Abstract
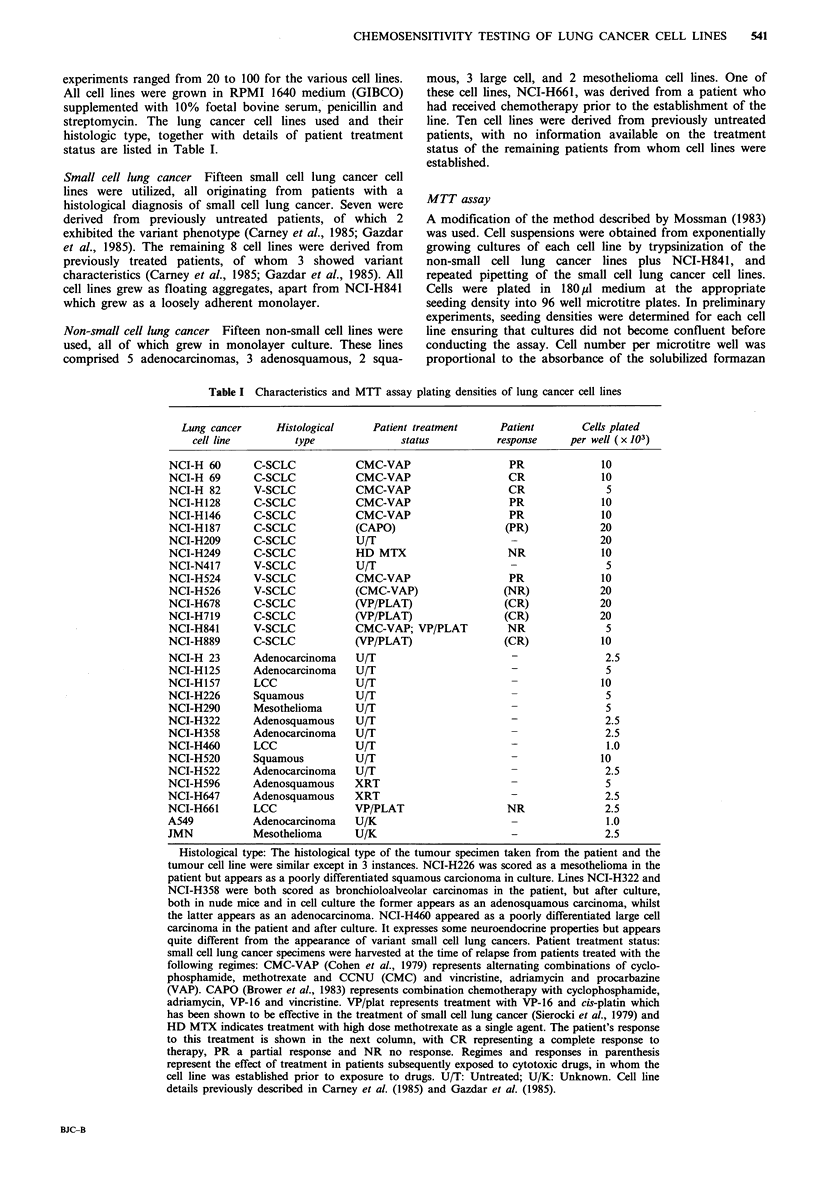
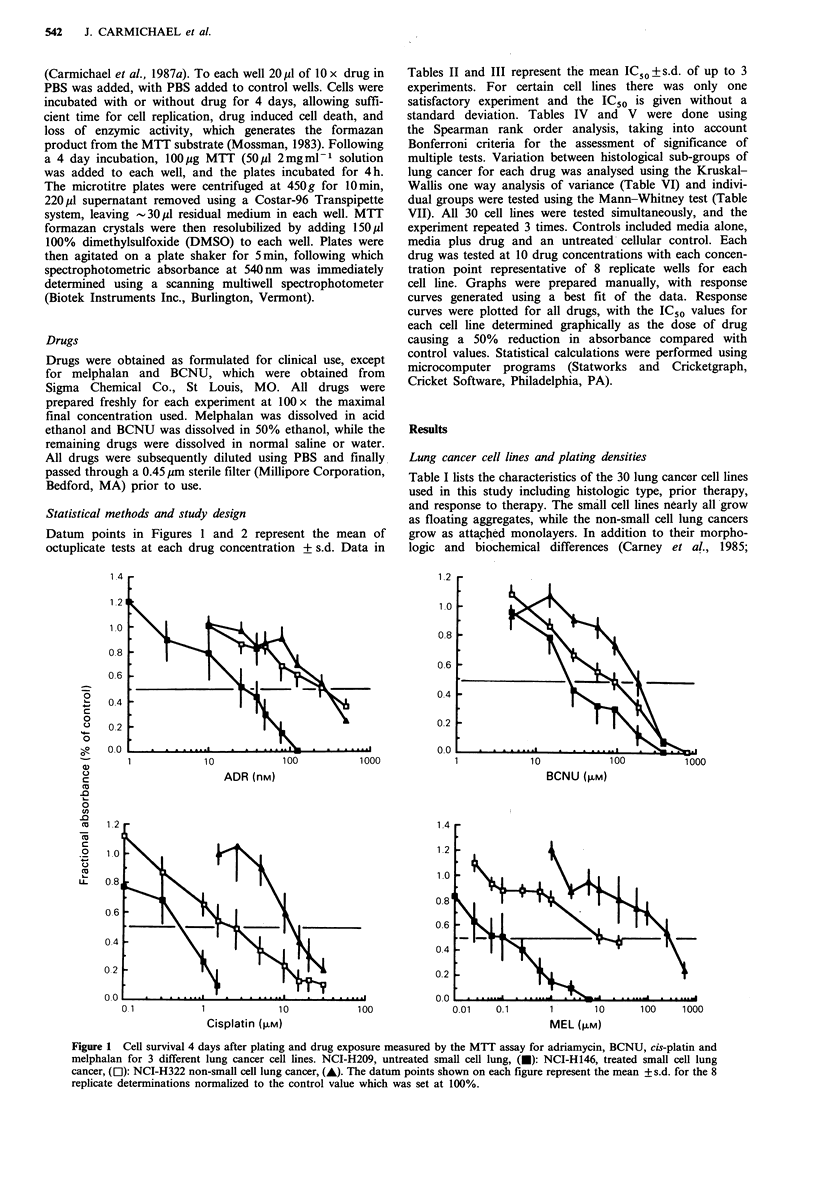
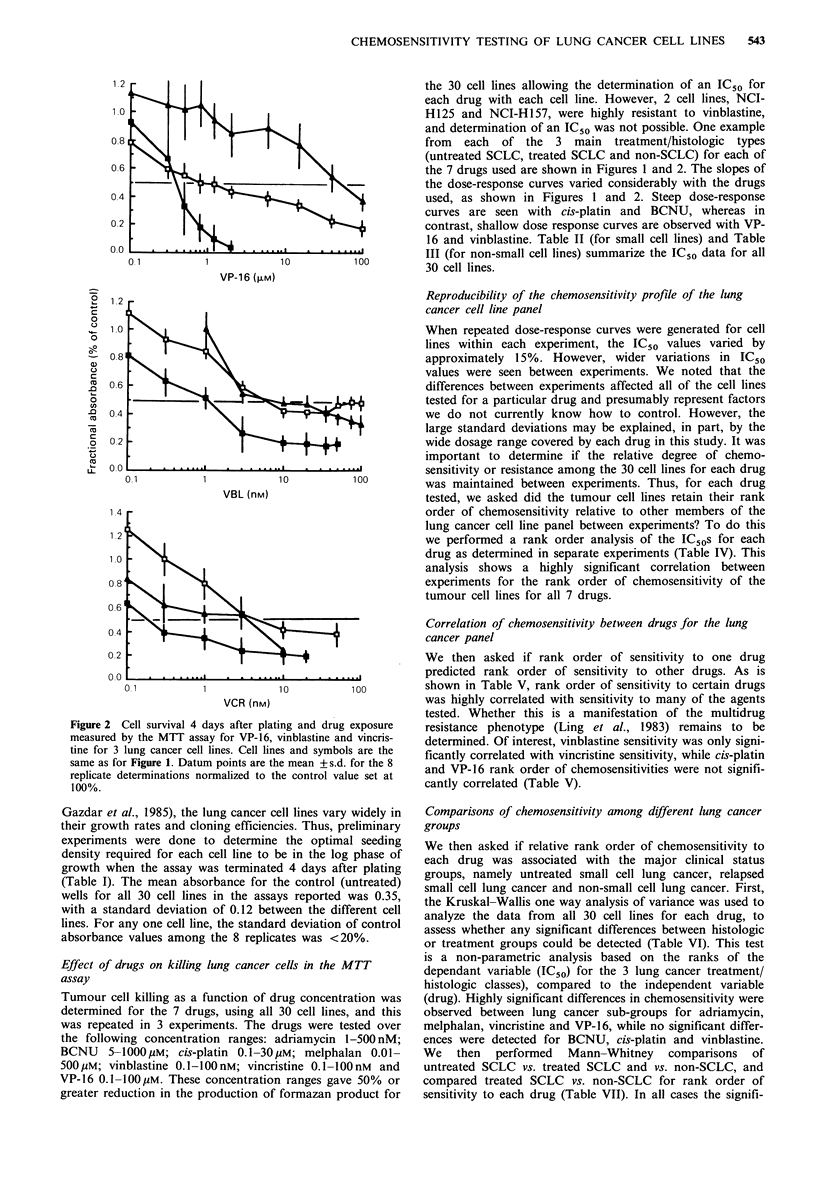
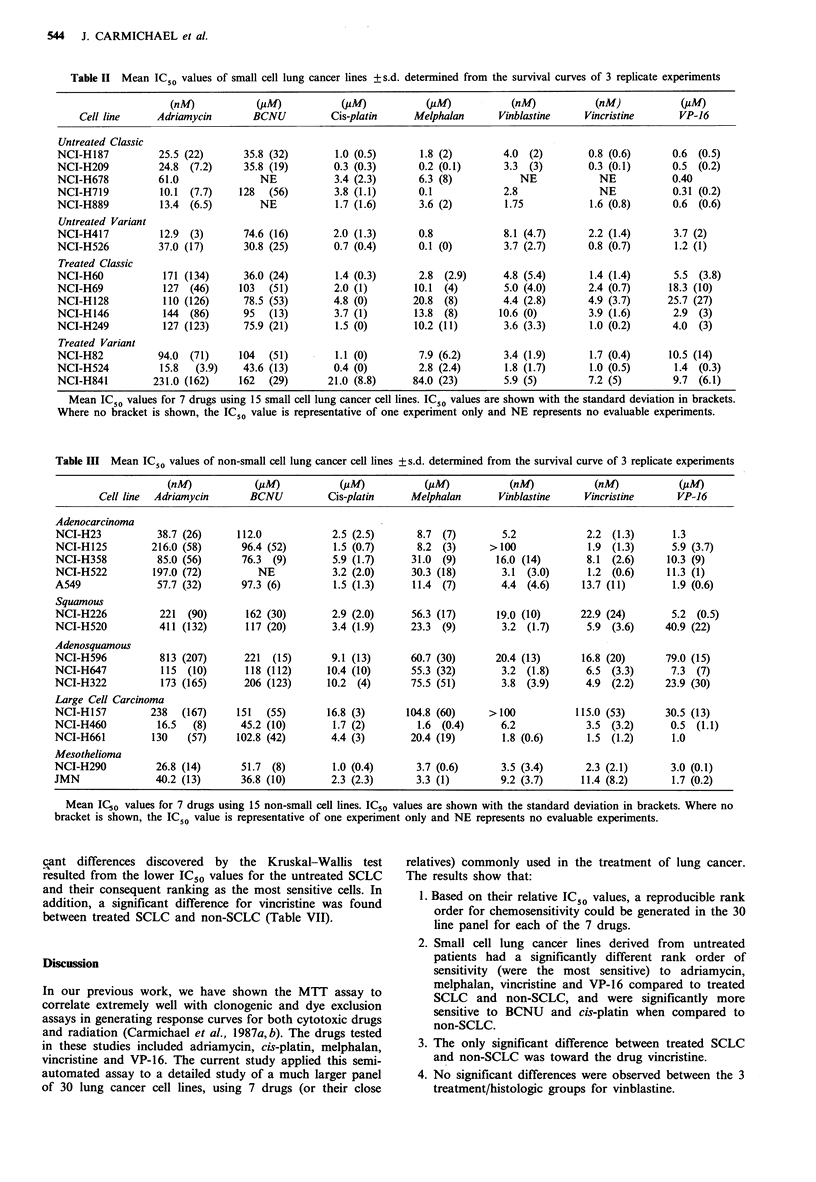
Thirty human lung cancer cell lines were tested for chemosensitivity using the semi-automated, non-clonogenic MTT assay. The tumour cell lines came from three major categories of patients: untreated small cell lung cancer (SCLC); SCLC relapsing on chemotherapy; and non-SCLC predominantly from untreated patients. From these data IC50 values were derived for each drug in each cell line. While some inter-experimental variability was observed, the rank order of chemosensitivity of each cell line within this panel was significantly correlated between experiments. These results show that tumour cell lines derived from untreated small cell lung cancer patients were the most chemosensitive for adriamycin, melphalan, vincristine and VP16 compared to the other cell types. In addition, untreated SCLC was more sensitive than non-SCLC to BCNU and cis-platin, while vincristine was the only drug to which treated SCLC was more sensitive compared to the non-SCLC lines. In contrast, no significant differences between the lung cancer types were observed for vinblastine. Thus, this panel of lung cancer cells exhibited a drug sensitivity profile paralleling that observed in clinical practice. These results suggest that this lung cancer cell line panel in combination with a relatively simple but reproducible chemosensitivity assay, such as the MTT assay, has potential for the testing of drug combinations and evaluating new anti-cancer agents in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK M. M., SPEER F. D. Further observations on the effects of cancer chemotherapeutic agents on the in vitro dehydrogenase activity of cancer tissue. J Natl Cancer Inst. 1954 Apr;14(5):1147–1158. [PubMed] [Google Scholar]
- Bertoncello I., Bradley T. R., Campbell J. J., Day A. J., McDonald I. A., McLeish G. R., Quinn M. A., Rome R., Hodgson G. S. Limitations of the clonal agar assay for the assessment of primary human ovarian tumour biopsies. Br J Cancer. 1982 Jun;45(6):803–811. doi: 10.1038/bjc.1982.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M. C., Bosanquet A. G., Gilby E. D. In vitro determination of tumour chemosensitivity in haematological malignancies. Hematol Oncol. 1985 Jan-Mar;3(1):1–10. doi: 10.1002/hon.2900030102. [DOI] [PubMed] [Google Scholar]
- Brower M., Ihde D. C., Johnston-Early A., Bunn P. A., Jr, Cohen M. H., Carney D. N., Makuch R. W., Matthews M. J., Radice P. A., Minna J. D. Treatment of extensive stage small cell bronchogenic carcinoma. Effects of variation in intensity of induction chemotherapy. Am J Med. 1983 Dec;75(6):993–1000. doi: 10.1016/0002-9343(83)90880-x. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987 Feb 15;47(4):943–946. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Cohen M. H., Ihde D. C., Bunn P. A., Jr, Fossieck B. E., Jr, Matthews M. J., Shackney S. E., Johnston-Early A., Makuch R., Minna J. D. Cyclic alternating combination chemotherapy for small cell bronchogenic carcinoma. Cancer Treat Rep. 1979 Feb;63(2):163–170. [PubMed] [Google Scholar]
- Cole S. P. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother Pharmacol. 1986;17(3):259–263. doi: 10.1007/BF00256695. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Nau M. M., Minna J. D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985 Jun;45(6):2924–2930. [PubMed] [Google Scholar]
- Gralla R. J., Casper E. S., Kelsen D. P., Braun D. W., Jr, Dukeman M. E., Martini N., Young C. W., Golbey R. B. Cisplatin and vindesine combination chemotherapy for advanced carcinoma of the lung: A randomized trial investigating two dosage schedules. Ann Intern Med. 1981 Oct;95(4):414–420. doi: 10.7326/0003-4819-95-4-414. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Johnson B. E., Ihde D. C., Bunn P. A., Becker B., Walsh T., Weinstein Z. R., Matthews M. J., Whang-Peng J., Makuch R. W., Johnston-Early A. Patients with small-cell lung cancer treated with combination chemotherapy with or without irradiation. Data on potential cures, chronic toxicities, and late relapses after a five- to eleven-year follow-up. Ann Intern Med. 1985 Sep;103(3):430–438. doi: 10.7326/0003-4819-103-3-430. [DOI] [PubMed] [Google Scholar]
- Klastersky J., Longeval E., Nicaise C., Weerts D. Etoposide and cis-platinum in non-small-cell bronchogenic carcinoma. Cancer Treat Rev. 1982 Jun;9 (Suppl):133–138. doi: 10.1016/s0305-7372(82)80092-3. [DOI] [PubMed] [Google Scholar]
- Kondo T., Ohkubo K. In vitro test for prediction of side effects of carcinostatic agents. Gan. 1967 Aug;58(4):349–354. [PubMed] [Google Scholar]
- Ling V., Kartner N., Sudo T., Siminovitch L., Riordan J. R. Multidrug-resistance phenotype in Chinese hamster ovary cells. Cancer Treat Rep. 1983 Oct;67(10):869–874. [PubMed] [Google Scholar]
- Livingston R. B. Combination chemotherapy of bronchogenic carcinoma. I. Non-oat cell. Cancer Treat Rev. 1977 Sep;4(3):153–165. doi: 10.1016/s0305-7372(77)80022-4. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Alberts D. S., Durie B. G., Meyskens F. L., Jones S. E., Soehnlen B., Chen H. S., Moon T. Clinical correlations of drug sensitivity in the human tumor stem cell assay. Recent Results Cancer Res. 1980;74:300–305. doi: 10.1007/978-3-642-81488-4_36. [DOI] [PubMed] [Google Scholar]
- Selby P., Buick R. N., Tannock I. A critical appraisal of the "human tumor stem-cell assay". N Engl J Med. 1983 Jan 20;308(3):129–134. doi: 10.1056/NEJM198301203080304. [DOI] [PubMed] [Google Scholar]
- Sierocki J. S., Hilaris B. S., Hopfan S., Martini N., Barton D., Golbey R. B., Wittes R. E. cis-Dichlorodiammineplatinum(II) and VP-16-213: an active induction regimen for small cell carcinoma of the lung. Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1593–1597. [PubMed] [Google Scholar]
- Von Hoff D. D., Casper J., Bradley E., Sandbach J., Jones D., Makuch R. Association between human tumor colony-forming assay results and response of an individual patient's tumor to chemotherapy. Am J Med. 1981 May;70(5):1027–1041. doi: 10.1016/0002-9343(81)90859-7. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Forseth B., Warfel L. E. Use of a radiometric system to screen for antineoplastic agents: correlation with a human tumor cloning system. Cancer Res. 1985 Sep;45(9):4032–4038. [PubMed] [Google Scholar]
- Weisenthal L. M., Marsden J. A., Dill P. L., Macaluso C. K. A novel dye exclusion method for testing in vitro chemosensitivity of human tumors. Cancer Res. 1983 Feb;43(2):749–757. [PubMed] [Google Scholar]


