Abstract
The angiotensin II type 1 (AT1) receptor is a G protein-coupled receptor that has a crucial role in the development of load-induced cardiac hypertrophy. Here, we show that cell stretch leads to activation of the AT1 receptor, which undergoes an anticlockwise rotation and a shift of transmembrane (TM) 7 into the ligand-binding pocket. As an inverse agonist, candesartan suppressed the stretch-induced helical movement of TM7 through the bindings of the carboxyl group of candesartan to the specific residues of the receptor. A molecular model proposes that the tight binding of candesartan to the AT1 receptor stabilizes the receptor in the inactive conformation, preventing its shift to the active conformation. Our results show that the AT1 receptor undergoes a conformational switch that couples mechanical stress-induced activation and inverse agonist-induced inactivation.
Keywords: cardiac hypertrophy, G protein-coupled receptor, inverse agonist, mechanical stress, molecular model
Introduction
Mechanical stress to cardiomyocytes is the most important stimulus that triggers hypertrophic responses (Komuro & Yazaki, 1993), and the hypertrophic responses to mechanical stretch are significantly inhibited by pretreatment with angiotensin II (AngII) type 1 (AT1) receptor blockers (ARB; Sadoshima et al, 1993; Yamazaki et al, 1995). Therefore, the AT1 receptor is crucial in the development of load-induced cardiac hypertrophy. We have recently shown that mechanical stress leads to activation of the AT1 receptor without the involvement of AngII (Zou et al, 2004). Mechanical stretch did not activate extracellular signal-regulated protein kinases (ERKs) in human embryonic kidney (HEK) 293 cells with no detectable expression of AT1 receptor, but forced expression of the AT1 receptor conferred the ability to respond to stretch. Candesartan, an ARB, inhibited mechanical stress-induced AT1 receptor activation and pressure overload-induced hypertrophy even in angiotensinogen-null mice. However, it remains unclear how AT1 receptor detects mechanical stress and translates it into biochemical signals inside the cells, and how candesartan inhibits AngII-independent activation of AT1 receptor.
Here, we show that there is a change in the conformation of the AT1 receptor when activated by mechanical stress. We refer to this as a ‘stretch-induced' conformational change, but whether the AT1 receptor directly absorbs the mechanical energy that drives this conformational change remains unclear. Studies using substituted cysteine accessibility mapping (SCAM) showed that transmembrane (TM) 7 of the AT1 receptor showed an anticlockwise rotation and a shift into the ligand-binding pocket in response to mechanical stretch. Candesartan suppressed the stretch-induced helical movement of TM7, and the binding of the carboxyl group of candesartan to Gln 257 in TM6 and Thr 287 in TM7 was responsible for the potent inverse agonism. Our results provide a previously unknown basis for the structural switch of the AT1 receptor that couples mechanical stress-induced activation and inverse agonist-induced inactivation.
Results And Discussion
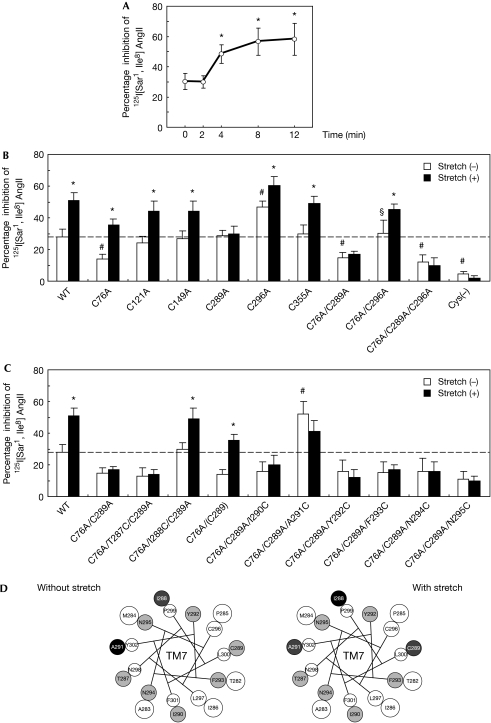
First, by using immunofluorescence analysis, we confirmed that the AT1 receptor was localized predominantly in the plasma membrane of HEK293 cells expressing this receptor (HEK293-AT1 cells) before and after stretch (supplementary Fig S1 online). Next, to examine whether mechanical stretch can induce changes in the conformation of the AT1 receptor, we carried out a SCAM study with or without stretch. The SCAM study has been used to investigate relative conformational changes by validating the presence of cysteine residues within the ligand pocket (see supplementary information online). As we reported previously (Miura et al, 2003), the percentage inhibition of 125I-labelled (Sar1, Ile8) AngII binding by methanethiosulphonate ethyl-ammonium (MTSEA+) reagent was approximately 30% in HEK293-AT1 cells, because Cys 76 in TM2 is accessible to water within the ligand pocket (Fig 1A). We found that the percentage inhibition of 125I-labelled (Sar1, Ile8) AngII gradually increased after stretch, reaching approximately 60% after 8 min (Fig 1A), indicating that stretch induces a conformational change in the AT1 receptor. To identify the native cysteine residues that gain accessibility to MTSEA+, we replaced individual cysteine residues with alanine and analysed the accessibility with or without stretch (Fig 1B). The affinities of these mutants for 125I-labelled (Sar1, Ile8) AngII were equivalent to that of the wild-type receptor (supplementary Table S1 online). Consistent with our previous results (Miura & Karnik, 2002; Miura et al, 2003), the reactions to MTSEA+ were enhanced in the Cys296Ala mutant, because this mutation increases the accessibility of Cys 289 without altering the accessibility of Cys 76 (Fig 1B). Interestingly, Cys289Ala, Cys76Ala/Cys289Ala, Cys76Ala/Cys289Ala/Cys296Ala and Cys(−) mutants, which contain a cysteine to alanine mutation at Cys 289 in TM7, did not show a stretch-induced increase in percentage inhibition of 125I-labelled (Sar1, Ile8) AngII binding. These results indicate that mechanical stretch increases the accessibility of Cys 289 by inducing a change in the conformation of TM7.
Figure 1.
Mechanical stress-induced anticlockwise rotation of TM7 in the AT1 receptor. (A) Alteration of cysteine accessibility by mechanical stretch in HEK293-AT1 cells. Cell membranes were prepared before (0 min) and after the indicated stretch time, and subjected to a SCAM study. *P<0.05 versus 0 min. (B) Alteration of cysteine accessibility by mechanical stretch in COS7 cells expressing wild-type (WT) and mutant AT1 receptors. The cells were stretched for 8 min. Cys(−) represents a mutant receptor in which all the cysteine residues were replaced with alanine. *P<0.05 versus stretch (−), #P<0.05 versus stretch (−) in wild-type, §P<0.05 versus stretch (−) in Cys76Ala/Cys289Ala. (C) Alteration of cysteine accessibility by mechanical stretch in COS7 cells expressing Cys76Ala/Cys289Ala mutant receptors in which TM7 residues ranging from Thr 287 to Asn 295 were successively substituted to cysteine. *P<0.05 versus stretch (−), #P<0.05 versus stretch (−) in Cys76Ala/Cys289Ala. (D) Helical wheel representation of TM7 reporter cysteine residues and the pattern of their reactivity to MTSEA+. Positions of MTSEA+-reacted cysteine residues in TM7 that affected 125I-labelled (Sar1, Ile8) AngII binding are shown in a helical wheel representation viewed from the extracellular side without (left) or with (right) stretch. Black circles correspond to the residues that inhibited 125I-labelled (Sar1, Ile8) AngII binding by 50% or more when substituted to cysteine, whereas dark grey circles indicate those that inhibited by around 30%. Light grey circles indicate those that had no inhibitory effect. White circles indicate receptors that were not examined. AngII, angiotensin II; AT1, AngII type 1; MTSEA+, methanethiosulphonate ethyl-ammonium; SCAM, substituted cysteine accessibility mapping; TM7, transmembrane 7.
To determine the stretch-induced helical movement of TM7, we carried out a series of SCAM experiments by using Cys76Ala/Cys289Ala mutant receptors in which TM7 residues ranging from Thr 287 to Asn 295 were substituted with cysteine one at a time. Cys76Ala/Ile288Cys/Cys289Ala and Cys76Ala/Cys289Ala/Ala291Cys mutants showed higher percentage inhibitions than Cys76Ala/Cys289Ala (Fig 1C), indicating that Ile 288 and Ala 291 are accessible to the ligand-binding pocket. Stretch increased accessibility in Cys76Ala (Cys 289) and in Cys76Ala/Ile288Cys/Cys289Ala mutants, but decreased it in the Cys76Ala/Cys289Ala/Ala291Cys mutant (Fig 1C). These results indicate that mechanical stress induces anticlockwise rotation of TM7 (Fig 1D). In general, G protein-coupled receptors (GPCRs) are maintained in an inactive conformation by interhelical interactions that constrain the receptor structure (Gether, 2000). Although interactions between TM3 and TM6 might be a conserved mechanism for conformational stabilization of GPCRs (Gether, 2000; Yao et al, 2006), stabilizing interactions between TM3 and TM7 have been reported in the AT1 receptor (Groblewski et al, 1997). Relaxation of the constraining interhelical interactions triggers activation of GPCRs when bound to agonists; therefore, we propose that the stabilizing interaction between TM3 and TM7 in the AT1 receptor might be disrupted by mechanical stress independently of AngII and that the anticlockwise rotation of TM7 might cause activation of intracellular signalling pathways.
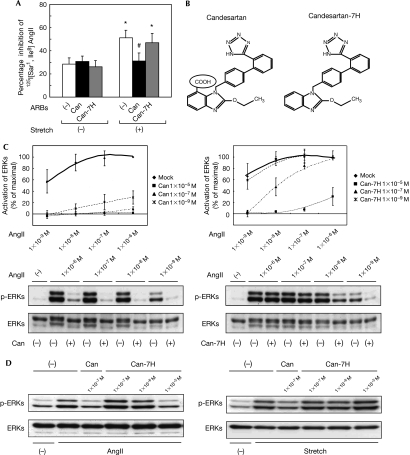
As shown in Fig 2A, candesartan completely suppressed a stretch-induced increase in the percentage inhibition of 125I-labelled (Sar1, Ile8) AngII binding in the SCAM experiments, indicating that candesartan blocked mechanical stress-induced conformational change in the AT1 receptor. ARBs show diverse inhibitory patterns ranging from surmountable inhibition (parallel rightward shift of agonist concentration–response curves) to insurmountable inhibition (waning of the maximal response; Vauquelin et al, 2001). We found that a derivative of candesartan (candesartan-7H), which lacks the carboxyl group at the benzimidazole ring (Fig 2B), showed a much lower inhibitory effect than candesartan on AngII-induced activation of ERKs in HEK293-AT1 cells, with a rightward shift of the concentration–response curve (Fig 2C). Importantly, 1 × 10−5 M of candesartan-7H inhibited almost equally the activation of ERKs induced by 1 × 10−7 M of AngII as did 1 × 10−7 M of candesartan (Fig 2D). However, stretch-induced ERK activations were inhibited by 1 × 10−7 M of candesartan, but not by candesartan-7H even at 1 × 10−5 M (Fig 2D). Consistently, candesartan-7H did not show a suppressive effect on stretch-induced increase in the percentage inhibition of 125I-labelled (Sar1, Ile8) AngII binding in the SCAM experiments (Fig 2A). In addition, candesartan, but not candesartan-7H, reduced the basal activity of wild-type AT1 receptor or a constitutively active AT1-N111G mutant, which contains an Asn 111 to glycine mutation (Boucard et al, 2003; supplementary Fig S2 online). These results indicate that the carboxyl group of candesartan is responsible both for the insurmountable inhibition of AngII-dependent receptor activation and for the potent inverse agonism against AngII-independent receptor activation.
Figure 2.
The carboxyl group is a crucial structure for inverse agonism of candesartan. (A) Alteration of cysteine accessibility by mechanical stretch with or without ARBs in HEK293-AT1 cells. The cells were pretreated with 1 × 10−7 M candesartan (Can) or its derivative, candesartan-7H (Can-7H), and then stretched for 0 or 8 min. *P<0.05 versus stretch (−), #P<0.05 versus stretch (+) without pretreatment of Can. (B) Chemical structures of Can and Can-7H. Can contains a carboxyl group at the benzimidazole ring (circled COOH), whereas Can-7H does not have this structure. (C) Response curves of AngII-mediated activation of ERKs (upper panels). HEK293-AT1 cells were pretreated with 1 × 10−7 M of Can or Can-7H and stimulated by AngII at the indicated concentrations (lower panels). The activation of ERKs was determined by using a polyclonal antibody against phosphorylated ERKs (p-ERKs). (D) HEK293-AT1 cells were pretreated with the indicated concentrations of Can or Can-7H and stimulated by AngII (left) or mechanical stretch (right). The activation of ERKs was determined. AngII, angiotensin II; ARB, AT1 receptor blocker; AT1, AngII type 1; ERK, extracellular signal-regulated protein kinase; HEK, human embryonic kidney cells.
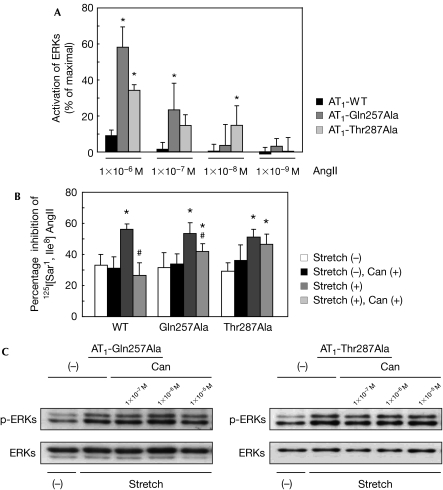
To establish the specific amino acids that bind to the the carboxyl group of candesartan, we selected candidate residues—His 256, Gln 257, Thr 287 and Tyr 292—on the basis of a molecular model of the AT1 receptor (Noda et al, 1995; Takezako et al, 2004) and examined the binding affinities of candesartan to AT1 mutant receptors with a substitution of each candidate residue to alanine. The affinities of candesartan were reduced by approximately tenfold in Gln257Ala and Thr287Ala mutants compared with the wild-type receptor (supplementary Table S2 online), indicating that the interactions of the carboxyl group of candesartan with Gln 257 and Thr 287 might be involved in a tight drug–receptor binding. Insurmountable inhibition by candesartan was not observed in these AT1 receptor mutants, because candesartan could not suppress the activation of ERKs mediated by higher concentration of AngII in HEK293 cells expressing these mutants (Fig 3A). Furthermore, we found that Gln257Ala and Thr287Ala mutants, similar to the wild-type receptor, showed an increase in the percentage inhibition of 125I-labelled (Sar1, Ile8) AngII binding after stretch, which was not significantly suppressed by candesartan (Fig 3B). In addition, inverse agonist activity of candesartan was also abolished in Gln257Ala and Thr287Ala mutants, because candesartan could not inhibit the stretch-induced activation of ERKs in HEK293 cells expressing these mutants (Fig 3C). Collectively, these results indicate that the tight binding of the carboxyl group of candesartan to Gln 257 and Thr 287 in AT1 receptor is crucial for the potent inverse agonism.
Figure 3.
Interactions of the carboxyl group of candesartan with Gln 257 and Thr 287 in the AT1 receptor. (A) HEK293 cells expressing wild-type AT1, Gln257Ala or Thr287Ala mutant receptors were pretreated with 1 × 10−7 M of candesartan (Can) and stimulated by AngII at the indicated concentrations. The activation of ERKs was determined. *P<0.05 versus wild-type AT1. (B) Alteration of cysteine accessibility by mechanical stretch in COS7 cells expressing wild-type AT1, Gln257Ala or Thr287Ala receptors. The cells were pretreated with or without 1 × 10−7 M Can and stretched for 0 or 8 min. *P<0.05 versus stretch (−), #P<0.05 versus stretch (+) in each receptor. (C) HEK293 cells expressing Gln257Ala (left) or Thr287Ala (right) mutant receptor were pretreated with the indicated concentrations of Can and stimulated by mechanical stretch. The activation of ERKs was determined. AngII, angiotensin II; AT1, AngII type 1; ERK, extracellular signal-regulated protein kinase; HEK, human embryonic kidney cells.
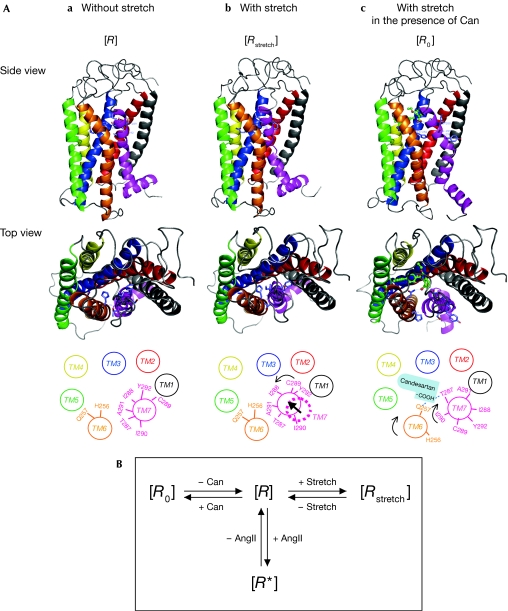
Finally, we constructed molecular models on three states: (i) AT1 receptor model without stretch, (ii) AT1 receptor model with stretch, and (iii) AT1 receptor with stretch in the presence of candesartan (Fig 4A; see supplementary information online). As shown in Fig 1C, mechanical stress induced anticlockwise rotation of TM7 and eventually the Cys 289 residue, originally faced in the direction of TM1, became accessible to the ligand-binding pocket. As Ile 288 becomes more accessible after stretch, TM7 might shift inside the ligand-binding pocket. By contrast, TM7 would shift away from the ligand-binding pocket in a constitutively active AT1-N111G mutant (Boucard et al, 2003). We reported previously that an amino–aromatic bonding interaction between Asn 111 in TM3 of the AT1 receptor and Tyr 4 of AngII triggers AngII-dependent receptor activation (Miura et al, 1999), and that the AT1-N111G receptor mimics the state of the wild-type receptor partly activated by AngII (Miura & Karnik, 2002). Therefore, an active conformation of the AT1 receptor induced by mechanical stress might be substantially different from that in AngII-dependent receptor activation. The ‘AT1 receptor model with stretch in the presence of candesartan' fulfils both conditions in which the tetrazole group of candesartan binds to Lys 199 (Noda et al, 1995; Takezako et al, 2004) and in which the carboxyl group of candesartan stably forms two hydrogen bonds with the side chains of Gln 257 and Thr 287.
Figure 4.
Molecular model of stretch-induced changes in the conformation of the AT1 receptor. (A) A molecular model was constructed with three states: AT1 receptor model without stretching, with stretch and with stretch in the presence of candesartan (Can). (B) The AT1 receptor is predicted to adopt distinct conformations. [R] is an unaligned inactive state and [R0] is an inactive state stabilized by an inverse agonist candesartan. [R*] is an active state stabilized by the agonist AngII and [Rstretch] is another active state stabilized by mechanical stretch. AngII, angiotensin II; AT1, AngII type 1.
According to a sequential binding and conformational model for the molecular mechanism of ligand action on GPCRs (Gether, 2000), the unaligned receptor exists in a unique state [R] that can undergo transitions to at least two other stabilized states, [R0] and [R*]. [R0] is an inactive state of the AT1 receptor that is stabilized by an inverse agonist candesartan, and [R*] is an active state stabilized by AngII (Fig 4A,B). Mechanical stretch might stabilize the AT1 receptor to another active state [Rstretch], independently of AngII (Fig 4A,B). In this study, the carboxyl group of candesartan was found to bind to Gln 257 in TM6 and to Thr 287 in TM7, and these interactions might constrain two TM domains until the receptor is stabilized in the inactive state [R0]. According to the model of mechanical stress in the presence of candesartan, TM6 rotates clockwise and TM7 moves to the same position in the inactive state [R] with clockwise rotation (Fig 4A). The clockwise rotations of TM6 and TM7 in this model were consistent with the result of a SCAM experiment showing a decrease in the accessibility of His 256, an increase in the accessibility of Ile 290 and a decrease in the accessibility of Ala 291 to the ligand-binding pocket (supplementary Fig S3 online). The distances the carboxyl group of candesartan from the hydroxyl group of Thr 287 and the carboxyl group of Gln 257 are 2.06 Å and 2.09 Å, respectively, which are reasonable for causing interactions through electrostatic and/or hydrogen bonds.
Here, we have shown compelling evidence that the AT1 receptor shows a conformational switch when mechanical stress of the whole cell leads to receptor activation. Recent evidence has shown that mechanical force directly alters the conformation or folding of cytoskeletal proteins, which enhances enzymatic activities or susceptibility to enzymatic reactions (Sawada et al, 2006). However, mechanical stretch activated the AT1 receptor even when the actin cytoskeleton was disorganized by treatment with cytochalasin D (supplementary Fig S4 online). Alternatively, stretch-activated ion channels (SACs) might trigger activation of the AT1 receptor after stretch. Although the rapid changes of membrane potential or intracellular Ca2+ within seconds of the initiation of stretching could not be measured, we found that treatment with GsMtx-4, a specific blocker for SACs, did not inhibit stretch-induced activation of the AT1 receptor (supplementary Fig S5 online). It will be of particular interest to describe the precise mechanism through which mechanical force is directly or indirectly transmitted to the AT1 receptor. Reconstitution of a mechanosensitive channel of large conductance from Escherichia coli (Perozo et al, 2002) in synthetic phosphatidylcholines with different chain lengths showed that a thin bilayer favoured the open state of channels, whereas a thick bilayer stabilized the closed state. In addition, a recent study using a fluorescence resonance energy transfer approach showed that membrane fluidity affected the conformational dynamics of the bradykinin B2 receptor in endothelial cells (Chachisvilis et al, 2006). It might be possible that membrane tension causes thinning of the lipid bilayer, which triggers tilting of TM7 in the AT1 receptor to avoid hydrophobic mismatch and to rectify a lateral pressure profile (Orr et al, 2006).
Furthermore, our present study provides a structural basis for how inverse agonists can inhibit receptor activation in the absence of agonists. According to our molecular model (Fig 4A,B), candesartan, as an inverse agonist, might forcibly induce a distinct transition from [R] to an inactive conformation [R0], preventing the shift of equilibrium to an active conformation [Rstretch], which translates mechanical stress into the activation of ERKs through phosphorylation of Janus kinase 2 and Gq protein coupling (Zou et al, 2004). This is consistent with the result of a recent study that used a fluorescence resonance energy transfer approach and showed that agonists and inverse agonists for α2A-adrenergic receptor induced distinct conformational changes in the receptor (Vilardaga et al, 2005). Recently, we reported that potent inverse agonism of olmesartan to suppress the constitutive activity of the AT1-N111G receptor required cooperative interactions between olmesartan and Tyr 113 in TM3 and His 256 in TM6 (Miura et al, 2006). Many drugs, previously considered to be neutral antagonists, have been shown to behave as an inverse agonist for GPCRs. Therefore, elucidation of the molecular basis of inverse agonism is of great importance to pharmacotherapy targeted GPCRs.
Methods
Application of mechanical stretch. The passive stretch of cultured cells by 20% was conducted as described previously (Zou et al, 2004; supplementary Fig S6 online).
Substituted cysteine accessibility mapping. SCAM was carried out as described previously (Miura & Karnik, 2002; Miura et al, 2003, 2005).
Molecular modelling of the AT1 receptor. Amino-acid sequence alignment between the human AT1 receptor and bovine rhodopsin was carried out using the CLUSTAL W program. Homology model structures of the human AT1 receptor were then constructed based on the crystal structure of bovine rhodopsin (Protein Data Bank ID: 1F88) by using the homology module in the Insight II program package (Accelrys Inc, San Diego, CA, USA). Conformations of extracellular loops were constructed by using the Search/Generate-Loops function of Insight II (see supplementary information online). The complete structure was subjected to energy minimization using the MMFF94 × force field in the programme MOE (version 2005.06, Chemical Computing Group) with a harmonic force constraint against the initial atomic positions to prevent the large movement of TM helices. Further methods can be found in the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Information
Acknowledgments
We are grateful to Dr S.S. Karnik for providing cDNAs. This work was supported, in part, by grants from the Japanese Ministry of Education, Science, Sports and Culture (to I.K., S.-i.M. and H.A.); from Health and Labor Sciences Research Grants, Japan Health Sciences Foundation, Takeda Medical Research Foundation, Takeda Science Foundation, Uehara Memorial Foundation, Kato Memorial Trust for Nambyo Research and the Japan Medical Association (to I.K.); and from Mochida Memorial Foundation, Japanese Heart Foundation/Novartis Research Award on Molecular and Cellular Cardiology, and Japan Intractable Diseases Research Foundation (to H.A.).
References
- Boucard AA, Roy M, Beaulieu ME, Lavigne P, Escher E, Guillemette G, Leduc R (2003) Constitutive activation of the angiotensin II type 1 receptor alters the spatial proximity of transmembrane 7 to the ligand-binding pocket. J Biol Chem 278: 36628–36636 [DOI] [PubMed] [Google Scholar]
- Chachisvilis M, Zhang YL, Frangos JA (2006) G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463–15468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gether U (2000) Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev 21: 90–113 [DOI] [PubMed] [Google Scholar]
- Groblewski T, Maigret B, Larguier R, Lombard C, Bonnafous JC, Marie J (1997) Mutation of Asn111 in the third transmembrane domain of the AT1A angiotensin II receptor induces its constitutive activation. J Biol Chem 272: 1822–1826 [DOI] [PubMed] [Google Scholar]
- Komuro I, Yazaki Y (1993) Control of cardiac gene expression by mechanical stress. Annu Rev Physiol 55: 55–75 [DOI] [PubMed] [Google Scholar]
- Miura S, Karnik SS (2002) Constitutive activation of angiotensin II type 1 receptor alters the orientation of transmembrane Helix-2. J Biol Chem 277: 24299–24305 [DOI] [PubMed] [Google Scholar]
- Miura S, Feng YH, Husain A, Karnik SS (1999) Role of aromaticity of agonist switches of angiotensin II in the activation of the AT1 receptor. J Biol Chem 274: 7103–7110 [DOI] [PubMed] [Google Scholar]
- Miura S, Zhang J, Boros J, Karnik SS (2003) TM2–TM7 interaction in coupling movement of transmembrane helices to activation of the angiotensin II type-1 receptor. J Biol Chem 278: 3720–3725 [DOI] [PubMed] [Google Scholar]
- Miura S, Karnik SS, Saku K (2005) Constitutively active homo-oligomeric angiotensin II type 2 receptor induces cell signaling independent of receptor conformation and ligand stimulation. J Biol Chem 280: 18237–18244 [DOI] [PubMed] [Google Scholar]
- Miura S et al. (2006) Molecular mechanism underlying inverse agonist of angiotensin II type 1 receptor. J Biol Chem 281: 19288–19295 [DOI] [PubMed] [Google Scholar]
- Noda K, Saad Y, Kinoshita A, Boyle TP, Graham RM, Husain A, Karnik SS (1995) Tetrazole and carboxylate groups of angiotensin receptor antagonists bind to the same subsite by different mechanisms. J Biol Chem 270: 2284–2289 [DOI] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA (2006) Mechanisms of mechanotransduction. Dev Cell 10: 11–20 [DOI] [PubMed] [Google Scholar]
- Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B (2002) Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418: 942–948 [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Xu Y, Slayter HS, Izumo S (1993) Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75: 977–984 [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP (2006) Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127: 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezako T, Gogonea C, Saad Y, Noda K, Karnik SS (2004) ‘Network leaning' as a mechanism of insurmountable antagonism of the angiotensin II type 1 receptor by non-peptide antagonists. J Biol Chem 279: 15248–15257 [DOI] [PubMed] [Google Scholar]
- Vauquelin G, Fierens F, Verheijen I, Vanderheyden P (2001) Insurmountable AT(1) receptor antagonism: the need for different antagonist binding states of the receptor. Trends Pharmacol Sci 22: 343–344 [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Steinmeyer R, Harms GS, Lohse MJ (2005) Molecular basis of inverse agonism in a G protein-coupled receptor. Nat Chem Biol 1: 25–28 [DOI] [PubMed] [Google Scholar]
- Yamazaki T et al. (1995) Angiotensin II partly mediates mechanical stress-induced cardiac hypertrophy. Circ Res 77: 258–265 [DOI] [PubMed] [Google Scholar]
- Yao X, Parnot C, Deupi X, Ratnala VR, Swaminath G, Farrens D, Kobilka B (2006) Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat Chem Biol 2: 417–422 [DOI] [PubMed] [Google Scholar]
- Zou Y et al. (2004) Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6: 499–506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information