Abstract
Phosphoinositides have crucial roles in cellular controls, many of which have been established through the use of small-molecule inhibitors. Here, we describe YM201636, a potent inhibitor of the mammalian class III phosphatidylinositol phosphate kinase PIKfyve, which synthesizes phosphatidylinositol 3,5-bisphosphate. Acute treatment of cells with YM201636 shows that the PIKfyve pathway is involved in the sorting of endosomal transport, with inhibition leading to the accumulation of a late endosomal compartment and blockade of retroviral exit. Inhibitor specificity is shown by the use of short interfering RNA against the target, as well as by rescue with the drug-resistant yeast orthologue Fab1. We concluded that the phosphatidylinositol 3,5-bisphosphate pathway is integral to endosome formation, determining morphology and cargo flux.
Keywords: phosphoinositides, PIKfyve, membrane transport, kinase inhibitor
Introduction
Phosphoinositides have crucial roles in a wide variety of cellular processes (reviewed by Parker, 2004; Lindmo & Stenmark, 2006), with the roles of phosphatidylinositol 3-phosphate (PtdIns3P), PtdIns(3,4)P2 and PtdIns(3,4,5)P3 largely explained by the use of LY294002 (Vlahos et al, 1994) and wortmannin (Arcaro & Wymann, 1993)—inhibitors of the PtdIns3P class of lipid kinases (Vanhaesebroeck et al, 2001). The related lipid phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2) has been shown to bind to several effector proteins (Parker, 2004; Michell et al, 2006); however, as yet there are no selective small-molecule inhibitors that block its production and this has hampered the understanding of the PtdIns(3,5)P2 pathway.
PIKfyve is the mammalian type III PtdInsP kinase that acts on PtdIns3P to generate PtdIns(3,5)P2 (McEwen et al, 1999). In yeast, inactivation of Fab1, the yeast type III PtdInsP kinase, causes a marked enlargement of the vacuole, which fails to acidify correctly, defects in the transport of proteins to the vacuole through the multivesicular body (MVB) and failure to grow at high temperature (Yamamoto et al, 1995; Odorizzi et al, 1998). These effects can be explained in part by a block in retrograde transport from the vacuole (Dove et al, 2004). Disruption of the PIKfyve orthologues in Caenorhabditis elegans and Drosophila melanogaster also shows a swollen endocytic compartment (Nicot et al, 2006; Rusten et al, 2006). In mammalian cells, overexpression of a catalytically inactive PIKfyve in COS7 or human embryonic kidney (HEK) 293 cells causes swelling of an endocytic vacuole-like compartment that resembles late endosomes (Ikonomov et al, 2003a), and a similar effect is shown by knockdown of PIKfyve using short interfering RNA (siRNA; Rutherford et al, 2006). PtdIns(3,5)P2 has also been shown to have a role in the retrieval of cargoes to the trans-Golgi network (TGN; Rutherford et al, 2006) and at the TGN (Ikonomov et al, 2003b), and has been implicated in insulin-regulated IRAP/GLUT4 vesicle transport (Berwick et al, 2004).
So far, overexpression of catalytically inactive PIKfyve and siRNA knockdown have been the only tools available to dissect the PtdIns(3,5)P2 pathways. Although clearly informative, these interventions have limitations owing to the gradual onset of PtdIns(3,5)P2 depletion. siRNA treatment of cells has different effects, depending on the extent of knockdown (Rutherford et al, 2006). Owing to the dynamic nature of many of the transport pathways likely to be regulated by PtdIns(3,5)P2, a small-molecule inhibitor of PIKfyve would be a valuable tool for investigating the acute effects of blocking PtdIns(3,5)P2 synthesis and the recovery process on release of the block. Here, we describe YM201636, a new, potent and selective inhibitor of PIKfyve.
Results And Discussion
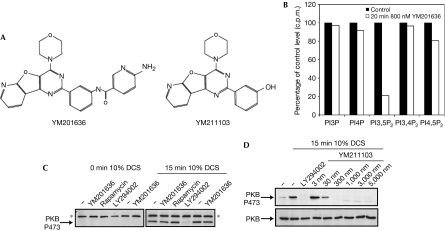
A drug discovery programme directed at phosphoinositide 3 kinase (Hayakawa et al, 2006) identified a pyridofuropyrimidine compound, YM201636 (Fig 1A), which was found to have potent in vitro inhibitory activity against PIKfyve, with a half-maximal inhibitory concentration (IC50) of 33 nM (Table 1). Notably, the yeast orthologue of PIKfyve, Fab1, was found to be insensitive to YM201636 (IC50>5 μM). Under the same assay conditions, an IC50 for PtdIns3P p110α was determined to be 3 μM, almost 100-fold higher than for PIKfyve (Table 1). YM201636 did not inhibit a type IIγ PtdInsP kinase even at 10 μM and inhibited a mouse type Iα PtdInsP kinase with an IC50>2 μM (data not shown). A different pyridofuropyrimidine, YM211103, showed a significant increase in potency towards p110α (IC50 2 nM), while showing a decreased ability to inhibit PIKfyve (Table 1).
Figure 1.
The specific inhibition of in vivo PtdIns(3,5)P2 production by YM201636. (A) Structures of the inhibitors. (B) PtdIns(3,5)P2 levels were measured as described in the Methods. The data points for inhibitor-treated cells represent the percentage of radiolabel incorporated into the lipids indicated, as a function of untreated cells (see raw data±s.d. data in Table 1). (C) NIH3T3 cells were serum-starved for 18 h (0.1% donor calf serum (DCS)) and then pretreated with vehicle (−) or inhibitors. Cells were then stimulated with 10% DCS, as indicated. Inhibitor concentrations were as follows: YM201636, 800 nM; rapamycin, 20 nM; LY294002, 10 μM. Blots were probed with PW88 to detect phosphorylation of PKB 473; this serum detects an additional nonspecific antigen at around 80 kDa. (D) Serum-starved NIH3T3 cells were serum-stimulated in the presence of increasing concentrations of YM211103, as indicated. The blot was probed for PKB 473 phosphorylation. Equal loading of samples was confirmed by probing for total PKB (lower panel). PKB, protein kinase B; PI3,5P2, PtdIns(3,5)P2, phosphatidylinositol 3,5-bisphosphate.
Table 1.
In vitro inhibitory properties of the pyridofuropyrimidine compound YM201636 and the related YM211103
| Inhibitor | PIKfyve IC50 | Fab1 IC50 | p110α IC50 |
|---|---|---|---|
| YM201636 | 0.033 μM (0.027–0.041) | >5 μM | 3.3 μM (2.8–3.9) |
| YM211103 | 0.069 μM (0.058–0.083) | >10 μM | 0.004 μM* |
| PIKfyve, Fab1 and p110α activities were assayed at 50 μM ATP under the conditions described in Methods. Data points were performed in triplicate; the 95% range of IC50 values are shown in parentheses. | |||
| *Data are from Workman et al (2004). | |||
| Fab1, yeast type III phosphatidylinositol kinase; IC5o, half-maximal inhibitory concentration; PIKfyve, mammalian type III phosphatidylinositol phosphate kinase. | |||
To test the in vivo effects of YM201636 on phosphoinositide production, serum-starved NIH3T3 cells were metabolically labelled with [32Pi]orthophosphate and serum stimulated in the presence or absence of YM201636. At 800 nM, YM201636 (see below) decreased PtdIns(3,5)P2 production by 80% (Fig 1B; Table 2). All other phosphoinositides identified remained largely unaltered, although PtdIns(4,5)P2 showed a modest decrease of around 20%. As the IC50 of YM201636 against type Iα PtdInsP kinase is around 100-fold greater than against PIKfyve, it is likely that this modest reduction in PtdIns(4,5)P2 is an indirect consequence of PIKfyve inhibition. Consistent with a lack of effect on PtdIns(3,4,5)P3, YM201636 had no influence on protein kinase B (PKB) Ser 473 phosphorylation at this concentration (Fig 1C). By contrast, the structurally related YM211103 decreased serum-stimulated phosphorylation of PKB (Fig 1D).
Table 2.
Effects of YM201636 treatment on phosphoinositide levels in NIH3T3 cells
| PtdIns3P | PtdIns4P | PtdIns(3,5)P2 | PtdIns(3,4)P2 | PtdIns(4,5)P2 | |
|---|---|---|---|---|---|
| Vehicle | 7,685±307 | 212,147±5,485 | 2,219±71 | 1,366±44 | 487,335±641 |
| YM201636 | 7,476±484 | 194,539±16,732 | 465±52 | 1,319±143 | 393,289±16,190 |
| PtdIns3P, phosphatidylinositol 3-phosphate. | |||||
| NIH3T3 cells were labelled with [32P]Pi and stimulated with serum in the presence of vehicle (dimethyl sulphoxide) or 800 nM YM201636. Cells were collected, phosphoinositides extracted and then analysed by high-performance liquid chromatography as described in the Methods. Total radiolabel incorporation in each phosphoinositide is given as c.p.m. and errors represent the range of data (n=2). | |||||
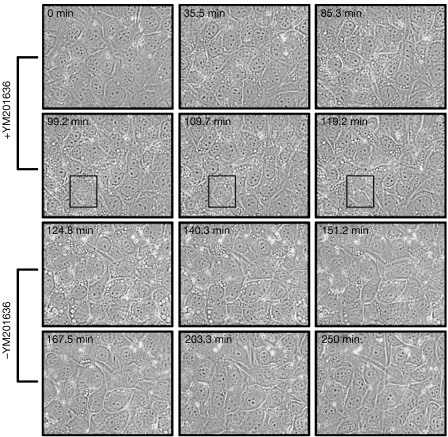
Acute treatment with YM201636 of various cell types, including mouse embryonic fibroblasts (MEFs), Madin–Darby canine kidney (MDCK), MCF10A, COS7 and NIH3T3 cells, causes the formation of large vesicular structures (Fig 2; supplementary Movie 1 online). The size and rate of formation are time- and concentration-dependent, with an A50 of around 400 nM. Withdrawal of YM201636 results in reversion of the swollen vesicle phenotype with kinetics similar to those of formation (Fig 2A). However, the presence of YM201636 (800 nM) does not inhibit cell division (Fig 2, boxed dividing cell). Growth curves for NIH3T3 cells over 7 days showed modest (25%) inhibitory effects (data not shown), which would indicate that other essential phosphoinositide pathways, for example, PtdIns(4,5)P2 synthesis, are not affected significantly.
Figure 2.
Time-lapse analysis of the swollen vesicles induced by YM201636. (A) Frames from a time-lapse film of NIH3T3 cells treated with 800 nM YM201636 (+YM201636). At 120 min, the cells were washed free of YM201636 and placed in fresh medium (−YM201636). The boxed region in panels 4–6 highlights a cell undergoing division.
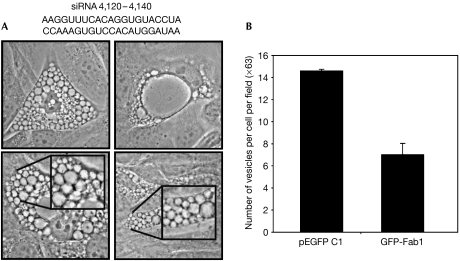
Transfection of any of four selected siRNA sequences targeting PIKfyve caused morphological changes similar to YM201636 treatment. Transfection of the most potent into NIH3T3 cells caused extensive vesicle swelling in 25% of cells (Fig 3A), consistent with the transfection efficiency and confirming previous observations (Rutherford et al, 2006).
Figure 3.
PIKfyve is the cellular target of YM201636. (A) Phase-contrast images of NIH3T3 cells transfected with siRNA to PIKfyve; the insets show magnified views. A 25% portion of the cells showed this vesiculation phenotype, which is consistent with the transfection efficiency; no such behaviour was observed with non-PIKfyve-directed siRNA. The siRNA used is shown above the images. (B) pEGFP or GFP-Fab1 fusion proteins were expressed in NIH3T3 cells and the cells were treated for 2 h with 800 nM YM201636. The data are from three independent transfections. EGFP, enhanced GFP; GFP, green fluorescent protein; PIKfyve, mammalian type III phosphatidylinositol phosphate kinase; siRNA, short interfering RNA.
To confirm further that PIKfyve is a target of YM201636, we expressed the YM201636-insensitive yeast type III PtdInsP kinase Fab1 in cells treated with the inhibitor. Fab1 is able to partly rescue the effects of YM201636, with a 50–60% reduction in the number and a reduction in the size of swollen vesicles induced (Fig 3B). Together, these data show that YM201636 induces the vesiculation phenotype by affecting PIKfyve and PtdIns(3,5)P2 production.
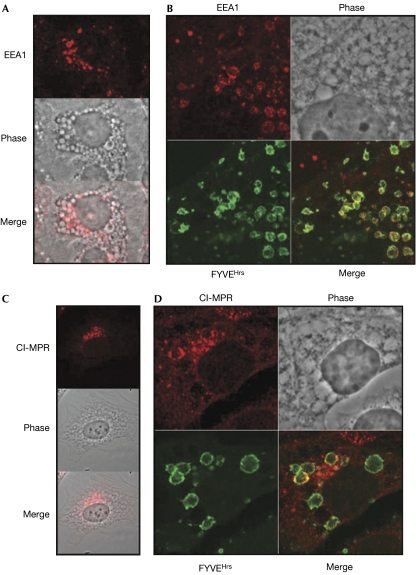
To analyse the origins and nature of the vesicle compartments expanded by acute inhibition of PIKfyve, we examined the localization of several compartmental markers. Not one of the Golgi markers (GM130 (Nakamura et al, 1995) and p230 (Erlich et al, 1996; Gleeson et al, 1996)), endoplasmic reticulum markers (p62 (Davis & Blobel, 1986), p115 (Barroso et al, 1995; Sapperstein et al, 1995), calnexin (Wada et al, 1991)) or the lysosomal marker LAMP1 was found on the swollen vesicle membrane or intralumenal vesicle (data not shown; supplementary Fig S1 online), indicating that Golgi, endoplasmic reticulum and lysosomes do not contribute directly to the dilated vesicles. Staining for the endosomal marker EEA1 (early endosomal antigen 1) showed a typical punctate cytoplasmic distribution (supplementary Fig S2 online), which, after treatment with 800 nM YM201636, became concentrated on the surface of a subset of enlarged vesicular structures and on some lumenal vesicles (Fig 4A). Generally, the distribution of EEA1 was enriched on smaller vesicles, covering their entire surface, and was mostly absent from larger vesicles (>5 μm in diameter). Transfection of a green fluorescent protein (GFP)-tagged tandem FYVEHrs (Gaullier et al, 1998) was used to track PtdIns3P on cellular membranes (Fig 4B). Co-staining for EEA1 showed localization to areas of PtdIns3P concentration and also that EEA1 was concentrated in subdomains on the surface of the swollen vesicles. Vesicles were found to be sheathed in actin filaments and to require an intact microtubule network for formation (supplementary Fig S3 online). Filming cells expressing GFP-Rab5 (supplementary Movie 2 online) provided clear evidence that Rab5-positive compartments contributed to the accumulating swollen vesicle phenotype. However, the heterogeneous nature of the swollen vesicles indicates that they are probably derived from multiple compartments, and electron microscopic analysis supported this (supplementary Fig S4 online).
Figure 4.
Endosomal markers localize to the swollen vesicles. (A) Localization of EEA1 in NIH3T3 cells treated with 800 nM YM201636 for 2 h. The phase image is merged with the fluorescent image in the bottom panel. (B) Localization of EEA1 and GFP-tandem-FYVEHrs domain (FYVEHrs) in NIH3T3 cells treated with YM201636 for 2 h. (C) Localization of the cation-independent mannose-6-phosphate receptor (Cl-MPR) in NIH3T3 cells treated with 800 nM YM201636 for 2 h. (D) Localization of Cl-MPR and transfected GFP-tandem-FYVEHrs domain in NIH3T3 cells treated with 800 nM YM201636 for 2 h. EEA1, early endosomal antigen 1; GFP, green fluorescent protein.
Expression of the PIKfyveK1831E kinase-dead mutant or depletion of PIKfyve by siRNA indicates that PIKfyve regulates cation-independent mannose 6-phosphate receptor (CI-MPR) recycling from the late endosome (Ikonomov et al, 2003a; Rutherford et al, 2006). NIH3T3 cells stained for CI-MPR showed a defined polarized perinuclear distribution (supplementary Fig S5A online). Treatment with 800 nM YM201636 did not substantially alter this, except that a relatively small number of enlarged vesicles in the same region stained positive for CI-MPR (Fig 4C). Most of the enlarged vesicles towards the periphery of the cell and in other areas of the perinuclear region were negative for CI-MPR, indicating either that they had lost CI-MPR or that they were derived from endocytic compartments not directly on the CI-MPR recycling pathway. Colocalization of CI-MPR and GFP-tandem-FYVEHrs domain was seen in the defined perinuclear region, but a clear separation existed in other perinuclear areas (Fig 4D). Thus, our data indicate that the PIKfyve-dependent pathway does not acutely influence CI-MPR transport; the previous, chronic inhibition studies (Ikonomov et al, 2003a; Rutherford et al, 2006) might represent a terminal phenotype.
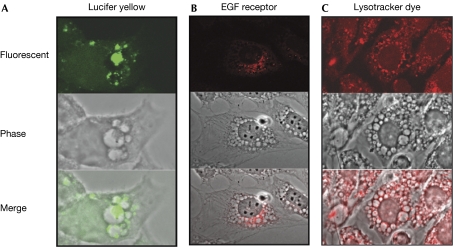
On exposure to Lucifer yellow and either pre- or post-treatment with YM201636, lumenal vesicles could be seen to retain the yellow stain; the bulk of the vesicles did not fluoresce (Fig 5A). Consistently, the distribution of epidermal growth factor (EGF) receptors in both COS7 and NIH3T3 cells after EGF stimulation in the presence of 800 nM YM201636 showed not only that endocytic vesicles are found in the lumen of the swollen vesicles, but also that the outer membrane of a subpopulation of swollen vesicles becomes highly enriched for EGF receptors (Fig 5B). The data suggest that both invagination and engulfment events were still occurring in the presence of the inhibitor; to account for the large increase in the surface area of the swollen vesicles, fusion events must also have been occurring (see also Rab5 in the supplementary Movie 2 online).
Figure 5.
Endosomal and lysosomal tracers are localized to the swollen vesicles. (A) NIH3T3 cells were incubated with 2 mg/ml Lucifer yellow for 30 min and then treated for 2 h with 800 nM YM201636. (B) Serum-starved NIH3T3 cells (20 h, 0.1% FCS) were treated with 800 nM YM201636 for 2 h and then stimulated with 10 nM EGF for 60 min. The EGF receptor was then stained with anti-EGF receptor and anti-mouse-cy3. (C) NIH3T3 cells were treated for 3 h with 800 nM YM201636; for the final 0.5 h, Lysotracker Red DND-99 was added to the medium at 100 nM. Lysotracker is acidotropic and accumulates in acidified organelles. EGF, epidermal growth factor.
It is known from yeast deficient in Fab1 that the aberrant enlarged vacuole formed is poorly acidified (Yamamoto et al, 1995). By using the pH-sensitive dye lysotracker (Invitrogen, Paisley, UK), it was found that the swollen vesicles in NIH3T3 cells treated with YM201636 were also poorly acidified (Fig 5C). However, surprisingly, some of the intralumenal vesicles that could be seen in the phase-contrast image were strongly fluorescent (Fig 5C), indicating either that the endocytic lumenal vesicles acidify post-entry or that existing acidified vesicles are engulfed by the swollen vesicles. This autophagic behaviour is characteristic of the response to amino-acid deprivation. To assess autophagic activity, the GFP-tagged autophagosome marker LC3 (Kabeya et al, 2000) was transfected into NIH3T3 cells. When these cells were treated with YM201636, it was apparent that most of LC3 accumulated inside the enlarged vesicles (supplementary Fig S7 online). This suggests that there is a degree of constitutive autophagy under these conditions and that the normal autophagic pathway is aberrant when PIKfyve is inhibited. Autophagic protein breakdown was indeed reduced in YM201636-treated cells (supplementary Fig S7 online), although whether this is a direct or indirect effect of PIKfyve inhibition remains unclear.
It has been shown that retroviruses bud from the cell by using the ESCRT (endosomal sorting complex required for transport) machinery that is normally used in protein sorting through the MVB (Martin-Serrano et al, 2003; Pornillos et al, 2003; Sherer et al, 2003), and that knockdown of PIKfyve by siRNA inhibits viral proliferation (Murray et al, 2005). We used TEGH human fibrosarcoma line (TEFLY Mo) producing ecotopic retrovirus containing pBabe-puro to test whether YM201636 has any effects on viral budding. Remarkably, treatment with 800 nM YM201636 reduced virus released into the medium by 80% (supplementary Fig S8 online). In control cells, the residual virus particles associated with cells were typically present in intercellular spaces. By contrast, in YM201636-treated cells, there was a substantial reduction in the number of virus particles associated with cellular sections, and where visible these were almost exclusively present in vacuolated structures. In two fields from YM201636-treated cells, a viral particle was observed connected to vacuolated membranes by an electron-dense material, probably unsevered membrane. The direct titre and ultrastructural analysis indicate that inhibition of PIKfyve blocks the formation and release of mature viral particles, as cellular viral particle accumulation was not observed in the context of reduced viral release.
In conclusion, YM201636 is a selective inhibitor of PIKfyve and PtdIns(3,5)P2 production in cells, acutely inducing swollen vesicles that derive in part from endosomal material and also from aberrant autophagosomal processes. It is evident that both typical, microtubule-dependent endosome fusion events and autophagic processes—although atypical—can be maintained despite the loss of PtdIns(3,5)P2 production. The accumulation of EGF receptors on the limiting membrane of the swollen vesicles is indicative of incorrect cargo sorting, similar to the block observed in the loss of function of Fab1 in yeast (Odorizzi et al, 1998) and in D. melanogaster (Rusten et al, 2006). The presence of some EGF receptors in the lumen is likely to reflect autophagic activity, as evidenced by the presence of Lucifer yellow-positive lumenal vesicles. However, whether the invagination is blocked as a primary event or secondary to the recycling of essential proteins, for example, ESCRT complex components, remains to be established.
Methods
Routine methods. Plasmid constructs, cell labelling and western blot protocols were as described previously (supplementary information online). Confocal, electron and video microscopic examinations were carried out as described previously (supplementary information online). siRNA knockdown and the protein turnover protocols are documented in the supplementary information online. Compounds were synthesized as described previously (Hayakawa et al, 2007).
In vitro lipid kinase assays. In vitro lipid kinase assays and lipid analysis were carried out as described previously (Cooke et al, 1998). IC50 values were determined by using Graphpad Prism and errors are given as ±95% confidence limit. PtdIns3P assays were carried out as for GST-Fab1 and GST-PIKfyve assays except that 100 μM PtdIns was used as a substrate.
In vivo measurement of phosphoinositide. In vivo levels of phosphoinositides were measured as described previously (Dove et al, 1997). Briefly, subconfluent 6 cm Petri dishes of NIH3T3 cells were washed twice in HBBSS (15 mM HEPES (Sigma-Aldrich, Haverhill, UK), 140 mM NaCl, 5 mM KCl, 2.8 mM NaHCO3, 1.5 mM CaCl2, 1 mM MgCl2, 0.06 mM MgSO4, 5.6 mM glucose, 0.1% (w/v) FAF BSA; pH 7.4 at 37°C) and incubated with 0.3 mCi per dish of [32P]Pi (GE Healthcare, Amersham, UK) for 1.5 h. The cells were washed once in HBBSS and challenged with 10% serum in HBBSS in the presence or absence of YM201636. After 10 min, the medium was removed from the cells and the cells were killed with 0.5 ml of ice-cold 1 M HCl. Phosphoinositides were extracted and analysed by using high-performance liquid chromatography as described previously (Dove et al, 1997).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Supplementary Movie 1
Supplementary Movie 2
Acknowledgments
We are grateful to Dr T. Jeffries for help with the Rab5 data, to C. Upton for the electron microscopy data, and to Professor R. Irvine and Dr J. Clarke (University of Cambridge) and Dr G. Thomas (University College London) for recombinant enzyme. F.T.C. acknowledges support of the Wellcome Trust.
References
- Arcaro A, Wymann MP (1993) Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 296: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso M, Nelson DS, Sztul E (1995) Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc Natl Acad Sci USA 92: 527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Dell GC, Welsh GI, Heesom KJ, Hers I, Fletcher LM, Cooke FT, Tavare JM (2004) Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J Cell Sci 117: 5985–5993 [DOI] [PubMed] [Google Scholar]
- Cooke FT, Dove SK, McEwen RK, Painter G, Holmes AB, Hall MN, Michell RH, Parker PJ (1998) The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr Biol 8: 1219–1222 [DOI] [PubMed] [Google Scholar]
- Davis LI, Blobel G (1986) Identification and characterization of a nuclear pore complex protein. Cell 45: 699–709 [DOI] [PubMed] [Google Scholar]
- Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH (1997) Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390: 187–192 [DOI] [PubMed] [Google Scholar]
- Dove SK et al. (2004) Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J 23: 1922–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich R, Gleeson PA, Campbell P, Dietzsch E, Toh BH (1996) Molecular characterization of trans-Golgi p230. A human peripheral membrane protein encoded by a gene on chromosome 6p12–22 contains extensive coiled-coil α-helical domains and a granin motif. J Biol Chem 271: 8328–8337 [DOI] [PubMed] [Google Scholar]
- Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R (1998) FYVE fingers bind PtdIns(3)P. Nature 394: 432–433 [DOI] [PubMed] [Google Scholar]
- Gleeson PA, Anderson TJ, Stow JL, Griffiths G, Toh BH, Matheson F (1996) p230 is associated with vesicles budding from the trans-Golgi network. J Cell Sci 109: 2811–2821 [DOI] [PubMed] [Google Scholar]
- Hayakawa M et al. (2006) Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110α inhibitors. Bioorg Med Chem 14: 6847–6858 [DOI] [PubMed] [Google Scholar]
- Hayakawa M et al. (2007) Synthesis and biological evaluation of pyrido[3′,2′:4,5]furo[3,2-d]pyrimidine derivatives as novel PI3 kinase p110α inhibitors. Bioorg Med Chem Lett 17: 2438–2442 [DOI] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Foti M, Carpentier JL, Shisheva A (2003a) PIKfyve controls fluid phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell 14: 4581–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Mlak K, Deeb R, Fligger J, Soans A, Finley RL Jr, Shisheva A (2003b) Active PIKfyve associates with and promotes the membrane attachment of the late endosome-to-trans-Golgi network transport factor Rab9 effector p40. J Biol Chem 278: 50863–50871 [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmo K, Stenmark H (2006) Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci 119: 605–614 [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J, Zang T, Bieniasz PD, Martin-Serrano J, Zang T, Bieniasz PD (2003) Role of ESCRT-I in retroviral budding. J Virol 77: 4794–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen RK, Dove SK, Cooke FT, Painter GF, Holmes AB, Shisheva A, Ohya Y, Parker PJ, Michell RH (1999) Complementation analysis in PtdInsP kinase-deficient yeast mutants demonstrates that Schizosaccharomyces pombe and murine Fab1p homologues are phosphatidylinositol 3-phosphate 5-kinases. J Biol Chem 274: 33905–33912 [DOI] [PubMed] [Google Scholar]
- Michell RH et al. (2006) Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem Sci 31: 52–63 [DOI] [PubMed] [Google Scholar]
- Murray JL et al. (2005) Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus. J Virol 79: 11742–11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G (1995) Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol 131: 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot AS, Fares H, Payrastre B, Chisholm AD, Labouesse M, Laporte J (2006) The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell 17: 3062–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD (1998) Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95: 847–858 [DOI] [PubMed] [Google Scholar]
- Parker PJ (2004) The ubiquitous phosphoinositides. Biochem Soc Trans 32: 893–898 [DOI] [PubMed] [Google Scholar]
- Pornillos O, Higginson DS, Stray KM, Fisher RD, Garrus JE, Payne M, He GP, Wang HE, Morham SG, Sundquist WI (2003) HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein. J Cell Biol 162: 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten TE, Rodahl LM, Pattni K, Englund C, Samakovlis C, Dove S, Brech A, Stenmark H (2006) Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol Biol Cell 17: 3989–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford AC, Pattni K, Bujny MV, Carlton JG, Stenmark H, Cullen PJ (2006) The mammalian phosphatidylinositol 3 monophosphate 5-kinase PIKfyve regulates retrograde transport for early endosomes. J Cell Sci 119: 3944–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein SK, Walter DM, Grosvenor AR, Heuser JE, Waters MG (1995) p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc Natl Acad Sci USA 92: 522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Ingmundson A, Horner SM, Cicchetti G, Allen PG, Pypaert M, Cunningham JM, Mothes W (2003) Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic 4: 785–801 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD (2001) Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602 [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269: 5241–5248 [PubMed] [Google Scholar]
- Wada I, Rindress D, Cameron PH, Ou WJ, Doherty JJ, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJ (1991) SSR α and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem 266: 19599–19610 [PubMed] [Google Scholar]
- Workman P, Raynaud F, Clarke P, te Poele R, Eccles S, Kelland L, Di Stefano F, Ahmadi K, Parker P, Waterfield MD (2004) Pharmacological properties and in vitro and in vivo antitumour activity of the potent and selective PI3 kinase inhibitor PI103. Eur J Cancer 40: 124 (414A) [Google Scholar]
- Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D (1995) Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell 6: 525–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Movie 1
Supplementary Movie 2