Abstract
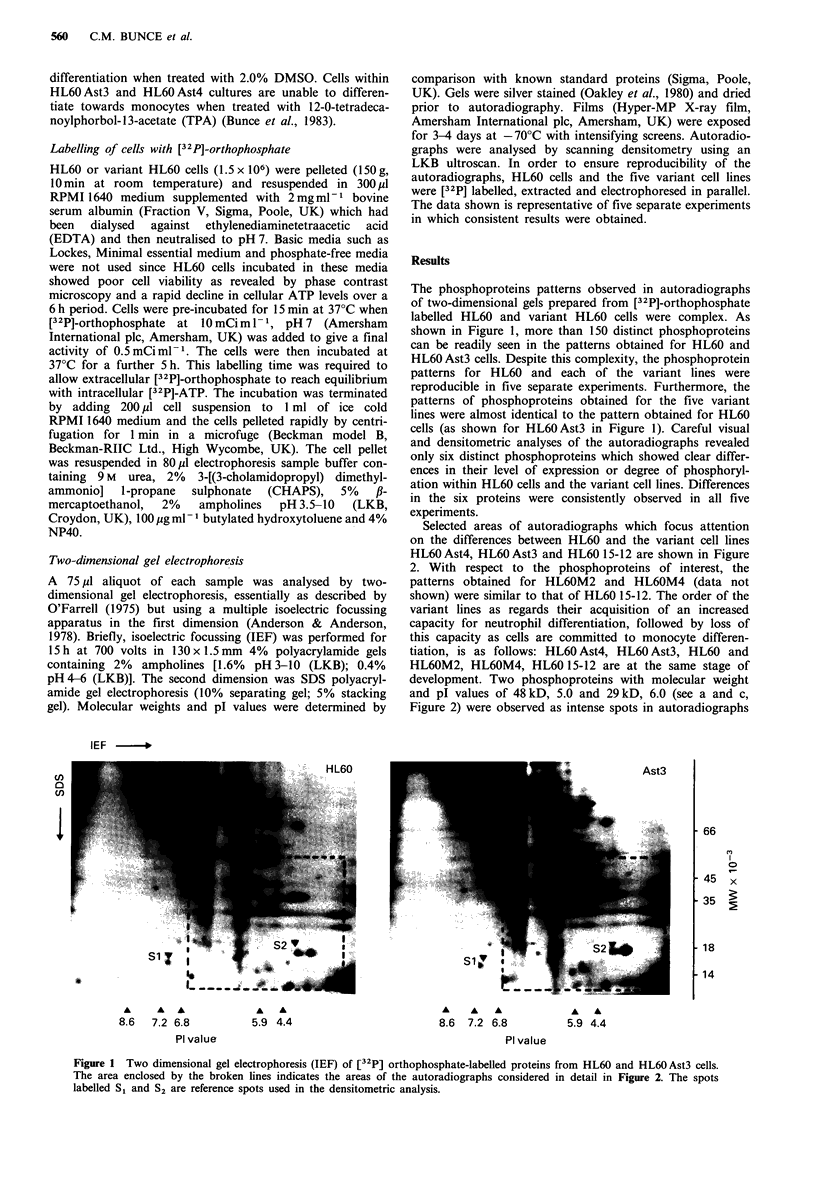
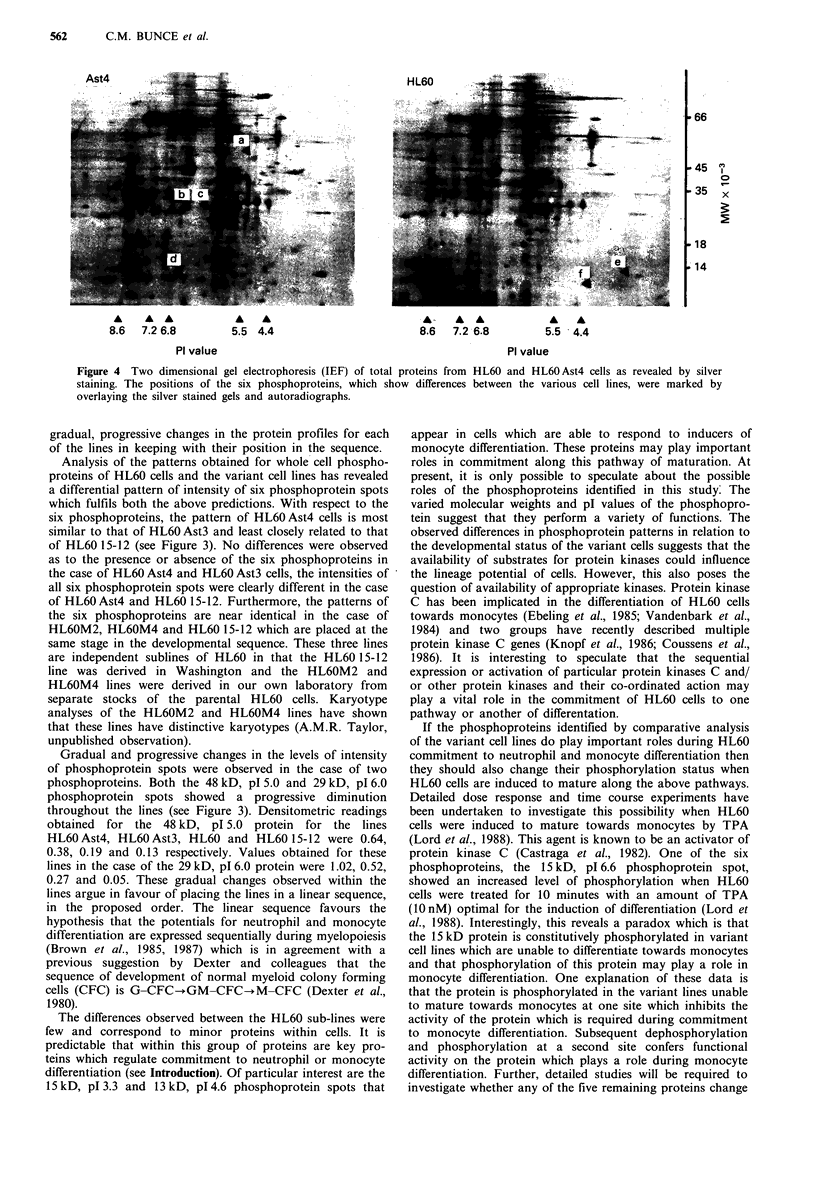
The human promyeloid cell line H60 can be induced to differentiate towards either neutrophils or monocytes. Variant cell lines, derived from HL60, which show reduced capacities for neutrophil and monocyte differentiation can be arranged in a developmental sequence which suggests that the potentials for neutrophil and monocyte differentiation are expressed sequentially by HL60 cells in this order. Analysis of the patterns of total cellular phosphoproteins within HL60 and 5 variant cell lines, by two-dimensional gel electrophoresis, has identified 6 distinct phosphoproteins which show progressive differences in the intensity of spots between the variant lines. The changes in these phosphoproteins relate to the position of the lines within the proposed development sequence. Similarly, lines placed close together in the sequence are more similar, as regards phosphoprotein profiles, than lines placed far apart. These studies provide direct evidence in favour of the hypothesis that the potentials for neutrophil and monocyte differentiation are expressed sequentially during myelopoiesis. Furthermore, two phosphoprotein spots were found to be restricted to lines able to differentiate towards monocytes. These proteins may play important roles during commitment to monocyte differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Brown G., Bunce C. M., Guy G. R. Sequential determination of lineage potentials during haemopoiesis. Br J Cancer. 1985 Nov;52(5):681–686. doi: 10.1038/bjc.1985.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G., Bunce C. M., Howie A. J., Lord J. M. Stochastic or ordered lineage commitment during hemopoiesis? Leukemia. 1987 Feb;1(2):150–153. [PubMed] [Google Scholar]
- Bunce C. M., Fisher A. G., Toksoz D., Brown G. Isolation and characterisation of dimethylsulphoxide resistant variants from the human promyeloid cell line HL60. Exp Hematol. 1983 Oct;11(9):828–833. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Chan L. C., Furley A. J., Watt S. M., Molgaard H. V. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986 Jan;67(1):1–11. [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Toksoz D., Bunce C. M., Stone P. C., Michell R. H., Brown G. Variant cell lines from the human promyelocyte line HL60. Leuk Res. 1982;6(4):491–498. doi: 10.1016/0145-2126(82)90006-6. [DOI] [PubMed] [Google Scholar]
- Vandenbark G. R., Kuhn L. J., Niedel J. E. Possible mechanism of phorbol diester-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1984 Feb;73(2):448–457. doi: 10.1172/JCI111231. [DOI] [PMC free article] [PubMed] [Google Scholar]





