982 Nov 15;30(5):669–673. doi: 10.1002/ijc.2910300520. [DOI] [PubMed] [Google Scholar]
- Teale D. M., Rees R. C., Clark A., Potter C. W. Properties of a herpesvirus-transformed hamster cell line: immunogenicity of sublines of high and low metastatic potential. Int J Cancer. 1984 May 15;33(5):701–708. doi: 10.1002/ijc.2910330523. [DOI] [PubMed] [Google Scholar]
- Teale D. M., Rees R. C., Clark A., Walker J. R., Potter C. W. Reduced susceptibility to natural killer cell lysis of hamster tumours exhibiting high levels of spontaneous metastasis. Cancer Lett. 1983 Jun;19(2):221–229. doi: 10.1016/0304-3835(83)90158-1. [DOI] [PubMed] [Google Scholar]
- Teale D. M., Rees R. C. Metastatic heterogeneity in a spontaneously metastatic HSV-2 induced hamster fibrosarcoma: association of phenotypic and genotypic properties with metastatic potential. Invasion Metastasis. 1987;7(3):129–143. [PubMed] [Google Scholar]
- Teale D. M., Rees R. C., Thorgeirsson U. P., Liotta L. A. Type IV collagenase activity of a primary HSV-2-induced hamster fibrosarcoma and its in vivo metastases and in vitro clones. Cancer. 1987 Sep 15;60(6):1263–1268. doi: 10.1002/1097-0142(19870915)60:6<1263::aid-cncr2820600617>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Robey P. G., Martin G. R. Biosynthesis of type IV procollagens. Biochemistry. 1980 Apr 1;19(7):1284–1289. doi: 10.1021/bi00548a003. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T., Thorgeirsson U. P., Hart I. R., Grant S. S., Liotta L. A. Expression of collagenase IV (basement membrane collagenase) activity in murine tumor cell hybrids that differ in metastatic potential. J Natl Cancer Inst. 1985 Jul;75(1):99–103. [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T., Thorgeirsson U. P., Rao C. N., Liotta L. A. Laminin increases the release of type IV collagenase from malignant cells. J Biol Chem. 1986 Feb 5;261(4):1883–1889. [PubMed] [Google Scholar]
- Walker J. R., Rees R. C., Teale D., Potter C. W. Properties of a Herpes virus-transformed hamster cell line--I. Growth and culture characteristics of sublines of high and low metastatic potential. Eur J Cancer Clin Oncol. 1982 Oct;18(10):1017–1026. doi: 10.1016/0277-5379(82)90251-6. [DOI] [PubMed] [Google Scholar]
- Wang B. S., McLoughlin G. A., Richie J. P., Mannick J. A. Correlation of the production of plasminogen activator with tumor metastasis in B16 mouse melanoma cell lines. Cancer Res. 1980 Feb;40(2):288–292. [PubMed] [Google Scholar]


