The FEBS Workshop on Invadopodia, Podosomes and Focal Adhesions in Tissue Invasion was organized by R. Buccione, S. Linder and M. Gimona, and took place in Ortona, Italy, between 8 and 13 September 2007. Further information about the meeting can be accessed at http://www.invadosomes.org/workshop.
Introduction
The mechanisms that cells use to polarize the machinery necessary to assemble, maintain and turnover lamellipodia, podosomes and invadopodia are still mysterious in many ways (Gimona & Buccione, 2006; Linder, 2007). These structures can be distinct in form and function depending on the context, although many common features were suggested in the talks and posters presented this year at the FEBS workshop on Invadopodia, Podosomes and Focal Adhesions in Tissue Invasion in Ortona, Italy. Some of the open questions in the field are: What does it take to make a focal contact? Or create a podosome or lamellipodium? Are these structures relevant in vivo in the three-dimensional extracellular space? How does the cell keep them in place, but allow them to be dynamic and functional? How does a cancer cell use variations on normal structures to become invasive and metastatic?
Podosomes, invadopodia, sealing zone: semantics or reality?
Three workshop sessions established the common features of and differences between podosomes, invadopodia and invasive podosomes—extending the usual attribution of podosomes to normal cells and of invadopodia to transformed cells. At present, podosomes are often studied in monocyte-derived cells and stimulated endothelial cells, whereas invadopodia are predominantly investigated in highly metastatic breast tumour cells or cells derived from other aggressive tumours. However, in culture, most of these cells show similar abilities to adhere, migrate, degrade the extracellular matrix (ECM), invade three-dimensional matrigels or transmigrate through cell layers and, most surprisingly, to also develop phagocytic activities. Despite these similarities, we now understand that morphological differences exist between these actin-containing structures, which vary from clustered actin networks in osteoclasts to ring-shaped rosettes made of individual podosomes in endothelial cells, or to more individualized structures in cancer-derived cells (Fig 1).
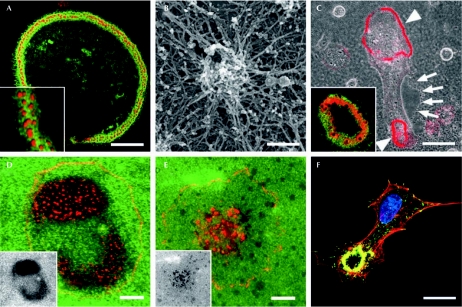
Figure 1.
Podosomes, invadopodia and the sealing zone. (A) Osteoclasts form an adhesion belt of podosomes on glass. (B) Podosomes exhibit a dense actin core surrounded by an actin cloud, as seen in scanning electron micrographs. (C) Osteoclasts form sealing zones (arrowheads) when resorbing bone ECM (arrows). Note the different arrangement of actin (red) and β3-integrin (green) structures in the insets of (A) and (C). (D) Cortactin-positive invadopodia (red) are formed by MDA cancer cells. (E) Actin-positive (red) podosomes are formed on a gelatin matrix (green) by macrophages. Invadopodia and podosomes are both active sites of ECM degradation (black areas in the insets of D and E).(F) In transforming growth factor-β1-induced bovine aortic endothelial cells, podosome rosettes (actin, red; vinculin, green) are the prominent degradation structures. Images were kindly provided by P. Jurdic (A, C), C. Luxenburg, L. Addadi and B. Geiger (B), S. Linder (D), P. Chavrier (E) and E. Génot (F). Scale bars, 40 μm in (A, C); 10 μm in (D–F); and 200 nm in (B). ECM, extracellular matrix.
Osteoclasts exhibit two different adhesion structures: podosomes when grown on glass (Fig 1A,B) and a sealing zone when resorbing bone (Fig 1C). F. Saltel (Bordeaux, France) showed that, in glass-adherent osteoclasts, podosomes are formed by two actin domains (Fig 1B): a dense actin core—associated with cortactin, actin-related protein (Arp)2/3 complex, Wiskott–Aldrich syndrome protein and CD44; and an actin cloud—a meshwork of F-actin cables that colocalize with vinculin and αvβ3-integrin—surrounding the core. Both domains have a role in osteoclast adhesion and the actin cloud also promotes contraction by associating with myosin II. P. Jurdic's group (Lyon, France) and B. Geiger (Rehovot, Israel) showed that the sealing zone—a prototype of structures involved in matrix degradation—is simply a contraction of these two actin domains.
S. Mueller (Washington, DC, USA) clarified the definition of invadopodia (Fig 1D) and investigated the different steps involved in their formation. She found that the lifetime of invadopodia by far exceeds the 2–3 min reported for podosomes in vitro (Fig 1E); moreover, invadopodia are devoid of vinculin, which is a characteristic of podosomes. By culturing MDA-MB-231 breast tumour cells in the presence of large gelatine beads, Muller compared the multi-step formation of invadopodia in a three-dimensional environment with that of related filopodia in two dimensions. The association of cortactin with phosphotyrosine proteins and actin condensation is triggered by contact with the substratum and has a crucial role in invadopodium initiation. However, cortactin is absent from the invadopodium tip where ECM degradation—predominantly mediated by the membrane type 1 matrix metalloproteinase (MT1-MMP)—takes place. E. Genot (Bordeaux, France) provided the first evidence that podosome rosettes (Fig 1F), which form in vitro in endothelial cells on transforming growth factor-β stimulation, are also detected after similar treatment in the endothelium of murine carotid artery as actin-rich degradation structures that contain focal adhesion kinase, vinculin and cortactin. Podosome rosettes might function as cellular devices that drill holes in the rigid blood vessel wall to create paths for endothelial cells. Results presented at this workshop and recently published findings on leukocyte transmigration through endothelial cell layers (Carman et al, 2007) strongly suggest that podosomes and invadopodia are invasive structures used to ‘palpate' and remodel the surrounding environment of cells.
Membrane transport at podosomes and invadopodia
Podosomes and invadopodia are busy places where ECM degradation and active protrusion take place. This meeting highlighted the importance of membrane transport in invadopodia and podosome formation. I. Maridonneau-Parini (Toulouse, France) showed that lysosomes and exocytic transport are both important mediators of macrophage migration—which is induced through a signalling cascade that involves the Src-related kinase haematopoietic cell kinase (Hck). They also contribute to ECM degradation, as presented by G. LeDez (Paris, France). G. Murphy (Cambridge, UK) provided evidence that tyrosine phosphorylated caveolin 1 (Cav1) associates with and regulates MT1-MMP activity in caveolae membranes. Notably, a reduction of the interaction between MT1-MMP and Cav1 also decreases cell locomotion. A. Weaver (Nashville, TN, USA) shed new light on the role of cortactin in invadopodia, providing compelling evidence that it does not act to regulate actin assembly in those structures, but instead to control the secretion of proteases. Weaver showed that cortactin is crucial for the membrane transport of several MMPs—such as MMP2, MMP9 and MT1-MMP—to invadopodia and for their secretion or surface display, as well as for global cell secretory activity. MMP transport to invadopodia is likely to occur through the Golgi complex, so it will be interesting to determine whether cortactin regulates Golgi transport.
Transport to and from the edge of migrating cells
The transport of membrane structures as well as the adhesion and actin assembly machinery is not only relevant for podosome formation, but also a major biological challenge for directional cell migration. Membrane transport is important in signal regulation at sites of ruffles and lamellipodia, as emphasized by A. Grande-Garcia (Madrid, Spain), who described the role of Cav1 in cell morphology and migration. Cav1-knockout mouse embryo fibroblasts (MEFs) seem to be non-polarized when grown in two dimensions, containing peripheral stress fibres and small focal adhesions (FAs). By contrast, wild-type MEFs seem more polarized and have prominent stress fibres and FAs. Grande-Garcia's data implicated Cav1 in the turnover of FAs perhaps through the regulation of the GTP-loading state of small GTPases. M. McNiven (Rochester, MN, USA) described the importance of Cav1 in the internalization of receptors and of E-cadherin, which contributes to the epithelial-to-mesenchymal transition in cancerous cells (Fig 2). Over the years, his group has shown that dorsal circular lamellipodia or ruffles are sites where epidermal growth factor or platelet-derived growth factor receptors are internalized through a tubulovesicular network (Orth & McNiven, 2006). The mechanisms that establish and maintain a polarized signal at the leading edge of a migrating cell remain some of the greatest mysteries about how cells move. Many types of signal have been proposed as leading-edge organizers and later found to be non-essential—such as phosphatidylinositol 3,4,5-trisphosphate (PIP3), which seems to be more important for the maximal speed of chemotaxis than for directional sensing (Hoeller & Kay, 2007). A. Huttenlocher (Madison, WI, USA) suggested that, at least in chemotaxing neutrophils, PIP3 has the role of a leading-edge organizer, whereas PIP2 organizes the detachment of the trailing edge. By using a new method to visualize PIP2 and PIP3 distinctively in living cells, Huttenlocher demonstrated that PIP3 accumulates only at the front of migrating neutrophils, whereas PIP2 is also enriched at the rear, where it is generated by the specifically localized PIP kinase PIPKIγ661. Consequently, PIPKIγ661-null neutrophils have an elongated morphology owing to problems in rear-end detachment, which validates the maxim that if you stick too long to the past, you cannot advance.
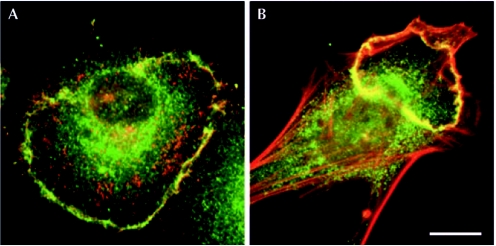
Figure 2.
Dorsal ruffles. (A) Cultured human pancreatic tumour cells internalize epidermal growth factor receptor (red) at sites positive for dynamin 2 (green), dorsal ruffles or circular lamellipodia. (B) Platelet-derived growth factor stimulates similar structures in fibroblasts, which are positive for actin-related protein 2/3 complex (green) and actin (red). Images are kindly provided by M. McNiven (A) and J.A. Legg and L. Machesky (B). Scale bars, 10 μm.
The importance of the urokinase-type plasminogen activator receptor (uPAR) for cell polarity was shown by C. Marshall (London, UK) and C. Madsen (Milan, Italy) independently. uPAR localizes uPA to the leading edges of some types of migrating cell and controls the activation of the small GTPase Rac. uPAR is not a transmembrane receptor and must therefore interact with other proteins—such as integrins—to have an effect on some of the processes involved in cell motility. Marshall showed that uPAR transmits signals to the cytoskeleton through association with vitronectin-binding integrins, and Madsen proposed that, although integrin signalling is required, the crucial event in uPAR signalling is its binding to vitronectin rather than uPAR binding to integrins. Therefore, these studies suggest a new mechanism of activation of Rac by uPAR.
But how is this kind of signalling maintained during prolonged cell migration events? An elegant model for how polarity might be reinforced and maintained at the leading edges of migrating cells was presented by K. Anderson (Glasgow, Scotland), who found that the leading edge constitutes a barrier to the diffusion of lipids. This effect is probably the result of a high concentration of peripherally associated membrane cytoskeleton proteins, such as the actin machinery. He presented a model for the tight packing of proteins in this region and gave compelling evidence that this self-reinforcing machinery may help to maintain cell persistence. M. Frame (Glasgow, Scotland) described another fascinating complex that might be responsible for the earliest actin nucleation events required in cell spreading. Her group discovered that focal adhesion kinase interacts transiently and in a phosphorylation-dependent manner with the Arp2/3 complex during migration (Serrels et al, 2007), and she proposed that this interaction releases Arp2/3 to drive lamellipodia formation and further spreading.
Focal adhesions from the inside and from above
The coordinated assembly and disassembly of FAs is as important for directed cell translocation as for protrusion and polarity. FAs do not seem so ‘focal' when one considers that more than 100 proteins could potentially localize to the FA, at least at some stage. How can we possibly cope with this daunting diversity and complexity? Geiger has been illuminating the inner life of FAs for decades and explained at this meeting that systems biology might fill our ‘lacunas of ignorance'. Geiger collected published data about how FA components interact and used smart cluster analysis software (Zaidel-Bar et al, 2007) to obtain general patterns. He showed that the effectors of small Rho GTPases—as opposed to the GTPases themselves—generally reside within FAs, that FA scaffolding proteins often bind to both signalling enzymes and their substrates, and that several proteins can be identified as molecular switches. This approach, combined with a related strategy that identifies gene products that regulate cell migration—presented by J. Brugge (Boston, MA, USA)—proves that systemic strategies might lead to solutions for complex biological problems (Lazebnik, 2002).
Integrins are found in all podosomes, invadopodia, sealing zones and FAs. J. Spatz (Stuttgart and Heidelberg, Germany) persuaded the audience that the time has come to manipulate adhesions at the nanoscale. Using block copolymer micelle nanolithography, he created nanoarrays of single αvβ3-integrin ligands and demonstrated the amazing adhesive sensitivity of cells. If integrins are placed more than 60 nm apart on a nanoarray, not only is the maturation of FAs inhibited and the ability of the cell to transmit forces reduced, but also cell-synthesizing activities—such as formation of a thick hyaluronan coat and secretion of fibronectin—are impaired. Cells grown on nanoarrays with an integrin ligand spacing gradient of 66 nm/mm can orient themselves and migrate directionally; hence, they can ‘feel' a spacing difference of approximately 3 nm between their leading and their trailing edges. Spatz explained that, when translated to a human length scale, this would correspond to the ability to feel 10 μm-sized structures—give it a try!
What happens on the cytoplasmic side of integrins? Talin has been suggested to promote inside-out integrin activation by binding to the NPXY motif in the cytoplasmic tail of αvβ3-integrin. B. Wehrle-Haller (Geneva, Switzerland) presented a new acidic motif—located in the membrane-proximal domain of the integrin tail—that recognizes basic residues in the talin FERM domain. When these basic residues are mutated to acidic residues, talin loses the ability to cluster integrins. Clustering can be elegantly restored by replacing the acidic integrin motif with a basic sequence. His view of the interaction between talin and integrin inside integrin clusters has important implications for how the cytoplasmic tails of the α- and β-integrin subunits interact. Wehrle-Haller believes that, in the absence of talin, they cannot separate, and supports this idea with a computational model that challenges existing nuclear magnetic resonance models.
R. Fässler (Martinsried, Germany) demonstrated that so-called kindlins (Kind) also have a FERM domain. Mice in which a haematopoietic system-specific isoform of Kind 3 has been knocked out die at birth, have osteopetrosis, do not develop B cells or lymph nodes, suffer from anaemia and have severe bleeding disorders during embryonic development. When haematopoietic foetal liver cells are transplanted from Kind 3-knockout mice to irradiated wild-type animals, the chimaeras exhibit excessive bleeding after injury. This bleeding is due to defects in blood clotting and platelet adhesion caused by the silencing of αIIbβ3-integrin in platelets. Silencing of αIIbβ3-integrin is a consequence of the lack of Kind 3 binding to a phosphotyrosine-containing domain in the αIIbβ3-integrin tail, distal from the NPXY motif. To achieve activation, talin must simultaneously bind to NPXY. It seems that signalling through the integrin tail is a complicated process, but necessarily so, as Fässler noted when he quoted Einstein: ‘Everything in life should be as simple as possible but not simpler.' Who would dare to contradict Einstein?
The versatility of stress fibre proteins
Contraction is often regarded as an antagonist to migration because a cell filled with huge contractile cables finds it difficult to translocate. What happens if biological cues tell such a cell to move? Several talks reported that proteins classically residing in contractile stress fibres (Fig 3) could attain different intracellular localizations and/or functions according to the level of cell stress. In his poster-prize-winning study, J. Colombelli (Heidelberg, Germany) literally punched holes in these structures and cut individual fibres using laser nanosurgery. In response to such stress release, zyxin redistributes from a sarcomeric organization and from the FAs closest to the injury site to the severed stress fibre ends. When stress fibres are locally stretched using atomic force microscopy, zyxin is increasingly recruited from the cytosol. The specific enrichment of zyxin at sites of high tension is typical of a mechanosensor. M. Beckerle (Salt Lake City, UT, USA), who previously reported a similar function for zyxin (Yoshigi et al, 2005), described its pro-apoptotic function at this meeting. Ultraviolet irradiation of fibroblasts leads to zyxin accumulation in the nucleus, where it induces apoptosis when bound to the cell cycle and apoptosis regulator 1 (CARP1). Consistently, zyxin-null cells and cells expressing a zyxin mutant that lacks the CARP1-binding domain have higher survival rates. Beckerle proposed that zyxin and CARP1 distribution and expression levels could be used as diagnostic tools to test the effect of apoptosis-inducing cancer therapies.
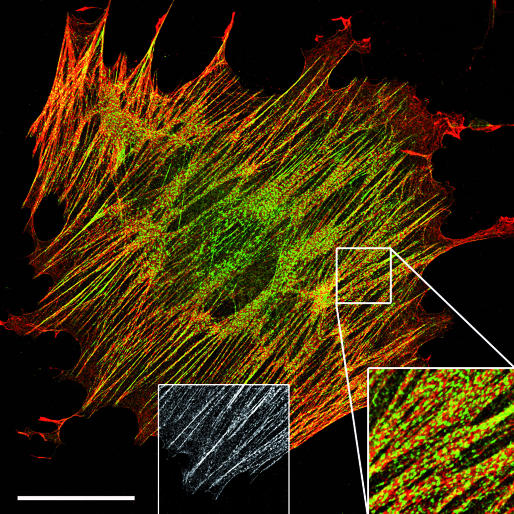
Figure 3.
Stress fibres: anti-motile structures? In fibroblasts, palladin (green) is expressed in stress fibres (phalloidin, red) in a sarcomeric pattern (right bottom inset) and in focal adhesions (greyscale inset). Image provided by B. Hinz. Scale bar, 50 μm.
C. Otey (Chapel Hill, NC, USA) showed that palladin—a protein found in stress fibres and FAs (Fig 3)—is involved in cancer cell metastasis. In metastatic cancer cell lines, palladin expression is positively correlated with invasive potential and palladin knockout reduces the formation of invadopodia. A mutation in the palladin gene correlates with a rare inherited form of pancreatic cancer, leading Otey to speculate that palladin has an important function in cancer progression. S. Creed (Sydney, Australia) showed that, similar to palladin, tropomyosin (TPM) has different isoforms that seem to exert different cellular functions. In particular, overexpression of the low-molecular-weight isoform TPM5NM1 promotes stress fibre formation in neuroepithelial cells, whereas the high-molecular-weight isoform TPM3 regulates the formation of motile cell structures such as filopodia and lamellipodia. These opposing functions seem to be regulated, in the case of TPM5NM1, by a specific interaction with the actin-bundling proteins fascin and α-actinin, and by proteins that regulate F-actin turnover such as actin-depolymerizing factor (ADF)/cofilin in the case of TPM3. Taken together, these findings imply that we need to revise our rather static view on how stress fibres and the proteins therein are organized.
Conclusion and perspectives
The interactive definition of podosomes, invadopodia and the sealing zone has been a major contribution of this meeting. The slow maturation process that has led to the characterization of these structures will ultimately set standards, just as with a good Italian wine. The meeting also clearly reflected the increasing communication between the adhesion and membrane-transport research fields. This is important, not only to understand how adhesion structures are used to digest the ECM, but also to explain how cells establish polarity and recycle membrane and receptors during directed migration. Finally, it is becoming apparent that numerous cytoplasmic proteins shuttle between ECM-adhesion structures, contractile stress fibres and even the nucleus; their localization has important implications for the migratory or invasive character of a cell. The next meeting, to be organized by L. Machesky and G. Jones in the UK, is eagerly awaited to discuss the remaining mysteries of cell adhesion.

Laura Machesky

Pierre Jurdic

Boris Hinz
Acknowledgments
P.J. thanks E. Genot for reading the manuscript. We are indebted to C. Otey and O. Carpen for providing the palladin antibodies used in Fig 3. The authors acknowledge the Cancer Research UK and the Medical Research Council UK, the Association Française contre le Cancer (ARC), the Agence Nationale de la Recherche (BLAN06-1_138145), and the Swiss National Science Foundation, grant no. #3100A0-113733/1. We apologize to all authors we could not cite due to space restrictions.
References
- Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA (2007) Transcellular diapedesis is initiated by invasive podosomes. Immunity 26: 784–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Buccione R (2006) Adhesions that mediate invasion. Int J Biochem Cell Biol 38: 1875–1892 [DOI] [PubMed] [Google Scholar]
- Hoeller O, Kay RR (2007) Chemotaxis in the absence of PIP3 gradients. Curr Biol 17: 813–817 [DOI] [PubMed] [Google Scholar]
- Lazebnik Y (2002) Can a biologist fix a radio?—Or, what I learned while studying apoptosis. Cancer Cell 2: 179–182 [DOI] [PubMed] [Google Scholar]
- Linder S (2007) The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol 17: 107–117 [DOI] [PubMed] [Google Scholar]
- Orth JD, McNiven MA (2006) Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res 66: 11094–11096 [DOI] [PubMed] [Google Scholar]
- Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC (2007) Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol 9: 1046–1056 [DOI] [PubMed] [Google Scholar]
- Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC (2005) Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol 171: 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9: 858–867 [DOI] [PMC free article] [PubMed] [Google Scholar]