Abstract
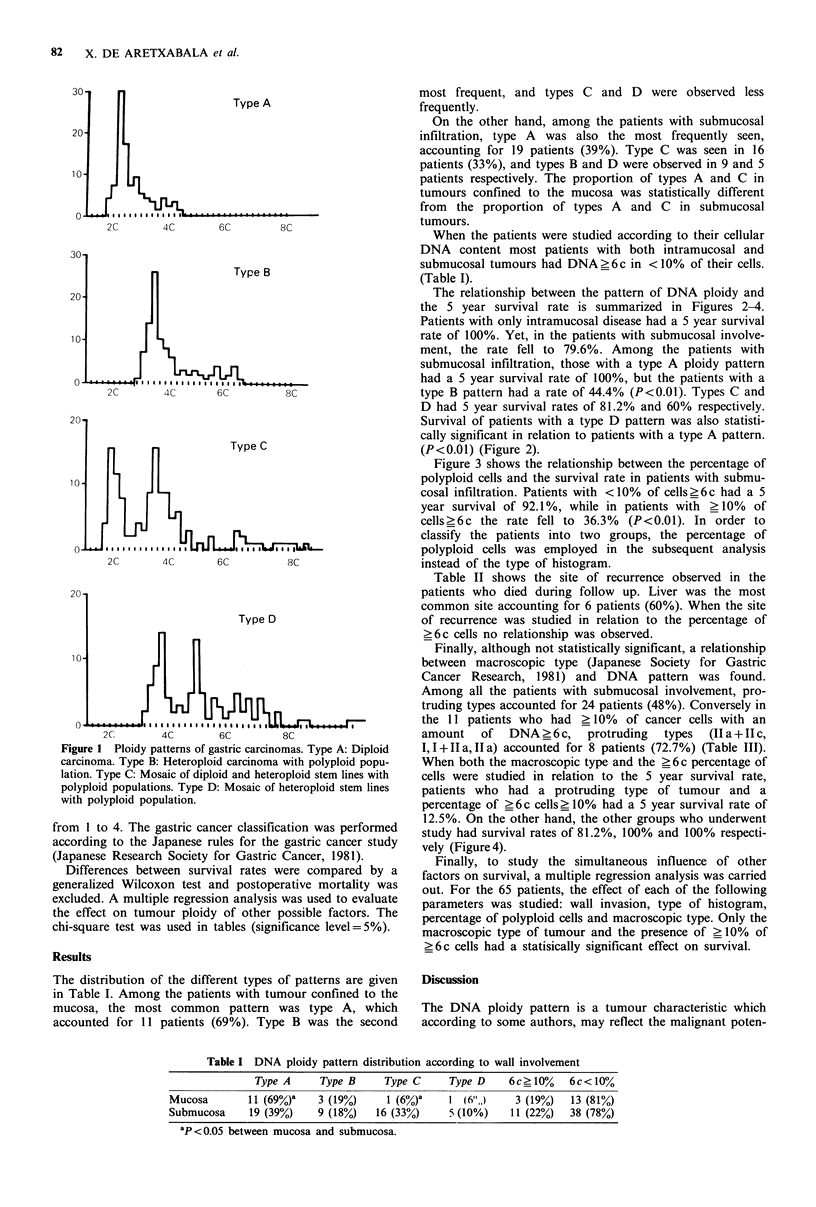
The relationship between DNA ploidy and clinical prognosis was determined in 65 patients who underwent gastroectomy for early gastric cancer. Of the 65 patients, 16 had intramucosal and 49 submucosal tumours. Five-year survival rates were 100 and 79.6% for patients with intramucosal and submucosal tumours respectively. Diploid tumours were observed more frequently among the patients with intramucosal neoplasms. Among the patients with submucosal invasion, the presence of polyploid cells (greater than or equal to 6c) in less than 10% of the malignant population was associated with a superior survival at 5 years, than those with greater than or equal to 10% of polyploid cells (92.1% vs. 36.3%). When the macroscopic type and the ploidy status were evaluated together, patients who had greater than or equal to 10% of cells with DNA greater than or equal to 6 c and a protruding type of tumour, had a 5 year survival rate of only 12.5%. Finally when factors such as the level of wall invasion, percentage of polyploid cells, type of histogram, and macroscopic type were evaluated by multiple regression analysis, macroscopic type and percentage of polyploid cells were the only significant prognostic factors. On the basis of these findings, the DNA ploidy pattern and the macroscopic type may be useful markers of patients who will develop recurrence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin N. B., Kay R. Prognostic significance of modal DNA value and other factors in malignant tumours, based on 1465 cases. Br J Cancer. 1979 Aug;40(2):210–221. doi: 10.1038/bjc.1979.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniak B., Herz F., Koss L. G. DNA distribution patterns in early gastric carcinomas. A Feulgen cytometric study of gastric brush smears. Cancer. 1987 Jan 1;59(1):113–117. doi: 10.1002/1097-0142(19870101)59:1<113::aid-cncr2820590124>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Frankfurt O. S., Chin J. L., Englander L. S., Greco W. R., Pontes J. E., Rustum Y. M. Relationship between DNA ploidy, glandular differentiation, and tumor spread in human prostate cancer. Cancer Res. 1985 Mar;45(3):1418–1423. [PubMed] [Google Scholar]
- Haraguchi M., Okamura T., Korenaga D., Tsujitani S., Marin P., Sugimachi K. Heterogeneity of DNA ploidy in patients with undifferentiated carcinomas of the stomach. Cancer. 1987 Mar 1;59(5):922–924. doi: 10.1002/1097-0142(19870301)59:5<922::aid-cncr2820590511>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Hattori T., Hosokawa Y., Fukuda M., Sugihara H., Hamada S., Takamatsu T., Nakanishi K., Tsuchihashi Y., Kitamura T., Fujita S. Analysis of DNA ploidy patterns of gastric carcinomas of Japanese. Cancer. 1984 Oct 15;54(8):1591–1597. doi: 10.1002/1097-0142(19841015)54:8<1591::aid-cncr2820540821>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Rugg C. A., Gelber R. D. Association of DNA index and S-phase fraction with prognosis of nodes positive early breast cancer. Cancer Res. 1987 Sep 1;47(17):4729–4735. [PubMed] [Google Scholar]
- Hedley D. W., Rugg C. A., Ng A. B., Taylor I. W. Influence of cellular DNA content on disease-free survival of Stage II breast cancer patients. Cancer Res. 1984 Nov;44(11):5395–5398. [PubMed] [Google Scholar]
- Inokuchi K., Kodama Y., Sasaki O., Kamegawa T., Okamura T. Differentiation of growth patterns of early gastric carcinoma determined by cytophotometric DNA analysis. Cancer. 1983 Mar 15;51(6):1138–1141. doi: 10.1002/1097-0142(19830315)51:6<1138::aid-cncr2820510627>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kennedy B. J. Staging of gastric cancer. Semin Oncol. 1985 Mar;12(1):19–20. [PubMed] [Google Scholar]
- Kodama Y., Inokuchi K., Soejima K., Matsusaka T., Okamura T. Growth patterns and prognosis in early gastric carcinoma. Superficially spreading and penetrating growth types. Cancer. 1983 Jan 15;51(2):320–326. doi: 10.1002/1097-0142(19830115)51:2<320::aid-cncr2820510226>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kodama Y., Sugimachi K., Soejima K., Matsusaka T., Inokuchi K. Evaluation of extensive lymph node dissection for carcinoma of the stomach. World J Surg. 1981 Mar;5(2):241–248. doi: 10.1007/BF01658301. [DOI] [PubMed] [Google Scholar]
- Koga S., Kaibara N., Nishidoi H., Kimura O., Tamura H., Takebayashi M., Yurugi E., Ikeguchi M., Okamoto T. Lymph node removal for advanced gastric cancer with special reference to peritoneal metastasis. Zentralbl Chir. 1983;108(21):1377–1381. [PubMed] [Google Scholar]
- Kokal W. A., Duda R. B., Azumi N., Sheibani K., Kemeny M. M., Terz J. J., Harada J. R. Tumor DNA content in primary and metastatic colorectal carcinoma. Arch Surg. 1986 Dec;121(12):1434–1439. doi: 10.1001/archsurg.1986.01400120084014. [DOI] [PubMed] [Google Scholar]
- Korenaga D., Okamura T., Sugimachi K., Inokuchi K. Prognostic study of intramucosal carcinoma of the stomach with DNA aneuploidy. Jpn J Surg. 1985 Nov;15(6):443–448. doi: 10.1007/BF02470089. [DOI] [PubMed] [Google Scholar]
- Matsusaka T., Kodama Y., Soejima K., Miyazaki M., Yoshimura K., Sugimachi K., Inokuchi K. Recurrence in early gastric cancer: a pathologic evaluation. Cancer. 1980 Jul 1;46(1):168–172. doi: 10.1002/1097-0142(19800701)46:1<168::aid-cncr2820460128>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]


